Abstract
Nuclear lamins form the lamina on the interior of the nuclear envelope, and are involved in the regulation of various cellular processes, including DNA replication and chromatin organization. Despite this evidence, little is known about potential alterations in nuclear metabolism, specifically lamin structure and integrity in isolated β-cells subjected to stress conditions, including chronic exposure to hyperglycemia [i.e., glucotoxicity]. Herein, we investigated effects of glucotoxic conditions on the catalytic activation of caspase 3 and the associated degradation of one of its substrate proteins, namely lamin-B. We report that incubation of insulin-secreting INS-1 832/13 cells, normal rat islets or human islets under glucotoxic conditions [20 mM; 12–48 hr] results in the degradation of native lamin B leading to accumulation of the degraded products in non-relevant cellular compartments, including cytosol. Moreover, the effects of high glucose on caspase 3 activation and lamin B degradation were mimicked by thapsigargin, a known inducer of endoplasmic reticulum stress [ER stress]. Nifedipine, a known blocker of calcium channel activation, inhibited high glucose-induced caspase 3 activation and lamin B degradation in these cells. 4-phenyl butyric acid, a known inhibitor of ER stress, markedly attenuated glucose-induced CHOP expression [ER stress marker], caspase 3 activation and lamin B degradation. We conclude that glucotoxic conditions promote caspase 3 activation and lamin B degradation, which may, in part, be due to increased ER stress under these conditions. We also provide further evidence to support beneficial effects of calcium channel blockers against metabolic dysfunction of the islet β-cell induced by hyperglycemic conditions.
Keywords: Caspase 3, Glucotoxicity, Nuclear lamin B, Pancreatic islet β-cell, nifedipine
1. Introduction
Apoptosis is the process of programmed cell death that is required for the maintenance of tissue homeostasis. It is characterized by specific changes in the morphology of the cell. The cell undergoes shrinkage, blebbing (bulges in the plasma membrane), nuclear fragmentation and chromatin condensation [1]. Basically, it is a natural process that balances cell growth and removal of damaged or injured cells. It is well established that β-cell failure is a characteristic feature of Type 1 and 2 diabetes. In both types of the disease, apoptosis is the most likely form of cell death. Therefore, it is necessary to study the intracellular events that the pancreatic β-cell undergoes during apoptosis, and understanding of such events will aid in searching for a possible drug target for the management and/or prevention of the disease [2, 3].
The caspase (cysteine dependent aspartate-directed proteases) family is one of the most important factors in the apoptotic pathway. Many proteins in the cell including a large number of structural proteins are cleaved by activated caspases which culminates in apoptosis of the cell. The caspase 8, 9 and 10 are initiator caspases whereas caspase 3, 6 and 7 are effector caspases. The initiator caspases cleave and activate the effector caspases leading to cell death [1]. These caspases exist in the form of inactive zymogens [also referred to as pro-caspases], which are activated by the process of cleavage by other upstream proteases or by auto- or trans-activation [4]. In the recent years a variety of substrates of caspases have been recognized. Caspase-dependent degradation of nuclear lamins has been identified as a precursor to nuclear collapse in programmed cell death [5]. The nuclear lamina lines the interior of the nuclear envelope and is composed of three proteins; lamins A, B and C. The A type lamins, which include lamins A and C, are products of alternative splicing of the same gene LMNA whereas lamin B is encoded by the LMNB1 gene [6]. These lamins are type V intermediate filament proteins and are lined up on the inner face of the inner nuclear membrane. The nuclear lamina is a fundamental part of major nuclear activities, namely mitosis, chromatin organization and in DNA replication. Besides, lamins also play key functional roles in providing structural support thereby contributing to the nuclear architecture [7].
Degradation of lamins leads to the breakdown of nuclear lamina which is a preliminary stage of apoptosis [8] as this is followed by DNA degradation and chromatin condensation [9]. Previous results from our laboratory indicated that IL-1β treatment causes an increase in lamin B degradation mediated by caspases [10]. In addition, a recent study highlighted the involvement of caspase 3 in the breakdown of the nuclear matrix by cleaving nuclear lamin B. It has been stated that this cleavage probably occurs by activation of caspase 3 directly or by other downstream proteases [11]. Recent evidence in PC12 and rat cortical cells also implicates cell apoptosis via caspase 3 activation and cleavage of lamin-B under lipotoxic conditions induced by saturated fatty acids, such as palmitic acid [12].
It is well established that chronic exposure of isolated β-cells to hyperglycemic conditions leads to metabolic dysfunction and demise [13,14]. Several intracellular signaling events have been identified as causal to HG-induced metabolic dysregulation of the islet. These include endoplasmic reticulum [ER] and oxidative stress. It has been suggested that both oxidative and ER stress lead to mitochondrial dysfunction, cytochrome-C release, and caspase activation resulting in the demise of the β-cell [15]. Despite this growing body of evidence, very little is known in the context of the islet β-cell, on potential detrimental effects of glucotoxic conditions on caspase activation and associated degradation of their respective substrate proteins. The overall objective of this study therefore is to investigate the role of caspase 3 on the degradation of nuclear lamins, specifically lamin-B in insulin-secreting INS-1 832/13 cells, rat islets and human islets under glucotoxic conditions. Our findings provide the first evidence to implicate significant caspase 3 mediated degradation of lamin B in these cells under glucotoxic environment. We also provide evidence to suggest novel roles for ER stress in glucose-induced caspase 3 activation and lamin B degradation, and associated alterations in the subcellular distribution of lamin B under glucotoxic conditions.
2. Materials and methods
2.1 Materials
Antisera directed against lamin-B, procaspase-3, and cleaved caspase-3 (Asp 175), CHOP were obtained from Cell Signaling (Danvers MA and Santa Cruz Biotechnology, Santa Cruz, CA). Anti-β Actin was from Sigma Aldrich (St. Louis, MO). Anti-mouse IgG and anti-rabbit IgG conjugated to horseradish peroxidase were from GE Healthcare UK. Glucose, nifedipine, thapsigargin, 4-Phenylbuyric acid were obtained from Sigma Aldrich (St. Louis, MO). Enhanced chemiluminescence (ECL) kit was from Amersham Biosciences (Piscataway, NJ). All other reagents were from Sigma Aldrich (St. Louis, MO) unless stated otherwise.
2.2 Insulin-secreting INS-1 832/13 cells, rat islets, human islets and culture conditions
INS-1 832/13 cells were cultured in RPMI-1640 medium containing 10% heat inactivated FBS supplemented with 100 IU/ ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 50 µM 2-mercaptoethanol and 10 mM HEPES (pH 7.4). The cultured cells were subcloned twice weekly following trypsinization and passages 53–61 were used for the study. Islets from normal 6 week-old male Sprague–Dawley rats (Harlan Laboratories, Oxford, MI) were isolated by the collagenase digestion method [16]. Human islets and islet culture medium were obtained from Prodo Laboratories, Irvine, CA. All protocols, including isolation of pancreatic islets from rats, were reviewed and approved by the Wayne State University and John D. Dingell VA Medical Center Institutional Animal Care and Use Committee.
INS-1 832/13 cells and normal rat islets were incubated in the presence of low [2.5 mM] and high glucose (20 mM or 30mM in case of human islets) for 12–48 hrs as indicated in the text. Low and high glucose solutions were prepared by supplementing glucose to glucose-free medium where appropriate. At the end of the incubation period the cells were harvested and lysed in RIPA buffer containing 1 mg/ml protease inhibitor cocktail, 1 mM NaF, 1 mM PMSF and 1 mM Na3VO4.
