Abstract
A recent theory of the control of human fetal hemoglobin synthesis, based on studies in cultured adult marrow, proposes that the phenotypic expression of fetal hemoglobin is largely dependent on the level of differentiation of the parental stem cells; that is, the earlier the progenitor, the greater the ability of its progeny to express fetal hemoglobin [Papayannopoulou, Th., Brice, M. & Stamatoyannopoulos, G. (1977) Proc. Natl. Acad. Sci. USA 74, 2923-2927]. To test this relationship with fetal tissue, we have studied hemoglobin synthesis in cultured human fetal liver, comparing γ chain synthesis in the descendants of the early progenitors (“bursts”) with that in the descendants of the later progenitors (“colonies”). Cells from the livers of midtrimester fetuses were cultured in methylcellulose with erythropoietin. The β/(β + γ) globin synthetic ratio on days 5 to 7, when colonies predominated, was 0.09-0.11, a value characteristic of fetal reticulocytes, and on days 11 and 12, when bursts predominated, was 0.15-0.17. Thus, in fetal liver, the descendants of the earlier progenitor, the burst-forming unit, may be making more β chains rather than more γ chains, compared to descendants of the later progenitor, the colonyforming unit. Our data on fetal liver, taken together with the data on adult marrow by others, suggest that the erythroid colonies express the gene characteristic of the age of the organism to a greater degree than bursts, which express β and γ genes less specifically. Thus, the capacity for highly selective gene expression characteristic of differentiated cells appears to be less well developed in the burst-forming unit than in the colony-forming unit.
Keywords: differentiation, erythropoietin, cell culture, abortus, gene expression
