SUMMARY
Obesity-related leptin resistance manifests in loss of leptin’s ability to reduce appetite and increase energy expenditure. Obesity is also associated with increased activity of the endocannabinoid system, and CB1 receptor (CB1R) inverse agonists reduce body weight and the associated metabolic complications, although adverse neuropsychiatric effects halted their therapeutic development. Here we show that in mice with diet-induced obesity (DIO), the peripherally restricted CB1R inverse agonist JD5037 is equieffective with its brain-penetrant parent compound in reducing appetite, body weight, hepatic steatosis, and insulin resistance, even though it does not occupy central CB1R or induce related behaviors. Appetite and weight reduction by JD5037 are mediated by resensitizing DIO mice to endogenous leptin through reversing the hyperleptinemia by decreasing leptin expression and secretion by adipocytes and increasing leptin clearance via the kidney. Thus, inverse agonism at peripheral CB1R not only improves cardiometabolic risk in obesity but has antiobesity effects by reversing leptin resistance.
INTRODUCTION
Endocannabinoids are lipid mediators that elicit a broad range of effects via G protein-coupled CB1 and CB2 receptors (Pacher et al., 2006). Activation of CB1R promotes food intake (Di Marzo et al., 2001), increases lipogenesis in adipose tissue (Cota et al., 2003; Matias et al., 2006) and liver (Osei-Hyiaman et al., 2005), and causes insulin resistance (Eckardt et al., 2009; Liu et al., 2012) and dyslipidemia (Ruby et al., 2008), which suggests that the endocannabinoid/CB1R system (ECS) is involved in obesity and its metabolic complications. Indeed, the ECS is overactive in obesity (Matias et al., 2006), and CB1R inverse agonists reduce body weight and improve metabolic abnormalities in obese subjects (Addy et al., 2008; Despres et al., 2005), although adverse neuropsychiatric effects halted their therapeutic development.
We reported that a CB1R neutral antagonist with reduced brain penetrance, AM6545, was equieffective with its parent compound rimonabant in reversing hepatic steatosis in diet-induced obesity (DIO), but was less effective than rimonabant in reducing body weight, adiposity, insulin resistance, and hyperleptinemia, and had minimal effect on food intake (Tam et al., 2010). As rimonabant is both brain penetrant and a CB1R inverse agonist, its greater efficacy could be due to either its central action or its inverse agonist properties. The present findings unequivocally prove the latter through the use of the peripherally restricted CB1R inverse agonist JD5037, which robustly reduces food intake, body weight, and adiposity in the absence of brain CB1R occupancy, as documented by positron emission tomography (PET).
Obesity is accompanied by resistance to the anorexic and weight-reducing effects of leptin (Dardeno et al., 2010), linked to a defect in leptin signaling, as reflected in decreased phosphorylation of the signaling protein STAT3 in the arcuate nucleus (Myers et al., 2010). Hyperleptinemia plays a key role in leptin resistance (Knight et al., 2010), and leptin sensitivity is restored when plasma leptin is reduced following dietary weight loss (Enriori et al., 2007). Chronic CB1R blockade attenuates hyperleptinemia in obese subjects (Despres et al., 2005) and rodents (Ravinet Trillou et al., 2003; Tam et al., 2010), and CB1R−/− mice exhibit increased leptin sensitivity (Ravinet Trillou et al., 2004). This suggests that reversal of leptin resistance contributes to the antiobesity effect of CB1 blockade. Indeed, AM6545 treatment of DIO mice restored leptin suppression of lipogenic gene expression in fat tissue, which may have contributed to a reduction in adiposity (Tam et al., 2010). However, leptin sensitivity in the hypothalamus and the mechanism by which peripheral CB1R blockade may affect it have not been explored. Here we show that peripheral CB1R inverse agonism reverses hypothalamic leptin resistance in DIO mice by rapidly reversing their hyperleptinemia, through decreasing leptin secretion by adipose tissue and increasing leptin clearance via the kidney.
RESULTS
JD5037 Is a Peripheral CB1R Inverse Agonist Devoid of Behavioral Effects
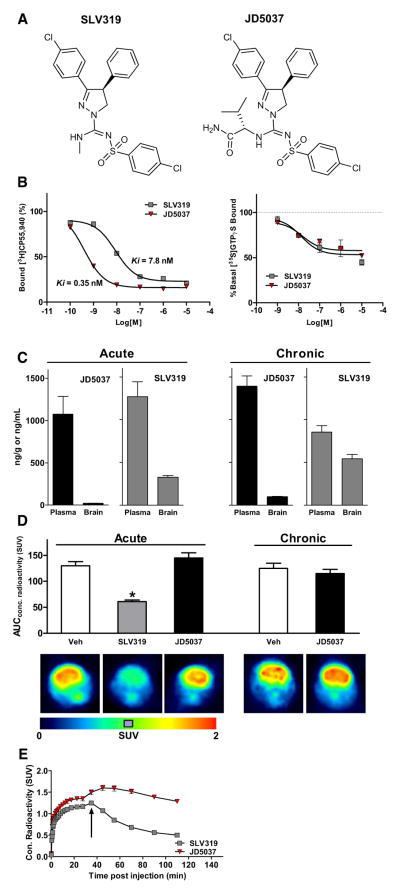
To reduce its brain penetrance, we modified the structure of the CB1R inverse agonist SLV319, yielding the compound JD5037 (Figure 1A). The absolute configuration of JD5037 as the S,S diastereomer was confirmed by X-ray diffraction (see Figure S1A online). JD5037 has high CB1R binding affinity (Ki 0.35 nM) and >700-fold CB1/CB2 selectivity. Both SLV319 and JD5037 are CB1R inverse agonists, verified by GTPγS binding (Figure 1B). CB1R specificity of JD5037 was confirmed as a potency ratio of >1,000 relative to a panel of 70 receptors, transporters, and ion channels (Table S1). Acute oral administration of 3 mg/kg JD5037 blocked the inhibition of gastrointestinal motility by a maximally effective dose of anandamide (Figure S1B), indicating full occupancy of CB1R on cholinergic terminals innervating the gut. Drug levels in plasma, brain, and liver were measured 1 hr after acute or 28 day oral dosing of JD5037 or SLV319 at 3 mg/kg/day. The brain/plasma ratio of JD5037 was <2% after acute and ~7% after chronic dosing, much lower than corresponding values for SLV319, and both compounds accumulated in liver (Figure 1C, legend). Furthermore, nonspecific binding to brain proteins, determined by equilibrium dialysis using CB1R−/− mouse brain, was 99.7%, resulting in subnanomolar free concentration of JD5037 in brain. The absence of in vivo brain CB1R occupancy following acute or chronic administration of JD5037 was documented by PET, showing lack of displacement of a CB1R PET ligand when JD5037 was given either before (Figure 1D) or after the radioligand (Figure 1E). Relative to SLV319, JD5037 has a larger polar surface area (117 versus 74), hydrogen bonding capacity (3 versus 1), and molecular weight (572 versus 487), limiting brain penetrance. P-glycoprotein- mediated extrusion of JD5037, indicated by moderately higher brain levels in Mdr1a/b−/− than wild-type mice (Figure S1C), also contributes to low brain penetrance.
Figure 1. JD5037 Is a Peripherally Restricted CB1 Receptor Inverse Agonist.
(A) Structure of JD5037 and its brain-penetrant parent SLV319.
(B) JD5037 has 20 times higher CB1 binding affinity than SLV319 (left), and both are inverse agonists, as tested by GTPγS binding (right). Each curve represents the mean of three experiments performed in triplicate.