2.3 Isolation of subcellular fractions
Extraction of cytosolic, membrane/organelle and nucleic protein fraction was carried out as per the manufacturer’s instructions using the ProteoExtract® Subcellular Proteome Extraction Kit. Briefly, INS-1 832/13 cells were incubated with low (2.5 mM) and high (20 mM) glucose for 24 hrs. The cells were scraped and suspended in wash buffer and pelleted by centrifugation for 10 min at 300×g at 4°C. The pellet obtained was then resuspended in the extraction buffer-I and protease inhibitor cocktail, provided in the kit. After incubation for 10 min at 4°C the cells were centrifuged for 10 min at 1,000×g, the supernatant obtained was the cytosolic fraction. The pellet was then resuspended in Extraction buffer-II and protease inhibitor cocktail and incubated for 30 min and then centrifuged for 10 min at 6,000×g. The supernatant thus obtained was the membrane/organelle fraction. Finally the pellet was resuspended in extraction buffer-III, protease inhibitor cocktail and benzonase, incubated for 10 min at 4°C and centrifuged at 6,800×g for 10 min. The supernatant obtained was the nucleic protein fraction and the remaining pellet, resuspended in buffer IV and protease inhibitor cocktail was the cytoskeletal matrix protein fraction.
2.4 Western blotting
Cellular lysate proteins (30–50 µg/lane) were separated by SDS-PAGE on 10% (w/v) polyacrylamide mini gels and electro transferred to a nitrocellulose membrane. The membranes were blocked with 5% milk in 10 mM Tris-HCl, pH 7.6, 1.5 M NaCl and 0.1% Tween 20 followed by incubation with primary antibodies (lamin B-1:250, cleaved caspase 3- 1:400) in TBS-T containing 5% BSA at room temperature for 1 h and washed 5 times for 5 min each with TBS-T. The membrane was then incubated with corresponding secondary antibodies conjugated to horseradish peroxidase (1:1000) in 5 % non fat dry milk in TBS-T at room temperature for 1 h. After washing, the protein signal was enhanced by chemiluminescence system and developed using Kodak Pro Image 400 R (New Haven, CT) and Carestream Molecular Imaging Software was used to measure the band density. The same blots were used to probe for β-actin to ensure equal loading and transfer of proteins.
2.5 Statistical analysis
The statistical significance of the differences between the experimental conditions was determined by t-test or ANOVA where appropriate. p value < 0.05 was considered significant.
3. Results
3.1 Exposure of INS-1 832/13 cells, normal rat islets and human islets to glucotoxic conditions induce caspase 3 activation and degradation of lamin B
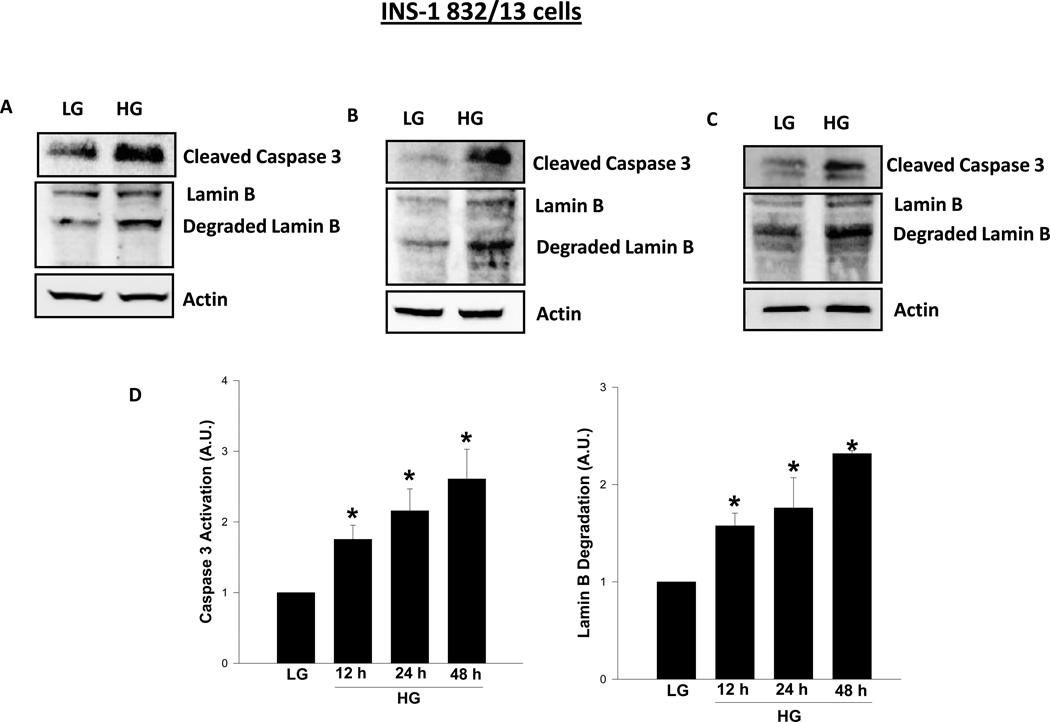
At the outset, INS-1 832/13 cells were incubated with either low [2.5 mM] or high [20 mM] glucose for 12, 24 and 48 hr, and caspase 3 activation, as evidenced by the emergence of caspase-3 degradation fragment, was monitored by Western blotting, and the data are then quantitated by densitometry. Data depicted in Figure 1 demonstrate a marked increase in caspase 3 activation as early as 12 hr [1.8 fold; Panel A], which continued to increase as a function of time [2.2 and 2.6 fold increase at 24 and 48 hr, respectively; Panels B and C]. Furthermore, we noticed a marked increase in the degradation of lamin B under these conditions [Figure 1]. For example the fold increase in lamin B degradation represented 1.6 fold at 12 hr [Panel A], 1.8 fold at 24 hr [Panel B] and 2.3 fold at 48 hr [Panel C]. Pooled data from multiple experiments are provided in Panel D. Together, data in Figure 1 suggested activation of caspase 3 and degradation of lamin B under glucotoxic conditions. It should be noted that the observed effects of glucose on caspase 3 activation and lamin B degradation are not due to osmotic effects of glucose since incubation of these cells with mannitol [20 mM], used as an osmotic control, did not elicit any clear effects on caspase-3 activation and lamin-B degradation under these conditions [n=2 experiments; additional data not shown].
Figure 1. Exposure of INS-1 832/13 cells to glucotoxic conditions results in caspase 3 activation and lamin B degradation.