Full text
PDF
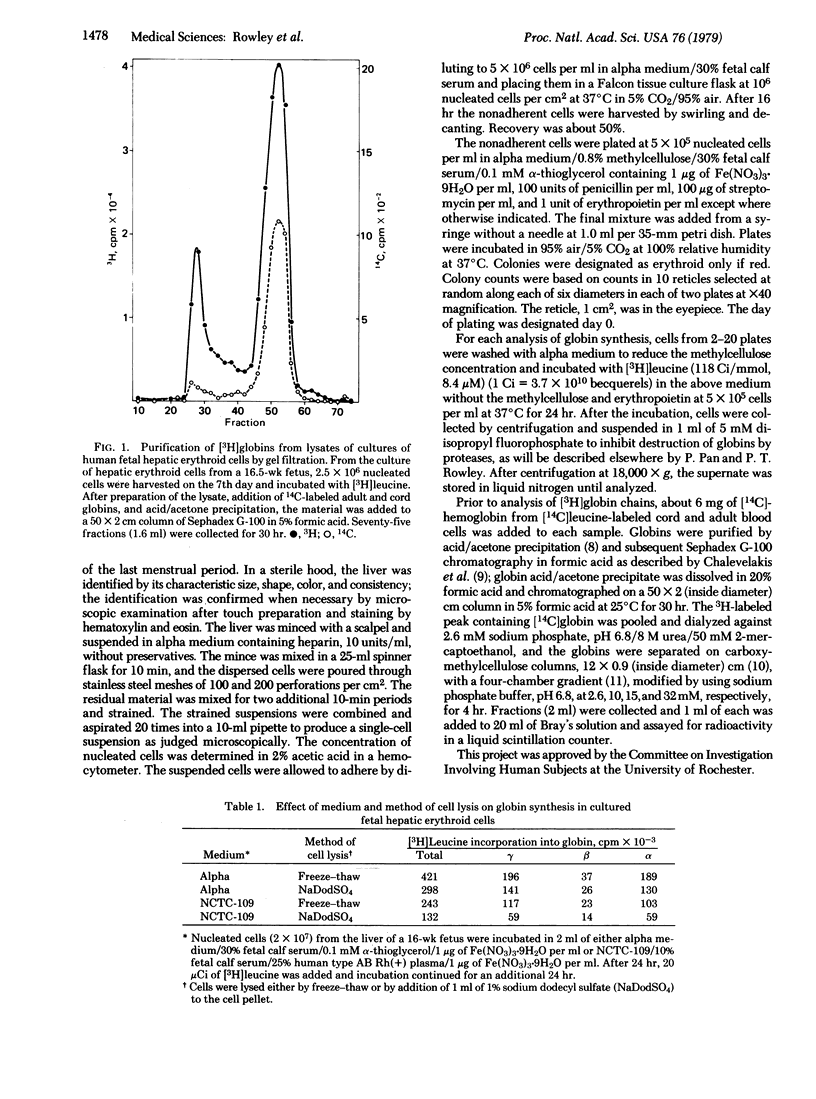
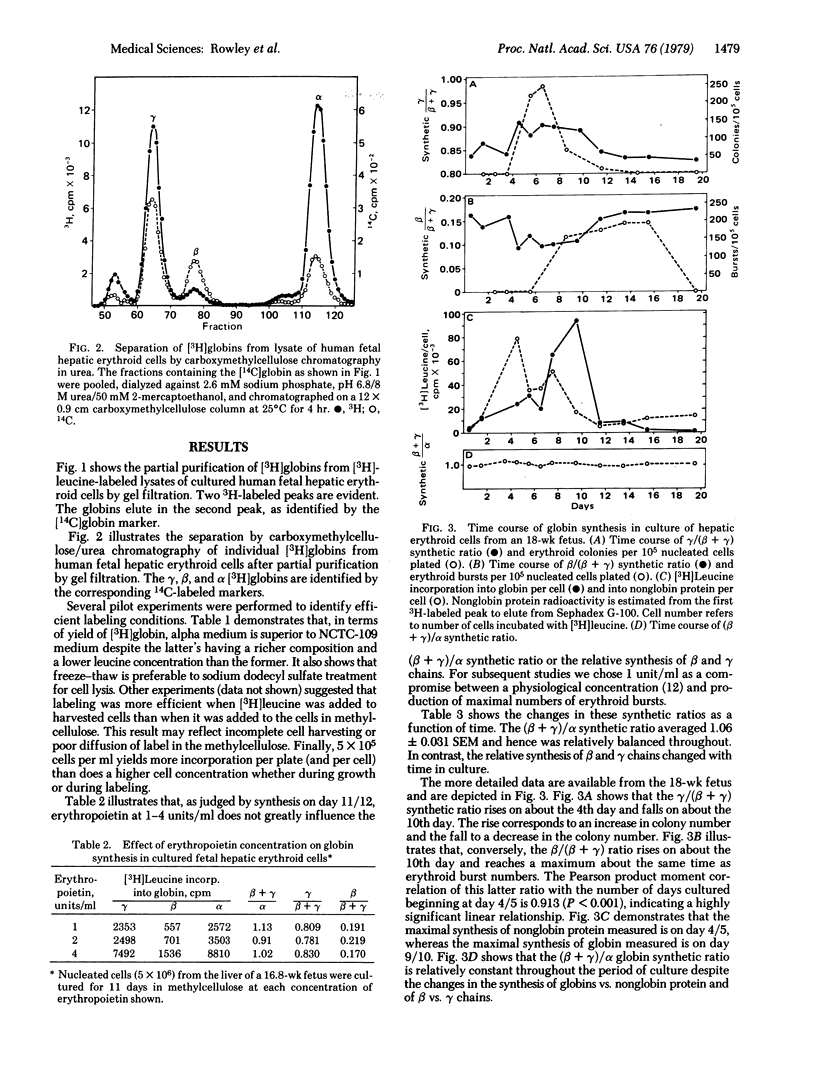
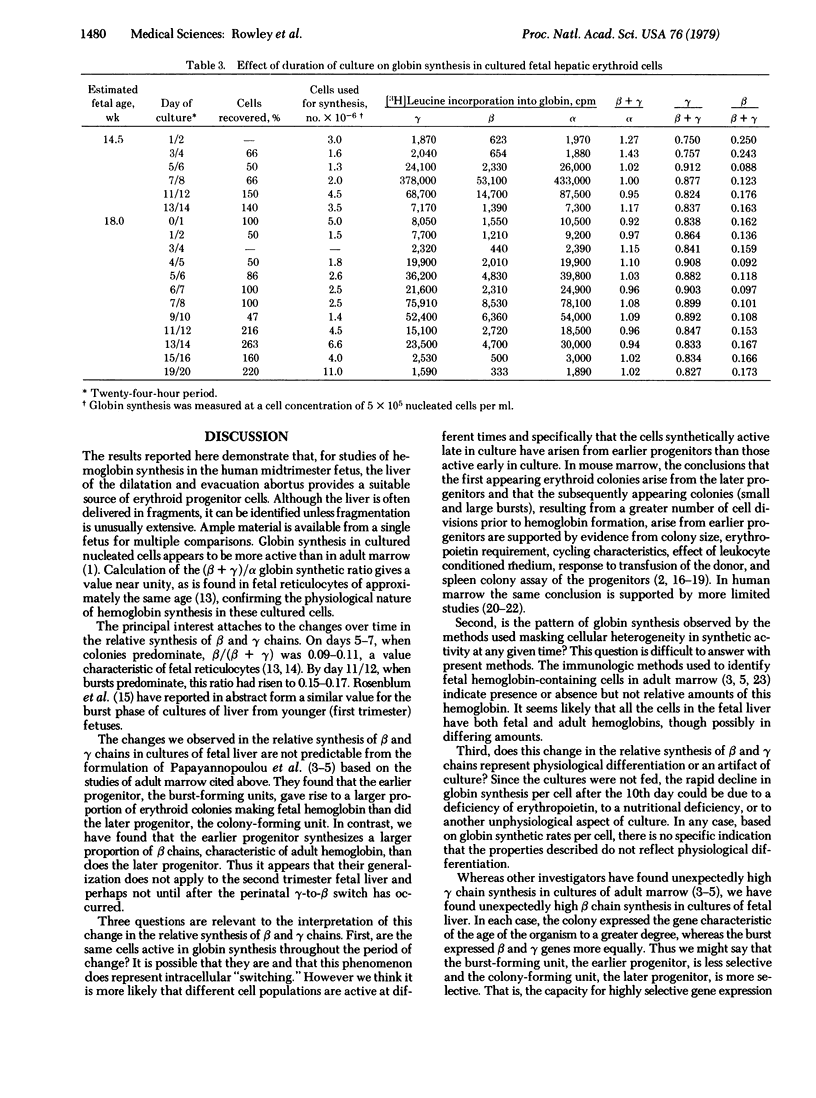

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Modell C. B., Fairweather D., Hobbins J. C., Mahoney M. J., Frigoletto F. D., Sherman A. S., Nathan D. G. Prenatal diagnosis of hemoglobinopathies. A review of 15 cases. N Engl J Med. 1976 Dec 23;295(26):1437–1443. doi: 10.1056/NEJM197612232952601. [DOI] [PubMed] [Google Scholar]
- Chalevelakis G., Clegg J. B., Weatherall D. J. Globin synthesis in normal human bone marrow. Br J Haematol. 1976 Dec;34(4):535–557. doi: 10.1111/j.1365-2141.1976.tb03600.x. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Graber S. E., Krantz S. B. Erythropoietin and the control of red cell production. Annu Rev Med. 1978;29:51–66. doi: 10.1146/annurev.me.29.020178.000411. [DOI] [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Human marrow cells capable of erythropoietic differentiation in vitro: definition of three erythroid colony responses. Blood. 1977 Jun;49(6):855–864. [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978 Mar;51(3):527–537. [PubMed] [Google Scholar]
- Gregory C. J. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. J Cell Physiol. 1976 Oct;89(2):289–301. doi: 10.1002/jcp.1040890212. [DOI] [PubMed] [Google Scholar]
- Gregory C. J., McCulloch E. A., Till J. E. Erythropoietic progenitors capable of colony formation in culture: state of differentiation. J Cell Physiol. 1973 Jun;81(3):411–420. doi: 10.1002/jcp.1040810313. [DOI] [PubMed] [Google Scholar]
- Iffy L., Jakobovits A., Westlake W., Wingate M., Caterini H., Kanofsky P., Menduke H. Early intrauterine development: I. The rate of growth of Caucasian embryos and fetuses between the 6th and 20th weeks of gestation. Pediatrics. 1975 Aug;56(2):173–186. [PubMed] [Google Scholar]