(C) Brain and plasma levels of JD5037 and SLV319 1 hr following acute or 28 day oral dosing at 3 mg/kg, n = 4–7 animals. Both compounds accumulate in liver, with hepatic levels after acute versus chronic administration of 3,676 ± 37 versus 6,262 ± 540 ng/g (JD5037) and 817 ± 88 versus 4,384 ± 386 ng/g (SLV319).
(D) Pretreatment of mice with SLV319, but not with JD5037 (3 mg/kg p.o. 1 hr prior to test), results in displacement of PET ligand from brain CB1R. Scans from typical experiments are shown, with statistics from three to six replicates. *p < 0.05 relative to values in vehicle pretreated mice.
(E) SLV319, but not JD5037 (3 mg/kg i.v. each), displaces bound CB1 PET ligand.
For crystallographic structure, physicochemical features, and behavioral profile of JD5037 see also Figure S1. Vertical bars represent SEM.
Accordingly, JD5037 was inactive in behavioral paradigms: unlike SLV319, JD5037 did not increase locomotor activity (Figure S1D), nor did it inhibit CB1R-mediated catalepsy (Figure S1E). Neither compound was anxiogenic in the elevated plus maze, whereas rimonabant was anxiogenic (Figure S1F). The lack of effect of SLV319 relative to rimonabant reflects the lower brain penetrance and resulting lower central CB1R occupancy by the former (Need et al., 2006).
Peripheral CB1R Inverse Agonism Is Fully Effective in Reducing Food Intake, Body Weight, and Hormonal/Metabolic Abnormalities in DIO Mice
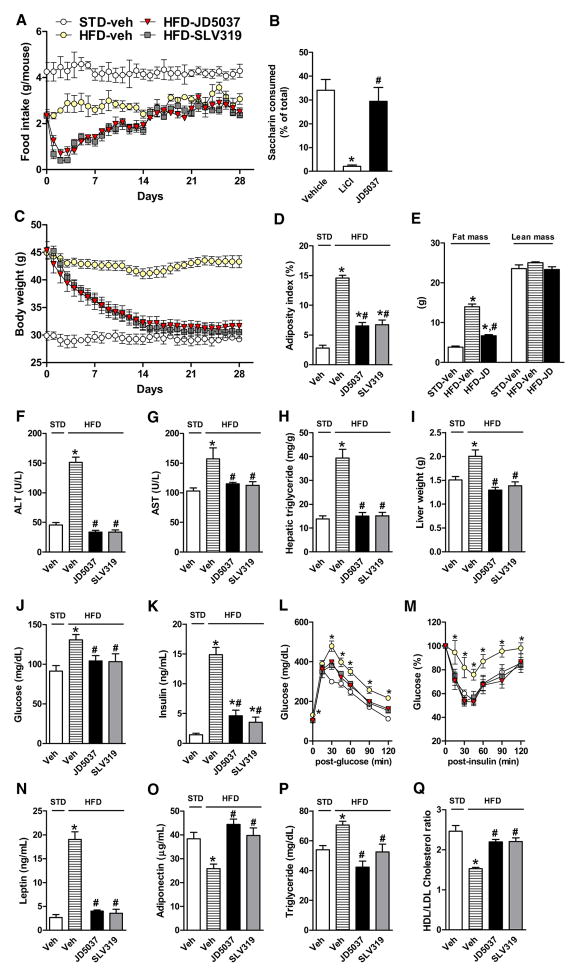
Male C57Bl/6J kept mice on a diet containing 60% of calories as fat became obese (body weight >42 g) after 14 weeks. In DIO mice treated with oral doses of 3 mg/kg/day for 28 days, JD5037 and SLV319 caused equal reductions in food intake (Figure 2A), body weight (Figure 2C), and adiposity (Figure 2D), with tolerance to the anorexic effect developing after 14 days. JD5037 was inactive in conditioned taste avoidance tests (Figure 2B); thus, the reduced food intake is not due to taste aversion. Off-target effects are unlikely, as JD5037 did not affect food intake or body weight in CB1R−/− mice (Figure S2). In contrast to the similar anorectic effect of global and peripheral CB1R blockade in unfasted DIO mice, which indicates a peripheral mechanism, only SLV319 and not JD5037 was able to reduce food intake in a fasting/refeeding paradigm tested in lean as well as ob/ob mice (Figure S3), indicating that the latter effect is centrally mediated and leptin independent.
Figure 2. SLV319 and JD5037 Cause Similar Metabolic Effects in DIO Mice.
(A) Transient reduction of daily food intake, n = 7 mice/group.
(B) JD5037 does not induce taste aversion, as tested in lean mice. n = 6–7/group. *p < 0.001 relative to vehicle. #p < 0.01 relative to LiCl group.
(C and D) (C) SLV319 and JD5037 (3 mg/kg/day for 28 days) normalize body weight and (D) reduce adiposity. n = 7/group, *p < 0.005 relative to STD, #p < 0.01 relative to HFD vehicle group.
(E) JD5037 treatment reduces body fat without affecting lean body mass, as assessed by MRI. N = 6/group. * and # as in (D).
(F–I) Reversal of HFD-induced steatosis (hepatic triglycerides) and hepatocellular damage (plasma ALT, AST). * and # as in (D).
(J–M) Reversal of HFD-induced hyperglycemia, hyperinsulinemia, glucose intolerance (ipGTT), and insulin resistance (ipIST). * and # as in (D).
(N and O) Reversal of HFD-induced hyperleptinemia and hypoadiponectinemia. * and # as in (D).
(P and Q) Improved plasma lipid profile, * and # as in (D).
For additional effects on substrate utilization and energy expenditure, see Figure S4. Vertical bars represent SEM.
Normalization of body weight was due to reduction in total body fat with no change in lean body mass, as determined by MRI (Figure 2E). The two compounds were also equieffective in reversing hepatic steatosis and hepatocellular damage (Figures 2F–2I), normalizing blood glucose and insulin levels (Figures 2J and 2K), attenuating glucose intolerance and insulin resistance (Figures 2L and 2M), reversing the hyperleptinemia and hypoadiponectemia (Figures 2N and 2O), and improving plasma lipid profile (Figures 2P and 2Q). Indirect calorimetry revealed increased total energy expenditure due to a selective increase in fat burning, as reflected in a reduction of the respiratory quotient, with JD5037 being somewhat more efficacious than SLV319 (Figure S4).
