Abstract
Background
Heterogeneous echogenicity of the thyroid gland has been associated with diffuse thyroid disease and benign and malignant nodules can coexist with diffuse thyroid disease. Underlying heterogeneous echogenicity might make it difficult to differentiate between benign and malignant nodules on US. Thus, the aim of this study was to evaluate the influence of underlying thyroid echogenicity on diagnosis of thyroid malignancies using US.
Methods
A total of 1,373 patients who underwent US-guided fine needle aspiration of 1,449 thyroid nodules from June 2009 to August 2009 were included. The diagnostic performance of US assessment for thyroid nodules was calculated and compared according to underlying thyroid echogenicity. The diagnostic performance of US assessments in the diagnosis of thyroid malignancy according to the underlying parenchymal echogenicity was compared using a logistic regression with the GEE (generalized estimating equation) method. Each US feature of malignant and benign thyroid nodules was analyzed according to underlying echogenicity to evaluate which feature affected the final diagnosis.
Results
Among the 1,449 nodules, 325 (22.4%) were malignant and 1,124 (77.6%) were benign. Thyroid glands with heterogeneous echogenicity showed significantly lower specificity, PPV, and accuracy compared to thyroid glands with homogeneous echogenicity, 76.3% to 83.7%, 48.7% to 60.9%, and 77.6% to 84.4%, respectively (P = 0.009, 0.02 and 0.005, respectively). In benign thyroid nodules, microlobulated or irregular margins were more frequently seen in thyroid glands with heterogeneous echogenicity than in those with homogenous echogenicity (P < 0.001).
Conclusion
Heterogeneous echogenicity of the thyroid gland significantly lowers the specificity, PPV, and accuracy of US in the differentiation of thyroid nodules. Therefore, caution is required during evaluation of thyroid nodules detected in thyroid parenchyma showing heterogeneous echogenicity.
Keywords: Ultrasonography, Thyroid gland, Diffuse thyroid disease, Thyroid malignancy, Thyroid nodule
Background
Heterogeneous echogenicity of the thyroid gland has been associated with diffuse thyroid disease (DTD) including Hashimoto thyroiditis (HT) and Graves’ disease [1-4]. Ultrasonographic (US) features of HT have been reported to show a broad spectrum of abnormal features ranging from focal ill-defined hypoechoic areas to diffuse homogeneous hypoechoic regions showing areas of internal echogenic fibrous septa or diffuse heterogeneous hypoechogenicity showing micronodular patterns [1-4].
Benign and malignant nodules can coexist with DTD [5,6]. In particular, the association between HT and papillary thyroid carcinoma (PTC) has been reported in many studies [5,7-9]. Although US features of malignant thyroid nodules with diffuse HT have been reported to be similar to typical malignant US features [10], underlying heterogeneous echogenicity might make it difficult to differentiate between benign and malignant nodules. Besides these considerations, there are no published reports on this topic: Does underlying thyroid parenchyma echogenicity affect the analysis of a thyroid nodule? If it does, what are the associated US features impacting the analysis of a thyroid nodule?
This study investigated the influence of underlying thyroid echogenicity on the diagnosis of thyroid malignancies.
Methods
This retrospective study was approved by the institutional review board (IRB) and ethics committee of Severance hospital, Seoul, Korea. Neither patient approval nor informed consent was required for review of medical records or images. Informed consent was signed and obtained from all patients before US-FNA or surgery prior to procedures as a daily practice.
Between June 2009 and August 2009, there were 1,534 consecutive patients with 1,632 thyroid nodules who underwent US-guided fine needle aspiration (US-FNA) on focal thyroid nodules larger than 5 mm in our institution (a referral center) in Korea. Among them, we retrospectively enrolled 1,373 patients with 1,449 thyroid nodules, from whom we could obtain cytopathologic results and follow-up data (Figure 1). There were 3 patients who underwent US-FNAs at 3 nodules, 70 patients who underwent US-FNAs at 2 nodules, and 1300 patients who underwent US-FNAs at 1 nodule. The mean age of patients included was 50.8 years (range, 15–95 years). Among the 1,373 patients, 1,126 were women (mean age, 50.5 years, range, 15–95 years) and 247 were men (mean age, 52.1 years, range, 25–80 years).
Figure 1.
Diagram of the study group. *Exclusion criteria in the result.