INS-1 832/13 cells were incubated in the presence of low (2.5mM; LG) or high (20mM; HG) glucose for 12 hr [Panel A], 24 hr [Panel B], and 48 hr [Panel C], and protein lysates [50 µg] were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed for cleaved [active] caspase 3 and degraded lamin B, and immune complexes were identified using ECL detection kit. To check equal protein loading, the membranes were stripped and reprobed for actin. Intensity of protein bands was quantitated by densitometry. The statistical significance of the differences between the control and experimental conditions was determined by t-test. Data in Panel D represent mean ± SEM from three to four independent experiments and expressed as fold change in caspase 3 activation and lamin B degradation. *p < 0.05 versus LG
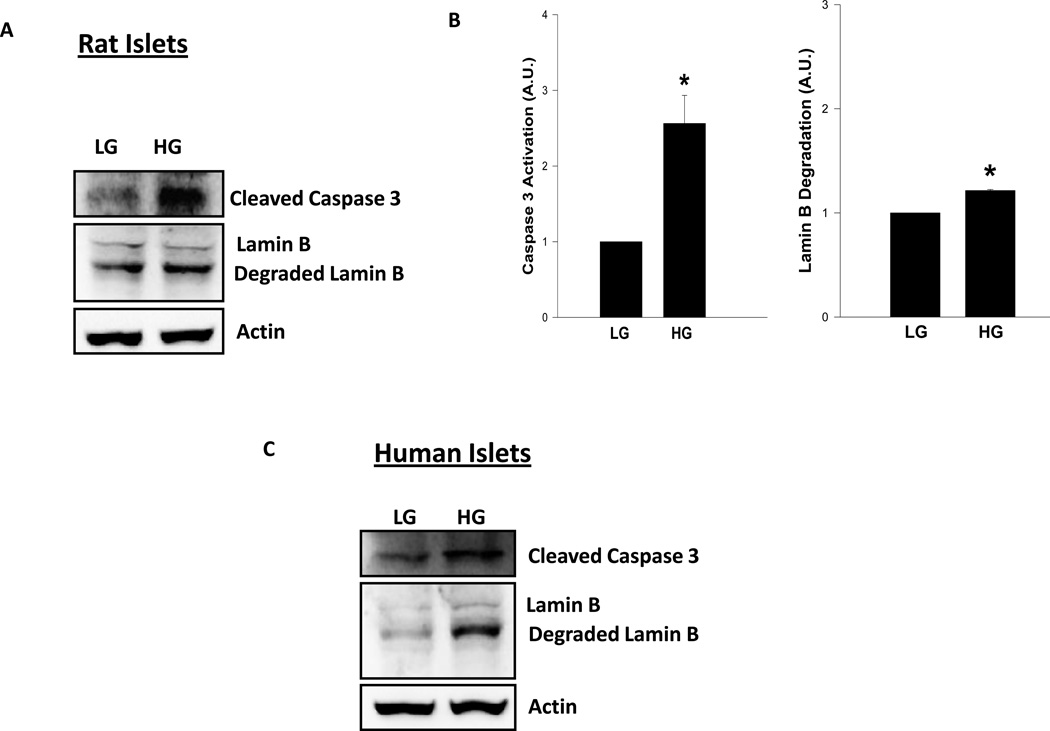
The above studies in INS-1 832/13 cells were repeated in normal rat islets to further validate the observed effects of glucotoxicity [20 mM glucose for 24 hrs] on caspase 3 activation and lamin B degradation are attributable to the primary islets as well. Data depicted in Figure 2 [Panels A and B] indicate a 2.6 fold increase in caspase 3 activation followed by a corresponding increase in lamin B degradation under these conditions [Figure 2; Panel A and B]. Likewise, we noticed a 1.9 fold increase in caspase-3 activation and 2 fold increase in lamin-B degradation in human islet preparations incubated with glucose [30 mM; 24 hr; Figure 2; Panel C]. These data in primary islets [rat and human] further support our observations in INS-1 832/13 cells [Figure 1].
Figure 2. Treatment of normal rat islets or human islets with high glucose results in caspase 3 activation and lamin B degradation.
Rat islets [Panels A and B] or human islets [Panel C] were incubated in the presence of low (2.5mM; LG) and high glucose (HG; 20mM for rat islets, 30mM for human islets) for 24 hr as described in the text. Twenty five µg lysate proteins were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed for cleaved caspase 3 and lamin B and immune complexes were identified using ECL detection kit. To check equal protein loading, the membrane for each protein was stripped and reprobed for actin. Quantitation of caspase 3 activation and lamin B degradation were carried out by densitometry. The statistical significance of the differences between the control and experimental conditions was determined by t-test. Data provided in Panel B represent mean ± SEM from three to four independent experiments and expressed as fold change for rat islets. Please note that human islet data [Panel C] were accrued from a single preparation. The ratios of cleaved caspase-3 to actin in LG and HG treated human islets were 0.068 vs. 0.120, respectively, and the ratios of degraded lamin-B to actin in LG and HG treated human islets were 0.142 vs. 0.283, respectively. * p < 0.05 versus LG.
3.2 Glucotoxic conditions promote alterations in the subcellular distribution of cleaved caspase 3 and lamin B in INS-1 832/13 cells
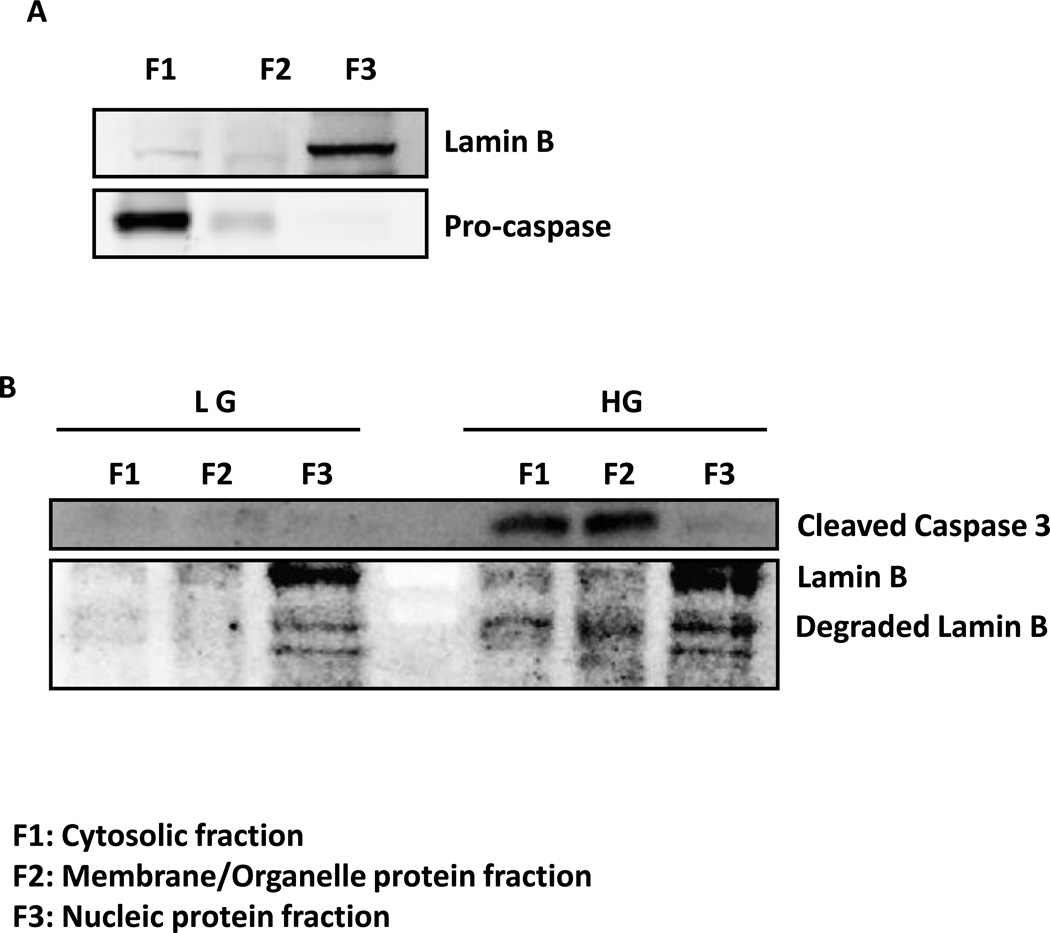
In these studies, we determined potential alterations, if any, in the subcellular localization of active caspase 3 fragment and lamin B degradation products in INS-1 832/13 cells following exposure to glucotoxic conditions. To determine this, INS-1 832/13 cells were incubated with low [2.5] or high glucose [20mM]. Individual subcellular fractions, namely the cytosolic fraction [fraction F1], membrane/organelle protein fraction [fraction F2] and the pure nucleic protein fraction [fraction F3] were isolated using a ProteoExtract subcellular proteome extraction kit [see Methods for additional details]. At the outset, we determined subcellular distribution of native caspase [pro-caspase] and lamin B in INS-1 832/13 lysates. Data depicted in Figure 3 [panel A], as expected, suggest that native lamin B and pro-caspase are localized predominantly in the nuclear [F3] and cytosolic [F1] fractions, respectively. However, glucotoxic conditions caused significant alterations in the subcellular distribution of biologically active [cleaved] caspase 3 and degraded lamin B [Figure 3; Panel B]. For example, we noticed significant accumulation of degraded lamin B in cytosolic [F1] and membrane/organelle protein [F2] fractions under high glucose-treatment conditions. Interestingly, we also noticed significant accumulation of cleaved caspase 3 in these fractions. Together, these observations demonstrate abnormal distribution of lamin B under glucotoxic conditions further suggestive of disassembly of nuclear structure under these conditions.