The Hypophagic and Weight-Reducing Effects of JD5037 Are Leptin Dependent
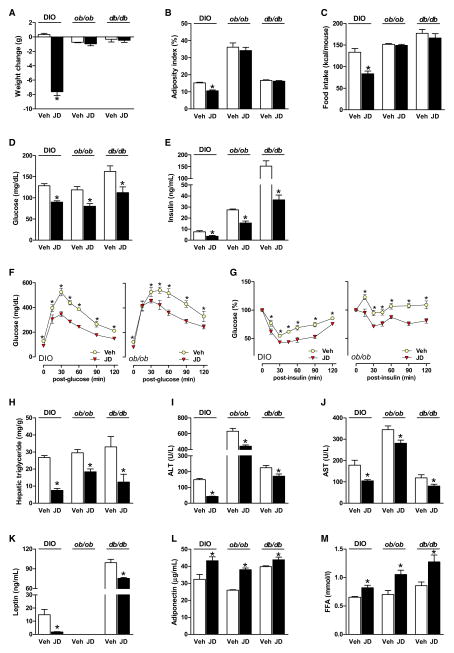
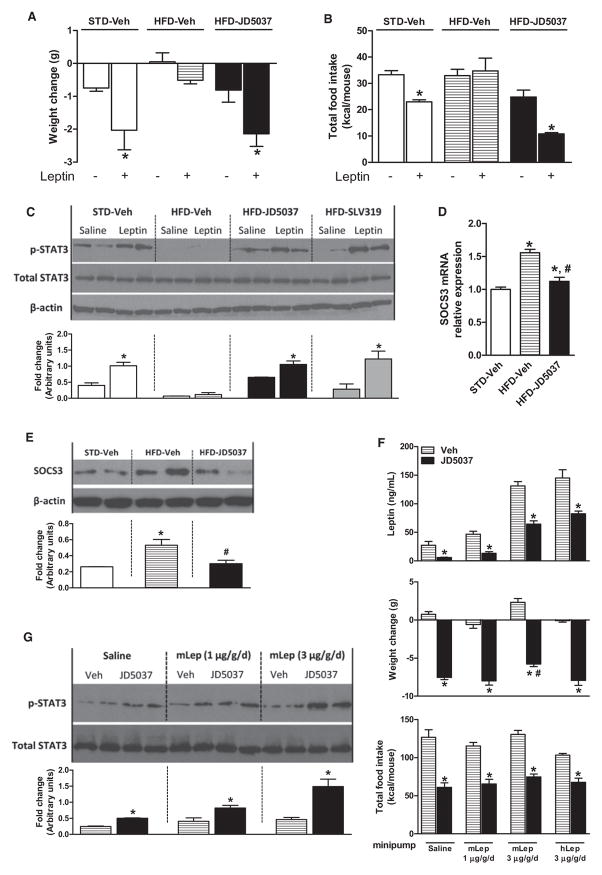
The above effects of JD5037 developed rapidly within 7 days of treatment of DIO mice (Figure 3, left pair of columns). Similar 7 day treatment of obese, leptin-deficient ob/ob or leptin receptor-defective db/db mice caused comparable changes in metabolic parameters (Figures 3D–3M) but did not affect food intake, body weight, or adiposity (Figures 3A–3C), suggesting that changes in the latter reflect resensitization of DIO mice to endogenous leptin. This was further supported by the effects of a pegylated superactive leptin antagonist that blocks the interaction of leptin with its receptor and, as a result, causes hyperphagia and body weight gain in lean mice (Shpilman et al., 2011) but has no such effects in mice made leptin resistant by viral overexpression of hypothalamic leptin (Scarpace et al., 2007). Accordingly, treatment of DIO mice with 5 μg/g of the leptin antagonist for 5 days did not affect food intake or body weight but abolished the weight reduction (Figure 4A) and hypophagia caused by JD5037 treatment, started on day 3 (Figure 4B).
Figure 3. Metabolic Effects of JD5037 in DIO, ob/ob, and db/db Mice.
(A–C) JD5037 (3 mg/kg/day, 7 days) reduces body weight, adiposity, and food intake in DIO but not in ob/ob or db/db mice. *p < 0.05 relative to vehicle, n = 5–6 mice/group.
(D and E) JD5037 treatment attenuates the hyperglycemia and hyperinsulinemia in DIO, ob/ob, and db/db mice.
(F and G) JD5037 attenuates the glucose intolerance (ipGTT) and insulin resistance (ipIST) in DIO and ob/ob mice. *p < 0.5 relative to corresponding vehicle values.
(H–J) JD5037 reduces hepatic triglycerides, plasma ALT, and AST in all three strains.
(K) JD5037 attenuates hyperleptinemia in DIO and db/db mice, no plasma leptin in ob/ob mice.
(L and M) JD5037 causes similar increases in plasma adiponectin and FFA in DIO, ob/ob, and db/db mice.
For the lack of an acute hypophagic effect of JD5037 in a fasting/refeeding paradigm, see Figure S3, and for the lack of effect of JD5037 on daily food intake and body weight in CB1R−/− mice, see Figure S2. Vertical bars represent SEM.
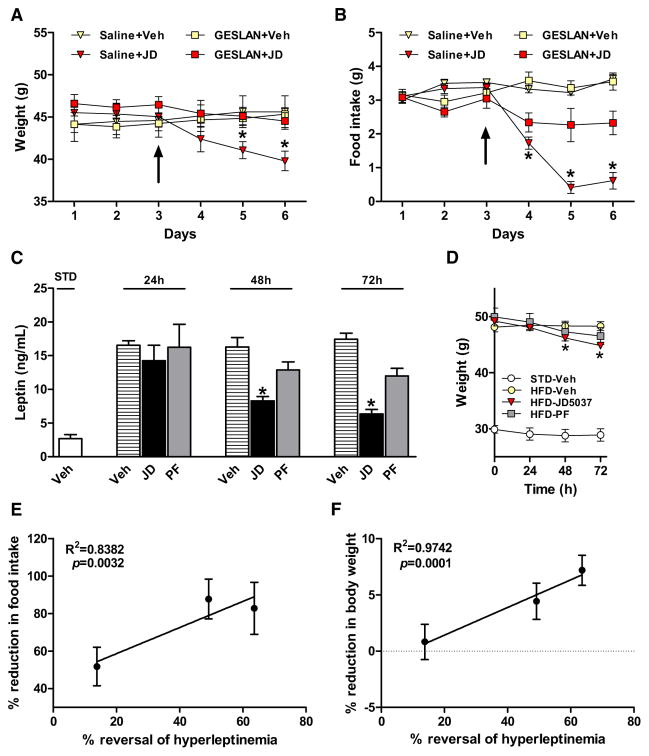
Figure 4. JD5037 Causes Hypophagia and Weight Loss in DIO Mice via Endogenous Leptin.
(A and B) Pretreatment of DIO mice with a leptin antagonist (GESLAN, 5 μg/g/day) prevents the weight loss and hypophagia caused by JD5037, 3mg/kg/day (treatment start at ↑). *p < 0.05 relative to GESLAN + vehicle group, n = 5 mice/group.
(C and D) Reversal of HFD-induced hyperleptinemia by JD5037, 3 mg/kg/day, but not by pair feeding, precedes reversal of the weight gain. *p < 0.05 relative to corresponding vehicle values, n = 8/group.
(E and F) Positive correlation between reversal of hyperleptinemia and reduction of either body weight or daily food intake, as calculated from data in (B)–(D).
Vertical bars represent SEM.
As hyperleptinemia is thought to be responsible for leptin resistance in DIO (Knight et al., 2010; Scarpace et al., 2002), we tested whether its reversal may restore leptin sensitivity in DIO mice. JD5037 rapidly reversed the hyperleptinemia: by the third day of treatment, >70% of the diet-induced increment of plasma leptin was eliminated, whereas only ~20% of the body weight gain was lost. These effects were due to a direct effect of CB1R blockade on the production and clearance of leptin, because they could not be reproduced by pair feeding (Figures 4C and 4D). Furthermore, the reversal of hyperleptinemia significantly and inversely correlated with the parallel decreases in food intake (Figure 4E) and body weight (Figure 4F).
Peripheral CB1R Blockade Reverses Leptin Resistance in DIO Mice
DIO mice are leptin resistant, as illustrated by the loss of leptin-induced anorexia, weight loss, and STAT3 phosphorylation in the arcuate nucleus. Chronic oral treatment with JD5037 restored all three parameters to levels in lean controls (Figures 5A–5C). The increased hypothalamic level of suppressor of cytokine signaling-3 (SOCS3), thought to account for reduced STAT3 phosphorylation (Howard and Flier, 2006), was also reversed (Figures 5D and 5E).
Figure 5. Inverse Agonism at Peripheral CB1R Reverses Leptin Resistance.