US and US-FNA
US examinations and US-FNA were performed by one of seven board-certified radiologists with 1 to 15 years of experience in thyroid imaging, using a 7- to 15- MHz linear probe (HDI 5000, Philips-Advanced Technology Laboratories, Bothell, WA, USA) or a 5- to 12- MHz linear probe (iU22, Philips-Advanced Technology Laboratories, Bothell, WA, USA). Compound imaging was performed for all US examinations. US features of the underlying thyroid parenchyma and thyroid nodule targeted for US-FNA were assessed at the time of US examination and US-FNA. Diffuse echogenicity of the thyroid parenchyma showing numerous micronodular appearances or echogenic septations was defined as ‘heterogeneous echogenicity’ of the thyroid gland [6,11,12]. Thyroid nodules were classified according to internal component, echogenicity, margin, calcification, and shape on US. Marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller than wide shape were considered suspicious malignant features of thyroid nodules on US (Figure 2) [13]. When thyroid nodules had one or more of the previously mentioned suspicious malignant US features, they were classified as “positive US”. When the thyroid nodules showed no suspicious malignant features, they were classified as “negative US”. After US, each US feature was recorded by the radiologists who performed the US on provided result sheets including the underlying echogenicity of the thyroid gland on US.
Figure 2.
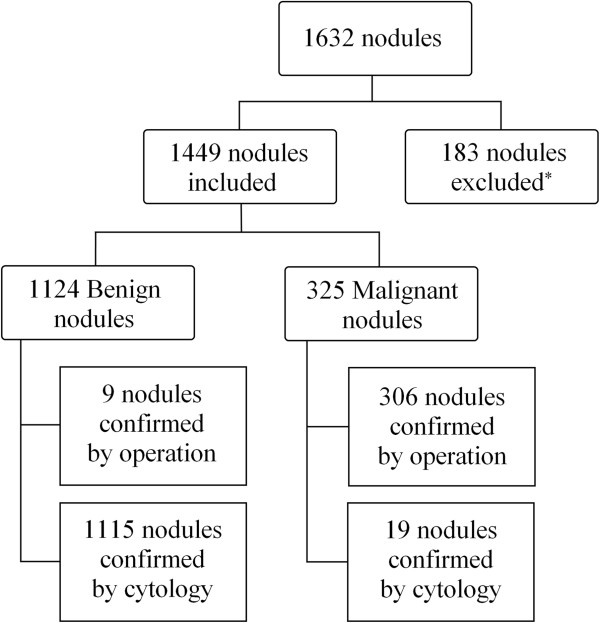
US findings of a malignant thyroid nodule in underlying homogenous thyroid echogenicity. (a) Transverse and (b) longitudinal US showed a 6-mm irregular, taller than wide nodule (arrows) with homogenous echogenicity of the underlying thyroid gland in the right thyroid gland. The lesion was diagnosed as papillary microcarcinoma on surgical histopathology.
At our institution, we do not routinely undergo FNA at thyroid nodules less than 5 mm. The US-FNAs were performed either on the thyroid nodule with suspicious US features or on the largest thyroid nodule if no suspicious US features were detected. However, FNAs were sometimes performed on multiple nodules in one patient because of multiple suspicious US features, physician’s or patient’s request. US-FNAs were performed using a freehand biopsy technique with a 23-gauge needle attached to a 2-ml disposable plastic syringe. Each lesion was aspirated at least twice. Aspirated material was expelled onto glass slides that were immediately placed in 95% alcohol for Papanicolaou staining. The remaining aspirated material in the syringe was rinsed with saline and processed for cell block preparation. Five experienced cytopathologists interpreted the cytology slides. In the study period, cytological reports were classified as (a) benign, (b) indeterminate, (c) suspicious for papillary thyroid carcinoma, (d) malignant, or (e) nondiagnostic. Among cases with benign cytology, lymphocytic thyroiditis was further diagnosed when the cytological specimen met the following criteria: the specimen showed grouped, monolayer sheets or scattered follicular and Hurthle cells with scattered lymphocytes; the colloid was scanty; and the follicular cells showed nuclear atypia with nuclear enlargement and clearing in the absence of nuclear grooves or inclusions [14]. A non-diagnostic cytology result was defined as the presence of less than six groups of cells, each containing at least ten cells [15,16]. Indeterminate cytology included follicular or Hurthle cell neoplasm. The “suspicious for papillary carcinoma” cytological result was designated when the specimen exhibited cytological atypia (nuclei are crowded and overlapping, enlarged, and pleomorphic) but showed insufficient cellularity for definite diagnosis of papillary carcinoma [17]. For this study, we recorded the results by retrospectively reviewing the cytological reports.
Measurement of serum anti-thyroid autoantibodies
Anti-thyroid antibodies were evaluated using venous blood samples from 938 patients. Serum thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TgAb) and TSH-binding inhibitory immunoglobulins (TBII) levels were measured by radioimmunoassay (Brahms, Hennigsdorf/Berlin, Germany). The existence of TPOAb and/or TgAb was defined by a serum concentration of the relevant thyroid autoantibody > 60 IU/L. Patients with HT were defined by positive results for TPOAb and/or TgAb [18]. A TBII exceeding 10% was considered positive. Patients with Graves’ disease were defined as positive for TBII.
