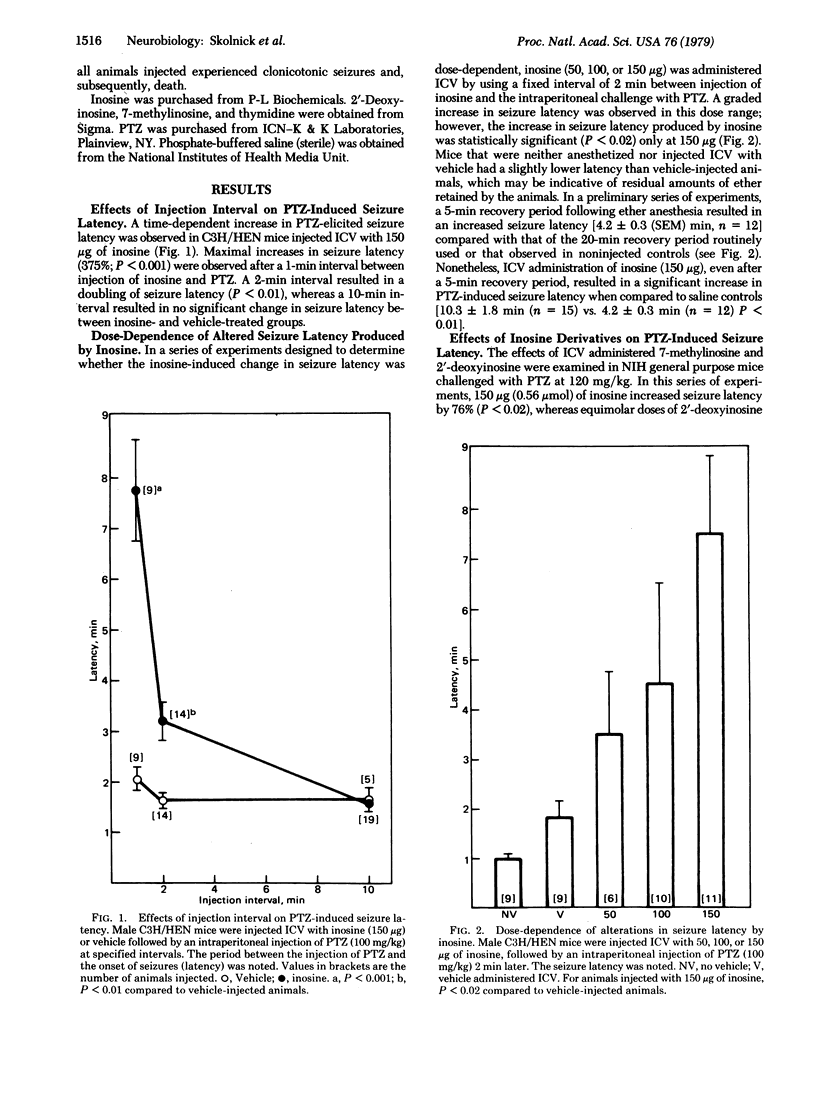
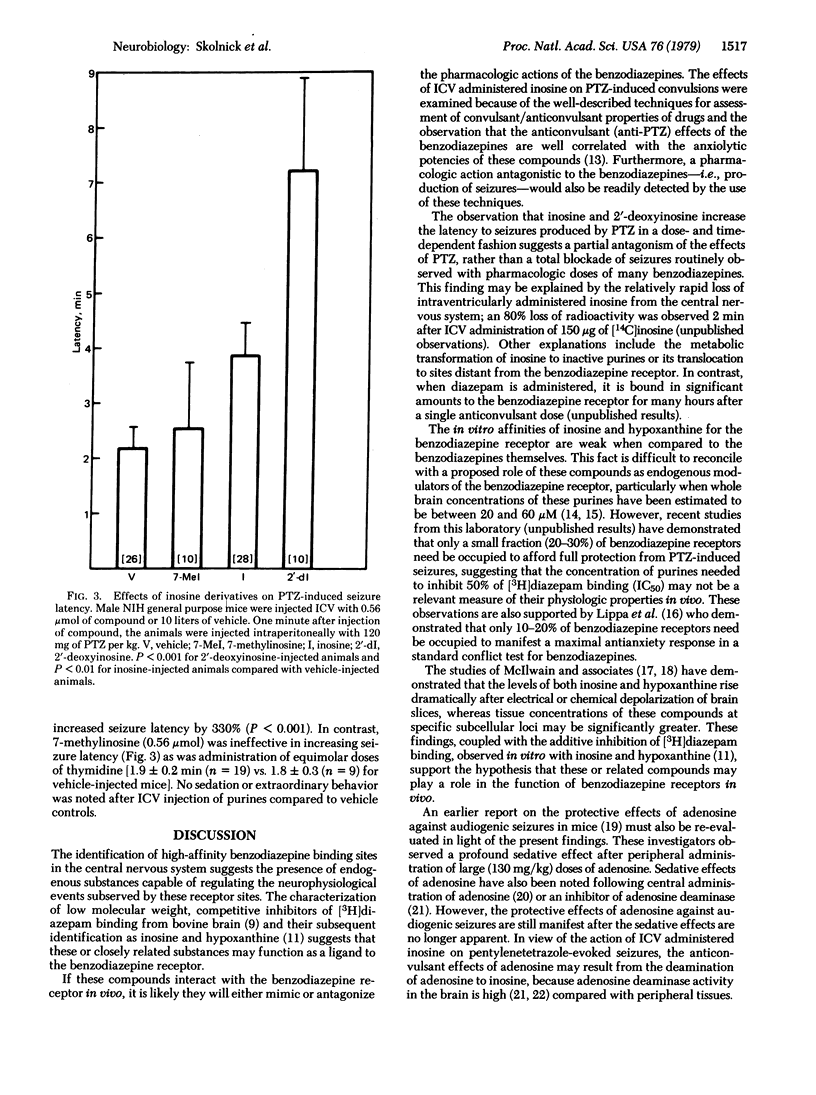
Abstract
Partially purified extracts of bovine brain were previously found to inhibit competitively the binding of [3H]-diazepam to rat brain synaptosomal membranes. The purines inosine and hypoxanthine were subsequently identified as the compounds responsible for this inhibitory activity. Intracerebroventricular administration of inosine to mice of the C3H/HEN and NIH general purpose strains caused a dose- and time-dependent increase in the latency to clonicotonic seizures produced by intraperitoneal administration of the convulsant pentylenetetrazole. Intracerebroventricular administration of equimolar doses of 2'-deoxyinosine, which is more potent than inosine in inhibiting the binding of [3H]diazepam in vitro, significantly increased pentylenetetrazole-evoked seizure latency. In contrast, both 7-methylinosine and thymidine were ineffective in inhibiting the in vitro binding of [3H]diazepam and increasing the latency to pentylenetetrazole-induced seizures in vivo. These results suggest that endogenously occurring purines such as inosine exhibit diazepam like effects when administered intracerebroventricularly, and these effects may be related to the interaction of inosine and related compounds with benzodiazepine receptors in the central nervous system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braestrup C., Albrechtsen R., Squires R. F. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977 Oct 20;269(5630):702–704. doi: 10.1038/269702a0. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Squires R. F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Snyder S. H. Benzodiazepine receptors: labeling in intact animals with [3H] flunitrazepam. Eur J Pharmacol. 1978 Mar 15;48(2):213–218. doi: 10.1016/0014-2999(78)90330-8. [DOI] [PubMed] [Google Scholar]
- Karobath M., Sperk G., Schönbeck G. Evidence for an endogenous factor interfering with 3H-diazepam binding to rat brain membranes. Eur J Pharmacol. 1978 Jun 1;49(3):323–326. doi: 10.1016/0014-2999(78)90111-5. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Kobayashi K., Hossmann K. A. Purine nucleotide metabolism in the cat brain after one hour of complete ischemia. J Neurochem. 1974 Aug;23(2):417–425. doi: 10.1111/j.1471-4159.1974.tb04374.x. [DOI] [PubMed] [Google Scholar]
- Maitre M., Chesielski L., Lehmann A., Kempf E., Mandel P. Protective effect of adenosine and nicotinamide against audiogenic seizure. Biochem Pharmacol. 1974 Oct 15;23(20):2807–2816. doi: 10.1016/0006-2952(74)90054-9. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Paul S. M., Greenlaw P., Goodwin F. K., Skolnick P. Demonstration of an endogenous, competitive inhibitor(s) of [3H] diazepam binding in bovine brain. Life Sci. 1978 Jun 5;22(21):1893–1900. doi: 10.1016/0024-3205(78)90476-9. [DOI] [PubMed] [Google Scholar]
