Abstract
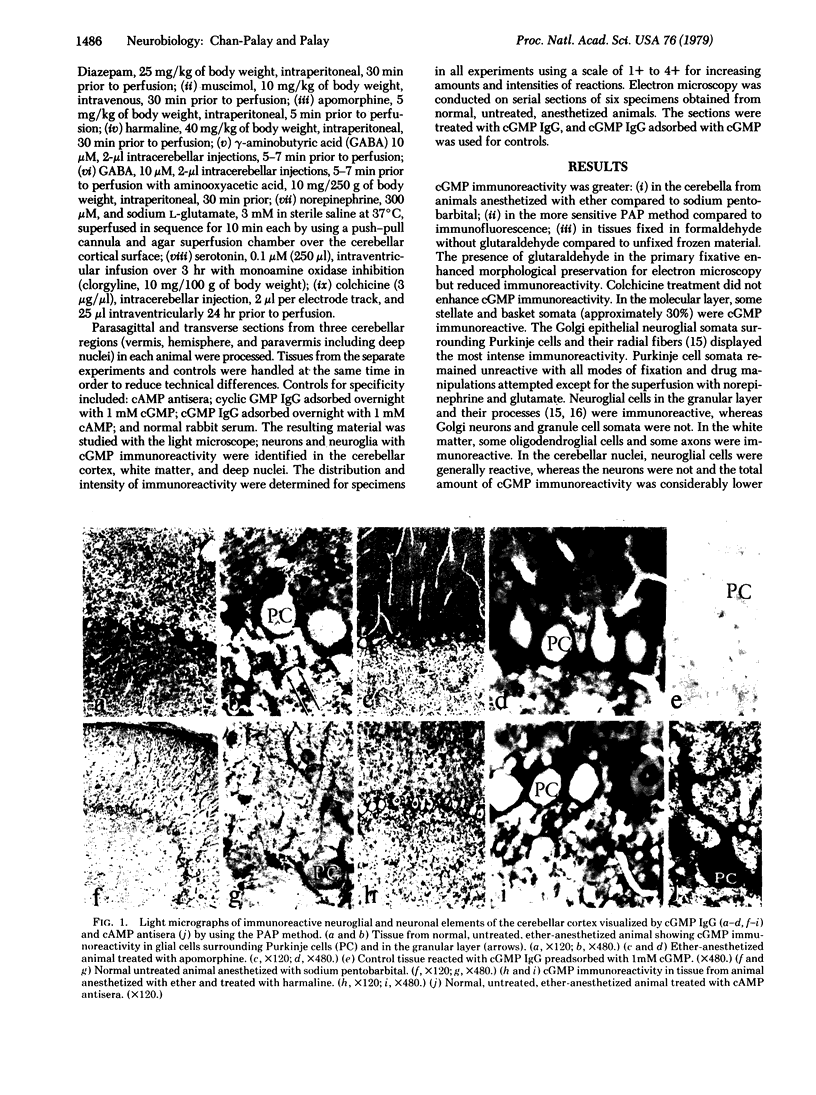
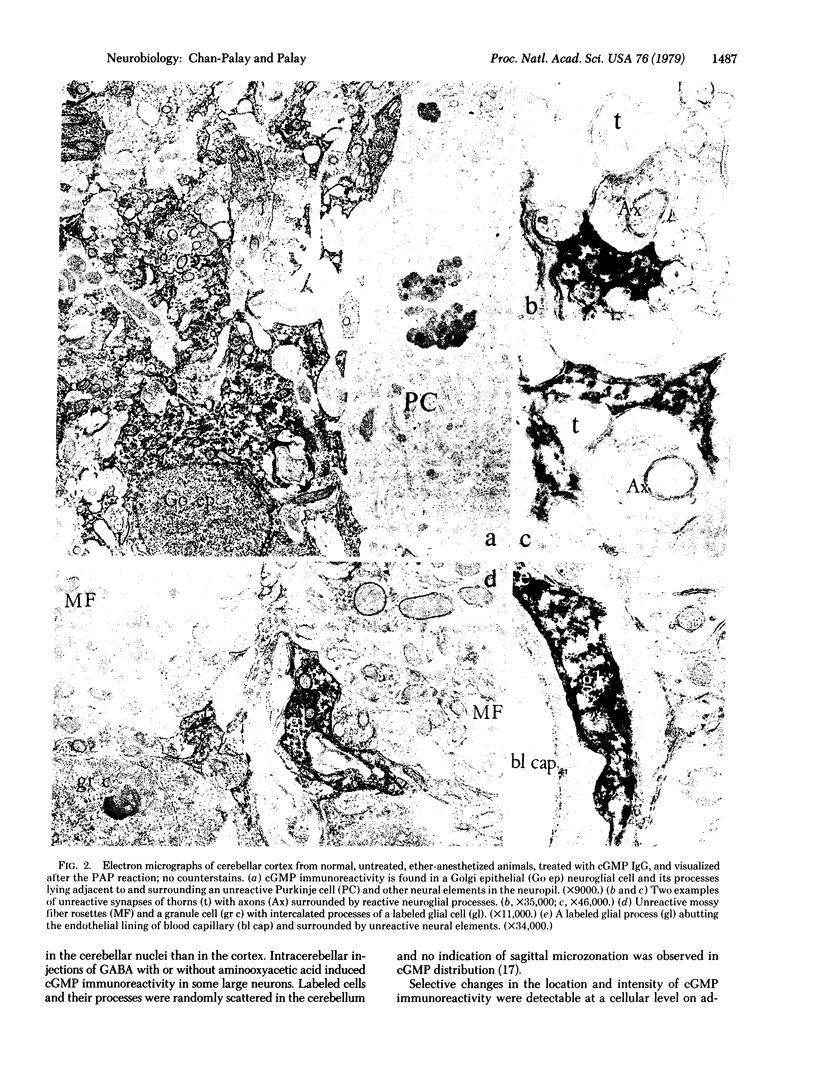
Guanosine 3′,5′-cyclic monophosphate (cGMP) immunoreactivity in the rat's cerebellum was studied with light and electron microscopy by the indirect fluorescence method and the peroxidase-antiperoxidase method. Labeled cells included neuroglial cells in the cerebellar cortex, white matter, and deep nuclei; some stellate and basket cells in the cortex; and some large neurons in the deep nuclei. No evidence was found for sagittal microzonation in the cGMP distribution. In the labeled cells, cGMP immunoreactive sites were localized to surface membranes, organelles, and the cytoplasmic matrix. Specificity was indicated by the same pattern of labeling after treatment with cGMP immunoglobulin that had been adsorbed with adenosine 3′,5′-cyclic monophosphate (cAMP) and by the failure to label after treatment with normal rabbit sera or with cGMP immunoglobulin that had been adsorbed with 1 mM cGMP. Cerebella treated with cAMP antisera, however, showed immunoreactivity in Purkinje cells, granule cells, and Golgi cells in addition to neuroglia in cortex and deep nuclei. Sequential norepinephrine and glutamate superfusions generally intensified cGMP immunoreactivity, not only in neuroglial cells but also in the background. Under these conditions some Purkinje cells and some granule cells were also labeled. Increased cGMP immunoreactivity was also obtained by treatment with harmaline, γ-aminobutyric acid and aminooxyacetic acid, muscimol, γ-aminobutyric acid, or apomorphine in order of decreasing effectiveness. Serotonin and colchicine produced no detectable increase of cGMP immunoreactivity above normal, and diazepam and sodium pentobarbital decreased it. In these experiments, diethyl ether was preferable to sodium pentobarbital for anesthesia on account of the depressive action of the latter on cGMP immunoreactivity. Thus, drugs that increase cerebellar activity enhance cGMP levels, whereas those that decrease cerebellar activity decrease cGMP levels. However, it is not clear whether these fluctuations in cGMP levels are a direct consequence of neurotransmitter function or are sequelae to other related events. The present study suggests that some neurons and many neuroglial cells are the major sites of cGMP in the cerebellum.