Statistical analysis
Histopathology results from surgery or US-FNA cytology were considered the standard reference of thyroid nodules. Statistical comparisons were performed using the Chi-square test for categorical variables and independent t-test for continuous variables. The diagnostic performance of US assessments of thyroid nodules according to the echogenicity of underlying thyroid parenchyma was calculated, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. The diagnostic performance of US assessments in the diagnosis of thyroid malignancy according to the underlying parenchymal echogenicity was compared using a logistic regression with the GEE (generalized estimating equation) method. We considered P-values less than 0.05 statistically significant. Statistical analysis was performed using commercial statistical software (SAS version 9.1, SAS Inc., Cary, NC, USA).
Results
We retrospectively enrolled 1,632 thyroid nodules from 1,534 patients, from whom we could obtain cytologic results. We excluded 125 nodules with non-diagnostic results of FNA, 16 nodules with atypical follicular epithelial cells, 2 nodules with results of parathyroid cells and lymph nodes, and 40 nodules without US findings available. Among the 1,449 nodules, 325 (22.4%) were malignant and 1,124 (77.6%) were benign (Figure 1). Histopathologic diagnoses of the 315 thyroid nodules are listed in Table 1. Patients (51.6 ± 11.8 years) diagnosed with benign nodules were significantly older than those (47.8 ± 12.6 years) diagnosed with malignant nodules (P < .001). The mean size of the benign nodules were 16.6 ± 10.5 mm, which was significantly larger than that of the malignant nodules, 11.8 ± 8.6 mm (P < .001). Gender was not associated with malignancy (P = 0.954).
Table 1.
Histopathologic diagnosis of 315 thyroid nodules
| Final pathology | n (%) |
|---|---|
|
Malignant (n = 306, 97.1%) |
|
| Papillary carcinoma, conventional |
264 (86.3) |
| Papillary carcinoma, follicular variant |
29 (9.5) |
| Papillary carcinoma, diffuse sclerosing variant |
5 (1.6) |
| Papillary carcinoma, oncocytic variant |
3 (1.0) |
| Medullary carcinoma |
3 (1.0) |
| Follicular carcinoma |
1 (0.3) |
| Hűrthle cell carcinoma |
1 (0.3) |
|
Benign (n = 9, 2.9%) |
|
| Adenomatous hyperplasia |
8 (88.9) |
| Hyalinizing trabecular adenoma | 1 (11.1) |
The mean age (52.3 ± 12.4 years) of the patients with underlying heterogeneous echogenicity of the thyroid gland was older than that (50.4 ± 12 years) of the patients with underlying homogeneous thyroid echogenicity (P = 0.015). In the underlying heterogeneous echogenicity group, 270 were women and 28 were men while in the underlying homogenous echogenicity group, 856 were female and 219 were male, exhibiting female predominancy in the underlying heterogeneous echogenicity group (P < .001). The mean size (15.6 ± 10.5 mm) of the nodules in the underlying homogenous echogenicity group was larger than that (14.7 ± 9.2 mm) of underlying heterogeneous echogenicity group, but it was not statistically significant (P = 0.119).
Of the 1,449 nodules included, 317 (21.9%) showed underlying heterogeneous echogenicity of the thyroid parenchyma on US. Table 2 shows the diagnostic performance of US in the differential diagnosis of thyroid nodules, comparing the two groups with and without underlying heterogeneous echogenicity of the thyroid parenchyma on US. The thyroid nodules in a thyroid gland with heterogeneous echogenicity had a significantly lower specificity, PPV and accuracy compared to those with homogeneous echogenicity. There were no significant differences in sensitivity and NPV between the two groups. To document the reason for different diagnostic performances of US according to the echogenicity of the thyroid parenchyma, we analyzed each US feature by the malignant and benign thyroid group according to the underlying echogenicity of the thyroid gland (Table 3). In benign thyroid nodules, microlobulated or irregular margins on US were more frequently seen in nodules in a thyroid gland with heterogeneous echogenicity than in those with homogenous echogenicity (P < 0.001) (Figure 3). On the other hand, in malignant thyroid nodules, there were no significant differences in US features according to the underlying echogenicity of the thyroid gland. Among a total of 1124 nodules diagnosed as benign thyroid nodules, 875 nodules were seen in the background of homogenous thyroid echogenicity and the other 249 nodules were found in that of heterogeneous thyroid echogenicity. The nodules that were diagnosed as lymphocytic thyroiditis occurred in 1.5% (13/875) of underlying homogenous thyroid echogenicity, while under heterogeneous thyroid echogenicity 14.9% (37/249) were found to be focal lymphocytic thyroiditis (Figure 4). The margins of nodules with lymphocytic thyroiditis were microlobulated or irregular in 8 nodules with underlying homogeneous thyroid echogenicity and 16 nodules with underlying heterogeneous thyroid echogenicity. We also analyzed each US feature of 1399 nodules according to underlying thyroid gland echogenicity while excluding nodules which were diagnosed with lymphocytic thyroiditis to eliminate the lymphocytic thyroiditis effect on US diagnostic performance (Table 4). In benign thyroid nodules, we could still more often find microlobulated or irregular margin on US in nodules with heterogeneous thyroid echogenicity than in those with homogenous thyroid echogenicity (P = 0.007).