Figure 3. Exposure to high glucose results in altered subcellular distribution of caspase 3 and degraded product of lamin B in INS-1 832/13 cells.
INS-1 832/13 cells were subjected to subcellular fractionation using ProteoExtract® Subcellular Proteome Extraction Kit at basal level (Panel A) and after treatment with low (2.5 mm; LG) and high (20 mM; HG) glucose for 24 hr (Panel B). The lysate proteins [~20–30 µg] were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed for native lamin B and pro-caspase [Panel A] and cleaved caspase 3 and degraded lamin B [Panel B], and immune complexes were identified using ECL detection kit. Data are representative of two experiments with identical results.
3.3 Thapsigargin, a known inducer of ER stress, markedly increases caspase 3 activation and lamin B degradation in INS 1-832/13 cells
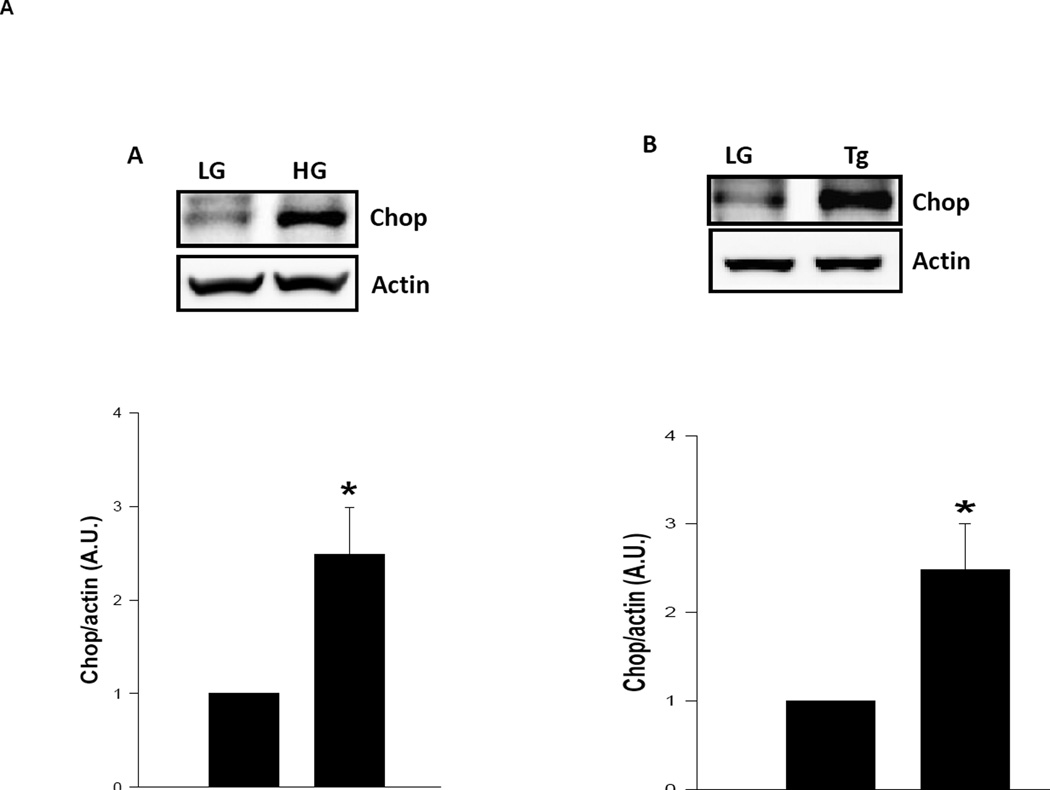
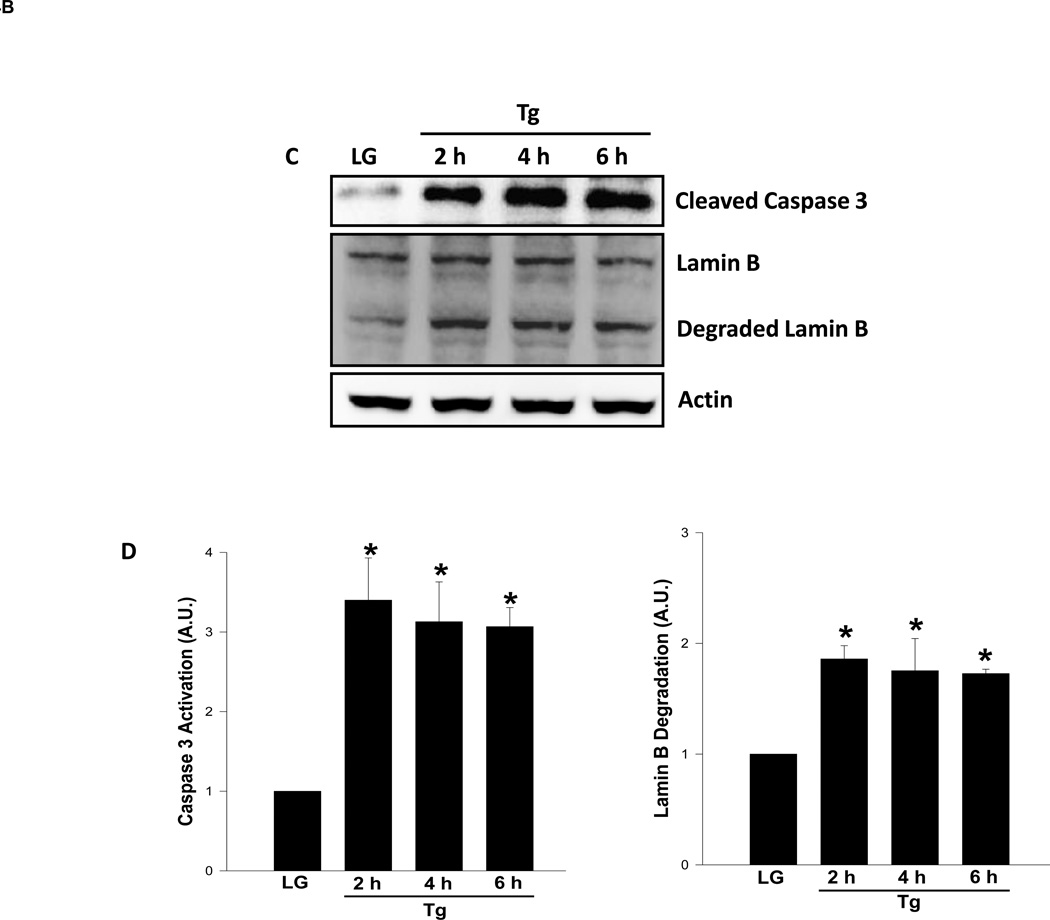
Several lines of experimental evidence implicate ER stress as one of intermediate steps involved in glucotoxicity of the islet β-cell [17–21]. To determine if high glucose induces ER stress in our current experimental model, we quantitated CHOP expression, an ER stress marker, in INS-1 832/13 cells exposed to low and high glucose conditions. Data depicted in Figure 4 [Panel A] demonstrate a significant increase in CHOP expression in cells exposed to glucotoxic conditions and a similar result was obtained with thapsigargin [Panel B]. To further assess if ER stress induces caspase 3 activation and lamin B degradation, we incubated INS-1 832/13 cells with thapsigargin and then quantitated caspase 3 activation and lamin B degradation in these cells. Data depicted in Figure 4 demonstrate significant increase in caspase 3 activation in cells treated with thapsigargin as early as 2 hr of incubation [3.4 fold; Figure 4; Panels C and D]. Note that thapsigargin effects were maximal at 2 hr since no further increase in caspase 3 activation was seen at 4 hr [3.1 fold activation] and 6 hr [3 fold activation; Figure 4; Panels C and D]. Under these conditions, we also noticed a significant increase on lamin B degradation induced by thapsigargin within 2 hr [1.9 fold], which remain plateued at 4 hr [1.8 fold] and 6 hrs [1.7 fold]. Together, our findings suggest ER stress as one of the mechanisms underlying glucose-mediated effects on caspase-3 activation and lamin-B degradation. This hypothesis was tested further using a pharmacological approach [see below]
Figure 4. Thapsigargin, a known inducer of ER stress, also promotes caspase 3 activation and lamin B degradation in INS-1 832/13 cells.
