Abstract
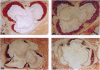
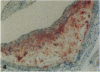
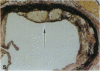
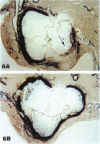
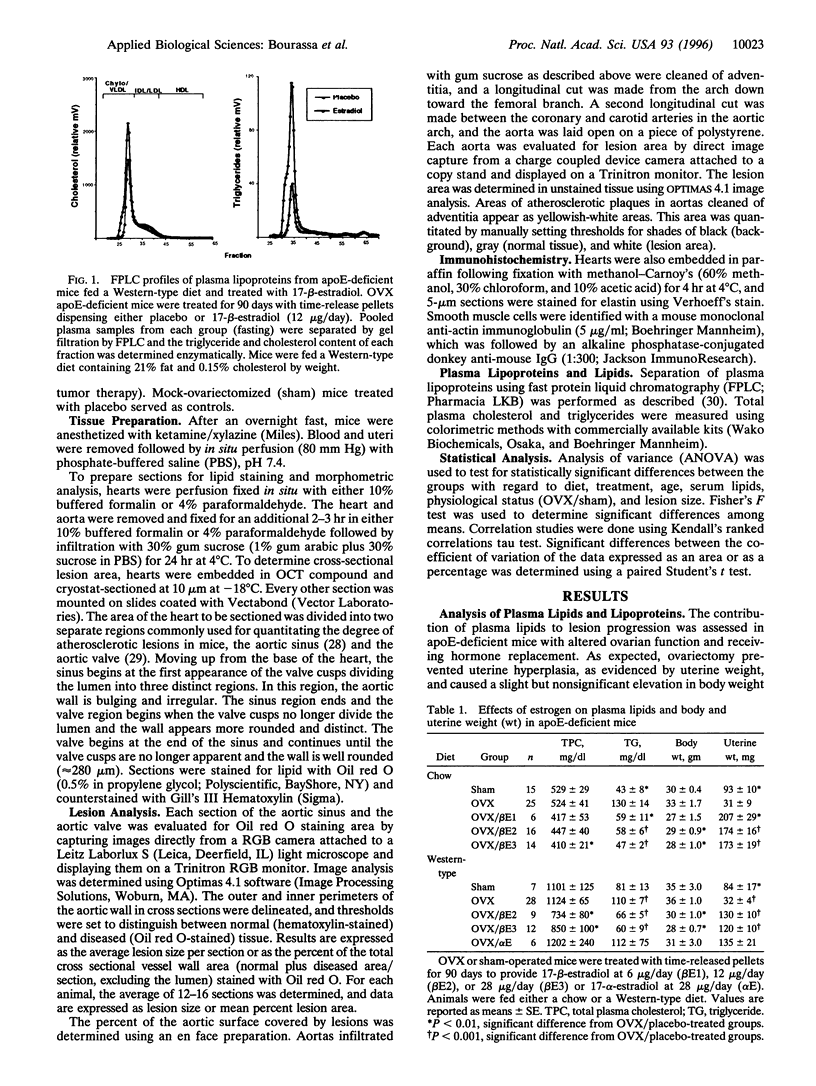
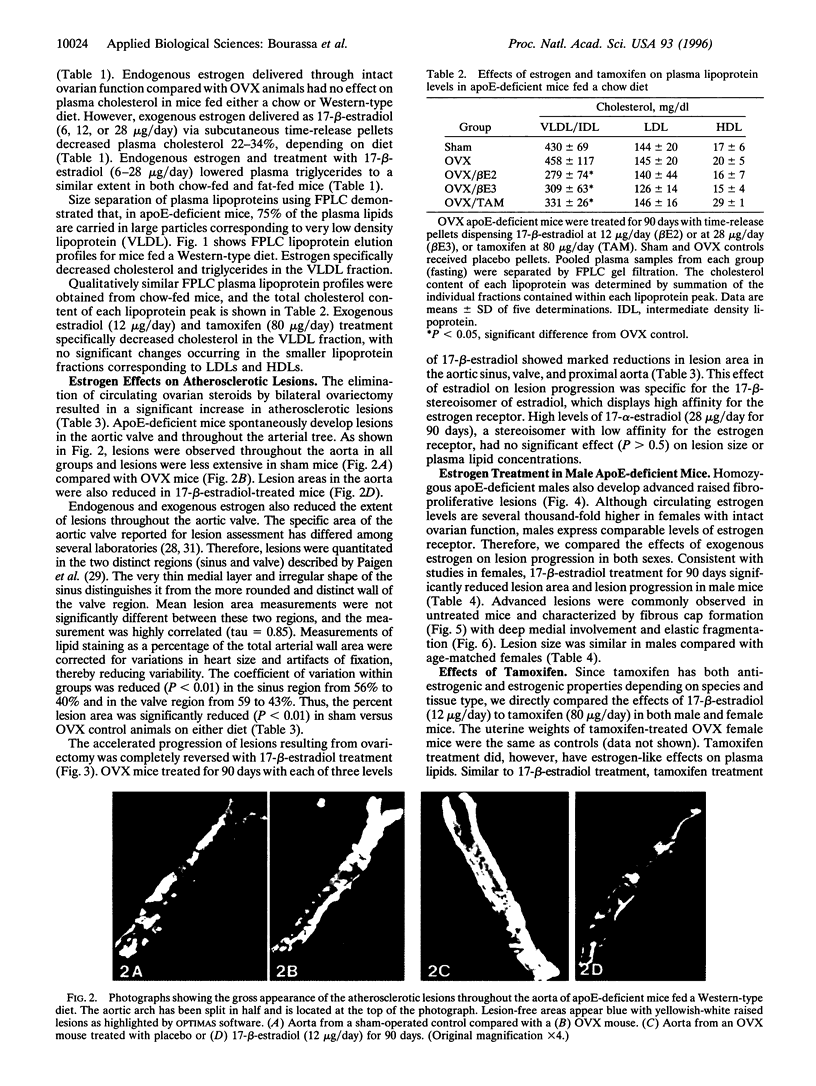
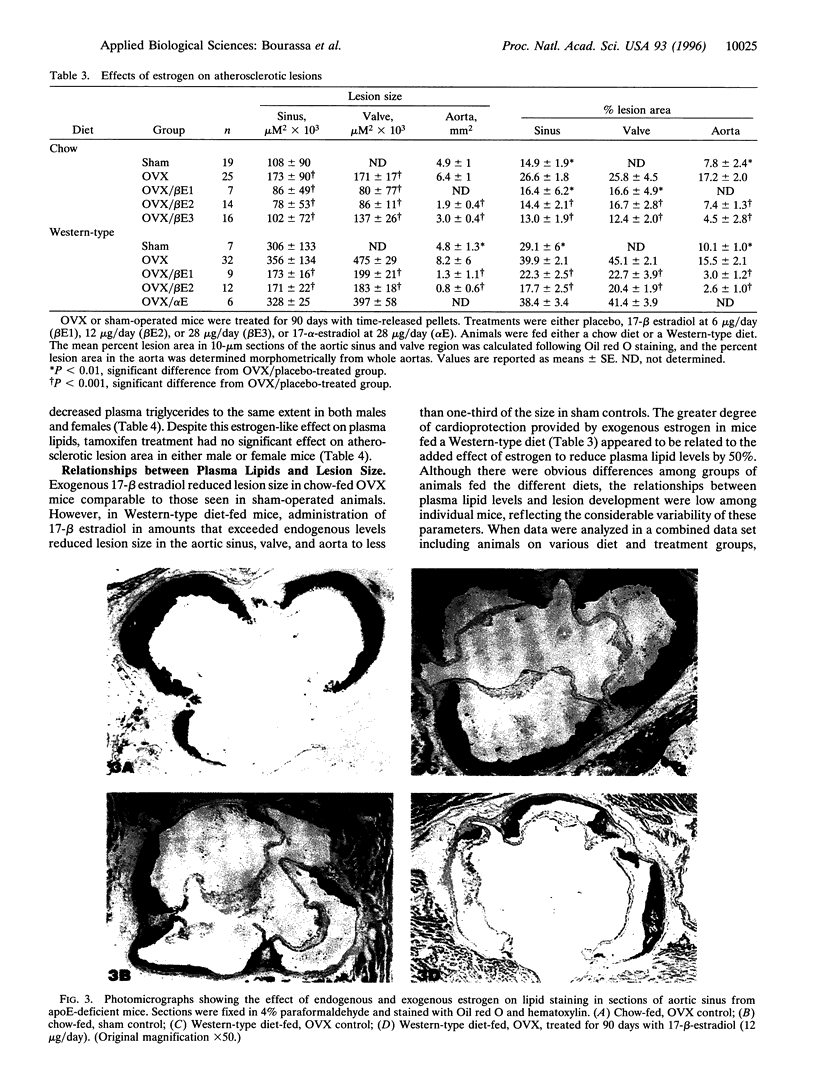
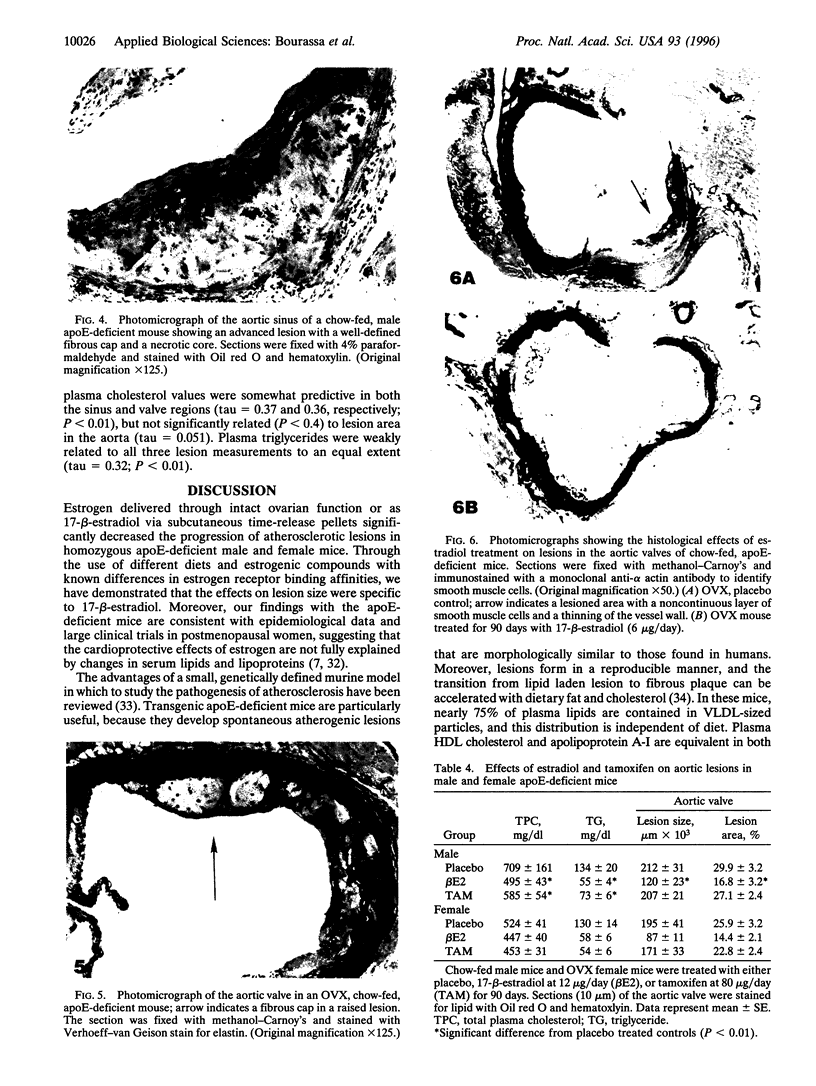
We have studied the effects of endogenous and exogenous estrogen on atherosclerotic lesions in apolipoprotein E-deficient mice. Female mice ovariectomized (OVX) at weaning displayed increases (P < 0.01) in fatty streak lesions in the proximal aorta and aortic sinus compared with female mice with intact ovarian function. These differences between the OVX and sham controls were apparent in both chow- and "Western-type" diet-fed mice. Moreover, increases in lesion size following OVX occurred without changes in plasma cholesterol. Hormone replacement with subdermal 17-beta-estradiol pellets releasing either 6, 14, or 28 micrograms/day significantly decreased (P < 0.001) atherosclerotic lesion area in both male and OVX female mice. In contrast, neither 17-alpha-estradiol (28 micrograms/day) or tamoxifen (85 micrograms/day) affected lesion progression in OVX female mice. In the Western diet-fed group, exogenous estradiol markedly reduced plasma cholesterol and triglycerides, whereas, in animals fed the chow diet, exogenous estrogen and tamoxifen treatment only decreased plasma and very low density lipoprotein