Table 2.
Diagnostic performance of US assessment in thyroid nodules according to the underlying echogenicity of the thyroid parenchyma
| Heterogeneous echogenicity on US | Homogeneous echogenicity on US | P -value | |
|---|---|---|---|
| Sensitivity |
82.4% (56/68) |
86.8% (223/257) |
0.354 |
| Specificity |
76.3% (190/249) |
83.7% (732/875) |
0.009 |
| Positive predictive value |
48.7% (56/115) |
60.9% (223/366) |
0.02 |
| Negative predictive value |
94.1% (190/202) |
95.6% (732/766) |
0.378 |
| Accuracy | 77.6% (246/317) | 84.4% (955/1132) | 0.005 |
Table 3.
Comparison of each US feature of 1449 thyroid nodules according to underlying echogenicity
| |
Total |
Malignant |
Benign |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Heterogeneous echogenicity | Homogeneous echogenicity | P -value | Heterogeneous echogenicity | Homogeneous echogenicity | P -value | Heterogeneous echogenicity | Homogeneous echogenicity | P -value | |
| Total number |
317 |
1132 |
|
68 |
257 |
|
249 |
875 |
|
| Echogenicity |
|
|
0.078 |
|
|
0.174 |
|
|
0.068 |
| Hyper/isoechogenicity |
125 (39.4) |
525 (46.4) |
|
3 (4.4) |
28 (10.9) |
|
122 (49.0) |
497 (56.8) |
|
| Hypoechogenicity |
176 (55.5) |
549 (48.5) |
|
56 (82.4) |
186 (72.4) |
|
120 (48.2) |
363 (41.5) |
|
| Marked hypoechogenicity |
16 (5.1) |
58 (5.1) |
|
9 (13.2) |
43 (16.7) |
|
7 (2.8) |
15 (1.7) |
|
| Margin |
|
|
0.005 |
|
|
0.314 |
|
|
<0.001 |
| Well circumscribed |
203 (64.0) |
818 (72.3) |
|
12 (17.7) |
60 (23.4) |
|
191 (76.7) |
758 (86.6) |
|
| microlobulated or irregular |
114 (36.0) |
314 (27.7) |
|
56 (82.4) |
197 (76.7) |
|
58 (23.3) |
117 (13.4) |
|
| Calcifications |
|
|
0.849 |
|
|
0.986 |
|
|
0.555 |
| Microcalcifications |
42 (13.3) |
141 (12.5) |
|
29 (42.7) |
108 (42.0) |
|
13 (5.2) |
33 (3.8) |
|
| Macrocalcifications |
39 (12.3) |
151 (13.3) |
|
9 (13.2) |
36 (14.0) |
|
30 (12.1) |
115 (13.1) |
|
| No calcifications |
236 (74.5) |
840 (74.2) |
|
30 (44.1) |
113 (44.0) |
|
206 (82.7) |
727 (83.1) |
|
| Shape |
|
|
0.737 |
|
|
0.790 |
|
|
0.245 |
| Wider than tall |
251 (79.2) |
906 (80.0) |
|
29 (42.7) |
105 (40.9) |
|
222 (89.2) |
801 (91.5) |
|
| Taller than wide | 66 (20.8) | 226 (20.0) | 39 (57.4) | 152 (59.1) | 27 (10.8) | 74 (8.5) | |||
Figure 3.
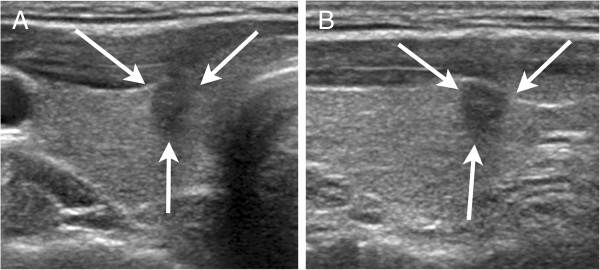
US finding of a false positive case with underlying heterogeneous thyroid gland. (a) Transverse and (b) longitudinal US showed 7-mm irregular, hypoechoic nodule (arrows) in heterogeneous echogenicity of the underlying thyroid gland in the left thyroid gland. The lesions was diagnosed with adenomatous hyperplasia on fine-needle aspiration biopsy and showed decrease in size on 5 years follow-up US.