(A) Effect of leptin (3 mg/kg twice daily for 4 days) on body weight in lean and DIO mice and in DIO mice treated for 7 days with JD5037, 3 mg/kg/day p.o. n = 4/group, *p < 0.05 relative to corresponding vehicle-treated group.
(B) Effect of leptin on food intake in the same groups as in (A).
(C) Leptin-induced phosphorylation of hypothalamic STAT3 is suppressed in DIO mice, but not in DIO mice pretreated with JD5037 or SLV379, 3/mg/kg/day for 28 days, as visualized by western blotting. Means ± SE from six mice per group were analyzed by densitometry.
(D and E) HFD-induced increase in hypothalamic SOCS3 mRNA and protein is reversed by chronic JD5037 treatment.
(F) Plasma leptin, food intake, and body weight change in DIO mice chronically infused with saline, 1 or 3 μg/g/day mouse leptin, or 3 μg/g/day human leptin, and treated for 7 days with vehicle or JD5037, 3 mg/kg/day. *p < 0.05 relative to vehicle.
(G) JD5037 increases basal pSTAT3 and restores leptin-induced pSTAT3 in DIO mice; densitometry as in (C).
For evidence against the role of the AP in leptin sensitization by CB1 blockade, see Figure S5. Vertical bars represent SEM.
To further test whether reversal of the hyperleptinemia is sufficient to restore leptin sensitivity, DIO mice were infused with leptin at different rates for 7 days and simultaneously treated with daily oral doses of 3 mg/kg JD5037 or vehicle. Plasma leptin was significantly reduced by JD5037, the reduction being progressively greater the higher the pretreatment level (Figure 5F). In each leptin dose group, JD5037 treatment fully restored leptin-induced appetite and weight reduction (Figure 5F) and increased hypothalamic pSTAT3 (Figure 5G), even though plasma leptin in the mice infused with 3 μg/g/day leptin remained above those in vehicle-treated, saline-infused DIO mice (Figure 5F). Another group of mice was infused with human leptin, levels of which were similarly reduced by JD5037 treatment as assayed using a human-specific ELISA, clearly indicating an effect on leptin clearance (Figure 5F). These findings indicate that it is the rapid reduction of plasma leptin rather than its absolute level that is critical in restoring leptin sensitivity.
The islet-derived anorexogenic peptide amylin also reverses leptin resistance by an action in the area postrema (AP) (Lutz, 2010). Because the AP contains CB1R and lacks a blood-brain barrier (Suarez et al., 2010), we tested whether it may also be the site of action of JD5037. In DIO mice with thermal ablation of the AP (APX), 7 day oral treatment with 3 mg/kg JD5037 caused the same reduction in food intake, body weight, and adiposity and restored leptin-induced anorexia and weight loss as in similarly treated sham-operated DIO mice. In contrast, 5 μg/kg amylin reduced food intake in sham-operated but not in APX DIO mice (Figure S5), as documented earlier (Lutz, 2010). Thus, the AP is not the site of action of JD5037.
Peripheral CB1R Blockade Suppresses Leptin Secretion
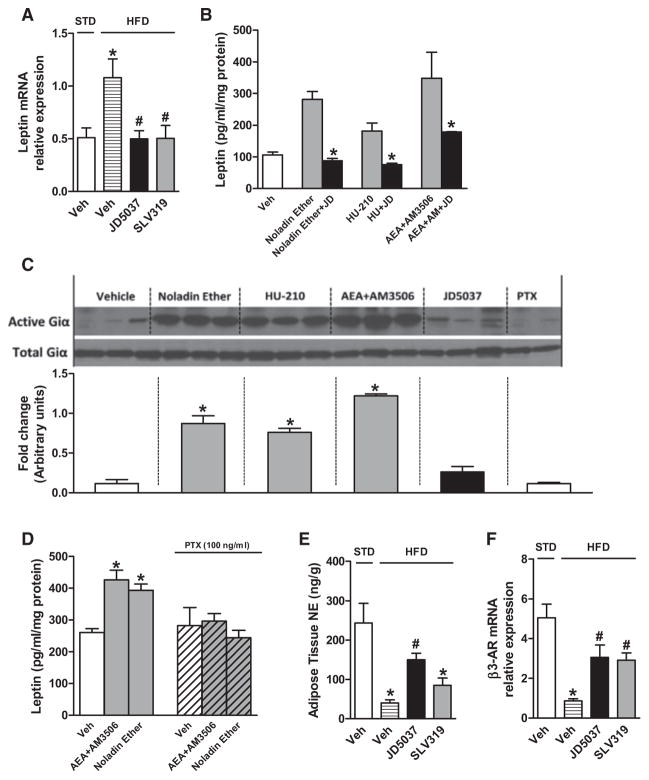
Because the reduction of plasma leptin by JD5037 treatment preceded the weight loss, it couldn’t have simply resulted from loss of fat mass. Indeed, CB1R blockade reversed the obesity-related increase in leptin mRNA in adipose tissue, suggesting a direct effect on leptin gene expression (Figure 6A). Furthermore, 24 hr incubation of differentiated 3T3 L1 adipocytes with different CB1R agonists increased leptin in the culture medium, which was inhibited by 100 nM JD5037 (Figure 6B). Gi protein involvement was indicated by increased expression of active Giα (Figure 6C) and by pertussis toxin blockade of CB1R-induced leptin secretion (Figure 6D).
Figure 6. Peripheral CB1R Inverse Agonism Reduces Leptin Expression and Secretion by Adipose Tissue.
(A) JD5037 or SLV319 (3 mg/kg/day, 28 days) reverses the HFD-induced increase in adipose tissue leptin mRNA. *p < 0.05 relative to STD vehicle, #p < 0.05 relative to HFD-vehicle. n = 6 mice/group.
(B) Activation of CB1R in 3T3 L1 adipocytes increases leptin secretion. Cells were incubated for 24 hr with 100 nM noladin ether, 100 nM HU-210, or 500 nM anandamide in the presence of the FAAH inhibitor AM3506, 100 nM (Godlewski et al., 2010), alone or in combination with 100 nM JD5037. *p < 0.05 relative to agonist alone.
(C) CB1 agonists increase active Giα in 3T3 L1 adipocytes, as quantified by densitometry of western blots.
(D) Pertussis toxin blocks CB1 agonist-induced leptin secretion by 3T3 L1 adipocytes.
(E and F) Peripheral CB1 blockade reverses obesity-induced reduction of adipose tissue NE content and β3-receptor mRNA. *p < 0.05 relative to STD, #p < 0.05 relative to HFD vehicle.
For the effect of JD5037 in denervated DIO mice, see Figure S6. Vertical bars represent SEM.
Leptin increases sympathetic tone, which promotes lipolysis and lipid oxidation in adipose tissue (Buettner et al., 2008). NE in adipose tissue was decreased by HFD, and the reduction was reversed by CB1 blockade (Figure 6E). JD5037 was more effective than SLV319, suggesting that their target was peripheral CB1R on sympathetic terminals innervating fat pads, blockade of which reverses CB1R-mediated inhibition of NE release. The reduced β3-adrenergic receptor gene expression in adipose tissue of DIO mice was also partially reversed by JD5037 (Figure 6F). The role of increased sympathetic tone in the reduction of leptin levels by JD5037 treatment was tested in DIO mice with chemical sympathetic denervation of the epididymal fat pad by local injection of 6-hydroxydopamine. As shown in Figure S6, NE was undetectable in the denervated fat pad of both vehicle- and JD5037-treated DIO mice. In parallel, JD5037-induced reductions in plasma leptin and body weight were significantly smaller in mice with denervated versus those with intact fat pads.