- Kan Y. W., Schwartz E., Nathan D. G. Globin chain synthesis in the alpha thalassemia syndromes. J Clin Invest. 1969 Nov;47(11):2512–2522. doi: 10.1172/JCI105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Trecartin R. F., Golbus M. S., Filly R. A. Prenatal diagnosis of beta-thalassaemia and sickle-cell anaemia. Experience with 24 cases. Lancet. 1977 Feb 5;1(8006):269–271. doi: 10.1016/s0140-6736(77)91821-9. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Parmley R. T., Bank H. L., Spicer S. S. Human marrow erythropoiesis in culture. I. Characterization of methylcellulose colony assay. Blood. 1976 Sep;48(3):407–417. [PubMed] [Google Scholar]
- Papayannopoulou T. H., Brice M., Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2033–2037. doi: 10.1073/pnas.73.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Nute P. E., Kurachi S., Stamatoyannopoulos G. Consistent activation of fetal hemoglobin synthesis in cultured adult bone marrow cells. Blood. 1978 Apr;51(4):671–679. [PubMed] [Google Scholar]
- Rowley P. T., Ohlsson-Wilhelm B. M., Farley B. A. Erythroid colony formation from human fetal liver. Proc Natl Acad Sci U S A. 1978 Feb;75(2):984–988. doi: 10.1073/pnas.75.2.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman A. D., Curtis J. E., McCulloch E. A. Erythropietic colonies in cultures of human marrow. Blood. 1974 Nov;44(5):659–669. [PubMed] [Google Scholar]
- Wood W. G., Clegg J. B., Weatherall D. J. Developmental biology of human hemoglobins. Prog Hematol. 1977;10:43–90. [PubMed] [Google Scholar]
- Wood W. G., Stamatoyannopoulos G., Lim G., Nute P. E. F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F. Blood. 1975 Nov;46(5):671–682. [PubMed] [Google Scholar]