triglycerides. However, lesion area was only weakly correlated with plasma cholesterol and triglycerides, 0.35 and 0.44 tau values, respectively (P < 0.01). In summary, in the apolipoprotein E-deficient mouse 17-beta-estradiol protects against atherosclerotic lesion formation, and this can only be partially explained through effects on plasma lipoprotein levels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. R., Kaplan J. R., Manuck S. B., Koritnik D. R., Parks J. S., Wolfe M. S., Clarkson T. B. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990 Nov-Dec;10(6):1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Bhatia A. J., Wade G. N. Effects of pregnancy and ovarian steroids on fatty acid synthesis and uptake in Syrian hamsters. Am J Physiol. 1991 Jan;260(1 Pt 2):R153–R158. doi: 10.1152/ajpregu.1991.260.1.R153. [DOI] [PubMed] [Google Scholar]
- Bush T. L., Barrett-Connor E., Cowan L. D., Criqui M. H., Wallace R. B., Suchindran C. M., Tyroler H. A., Rifkind B. M. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987 Jun;75(6):1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- Bush T. L., Fried L. P., Barrett-Connor E. Cholesterol, lipoproteins, and coronary heart disease in women. Clin Chem. 1988;34(8B):B60–B70. [PubMed] [Google Scholar]
- Chang S., Zhang S. H., Maeda N., Borensztajn J. Hepatic clearance of chylomicron remnants in mice lacking apoprotein E. Biochim Biophys Acta. 1994 Nov 17;1215(1-2):205–208. doi: 10.1016/0005-2760(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Colditz G. A., Willett W. C., Stampfer M. J., Rosner B., Speizer F. E., Hennekens C. H. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987 Apr 30;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- Cummings S. R. Evaluating the benefits and risks of postmenopausal hormone therapy. Am J Med. 1991 Nov 25;91(5B):14S–18S. doi: 10.1016/0002-9343(91)90241-o. [DOI] [PubMed] [Google Scholar]
- Demer L. L. A skeleton in the atherosclerosis closet. Circulation. 1995 Oct 15;92(8):2029–2032. doi: 10.1161/01.cir.92.8.2029. [DOI] [PubMed] [Google Scholar]
- Edwards M. W., Bain S. D., Bailey M. C., Lantry M. M., Howard G. A. 17 beta estradiol stimulation of endosteal bone formation in the ovariectomized mouse: an animal model for the evaluation of bone-targeted estrogens. Bone. 1992;13(1):29–34. doi: 10.1016/8756-3282(92)90358-4. [DOI] [PubMed] [Google Scholar]
- Foegh M. L., Asotra S., Howell M. H., Ramwell P. W. Estradiol inhibition of arterial neointimal hyperplasia after balloon injury. J Vasc Surg. 1994 Apr;19(4):722–726. doi: 10.1016/s0741-5214(94)70047-8. [DOI] [PubMed] [Google Scholar]
- Frazier-Jessen M. R., Kovacs E. J. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J Immunol. 1995 Feb 15;154(4):1838–1845. [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor B. J., Sand T., Clark R. W., Aiello R. J., Bamberger M. J., Moberly J. B. Inhibition of cholesteryl ester transfer protein activity in hamsters alters HDL lipid composition. Atherosclerosis. 