Abstract
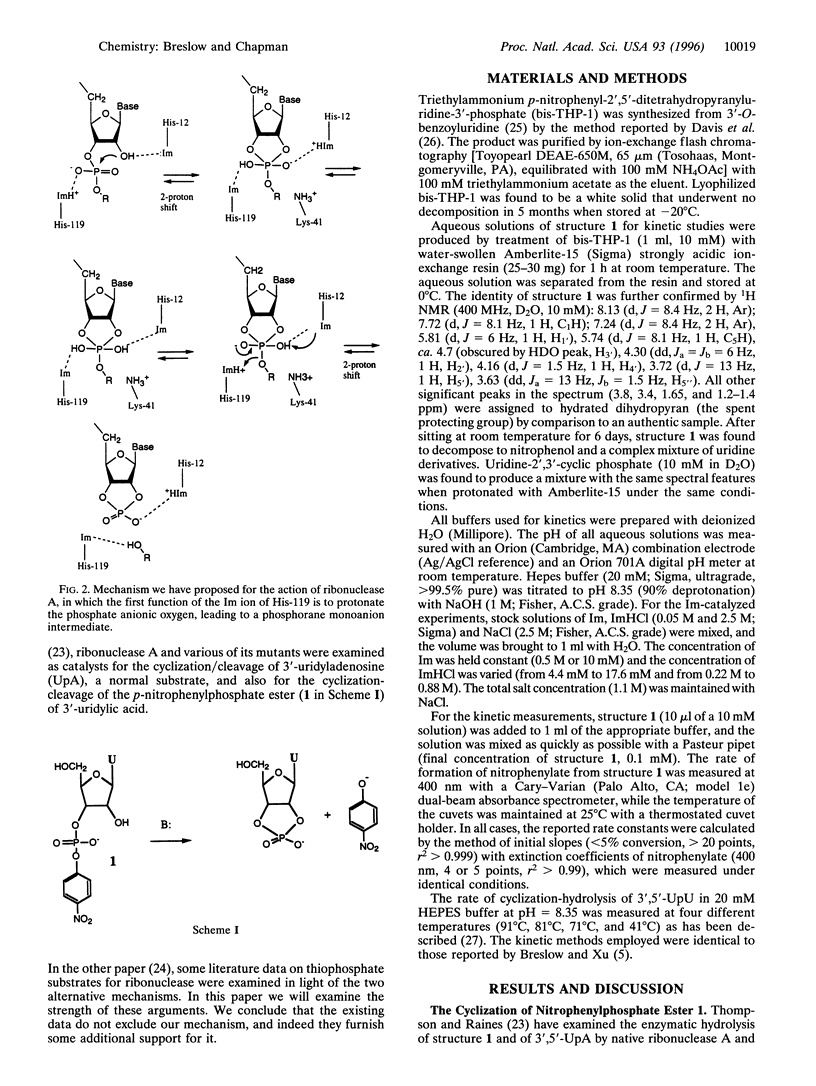
It has been reported that His-119 of ribonuclease A plays a major role as an imidazolium ion acid catalyst in the cyclization/cleavage of normal dinucleotides but that it is not needed for the cyclization/cleavage of 3'-uridyl p-nitrophenyl phosphate. We see that this is also true for simple buffer catalysis, where imidazole (as in His-12 of the enzyme), but not imidazolium ion, plays a significant catalytic role with the nitrophenyl substrate, but both are catalytic for normal dinucleotides such as uridyluridine. Rate studies show that the enzyme catalyzes the cyclization of the nitrophenylphosphate derivative 47,000,000 times less effectively (kcat/kuncat) than it does uridyladenosine, indicating that approximately 50% of the catalytic free energy change is lost with this substrate. This suggests that the nitrophenyl substrate is not correctly bound to take full advantage of the catalytic groups of the enzyme and is thus not a good guide to the mechanism used by normal nucleotides. The published data on kinetic effects with ribonuclease A of substituting thiophosphate groups for the phosphate groups of normal substrates has been discussed elsewhere, and it was argued that these effects are suggestive of the classical mechanism for ribonuclease action, not the novel mechanism we have recently proposed. The details of these rate effects, including stereochemical preferences in the thiophosphate series, can be invoked as support for our newer mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslow R., Xu R. Recognition and catalysis in nucleic acid chemistry. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1201–1207. doi: 10.1073/pnas.90.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. Diastereomers of 5'-O-adenosyl 3'-O-uridyl phosphorothioate: chemical synthesis and enzymatic properties. Biochemistry. 1979 Feb 20;18(4):592–596. doi: 10.1021/bi00571a007. [DOI] [PubMed] [Google Scholar]
- Campbell R. L., Petsko G. A. Ribonuclease structure and catalysis: crystal structure of sulfate-free native ribonuclease A at 1.5-A resolution. Biochemistry. 1987 Dec 29;26(26):8579–8584. doi: 10.1021/bi00400a013. [DOI] [PubMed] [Google Scholar]
- Davis A. M., Regan A. C., Williams A. Experimental charge measurement at leaving oxygen in the bovine ribonuclease A catalyzed cyclization of uridine 3'-phosphate aryl esters. Biochemistry. 1988 Dec 13;27(25):9042–9047. doi: 10.1021/bi00425a024. [DOI] [PubMed] [Google Scholar]