Peripheral CB1R Blockade Increases Leptin Clearance
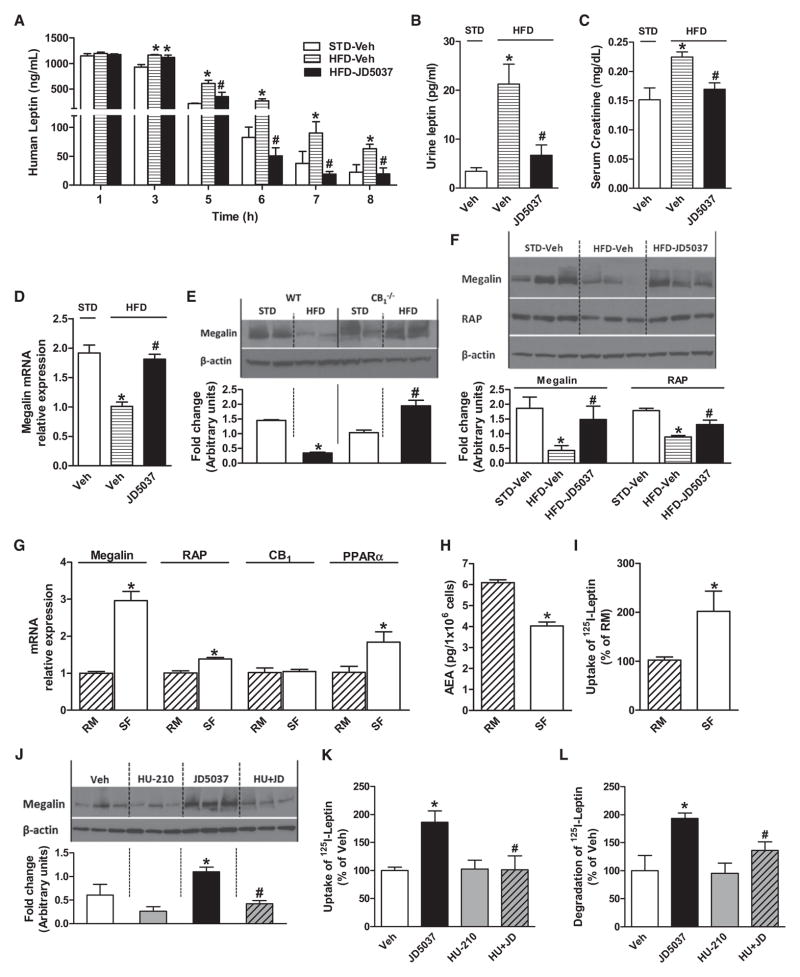
The reversal of hyperleptinemia by CB1 blockade also involves increased leptin clearance. The elimination half-life of leptin was increased 2-fold in DIO mice, which was reversed by JD5037 treatment (Figure 7A). Leptin is removed by glomerular filtration followed by metabolic degradation in the renal tubules (Cumin et al., 1997). The increase in serum creatinine in DIO mice suggests that leptin removal by glomerular filtration is reduced in DIO, and reversal of this change by JD5037 (Figure 7C) implicates CB1R.
Figure 7. Reduced Leptin Clearance in DIO Mice Is Normalized by Peripheral CB1 Blockade.
(A) Pharmacokinetics of human leptin (10 mg/kg i.p.) injected into lean (STD-veh, t1/2 33.5 ± 0.1 min) and DIO mice (HFD-veh, t1/2 50.2 ± 4.8 min, p < 0.05) and DIO mice treated with JD5037, 3 mg/kg/day p.o. for 7 days (t1/2 28.8 ± 0.9 min). Note that the increase in t1/2 in DIO mice is reversed by JD5037, *p < 0.05 relative to STD, #p < 0.05 relative to HFD vehicle.
(B) Leptin microuria in DIO mice is reversed by JD5037 treatment, * and # as in (A).
(C) JD5037 reverses the diet-induced increase in serum creatinine levels, * and # as in (A).
(D) Reduction in megalin mRNA in kidney of DIO mice is reversed by JD5037 treatment.
(E) HFD reduces renal megalin protein in wild-type but not in CB1−/− mice (densitometry of western blots). *p < 0.05 relative to WT-STD, #p < 0.05 relative to CB1−/−-STD.
(F) Reduced megalin and RAP protein in the DIO kidney are normalized by JD5037, 3 mg/kg/day for 28 days, as quantified by densitometry * and # as in (A).
(G–I) Switching RPTEC from regular (RM) to serum-free medium (SF) increases megalin, RAP, and PPARα expression; reduces anandamide content; and increases leptin uptake.
(J–L) In RPTEC maintained in regular medium, JD5037 (1 μM, 24 hr) increases megalin protein levels, leptin uptake, and leptin degradation, effects reversed by coincubation with 100 nM HU-210. *p < 0.05 relative to vehicle, #p < 0.05 relative to JD5037.
For CB1/megalin interaction in the choroid plexus, see Figure S7. Vertical bars represent SEM.
The subsequent tubular uptake and metabolism of leptin is also reduced in DIO and reversed by CB1R blockade. The renal levels of megalin, a multiligand endocytic receptor that mediates the tubular uptake of leptin (Ceccarini et al., 2009; Hama et al., 2004), was reduced by HFD in wild-type but not in CB1−/− mice, and JD5037 increased megalin mRNA and protein levels in the kidney of DIO mice (Figures 7D–7F). Proper folding and export of megalin from the endoplasmic reticulum is dependent on the chaperone, receptor-associated protein (RAP) (Birn et al., 2000). Renal levels of RAP were also reduced in DIO mice and normalized by JD5037 treatment (Figure 7F).
Direct evidence for the role of CB1R in megalin-mediated leptin clearance was obtained in cultured renal proximal tubular epithelial cells (RPTECs), which express CB1R (Jenkin et al., 2010; Lim et al., 2010). Removing the serum from the culture medium reduced the cellular levels of anandamide (Figure 7H), whereas 125I-leptin uptake was increased (Figure 7I), which paralleled the increase in megalin, RAP, and PPARα, a positive regulator of megalin expression (Cabezas et al., 2011) (Figure 7G). This suggests that in cells maintained in serum-containing medium, the higher levels of anandamide tonically inhibit megalin expression and, consequently, leptin degradation. Accordingly, megalin expression, 125I-leptin uptake, and degradation in such cells were increased by JD5037 in a CB1R agonist-reversible manner (Figures 7J–7L).
Megalin is present in choroid plexus endothelial cells, where it has been implicated in leptin transport from blood to CSF (Dietrich et al., 2008). Interestingly, megalin expression was reduced by HFD in parallel with a reduced CSF:plasma leptin ratio, and both changes were reversed by JD5037 treatment (Figure S7).
DISCUSSION
We report that a peripherally restricted CB1R inverse agonist, JD5037, is equieffective with its brain-penetrant parent compound in decreasing daily food intake, body weight, and adiposity in DIO mice. This clearly indicates that inverse agonism at peripheral CB1R rather than a central action mediates these effects, confirming earlier suggestions that the hypophagic effect of brain-penetrant CB1 inverse agonists may be mediated by peripheral CB1R (Gomez et al., 2002; Need et al., 2006). The role of leptin in these effects is supported by their absence in ob/ob and db/db mice and in DIO mice pretreated with a leptin antagonist. In contrast, the hunger-induced food intake on refeeding fasted nonobese mice was suppressed only by SLV319 and not by JD5037, and SLV319 retained its efficacy in ob/ob mice (Figure S3), indicating that this latter effect is centrally mediated and leptin independent, as reported earlier (Di Marzo et al., 2001). However, this acute effect, which occurs in the first hour after refeeding and thus reflects meal initiation rather than consummatory food intake, is unlikely to contribute to weight loss in nonfasted DIO mice treated with a CB1R inverse agonist. The most likely mechanism underlying this latter effect is reversal of the obesity-related leptin resistance due to a rapid reversal of hyperleptinemia, which results from reduced leptin secretion by adipocytes and increased leptin clearance via the kidneys, as discussed below. Nevertheless, we can’t exclude the possibility that other mechanisms may also contribute to the effects of peripheral CB1R blockade on consummatory food intake and body weight.