1994 Sep 30;110(1):101–109. doi: 10.1016/0021-9150(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Gerhard M., Ganz P. How do we explain the clinical benefits of estrogen? From bedside to bench. Circulation. 1995 Jul 1;92(1):5–8. doi: 10.1161/01.cir.92.1.5. [DOI] [PubMed] [Google Scholar]
- Gisclard V., Miller V. M., Vanhoutte P. M. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988 Jan;244(1):19–22. [PubMed] [Google Scholar]
- Grainger D. J., Witchell C. M., Metcalfe J. C. Tamoxifen elevates transforming growth factor-beta and suppresses diet-induced formation of lipid lesions in mouse aorta. Nat Med. 1995 Oct;1(10):1067–1073. doi: 10.1038/nm1095-1067. [DOI] [PubMed] [Google Scholar]
- Gruchow H. W., Anderson A. J., Barboriak J. J., Sobocinski K. A. Postmenopausal use of estrogen and occlusion of coronary arteries. Am Heart J. 1988 May;115(5):954–963. doi: 10.1016/0002-8703(88)90063-4. [DOI] [PubMed] [Google Scholar]
- Hough J. L., Zilversmit D. B. Effect of 17 beta estradiol on aortic cholesterol content and metabolism in cholesterol-fed rabbits. Arteriosclerosis. 1986 Jan-Feb;6(1):57–63. doi: 10.1161/01.atv.6.1.57. [DOI] [PubMed] [Google Scholar]