Figure 4.
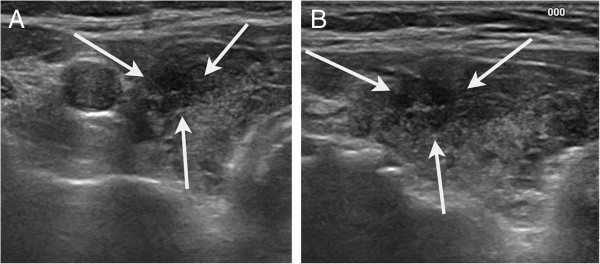
US finding of a false positive case with underlying heterogenous thyroid gland echogenicity. (a) Transverse and (b) longitudinal US showed an 8-mm microlobulated, marked hypoechoic nodule (arrows) with heterogenous echogenicity of the underlying thyroid gland. This lesion was later found to be lymphocytic thyroditis on fine-needle aspiration biopsy and no longer detectable on 2 years follow up US.
Table 4.
Comparison of each US feature of 1399 thyroid nodules excluding thyroid nodules with cytologic results of lymphocytic thyroiditis according to underlying echogenicity
| |
Total |
Malignant |
Benign |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Heterogeneous echogenicity | Homogeneous echogenicity | P -value | Heterogeneous echogenicity | Homogeneous echogenicity | P -value | Heterogeneous echogenicity | Homogeneous echogenicity | P -value | |
| Total number |
280 |
1119 |
|
68 |
257 |
|
212 |
862 |
|
| Echogenicity |
|
|
0.209 |
|
|
0.174 |
|
|
0.366 |
| Hyper/isoechogenicity |
114 (40.7) |
520 (46.5) |
|
3 (4.4) |
28 (10.9) |
|
111 (52.4) |
492 (57.1) |
|
| Hypoechogenicity |
152 (54.3) |
543 (48.5) |
|
56 (82.4) |
186 (72.4) |
|
96 (45.3) |
357 (41.4) |
|
| Marked hypoechogenicity |
14 (5.0) |
56 (5.0) |
|
9 (13.2) |
43 (16.7) |
|
5 (2.4) |
13 (1.5) |
|
| Margin |
|
|
0.012 |
|
|
0.314 |
|
|
0.007 |
| Well circumscribed |
182 (65.0) |
813 (72.7) |
|
12 (17.6) |
60 (23.3) |
|
170 (80.2) |
753 (87.4) |
|
| microlobulated or irregular |
98 (35.0) |
306 (27.3) |
|
56 (82.4) |
197 (76.7) |
|
42 (19.8) |
109 (12.6) |
|
| Calcifications |
|
|
0.728 |
|
|
0.986 |
|
|
0.613 |
| Microcalcifications |
40 (14.3) |
140 (12.5) |
|
29 (42.7) |
108 (42.0) |
|
11 (5.2) |
32 (3.7) |
|
| Macrocalcifications |
37 (13.2) |
149 (13.3) |
|
9 (13.2) |
36 (14.0) |
|
28 (13.2) |
113 (13.1) |
|
| No calcifications |
203 (72.5) |
830 (74.2) |
|
30 (44.1) |
113 (44.0) |
|
173 (81.6) |
717 (83.2) |
|
| Shape |
|
|
0.306 |
|
|
0.790 |
|
|
0.124 |
| Wider than tall |
217 (77.5) |
898 (80.3) |
|
29 (42.6) |
105 (40.9) |
|
188 (88.7) |
793 (92.0) |
|
| Taller than wide | 63 (22.5) | 221 (19.7) | 39 (57.4) | 152 (59.1) | 24 (11.3) | 69 (8.0) | |||
The diagnosis of DTD was based on either histopathologic reports (n = 51) or serum antibody testing (n = 369). Three hundred and sixty nine patients underwent serum TPOAb and TBII tests at least three months prior to US-FNA. Patients with DTD showed significantly more heterogeneous echogenicity (39.8%) of the thyroid parenchyma on US compared with patients without DTD (13.7%, P < 0.001).
Discussion
DTD encompasses diverse clinical entities including Graves’ disease and HT and it is commonly observed throughout the population. Annually around 0.5 per 1000 women develop Graves’ disease and a further 1-2% have autoimmune hypothyroidism including HT [19,20]. These disorders are 5 – 10 times more frequent in females [21] and our study also exhibits female predominancy (F:M = 4.7 :1). Both disorders share a cognate etiology with susceptibility determined by genetic factors and environmental factors but present with different clinical symptoms [21]. The most common cause of hypothyroidism is environmental iodine deficiency [22]. In areas of iodine sufficiency such as the United States and Korea, HT is the most common cause of hypothyroidism [23]. On the other hand, in European countries the atrophic variant of HT is much more common and mostly leads to hypothyroidism slowly [24]. Graves’ disease manifests as any form of hyperthyroidism with specific symptoms of Graves’ disease such as ophthalmopathy [20].