Panel A: INS-1 832/13 cells are incubated with low glucose (LG; 2.5 mM) or high glucose (HG; 20 mM) for 24 hr. Expression of CHOP was determined in cell lysates by Western blotting. To check equal protein loading, the membrane for each proteins was stripped and reprobed for actin. Quantification of CHOP expression was carried out by densitometry. The statistical significance of the differences between the control and the experimental groups was determined by t-test. Data represent mean ± SEM from three independent experiments and expressed as fold change. *p <0.05 vs. LG.
Panel B: INS-1 832/13 cells are incubated with low glucose (LG; 5 mM) or thapsigargin (Tg; 0.25 µM for 6 hr). Expression of CHOP was determined by Western blotting. To check equal protein loading, the membrane for each proteins was stripped and reprobed for actin. Quantification of CHOP expression was carried out by densitometry. The statistical significance of the differences between the control and the experimental groups was determined by t-test. Data represent mean ± SEM from three independent experiments and expressed as fold change. *p <0.05 vs. LG.
Panel C: INS-1 832/13 cells were incubated in the presence of low glucose (2.5 mM) or thapsigargin (Tg; 0.25 µM) for 2, 4 and 6 hr as indicated in the figure. Forty µg lysate proteins were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed for cleaved caspase 3 and degraded lamin B, and immune complexes were identified using ECL detection kit. To check equal protein loading, the membrane for each proteins was stripped and reprobed for actin. Quantitation of caspase 3 activation and lamin B degradation were carried out by densitometry. The statistical significance of the differences between the control and experimental conditions was determined by ANOVA followed by Student-Newman-Keuls post hoc test. Data in Panel D represent mean ± SEM from three independent experiments and expressed as fold change. *P < 0.05 versus LG.
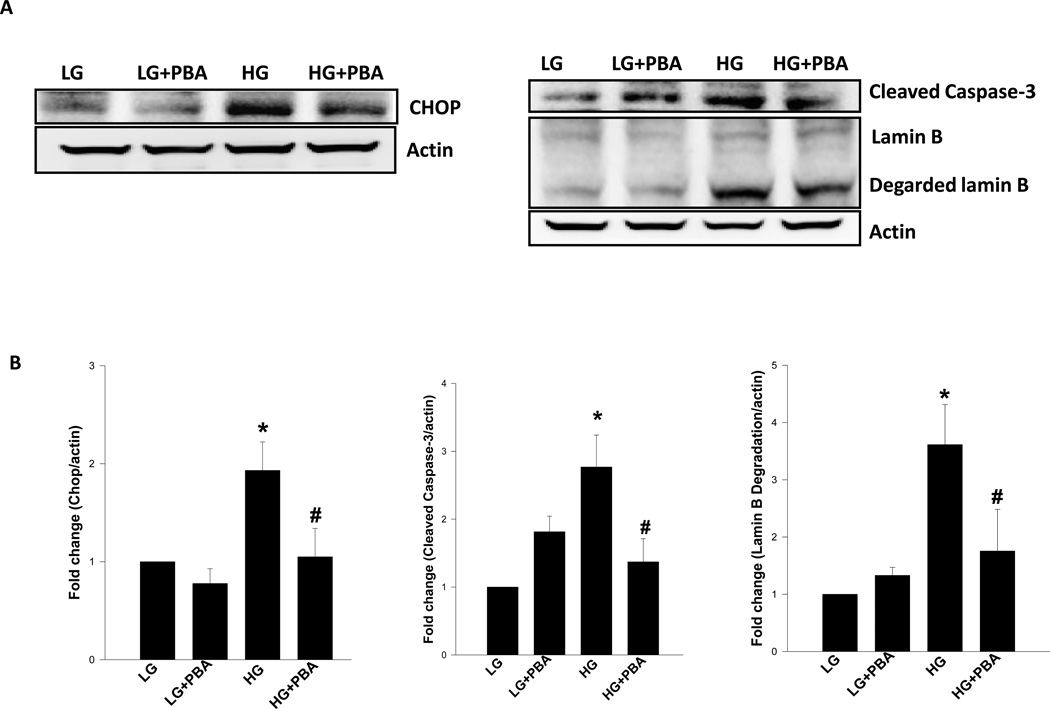
3.4 4-Phenylbutyric acid [PBA], a known inhibitor of ER-stress, markedly attenuates glucose-induced CHOP expression, caspase 3 activation and lamin B degradation
As a logical extension to the above studies, we attempted to establish a causal role for ER-stress in high glucose-induced caspase 3 activation and lamin B degradation. To accomplish this, we used PBA, a known inhibitor of ER-stress, on glucose-induced caspase 3 activation and lamin B degradation. We also quantified CHOP expression in these cells to affirm that PBA inhibits glucose-induced ER-stress under current experimental conditions. Data depicted in Figure 5 demonstrate a significant increase in CHOP expression in glucose-treated cells. We also observed complete inhibition of glucose-induced CHOP expression by PBA [Figure 5]. These data thus validate the use of PBA as an inhibitor of ER-stress in INS-1 832/13 cells. More importantly, we also noticed a complete inhibition of glucose-induced caspase 3 activation and lamin B degradation by PBA in these cells [Figure 5; Panels A and B]. Taken together, these data provide the first evidence that glucotoxicity promotes caspase 3 activation and lamin B degradation in an ER-stress sensitive fashion.
Figure 5. 4-Phenylbutyric acid [PBA], a known inhibitor of ER-stress, markedly attenuates glucose-induced CHOP expression, caspase 3 activation and lamin B degradation in INS-1 832/13 cells.
Panel A: INS-1 832/13 Cells were incubated in the presence of low (2.5 mM; LG) or high (20 mM; HG) glucose for 24 hrs in the presence and absence of PBA (0.5 mM). Lysate proteins [40 µg] were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed for CHOP, active caspase 3 and degraded lamin B and immune complexes were identified using ECL detection kit. To check equal protein loading, the membranes were stripped and reprobed for actin. Intensity of protein bands was quantitated by densitometry [Panel B]. The statistical significance of the differences between the experimental conditions was determined by ANOVA followed by Student-Newman-Keuls post hoc test. Data represent mean ± SEM from three independent experiments and expressed as fold change in CHOP expression, caspase 3 activation and lamin B degradation. *p < 0.05 versus LG, # p < 0.05 versus HG.