The gold standard marker of cellular leptin action is pSTAT3 in the arcuate nucleus (Myers et al., 2010). JD5037 treatment of DIO mice restored leptin-induced hypothalamic pSTAT3 to levels in lean mice, and also increased basal pSTAT3 (Figure 5C), suggesting sensitization to endogenous leptin. JD5037 treatment also restored the appetite- and weight-reducing effects of leptin. Because hyperleptinemia is required for leptin resistance in DIO (Knight et al., 2010), these effects likely resulted from the rapid reversal of hyperleptinemia. If reversing leptin resistance reduces obesity, one might also expect that leptin resistance promotes obesity. Indeed, viral overexpression of leptin in the hypothalamus induces leptin resistance and exacerbates DIO (Scarpace and Zhang, 2007), whereas preventing leptin resistance may have the opposite effect. In the study of Knight et al. (2010), mice with maintained leptin sensitivity achieved the same weight on HFD as wild-type mice that became leptin resistant. However, their prediet weight was substantially higher, so they gained less weight despite a trend for reduced locomotor activity, which suggests partial resistance to DIO.
Resensitization to leptin by JD5037 was independent of the degree of hyperleptinemia in DIO mice chronically infused with leptin (Figures 5F and 5G). This is compatible with the asymmetric nature of leptin action, whereby a reduction of plasma leptin in conditions of negative energy balance powerfully stimulates appetite and energy conservation, whereas increased leptin in states of weight gain is less effective as a counterregulatory signal (Leibel, 2008). In this model, leptin resistance manifests as an increase in set point for adipose mass, as reflected by plasma leptin, below which the anabolic counterregulatory response is activated to protect body weight (Leibel, 2008). Accordingly, leptin infusion to DIO mice increased the set point to higher plasma leptin levels, from which comparable reductions by JD5037 resulted in similar increases in leptin sensitivity (Figures 5F and 5G).
Reduction of plasma leptin by JD5037 was the combined result of decreased leptin expression and secretion by adipocytes and increased leptin clearance. The decrease in leptin secretion by JD5037 likely involves two mechanisms. First, differentiated adipocytes express CB1R (Bensaid et al., 2003; Cota et al., 2003), which may regulate leptin expression and secretion. Indeed, JD5037 reduced ob mRNA in adipose tissue in vivo and reversed CB1R-mediated increases in leptin secretion in adipocytes in vitro (Figures 6A and 6B). Second, inhibition of NE release by presynaptic CB1R can be removed by pharmacological blockade (Ishac et al., 1996) or genetic deletion of CB1R in sympathetic neurons (Quarta et al., 2010), and the resulting increase in sympathetic tone accounts for the increased NE content of adipose tissue of JD5037-treated DIO mice (Figure 6E). JD5037 also increased β3-adrenergic receptor expression, and NE acting via β3-receptors in adipocytes suppresses leptin secretion (Gettys et al., 1996; Mantzoros et al., 1996). Furthermore, peripheral CB1R blockade resensitizes DIO mice to leptin, and leptin itself increases sympathetic tone through a central action (Buettner et al., 2008). Both of these mechanisms could contribute to the increased NE levels in fat pads of JD5037-treated DIO mice, which reflects reversal of the well-established decrease of sympathetic tone in DIO (Cruciani-Guglielmacci et al., 2005). Finally, the increased sympathetic tone to adipose tissue also mediates the increased energy expenditure of JD5037-treated DIO mice (Figure S4) by increasing lipolysis and fatty acid oxidation via adipocyte β-adrenergic receptors (Bachman et al., 2002; Song et al., 2010). Thus, blockade of CB1R on adipocytes and on sympathetic nerve terminals innervating fat pads may both contribute to reduced leptin secretion.
As to the relative contribution of these two mechanisms, the role of presynaptic CB1R on sympathetic nerves is indicated by our finding that chemical denervation of the major visceral fat pad in DIO mice significantly attenuated JD5037-induced reductions in plasma leptin and body weight (Figure S6). In turn, the role of adipocyte CB1R is supported by the observed effects of CB1R ligands in cultured adipocytes (Figure 6B). Additional in vivo evidence may be obtained in mice with selective deletion of CB1R in adipocytes (Mancini et al., 2010).
The slower rate of leptin elimination in DIO mice (Figure 7A) is similar to that in obese humans (Wong et al., 2004), which contributes to hyperleptinemia. In turn, reversal of hyperleptinemia by CB1R blockade involves increased leptin clearance (Figure 7A). Leptin is removed by glomerular filtration followed by metabolic degradation in the proximal tubules via uptake by the multifunctional endocytic receptor megalin (Ceccarini et al., 2009). Here we report that CB1R receptors in RPTECs regulate megalin expression and leptin uptake and degradation. First, primary-cultured, serum-stimulated RPTECs generate anandamide and express CB1R (Figures 7G and 7H), blockade of which increases megalin expression as well as leptin uptake and degradation (Figures 7J–7L). Second, DIO mice have reduced renal megalin and RAP levels, which are normalized by JD5037 (Figures 7D–7F).
Despite its reduced tubular uptake and metabolism, only trace amounts of leptin are present in the urine of DIO mice (Figure 7B). This suggests that reduced amounts of leptin reach the tubules by filtration, and/or unmetabolized leptin may be reabsorbed into the circulation. As for leptin filtration, CB1R are present in glomerular podocytes, where their overexpression promotes apoptosis (Barutta et al., 2010), and in arterioles, where their stimulation reduces glomerular filtration rate (Koura et al., 2004). Glomerular filtration is also reduced in obese Zucker rats and normalized by chronic rimonabant treatment (Janiak et al., 2007). In the present study, JD5037 treatment normalized the elevated plasma creatinine of DIO mice (Figure 7C), suggesting improved glomerular filtration. Furthermore, renal fractional extraction of leptin is reduced in hyperleptinemia (Meyer et al., 1997), and it may also be reversed by CB1R blockade.
Additionally, at low tubular levels of megalin, intact leptin may be reabsorbed into the circulation contributing to the hyperleptinemia, and CB1 blockade would limit such transcytosis by restoring megalin-mediated leptin degradation. Indeed, in polarized cells, such as RPTECs, leptin transport in apical to basolateral direction can occur via the short form of the leptin receptor (ObRa) (Hileman et al., 2000), which is highly expressed in the kidney (Fei et al., 1997).
Megalin is present in the choroid plexus epithelium, which also expresses CB1R (Maccarrone et al., 2006), providing another mechanism by which CB1 ligands could modulate a peripheral form of leptin resistance. The parallel reductions of megalin in the choroid plexus and of the CSF:plasma ratio of leptin, and their reversal by chronic JD5037 treatment (Figure S6), are compatible with such a mechanism.