On US, a change in the underlying thyroid echotexture involving diffuse thyroid glands can help guide the diagnosis of DTD [1,25,26]. Characteristic US features of HT consist of numerous tiny hypoechoic nodulations or diffuse homogeneous hypoechogenicity with echogenic fibrous bands [1-4]. An abnormal thyroid gland pattern on US not only helps the diagnosis of asymptomatic DTD [16,27] but it can also be a good diagnostic predictor in patients with subclinical to overt hypothyroidism when combined with TPOAb and TgAb [28,29]. Furthermore, US findings of the thyroid gland can predict outcomes of levothyroxine treatment in patients with subclinical hypothyroidism [29].
Thyroid cancer is one of the most common cancers in the Korean population, and reported to be 64.4/100,000 [30]. Recently, the incidence of thyroid cancer has rapidly increased in Korea because the increasing use of high-resolution US and US-FNAs have enabled the detection of subclinical disease [30-32]. On the other hand, thyroid cancer is very rare in central Europe and comprises only a 3/100,000 incidence rate with high incidence of benign nodules [33]. Well acknowledged suspicious US features suggesting malignancy in thyroid nodules are microlobulated or irregular margins, microcalcifications, hypoechogenicity, and taller than wide shape [13]. Although US is a powerful modality for differentiating malignancy from benign focal thyroid nodules [13,15,34], some studies have speculated that it might be difficult to detect malignant nodules in patients with HT on US because the heterogeneous hypoechogenicity and micronodulation seen in HT are somewhat similar to features seen in malignant thyroid nodules [2,12]. In this study, we evaluated whether the underlying thyroid parenchyma echogenicity affects the analysis of a thyroid nodule and which associated US features impact the analysis of a thyroid nodule on US. This study reveals that the underlying heterogeneous echogenicity of the background thyroid gland influences differentiation between benign and malignant thyroid nodules on US. The diagnostic performance of US had a more superior specificity, PPV and accuracy for diagnosing malignant nodules when thyroid glands showed underlying homogeneous echogenicity rather than heterogeneous echogenicity. Because the underlying heterogeneous echogenicity of the thyroid gland did affect the diagnostic performance of US, we wanted to evaluate which associated US features influenced the analysis of thyroid nodules on US. Among the benign nodules, microlobulated or irregular margins were more frequently seen in thyroid nodules with underlying heterogeneous echogenicity of the thyroid gland. In other word, physicians may have a higher chance to interpret a benign nodule as microlobulated or irregular margin on US in the underlying heterogeneous thyroid echogenicity group than in the underlying homogeneous thyroid echogenicity group, which explains the lower specificity of US in the underlying heterogeneous thyroid echogenicity group.
The US feature of focal lymphocytic thyroditis is variable; they can present either as hyperechoic nodules with ill-defined margins, ill-defined hypoechoic nodules, or solid hypoechoic nodules with well-defined margins [6,11,14,35]. However, a majority of studies reveal that margins of such nodules are often irregular [6,35] which can mimic suspicious malignant nodules on US and consequently increase the false positive rate of US. Even after excluding the nodules which were diagnosed with lymphocytic thyroditis, microlobulated or irregular margins were still more frequently observed in benign thyroid nodules with underlying heterogeneous thyroid echogenicity than in those with underlying homogeneous thyroid echogenicity. Therefore, our result supports the hypothesis that the underlying heterogeneous echogenicity of the thyroid gland can influence the differentiation of benign and malignant nodules, especially the US analysis of margins of thyroid nodules, a conclusion which needs verification with further studies.
There are some limitations to this study. First, some of the lesions that had undergone US-FNA only once were also included and considered benign or malignant. Although we believe that false-negative and false-positive results were negligible in our institution [36], the results of our study may be affected. Second, seven radiologists with varied experience performed US examinations and US-FNA, and interobserver variability among the radiologists may exist [34,37]. Third, the underlying parenchymal echogenicity of the thyroid gland was only classified into two categories in this study – homogeneous echogenicity and heterogeneous hypoechogenicity – and subcategories were not considered. Furthermore, due to interobserver variability, these categories may depend and vary among US performers. Fourth, this study population only included thyroid nodules which had been performed US-FNA. In our institution, US-FNAs are usually performed either on the thyroid nodule with suspicious US features or on the largest nodule if there are no suspicious US features. In this study, US-FNA had been performed on only one nodule in most cases. Therefore, a selection bias may exist. However, this study focused on the impact on underlying thyroid echogenicity for diagnosing thyroid malignancies using US, not the effect on several US features for diagnosing thyroid malignancies according to the multiplicity. Therefore, we do not think that this limitation has a strong influence on the value or result of this study.