Keywords: cerebellum, cyclic AMP, γ-aminobutyric acid, harmaline, diazepam
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggio G., Costa E., Guidotti A. Pharmacologically induced changes in the 3':5'-cyclic guanosine monophosphate content of rat cerebellar cortex: difference between apomorphine, haloperidol and harmaline. J Pharmacol Exp Ther. 1977 Jan;200(1):207–215. [PubMed] [Google Scholar]
- Biggio G., Guidotti A. Climbing fiver activation and 3', 5'-cyclic guanosine monophosphate (cGMP) content in cortex and deep nuclei of cerebellum. Brain Res. 1976 May 7;107(2):365–373. doi: 10.1016/0006-8993(76)90233-x. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Hoffer B. J., Battenberg E. R., Siggins G. R., Steiner A. L., Parker C. W., Wedner H. J. Adenosine 3',5'-monophosphate is localized in cerebellar neurons: immunofluorescence evidence. Science. 1972 Aug 4;177(4047):436–438. doi: 10.1126/science.177.4047.436. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L., Brown J. T., Van Itallie C. Sagittal organization of olivocerebellar and reticulocerebellar projections: autoradiographic studies with 35S-methionine. Exp Brain Res. 1977 Dec 19;30(4):561–576. doi: 10.1007/BF00237645. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L. The form of velate astrocytes in the cerebellar cortex of monkey and rat: high voltage electron microscopy of rapid Golgi preparations. Z Anat Entwicklungsgesch. 1972;138(1):1–19. doi: 10.1007/BF00519921. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L. Ultrastructural identification of substance P cells and their processes in rat sensory ganglia and their terminals in the spinal cord by immunocytochemistry. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4050–4054. doi: 10.1073/pnas.74.9.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming R., Eccleston D., Steiner A. Immunohistochemical localization of cyclic GMP in rat cerebellum. J Cyclic Nucleotide Res. 1977 Aug;3(4):275–282. [PubMed] [Google Scholar]
- Ferrendelli J. A., Chang M. M., Kinscherf D. A. Elevation of cyclic GMP levels in central nervous system by excitatory and inhibitory amino acids. J Neurochem. 1974 Apr;22(4):535–540. doi: 10.1111/j.1471-4159.1974.tb06890.x. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A., Kinscherf D. A., Chang M. M., Morgan J. F. Comparison of the effects of biogenic amines on cyclic GMP and cycle AMP levels in mouse cerebellum in vitro. Brain Res. 1975 Jan 24;84(1):63–73. doi: 10.1016/0006-8993(75)90800-8. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A., Steiner A. L., McDougal D. B., Jr, Kipnis D. M. The effect of oxotremorine and atropine on cGMP and cAMP levels in mouse cerebral cortex and cerebellum. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1061–1067. doi: 10.1016/0006-291x(70)90193-2. [DOI] [PubMed] [Google Scholar]
- Kimura H., Thomas E., Murad F. Effects of decapitation, ether and pentobarbital on guanosine 3',5'-phosphate and adenosine 3',5'-phosphate levels in rat tissues. Biochim Biophys Acta. 1974 May 24;343(3):519–528. doi: 10.1016/0304-4165(74)90269-4. [DOI] [PubMed] [Google Scholar]
- Mao C. C., Guidotti A., Costa E. Evidence for an involvement of GABA in the mediation of the cerebellar cGMP decrease and the anticonvulsant action diazepam. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(4):369–378. doi: 10.1007/BF00508411. [DOI] [PubMed] [Google Scholar]
- Mao C. C., Guidotti A., Costa E. The regulation of cyclic guanosine monophosphate in rat cerebellum: possible involvement of putative amino acid neurotransmitters. Brain Res. 1974 Oct 25;79(3):510–514. doi: 10.1016/0006-8993(74)90449-1. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Cyclic nucleotides and nervous system function. Physiol Rev. 1977 Apr;57(2):157–256. doi: 10.1152/physrev.1977.57.2.157. [DOI] [PubMed] [Google Scholar]
- Redos J. D., Catravas G. N., Hunt W. A. Ethanol-induced depletion of cerebellar guanosine 3',5' -cyclic monophosphate. Science. 1976 Jul 2;193(4247):58–59. doi: 10.1126/science.180596. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]