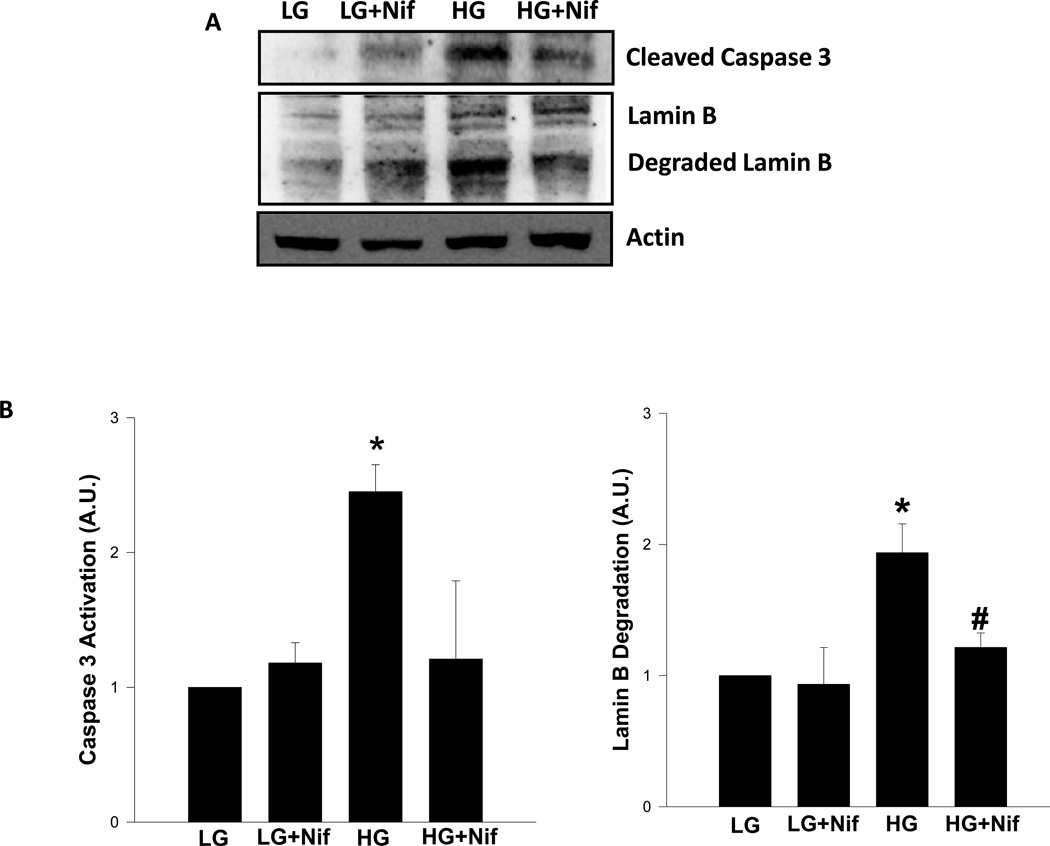
3.5 Nifedipine, a calcium channel blocker, inhibits glucose-induced caspase 3 activation and lamin B degradation in INS-1 832/13 cells and normal rat islets
We recently reported that calcium overload may represent one of the signaling mechanisms involved in caspase-3 activation under conditions of cellular apoptosis in isolated β-cells [21]. For example, using etoposide, a known inducer of loss in metabolic viability in β-cells, we reported a marked increase in caspase 3 activity in INS-1 832/13 cells and normal rat islets. More importantly, we have been able to markedly prevent etoposide-induced metabolic dysfunction in these cells by nifedipine, a known blocker of calcium channel activation and calcium entry [22]. Furthermore, recent findings from the laboratory of Wang and associates [23] have also demonstrated significant protective effects of nifedipine against high glucose-induced ER stress and apoptosis. Therefore, as a logical extension to findings that we reported above, we undertook a study to see if glucose-induced caspase 3 activation and lamin B degradation are rescued by nifedipine. Data presented in Figure 6 indicate a modest, but insignificant [1.2 fold] increase in caspase 3 activation in INS-1 832/13 cells incubated with nifedipine alone under basal conditions. No significant effects of nifedipine were demonstrable on lamin B degradation under these conditions. As shown above, high glucose-treatment markedly enhanced caspase 3 activation [2.5 fold] and lamin B degradation [1.9 fold; Figure 6]. Interestingly, coprovision of nifedipine with glucose markedly reduced high glucose-induced effects on caspase 3 activation [2.5 fold vs. 1.2 fold in the absence and presence of nifedipine, respectively]. In a manner akin to these findings, nifedipine also attenuated lamin B degradation induced by glucose [1.9 fold in the absence of nifedipine and 1.2 fold in its presence; Figure 6].
Figure 6. Nifedipine, a calcium channel blocker, inhibits glucose-induced caspase 3 activation and lamin B degradation in INS-1 832/13 cells.
Panel A: INS-1 832/13 cells were incubated with low glucose (LG; 2.5mM) or high glucose (HG; 20mM) for 24 hrs in the absence [diluent] or presence of nifedipine (Nif; 10 µM). Lysate proteins [40 µg] were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed for degraded lamin B and cleaved caspase 3, and immune complexes were identified using ECL detection kit. To check equal protein loading, the membrane for each proteins was stripped and reprobed for actin. Quantitation of caspase 3 activation and lamin B degradation were carried out by densitometry. The statistical significance of the differences between control and the experimental conditions was determined by ANOVA followed by Student-Newman-Keuls post hoc test. Data represent mean ± SEM from three independent experiments and expressed as fold change [Panel B]. *p < 0.05 vs. LG and # p <0.05 vs. HG.
We next assessed protective effects of nifedipine against high glucose-induced caspase 3 activation and lamin B degradation in normal rodent islets. Further, we examined whether protection of these cellular events by nifedipine is mediated via inhibition of glucose-induced ER stress. To accomplish this, normal rat islets were incubated in the presence of low or high glucose in the absence or presence of nifedipine, and expression of CHOP, caspase 3 activation and lamin B degradation were quantified in cell lysates as described above. Data presented in Table I demonstrate a significant increase in the expression of CHOP in rat islets exposed to high glucose, which was inhibited by nifedipine. These data suggest that glucose-mediated effects involve increase in ER stress, which is sensitive to inhibition of calcium channels. In a manner akin to these effects, nifedipine significantly prevented glucose-induced activation of caspase 3 in rat islets. The extent of lamin B degradation reached to basal values in cells incubated simultaneously with high glucose and nifedipine [Table I]. It is important to note that nifedipine, by itself, increased caspase 3 activation and lamin B degradation under low glucose conditions; such effects might have masked its effects on glucose-induced caspase 3 activation and lamin B degradation [Table I].
Table I.
Effect of nifedipine on glucose-induced CHOP expression, caspase 3 activation and lamin B degradation in normal rat islets
| Treatment conditions | CHOP expression | Caspase 3 activation | Lamin-B degradation |
|---|---|---|---|
| Low glucose | 1.00 | 1.00 | 1.00 |
| Low glucose + nifedipine | 1.11±0.16 | 1.42±0.21 | 1.33±0.39 |
| High glucose | 1.62±0.11* | 1.67±0.12* | 1.28±0.17 |
| High glucose+ nifedipine | 1.21±0.10** | 0.63±0.31** | 0.93±0.19 |
Rat islets were incubated in the presence of low (2.5 mM) or high (20 mM) glucose for 24 hrs in the presence and absence of nifedipine (10 µM) as indicated. Lysate proteins [40 µg] were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed for CHOP, cleaved caspase 3 and degraded lamin B, and immune complexes were identified using ECL detection kit. To check equal protein loading, the membranes were stripped and reprobed for actin. Intensity of protein bands was quantified by densitometry. The statistical significance of the differences between the experimental conditions was determined by t-test. Data represent mean ± SEM from three to four independent experiments and expressed as fold change over low glucose exposure condition.
P < 0.05 vs. low glucose and
P < 0.05 vs. high glucose.
Taken together, our findings provide the first direct evidence to suggest that glucotoxic conditions induce ER stress to promote caspase 3 activation and lamin B degradation in INS-1 832/13 cells and normal rodent islets. Our data also suggest significant alterations in subcellular distribution of lamin B and its degradation products in these cells under the duress of glucotoxicity.
4. Discussion
Several lines of evidence in clonal β-cells, normal rodent islets and human islets implicate that chronic exposure of these cells to elevated glucose induces metabolic dysfunction that leads to loss in their viability culminating in apoptosis [14,24–26]. Several mechanisms have been postulated for metabolic dysregulation of the islet including generation of oxidative stress and ER stress culminating in mitochondrial abnormalities [14,27,28]. However, very little is known to date with regard to alterations in the nuclear compartment induced by chronic hyperglycemic conditions. Our findings suggested that; (i) high glucose induces caspase 3 activation, which in turn, degrades nuclear lamin B; (ii) high glucose also induces significant alterations in the subcellular distribution of lamin B; (iii) thapsigargin, a known inducer of ER stress in many cell types, including the islet β-cell, also induces caspase 3 activation and lamin B degradation ; (iv) nifedipine, a known inhibitor of plasma membrane-associated calcium channels, markedly attenuates glucose-induced caspase-3 activation and lamin-B degradation; and (v) PBA, a known inhibitor of ER-stress, markedly reduced glucose-induced CHOP expression, caspase 3 activation and lamin B degradation. Based on these findings we propose that nifedipine-sensitive influx and intracellular accumulation of calcium as one of the signaling events leading to glucose-induced metabolic dysfunction of the islet β-cell, leading to the activation of caspase- 3 and lamin-B degradation (see below).