Conclusions
The underlying heterogeneous echogenicity of the thyroid gland significantly lowers the specificity, PPV and accuracy of US in the differentiation of thyroid nodules. Therefore, caution is required during evaluation of thyroid nodules detected among thyroid parenchyma showing heterogeneous echogenicity on US.
Abbreviations
DTD: Diffuse thyroid disease; HT: Hashimoto thyroiditis; US: Ultrasonography; PTC: Papillary thyroid carcinoma; US-FNA: US-guided fine needle aspiration; TPOAb: Thyroid peroxidase antibody; TgAb: Thyroglobulin antibody; TBII: TSH-binding inhibitory immunoglobulins; PPV: Positive predictive value; NPV: Negative predictive value.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MP was involved in acquisition of data, analysis and interpretation of data and manuscript construction. SHP was involved in acquisition of data and revision. E-KK was involved in manuscript drafting and revision. JHY participated in study design and manuscript revision. HJM was involved in manuscript drafting and revision. HSL was involved in analysis and interpretation of data and revision. JYK mainly contributed to conception and decision, drafting the manuscript and final approval of the version to be published. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Mina Park, Email: to.minapark@yuhs.ac.
So Hee Park, Email: lunar38@hanmail.net.
Eun-Kyung Kim, Email: ekkim@yuhs.ac.
Jung Hyun Yoon, Email: lvjenny@yuhs.ac.
Hee Jung Moon, Email: artemis4u@yuhs.ac.
Hye Sun Lee, Email: hslee1@yuhs.ac.
Jin Young Kwak, Email: docjin@yuhs.ac.
References
- Marcocci C, Vitti P, Cetani F, Catalano F, Concetti R, Pinchera A. Thyroid ultrasonography helps to identify patients with diffuse lymphocytic thyroiditis who are prone to develop hypothyroidism. J Clin Endocrinol Metab. 1991;72:209–213. doi: 10.1210/jcem-72-1-209. [DOI] [PubMed] [Google Scholar]
- Ohmori N, Miyakawa M, Ohmori K, Takano K. Ultrasonographic findings of papillary thyroid carcinoma with Hashimoto’s thyroiditis. Intern Med. 2007;46:547–550. doi: 10.2169/internalmedicine.46.1901. [DOI] [PubMed] [Google Scholar]
- Set PA, Oleszczuk-Raschke K, von Lengerke JH, Bramswig J. Sonographic features of Hashimoto thyroiditis in childhood. Clin Radiol. 1996;51:167–169. doi: 10.1016/S0009-9260(96)80317-5. [DOI] [PubMed] [Google Scholar]
- Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999;126:1070–1076. doi: 10.1067/msy.2099.101431. [DOI] [PubMed] [Google Scholar]
- Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzzo A. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science. 1997;275:960. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- Takashima S, Matsuzuka F, Nagareda T, Tomiyama N, Kozuka T. Thyroid nodules associated with Hashimoto thyroiditis: assessment with US. Radiology. 1992;185:125–130. doi: 10.1148/radiology.185.1.1523294. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291–297. doi: 10.1001/archsurg.1955.01270080137023. [DOI] [PubMed] [Google Scholar]
- Hirabayashi RN, Lindsay S. The relation of thyroid carcinoma and chronic thyroiditis. Surg Gynecol Obstet. 1965;121:243–252. [PubMed] [Google Scholar]
- Okayasu I, Fujiwara M, Hara Y, Tanaka Y, Rose NR. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer. 1995;76:2312–2318. doi: 10.1002/1097-0142(19951201)76:11<2312::AID-CNCR2820761120>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Anderson L, Middleton WD, Teefey SA, Reading CC, Langer JE, Desser T, Szabunio MM, Mandel SJ, Hildebolt CF, Cronan JJ. Hashimoto thyroiditis: part 2, sonographic analysis of benign and malignant nodules in patients with diffuse hashimoto thyroiditis. AJR Am J Roentgenol. 2010;195:216–222. doi: 10.2214/AJR.09.3680. [DOI] [PubMed] [Google Scholar]
- Anderson L, Middleton WD, Teefey SA, Reading CC, Langer JE, Desser T, Szabunio MM, Hildebolt CF, Mandel SJ, Cronan JJ. Hashimoto thyroiditis: part 1, sonographic analysis of the nodular form of hashimoto thyroiditis. AJR Am J Roentgenol. 2010;195:208–215. doi: 10.2214/AJR.09.2459. [DOI] [PubMed] [Google Scholar]
- Yeh HC, Futterweit W, Gilbert P. Micronodulation: ultrasonographic sign of hashimoto thyroiditis. J Ultrasound Med. 1996;15:813–819. doi: 10.7863/jum.1996.15.12.813. [DOI] [PubMed] [Google Scholar]
- Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- Moon HJ, Kim EK, Kim MJ, Kwak JY. Lymphocytic thyroiditis on fine-needle aspiration biopsy of focal thyroid nodules: approach to management. AJR Am J Roentgenol. 2009;193:W345–349. doi: 10.2214/AJR.09.2413. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M. Revised american thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- Kwak JY, Kim EK, Kim HJ, Kim MJ, Son EJ, Moon HJ. How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur Radiol. 2009;19:1923–1931. doi: 10.1007/s00330-009-1369-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Teng D, Shan Z, Teng X, Guan H, Yu X, Fan C, Chong W, Yang F, Dai H, Gu X, Yu Y, Mao J, Zhao D, Li J, Chen Y, Yang R, Li C, Teng W. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab. 2008;93:1751–1757. doi: 10.1210/jc.2007-2368. [DOI] [PubMed] [Google Scholar]
- Vanderpump MPJ, Tunbridge WMG. The epidemiology of autoimmune thyroid disease. Autoimmune endocrinopathies. 1999;15:141–162. [Google Scholar]
- Weetman AP. Graves’ disease. N Engl J Med. 2000;343:1236–1248. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–340. doi: 10.1080/08916930410001705394. [DOI] [PubMed] [Google Scholar]
- Andersson M, De Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10:1606–1611. doi: 10.1017/S1368980007361004. [DOI] [PubMed] [Google Scholar]
- Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein IL, Mechanick JI, Pessah-Pollack R, Singer P, Woeber KA. Clinical Practice Guidelines for Hypothyroidism in Adults Co-sponsored by the American Association of Clinical Endocrinologists (AACE) and the American Thyroid Association, Inc.(ATA) Thyroid. 2012;22:1200–1235. doi: 10.1089/thy.2012.0205. [DOI] [PubMed] [Google Scholar]
- Blank W, Braun B. Sonography of the thyroid--part 2: thyroid inflammation, impairmant of thyroid function and interventions. Ultraschall Med. 2008;29:128. doi: 10.1055/s-2008-1027319. [DOI] [PubMed] [Google Scholar]
- Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000;10:251–259. doi: 10.1089/thy.2000.10.251. [DOI] [PubMed] [Google Scholar]
- Raber W, Gessl A, Nowotny P, Vierhapper H. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hundred fifty-one subjects. Thyroid. 2002;12:725–731. doi: 10.1089/105072502760258712. [DOI] [PubMed] [Google Scholar]
- Kwak JY, Koo H, Youk JH, Kim MJ, Moon HJ, Son EJ, Kim EK. Value of US correlation of a thyroid nodule with initially benign cytologic Results1. Radiology. 2010;254:292–300. doi: 10.1148/radiol.2541090460. [DOI] [PubMed] [Google Scholar]
- Rosário PWS, Bessa B, Valadão MMA, Purisch S. Natural history of mild subclinical hypothyroidism: prognostic value of ultrasound. Thyroid. 2009;19:9–12. doi: 10.1089/thy.2008.0221. [DOI] [PubMed] [Google Scholar]
- Shin D, Kim E, Lee E. Role of ultrasonography in outcome prediction in subclinical hypothyroid patients treated with levothyroxine. Endocr J. 2010;57:15. doi: 10.1507/endocrj.K09E-154. [DOI] [PubMed] [Google Scholar]
- Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank W, Braun B. Sonography of the thyroid--Part 1. Ultraschall Med. 2007;28:554–568. doi: 10.1055/s-2007-963722. [DOI] [PubMed] [Google Scholar]
- Park SH, Kim SJ, Kim EK, Kim MJ, Son EJ, Kwak JY. Interobserver agreement in assessing the sonographic and elastographic features of malignant thyroid nodules. Am J Roentgenol. 2009;193:W416–W423. doi: 10.2214/AJR.09.2541. [DOI] [PubMed] [Google Scholar]
- Langer JE, Khan A, Nisenbaum HL, Baloch ZW, Horii SC, Coleman BG, Mandel SJ. Sonographic appearance of focal thyroiditis. Am J Roentgenol. 2001;176:751–754. doi: 10.2214/ajr.176.3.1760751. [DOI] [PubMed] [Google Scholar]
- Kim D, Eun C, In H, Kim M, Jung S, Bae S. Sonographic differentiation of asymptomatic diffuse thyroid disease from normal thyroid: a prospective study. AJNR Am J Neuroradiol. 2010;31:1956–1960. doi: 10.3174/ajnr.A2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim EK, Kwak JY, Kim MJ, Son EJ. Interobserver and intraobserver variations in ultrasound assessment of thyroid nodules. Thyroid. 2010;20:167–172. doi: 10.1089/thy.2008.0354. [DOI] [PubMed] [Google Scholar]