As to alternative mechanisms for leptin sensitization by peripheral CB1 blockade, the possible role of the AP, a brain structure with defective blood-brain barrier and the site of action of the leptin-sensitizing peptide amylin (Lutz, 2010) could be ruled out (see Figure S5). The proposed role of afferent nerve terminals in the gut in the anorectic and weight-reducing effects of CB1 blockade (Gomez et al., 2002) is also unlikely, because sub-diaphragmatic vagal deafferentation or complete celiac/superior mesenteric gangliectomy had no effect on the hypophagic response of rodents to rimonabant (Madsen et al., 2009).
Mice with selective genetic deletion (Quarta et al., 2010) or desensitization of CB1R (Jung et al., 2012) in CaMKIIα-containing neurons are lean and resistant to DIO, similar to mice with global CB1R knockout. Because CaMKIIα is expressed predominantly (although not exclusively) in the brain, these findings suggest that CB1R in the CNS are required for the development of DIO. This does not negate the role of peripheral CB1R in maintaining or reversing obesity, as obesity upregulates CB1R in fat tissue (Bensaid et al., 2003), liver (Jourdan et al., 2010; Osei-Hyiaman et al., 2008; Quarta et al., 2010), and skeletal muscle (Pagotto et al., 2006), and CB1R blockade reduces body weight in obese but not in lean mice (Jbilo et al., 2005).
A key finding of the present study is the greater efficacy of peripheral CB1R inverse agonism compared to neutral antagonism in reducing food intake and body weight. Hyperleptinemia, which is the target of these effects, was partially reduced by the neutral antagonist AM6545 (Tam et al., 2010) but completely normalized by JD5037 (Figure 2N). It is possible that in obesity the fraction of CB1R present in the active conformation is increased in peripheral tissues involved in metabolic regulation, which could account for the greater efficacy of inverse agonists over neutral antagonists.
JD5037-induced hypophagia and weight loss were absent in ob/ob and db/db mice and in DIO mice treated with a leptin antagonist, indicating the role of leptin in these effects. In contrast, JD5037 caused comparable improvements in glucose tolerance and insulin sensitivity in all three strains of mice, thus these effects are leptin independent. Diet-induced hepatic insulin resistance is mediated by hepatic CB1R (Liu et al., 2012; Osei-Hyiaman et al., 2008), and CB1 agonists induce muscle insulin resistance in lean mice, reversible by CB1 blockade (Song et al., 2011). Thus, the insulin-sensitizing effect of JD5037 is likely mediated via CB1R in liver and skeletal muscle.
In summary, peripherally restricted CB1R inverse agonists have therapeutic potential in obesity. Their high efficacy in normalizing the related metabolic abnormalities, including insulin resistance and fatty liver, would warrant their testing as monotherapy in these conditions. A key component in the mode of action of CB1R inverse agonism is reversal of leptin resistance, as illustrated in the graphical abstract. An important issue in obesity treatment is inability to maintain weight loss, due to the perception by the brain of weight loss as a relative leptin-deficient state, which triggers an anabolic response promoting the regain of lost weight (Rosenbaum and Leibel, 2010). Thus, by sensitizing the body to endogenous leptin, peripheral CB1R blockade may not only promote weight loss, but could help maintain it.
EXPERIMENTAL PROCEDURES
Drugs
JD5037, a structurally modified analog of SLV319 (ibipinabant), was synthesized and its diastereomeric structure verified by X-ray crystallography, as detailed in the Supplemental Experimental Procedures.
Experimental Protocol
Six-week-old male C57Bl/6J mice received a diet containing 60% of calories as fat, resulting in body weights >42 g in 12–14 weeks. Mice with DIO then received vehicle, JD5037, or SLV319 daily for 7–28 days by oral gavage of 3 mg/kg. Age-matched control mice on standard diet (STD) received vehicle daily. Body weight and food intake were monitored daily. At the end of the treatment period, groups of mice were subjected to i.p. glucose tolerance and insulin sensitivity tests or indirect calorimetry. Mice were then sacrificed by cervical dislocation, and total body fat content and lean mass were determined by MRI. In other mice the brain, liver, kidney, and combined fat pads were removed, weighed, and snap frozen, and trunk blood was collected for determining endocrine and biochemical parameters. Adiposity index was defined as the combined weight of the epidydimal, retroperitoneal, and inguinal fat pads, expressed as percentage of total body weight. Adult male ob/ob and db/db mice were treated with JD5037 or vehicle for 7 days, during which food intake and body weight had been monitored. Hormonal and metabolic parameters were determined as in mice with DIO.
Leptin Sensitivity
Leptin sensitivity was assessed in two paradigms: (1) STAT3 phosphorylation in the hypothalamus in response to acute in vivo leptin treatment was quantified ex vivo by western blotting; (2) reductions in food intake and body weight in response to subacute leptin treatment were monitored in vivo.
Leptin Clearance
Recombinant human leptin (R&D Systems) was injected i.p. at a dose of 10 mg/kg to mice fed STD or HFD for 14 weeks, or to HFD-fed mice treated with JD5037 (3 mg/kg p.o.) for 7 days. Blood samples were taken from the mandibular vein at 60, 180, 300, 360, 420, and 480 min. Serum was immediately separated, and human leptin level was determined by ELISA (Millipore Corporation). Terminal elimination rate constant was determined by linear regression of the last three measurable concentrations and the terminal half-life of leptin calculated as described (Wong et al., 2004).
Statistical Analyses
Values are expressed as mean ± SEM. Unpaired two-tailed Student’s t test was used to determine differences between vehicle- and drug-treated groups. Time-dependent variables were analyzed and results in multiple groups were compared by ANOVA followed by Bonferroni test. Nonlinear regression analysis was used to determine the Ki values of ligands in binding assays (Graph-Pad Prism version 5 for windows). Significance was at p < 0.05.
All animal experiments have been approved by the Animal Care and Use Committee of NIAAA.
Supplementary Material
Acknowledgments
This work was supported by intramural funds from the National Institute on Alcohol Abuse and Alcoholism, NIH. Design and synthesis of JD5037 was funded by Jenrin Discovery, Inc. J.F.M. and R.J.C. hold a patent on JD5037. We thank V. Pike and C. Morris for support and advice with the PET studies, and M. Allen, R. Schwartzbeck, A. Noguchi, D. Springer, and R. Kechrid for their assistance with the animal studies.
Footnotes
Supplemental Information includes seven figures, one table, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at http://dx.doi.org/10.1016/j.cmet.2012.07.002.