Lamins are intermediate filament proteins that constitute the main components of the lamina underlying the inner-nuclear membrane and serve to organize chromatin. Our current findings suggested significant alterations in the subcellular distribution of lamin B in INS-1 832/13 cells under conditions of exposure to high glucose. What then might be the mechanisms underlying abnormal distribution of lamin B that we observed in beta-cells exposed to high glucose conditions? It is well established that in a manner akin to small molecular weight G-proteins and the γ-subunits of trimeric G-proteins, nuclear lamins undergo post-translational farnesylation, catalyzed by the farnesyltransferase [FTase]. Along these lines, we have recently reported caspase 3-mediated degradation of the α-subunit of FTase in insulin-secreting cells [22]. It has been shown in other cells that caspase-mediated degradation of FTase results in functional inactivation of the enzyme, which presumably could lead to impaired farnesylation of substrate proteins culminating in mistargeting of these proteins [29]. It is likely that similar alterations in the farnesylation of lamin B might be taking place in the beta-cell under high glucose-treatment conditions. Additional studies are needed to test such a possibility. Along these lines, recent studies by Chang and associates have demonstrated that inhibition of farnesylation using FTIs led to a marked reduction in the biogenesis of mature lamin A culminating in the accumulation of prelamin A intracellularly. Further, their data suggested a critical requirement for a geranylgeranylation signaling step in the conversion of farnesylated lamin A to its mature form mediated by a zinc metalloprotease [30]. Together, these findings implicate novel roles for post-translational prenylation steps in the functional regulation of lamins. It remains to be determined if glucotoxic conditions impair normal processing of lamins due to degradation of prenylating enzymes under glucotoxic conditions, and if so, whether unprenylated lamins are more vulnerable to degradation by caspase 3.
It is also noteworthy that our current observations also suggested regulatory roles for accumulation of cytosolic calcium in glucose-induced effects on caspase 3 activation and lamin B degradation. As described above, our findings are in agreement with observations by Wang et al. [23] who reported cytoprotective effects, by nifedipine, against endoplasmic reticulum stress and apoptosis induced by glucotoxic conditions in pancreatic β-cells. Therefore, it is likely that such conditions could also promote β-cell dysfunction via calcium-mediated mitochondrial dysregulation, caspase 3 activation and lamin B degradation pathways leading to loss in cell viability. This possibility needs to be verified experimentally. Indeed, recent observations by Xu and associates [31] have provided compelling evidence to suggest a marked reduction by verapamil, a calcium channel blocker, of increased expression of pro-apoptotic thioredoxin-interacting protein expression and restoration of normal β-cell survival and function in the BTBR ob/ob mice. Our current findings provide further evidence in support of the formulation that influx of extracellular calcium and subsequent overload of mitochondrial calcium leads to dysregulation of cellular function. Thus, calcium channel blockers such as verapamil [22,23,31] and nifedipine (current study) appear to exert cytoprotective effects in β-cells against noxious stimuli and improve β-cell survival and function.
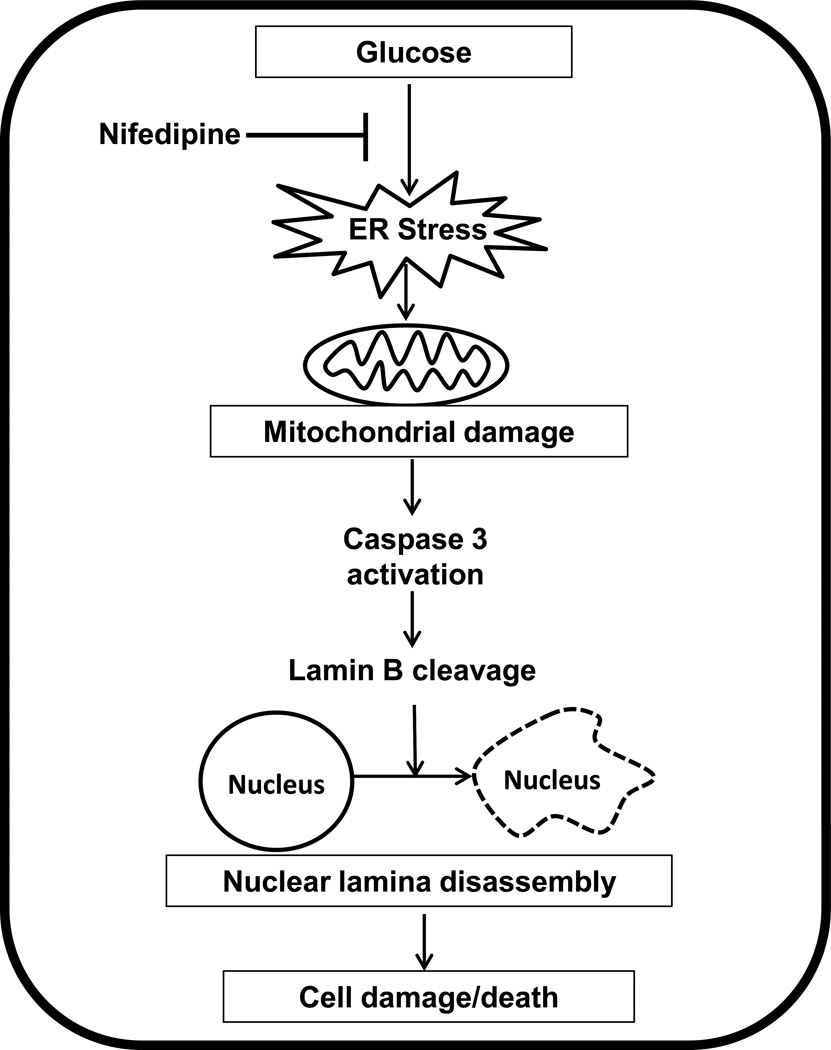
Based on the published evidence and data accrued in the current study we propose that exposure of isolated β-cells to hyperglycemic conditions leads to increased oxidative and ER stress leading to mitochondrial dysfunction [Figure 7]. Consequentially, cytosolic caspase 3 is activated by cytochrome C released from the dysregulated mitochondria. As demonstrated in the current study, caspase 3 activation leads to degradation of lamin B culminating in nuclear damage and altered distribution of degraded lamin B into various subcellular compartments, and nuclear lamina disassembly and associated loss in cell viability. We also propose that glucose-induced metabolic dysfunction in these cells may, in part, be due to increase in the ER stress as evidenced by significant inhibition of glucose-induced CHOP expression, caspase 3 activation and lamin B degradation by PBA, a known inhibitor of ER stress. Our findings also suggest cytoprotective effects of calcium channel blockers [e.g., nifedipine] against noxious effects of glucose as demonstrated in this study and data from other laboratories [22, 23,31]. Lastly, our studies provide further support to the recent observations by Tang and associates establishing clear roles for the oxidative stress-ER stress signaling axis in glucose-induced β-cell dysfunction in vivo in rats [34]
Figure 7. Proposed model for high glucose-mediated, ER-stress-induced β-cell damage/death via caspase 3 mediated degradation of lamin B in pancreatic β-cells.