- Möhler H., Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977 Nov 25;198(4319):849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- Möhler H., Okada T. Properties of 3H-diazepam binding to benzodiazepine receptors in rat cerebral cortex. Life Sci. 1977 Jun 15;20(12):2101–2110. doi: 10.1016/0024-3205(77)90191-6. [DOI] [PubMed] [Google Scholar]
- Noble E. P., Wurtman R. J., Axelrod J. A simple and rapid method for injecting H3-norepinephrine into the lateral ventricle of the rat brain. Life Sci. 1967 Feb 1;6(3):281–291. doi: 10.1016/0024-3205(67)90157-9. [DOI] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Adenine derivatives as neurohumoral agents in the brain. The quantities liberated on excitation of superfused cerebral tissues. Biochem J. 1972 Dec;130(4):975–981. doi: 10.1042/bj1300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Rat cerebral-cortex adenosine deaminase activity and its subcellular distribution. Biochem J. 1974 Oct;144(1):37–41. doi: 10.1042/bj1440037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad O. D., Schrader H. The determination of inosine and hypoxanthine in the rat brain during normothermic and hypothermic anoxia. Acta Neurol Scand. 1978 Apr;57(4):281–288. doi: 10.1111/j.1600-0404.1978.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Skolnick P., Marangos P. J., Goodwin F. K., Edwards M., Paul S. Identification of inosine and hypoxanthine as endogenous inhibitors of [3H] diazepam binding in the central nervous system. Life Sci. 1978 Oct 9;23(14):1473–1480. doi: 10.1016/0024-3205(78)90128-5. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Brastrup C. Benzodiazepine receptors in rat brain. Nature. 1977 Apr 21;266(5604):732–734. doi: 10.1038/266732a0. [DOI] [PubMed] [Google Scholar]
- Sun M. C., McIlwain H., Pull I. The metabolism of adenine derivatives in different parts of the brain of the rat, and their release from hypothalamic preparations on excitation. J Neurobiol. 1976 Mar;7(2):109–122. doi: 10.1002/neu.480070204. [DOI] [PubMed] [Google Scholar]
- Williamson M. J., Paul S. M., Skolnick P. Demonstration of [3H] diazepam binding to benzodiazepine receptors in vivo. Life Sci. 1978 Nov 9;23(19):1935–1940. doi: 10.1016/0024-3205(78)90560-x. [DOI] [PubMed] [Google Scholar]
- Williamson M. J., Paul S. M., Skolnick P. Labelling of benzodiazepine receptors in vivo. Nature. 1978 Oct 12;275(5680):551–553. doi: 10.1038/275551a0. [DOI] [PubMed] [Google Scholar]