References
- Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohorquez SM, Stoch A, et al. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab. 2008;7:68–78. doi: 10.1016/j.cmet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. BetaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Barutta F, Corbelli A, Mastrocola R, Gambino R, Di Marzo V, Pinach S, Rastaldi MP, Perin PC, Gruden G. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes. 2010;59:1046–1054. doi: 10.2337/db09-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Birn H, Vorum H, Verroust PJ, Moestrup SK, Christensen EI. Receptor-associated protein is important for normal processing of megalin in kidney proximal tubules. J Am Soc Nephrol. 2000;11:191–202. doi: 10.1681/ASN.V112191. [DOI] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas F, Lagos J, Cespedes C, Vio CP, Bronfman M, Marzolo MP. Megalin/LRP2 expression is induced by peroxisome proliferator- activated receptor -alpha and -gamma: implications for PPARs’ roles in renal function. PLoS ONE. 2011;6:e16794. doi: 10.1371/journal.pone.0016794. http://dx.doi.org/10.1371/journal.pone.0016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini G, Flavell RR, Butelman ER, Synan M, Willnow TE, Bar-Dagan M, Goldsmith SJ, Kreek MJ, Kothari P, Vallabhajosula S, et al. PET imaging of leptin biodistribution and metabolism in rodents and primates. Cell Metab. 2009;10:148–159. doi: 10.1016/j.cmet.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani-Guglielmacci C, Vincent-Lamon M, Rouch C, Orosco M, Ktorza A, Magnan C. Early changes in insulin secretion and action induced by high-fat diet are related to a decreased sympathetic tone. Am J Physiol Endocrinol Metab. 2005;288:E148–E154. doi: 10.1152/ajpendo.00225.2004. [DOI] [PubMed] [Google Scholar]
- Cumin F, Baum HP, Levens N. Mechanism of leptin removal from the circulation by the kidney. J Endocrinol. 1997;155:577–585. doi: 10.1677/joe.0.1550577. [DOI] [PubMed] [Google Scholar]
- Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–393. doi: 10.1016/j.yfrne.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Spuch C, Antequera D, Rodal I, de Yebenes JG, Molina JA, Bermejo F, Carro E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging. 2008;29:902–912. doi: 10.1016/j.neurobiolaging.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Eckardt K, Sell H, Taube A, Koenen M, Platzbecker B, Cramer A, Horrighs A, Lehtonen M, Tennagels N, Eckel J. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia. 2009;52:664–674. doi: 10.1007/s00125-008-1240-4. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tisssues. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Alapafuja SO, Batkai S, Nikas SP, Cinar R, Offertaler L, Osei-Hyiaman D, Liu J, Mukhopadhyay B, Harvey-White J, et al. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol. 2010;17:1256–1266. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Saito A, Takeda T, Tanuma A, Xie Y, Sato K, Kazama JJ, Gejyo F. Evidence indicating that renal tubular metabolism of leptin is mediated by megalin but not by the leptin receptors. Endocrinology. 2004;145:3935–3940. doi: 10.1210/en.2004-0074. [DOI] [PubMed] [Google Scholar]
- Hileman SM, Tornoe J, Flier JS, Bjorbaek C. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby canine kidney cells. Endocrinology. 2000;141:1955–1961. doi: 10.1210/endo.141.6.7450. [DOI] [PubMed] [Google Scholar]
- Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023– 2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, Barbosa I, Dedio J, Maffrand JP, Le Fur G, et al. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007;72:1345–1357. doi: 10.1038/sj.ki.5002540. [DOI] [PubMed] [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- Jenkin KA, McAinch AJ, Grinfeld E, Hryciw DH. Role for cannabinoid receptors in human proximal tubular hypertrophy. Cell Physiol Biochem. 2010;26:879–886. doi: 10.1159/000323997. [DOI] [PubMed] [Google Scholar]
- Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59:926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Clapper JR, Fu J, D’Agostino G, Guijarro A, Thongkham D, Avanesian A, Astarita G, DiPatrizio NV, Frontini A, et al. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012;15:299–310. doi: 10.1016/j.cmet.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. http://dx.doi.org/10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ, Hayashi M, Saruta T. Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol. 2004;15:1488–1494. doi: 10.1097/01.asn.0000130561.82631.bc. [DOI] [PubMed] [Google Scholar]
- Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 2008;32(Suppl 7):S98–S108. doi: 10.1038/ijo.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JC, Lim SK, Han HJ, Park SH. Cannabinoid receptor 1 mediates palmitic acid-induced apoptosis via endoplasmic reticulum stress in human renal proximal tubular cells. J Cell Physiol. 2010;225:654–663. doi: 10.1002/jcp.22255. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, Yin S, Gao P, Shan X, Pickel J, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142:1218–1228. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA. The role of amylin in the control of energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1475–R1484. doi: 10.1152/ajpregu.00703.2009. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Fiori A, Bari M, Granata F, Gasperi V, De Stefano ME, Finazzi-Agro A, Strom R. Regulation by cannabinoid receptors of anandamide transport across the blood-brain barrier and through other endothelial cells. Thromb Haemost. 2006;95:117–127. [PubMed] [Google Scholar]
- Madsen AN, Jelsing J, van de Wall EH, Vrang N, Larsen PJ, Schwartz GJ. Rimonabant induced anorexia in rodents is not mediated by vagal or sympathetic gut afferents. Neurosci Lett. 2009;449:20–23. doi: 10.1016/j.neulet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Mancini G, Quarta C, Srivastava RK, Klaus S, Pagotto U, Lutz B. Adipocyte-specific CB1 conditional knock-out mice: new insights in the study of obesity and metabolic syndrome. 20th Annual Symposium on the Cannabinoids; Research Triangle Park, NC, USA: International Cannabinoid Research Society; 2010. p. 17. [Google Scholar]
- Mantzoros CS, Qu D, Frederich RC, Susulic VS, Lowell BB, Maratos-Flier E, Flier JS. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45:909–914. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Meyer C, Robson D, Rackovsky N, Nadkarni V, Gerich J. Role of the kidney in human leptin metabolism. Am J Physiol. 1997;273:E903–E907. doi: 10.1152/ajpendo.1997.273.5.E903. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AB, Davis RJ, Alexander-Chacko JT, Eastwood B, Chernet E, Phebus LA, Sindelar DK, Nomikos GG. The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats. Psychopharmacology (Berl) 2006;184:26–35. doi: 10.1007/s00213-005-0234-x. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C, Kunos G. Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, Braulke LJ, Fekete C, Latorre R, Nanni C, Bucci M, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11:273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby MA, Nomura DK, Hudak CS, Mangravite LM, Chiu S, Casida JE, Krauss RM. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proc Natl Acad Sci USA. 2008;105:14561–14566. doi: 10.1073/pnas.0807232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpace PJ, Zhang Y. Elevated leptin: consequence or cause of obesity? Front Biosci. 2007;12:3531–3544. doi: 10.2741/2332. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tumer N. Leptin-induced leptin resistance reveals separate roles for the anorexic and thermogenic responses in weight maintenance. Endocrinology. 2002;143:3026–3035. doi: 10.1210/endo.143.8.8966. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zhang Y, Cheng KY, Tumer N. Leptin antagonist reveals an uncoupling between leptin receptor signal transducer and activator of transcription 3 signaling and metabolic responses with central leptin resistance. J Pharmacol Exp Ther. 2007;320:706–712. doi: 10.1124/jpet.106.112813. [DOI] [PubMed] [Google Scholar]
- Shpilman M, Niv-Spector L, Katz M, Varol C, Solomon G, Ayalon-Soffer M, Boder E, Halpern Z, Elinav E, Gertler A. Development and characterization of high affinity leptins and leptin antagonists. J Biol Chem. 2011;286:4429–4442. doi: 10.1074/jbc.M110.196402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, Kim JH, Goode J, Igata M, Paz JC, et al. CRTC3 links catecholamine signalling to energy balance. Nature. 2010;468:933–939. doi: 10.1038/nature09564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Bandsma RH, Xiao C, Xi L, Shao W, Jin T, Lewis GF. Acute cannabinoid receptor type 1 (CB1R) modulation influences insulin sensitivity by an effect outside the central nervous system in mice. Diabetologia. 2011;54:1181–1189. doi: 10.1007/s00125-011-2082-z. [DOI] [PubMed] [Google Scholar]
- Suarez J, Romero-Zerbo SY, Rivera P, Bermudez-Silva FJ, Perez J, De Fonseca FR, Fernandez-Llebrez P. Endocannabinoid system in the adult rat circumventricular areas: an immunohistochemical study. J Comp Neurol. 2010;518:3065–3085. doi: 10.1002/cne.22382. [DOI] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab. 2004;89:2672–2677. doi: 10.1210/jc.2003-031931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.