We propose that exposure of insulin-secreting cells to high glucose leads to increased oxidative and ER stress leading to mitochondrial dysfunction [14,32,33]. As a consequence, cytosolic caspase 3 is activated by the released cytochrome C from the dysregulated mitochondria. As shown in this study, caspase 3 activation leads to degradation of lamin B culminating in nuclear damage and altered distribution of degraded lamin B into various subcellular compartments leading to nuclear lamina disassembly. Our current findings suggest that glucose-induced metabolic dysfunction in these cells may, in part, be due to increase in the ER stress. Our model also highlights potential cytoprotective effects of calcium channel blockers [e.g., nifedipine] against noxious effects of hyperglycemia as demonstrated in this study, and by other recent studies [22,23,31].
Acknowledgement
This research was supported in part by a Merit Review Award (to AK; 1BX000469) from the Department of VA, and the National Institutes of Health [RO1 DK74921]. AK is also the recipient of a Senior Research Career Scientist Award from the Department of VA. KS is the recipient of Rumble Fellowship from Wayne State University. AMM is the recipient of Rumble Fellowship from Wayne State University and George Fuller Endowed Fellowship from the Eugene Applebaum College of Pharmacy and Health Sciences. We thank Prof. Chris Newgard for kindly providing INS-1 832/13 cells.
Abbreviations
- CHOP
C/EBP homologous protein
- ER
Endoplasmic reticulum
- FTase
Farnesyl transferase
- FTI
Farnesyl transferase inhibitor
- Nif
Nifedipine
- PBA
4-Phenylbutyric acid
- Tg
Thapsigargin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bantel H, Schulze-Osthoff K. Mechanisms of cell death in acute liver failure. Front Physiol. 2012;3:79. doi: 10.3389/fphys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 3.Chandra J, Zhivotovsky B, Zaitsev S, Juntti-Berggren L, Berggren PO, Orrenius S. Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes. 2001;50:S44–S47. doi: 10.2337/diabetes.50.2007.s44. [DOI] [PubMed] [Google Scholar]
- 4.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 5.Lazebnik YA, Takahashi A, Moir RD, Goldman RD, Poirier GG, Kaufmann SH, et al. Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc Natl Acad Sci USA. 1995;92:9042–9046. doi: 10.1073/pnas.92.20.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke B, Stewart CL. The nuclear lamins: flexibility in Function. Nat Rev Mol Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 7.Gruenbaum Y, Wilson KL, Harel A, Goldberg M, Cohen M. Review: nuclear lamins--structural proteins with fundamental functions. J Struct Biol. 2000;129:313–323. doi: 10.1006/jsbi.2000.4216. [DOI] [PubMed] [Google Scholar]
- 8.Rao L, Perez D, White E. Lamin Proteolysis Facilitates Nuclear Events During Apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neamati N, Fernandez A, Wright S, Kiefer J, McConkey DJ. Degradation of Lamin B, Precedes Oligonucleosomal DNA Fragmentation in Apoptotic Thymocytes and Isolated Thymocyte Nuclei. J Immunol. 1995;154:3788–3795. [PubMed] [Google Scholar]
- 10.Veluthakal R, Amin R, Kowluru A. Interleukin-1 induces posttranslational carboxymethylation and alterations in subnuclear distribution of lamin B in insulin-secreting RINm5F cells. Am J Physiol Cell Physiol. 2004;287:C1152–C1162. doi: 10.1152/ajpcell.00083.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kivinen K, Kallajoki M, Taimen P. Caspase-3 is required in the apoptotic disintegration of the nuclear matrix. Exp Cell Res. 2005;311:62–73. doi: 10.1016/j.yexcr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Almaguel FG, Liu JW, Pacheco FJ, Casiano CA, De Leon M. Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. J Neurosci Res. 2009;87:1207–1218. doi: 10.1002/jnr.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos C. Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae. Postgrad Med. 2012;124:90–97. doi: 10.3810/pgm.2012.11.2615. [DOI] [PubMed] [Google Scholar]
- 14.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonas JC, Bensellam M, Duprez J, Elouil H, Guiot Y, Pascal SM. Glucose regulation of islet stress responses and beta-cell failure in type 2 diabetes. Diabetes Obes Metab. 2009;11:65–81. doi: 10.1111/j.1463-1326.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- 16.Jayaram B, Syed I, Kyathanahalli CN, Rhodes CJ, Kowluru A. Arf nucleotide binding site opener [ARNO] promotes sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 β-cells and rat islets. Biochem Pharmacol. 2011;81:1016–1027. doi: 10.1016/j.bcp.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon MJ, Chung HS, Yoon CS, Lee EJ, Kim TK, Lee SH, et al. Low glibenclamide concentrations affect endoplasmic reticulum stress in INS-1 cells under glucotoxic or glucolipotoxic conditions. Korean J Intern Med. 2013;28:339–346. doi: 10.3904/kjim.2013.28.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachar E, Ariav Y, Ketzinel-Gilad M, Cerasi E, Kaiser N, Leibowitz G. Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic beta-cells via activation of mTORC1. PLoS One. 2009;4:e4954. doi: 10.1371/journal.pone.0004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian B, Wang H, Men X, Zhang W, Cai H, Xu S, et al. TRIB3 is implicated in glucotoxicity- and endoplasmic reticulum-stress-induced beta-cell apoptosis. J Endocrinol. 2008;199:407–416. doi: 10.1677/JOE-08-0331. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 21.Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 22.Arora DK, Mohammed AM, Kowluru A. Nifedipine prevents etoposide-induced caspase-3 activation, prenyl transferase degradation and loss in cell viability in pancreatic β-cells. Apoptosis. 2013;18:1–8. doi: 10.1007/s10495-012-0763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Gao L, Li Y, Chen H, Sun Z. Nifedipine Protects INS-1 β-Cell from High Glucose-Induced ER Stress and Apoptosis. Int J Mol Sci. 2011;12:7569–7580. doi: 10.3390/ijms12117569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim WH, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, et al. Exposure to chronic high glucose induces beta-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic beta-cells. Diabetes. 2005;54:2602–2611. doi: 10.2337/diabetes.54.9.2602. [DOI] [PubMed] [Google Scholar]
- 25.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 26.Piro S, Anello M, Di Pietro C, Lizzio MN, Patanè G, Rabuazzo AM, et al. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism. 2002;51:1340–1347. doi: 10.1053/meta.2002.35200. [DOI] [PubMed] [Google Scholar]
- 27.Leem J, Koh EH. Interaction between mitochondria and the endoplasmic reticulum: implications for the pathogenesis of type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:242984. doi: 10.1155/2012/242984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol Metab. 2012;23:477–487. doi: 10.1016/j.tem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Kim KW, Chung HH, Chung CW, Kim IK, Miura M, Wang S, et al. Inactivation of farnesyltransferase and geranylgeranyltransferase I by caspase-3: cleavage of the common alpha subunit during apoptosis. Oncogene. 2001;20:358–366. doi: 10.1038/sj.onc.1204099. [DOI] [PubMed] [Google Scholar]
- 30.Chang SY, Hudon-Miller SE, Yang SH, et al. Inhibitors of protein geranylgeranyltransferase-I lead to prelamin A accumulation in cells by inhibiting ZMPSTE24. J Lipid Res. 2012;53:1176–1182. doi: 10.1194/jlr.M026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu G, Chen J, Jing G, Shalev A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;6:848–856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syed I, Kyathanahalli CN, Jayaram B, Govind S, Rhodes CJ, Kowluru RA, Kowluru A. Increased phogocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes. 2011;60:2843–2852. doi: 10.2337/db11-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang C, Koulajian K, Schuiki I, Zhang L, Desai T, Ivovic A, Wang P, Robson-Doucette C, Wheeler MB, Minassian B, Volchuk A, Giacca A. Glucose-induced beta cell dysfunction in in vivo in rats: link between oxidative stress and endoplasmic reticulum stress. Diabetologia. 2012;55:1366–1379. doi: 10.1007/s00125-012-2474-8. [DOI] [PubMed] [Google Scholar]