- Jiang C. W., Sarrel P. M., Lindsay D. C., Poole-Wilson P. A., Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br J Pharmacol. 1991 Dec;104(4):1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilma B., Eichler H. G., Breiteneder H., Wolzt M., Aringer M., Graninger W., Röhrer C., Veitl M., Wagner O. F. Effects of 17 beta-estradiol on circulating adhesion molecules. J Clin Endocrinol Metab. 1994 Dec;79(6):1619–1624. doi: 10.1210/jcem.79.6.7527406. [DOI] [PubMed] [Google Scholar]
- Jordan V. C. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984 Dec;36(4):245–276. [PubMed] [Google Scholar]
- Jordan V. C., Murphy C. S. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev. 1990 Nov;11(4):578–610. doi: 10.1210/edrv-11-4-578. [DOI] [PubMed] [Google Scholar]
- Keaney J. F., Jr, Shwaery G. T., Xu A., Nicolosi R. J., Loscalzo J., Foxall T. L., Vita J. A. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation. 1994 May;89(5):2251–2259. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- Liu M. S., Jirik F. R., LeBoeuf R. C., Henderson H., Castellani L. W., Lusis A. J., Ma Y., Forsythe I. J., Zhang H., Kirk E. Alteration of lipid profiles in plasma of transgenic mice expressing human lipoprotein lipase. J Biol Chem. 1994 Apr 15;269(15):11417–11424. [PubMed] [Google Scholar]
- Ma P. T., Gil G., Südhof T. C., Bilheimer D. W., Goldstein J. L., Brown M. S. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8370–8374. doi: 10.1073/pnas.83.21.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoki J. J., Zavala E., Silva G., Mendoza-Patiño N., Rubio-Póo C., Medina-Martínez S., Domínguez-Escoto P. The dual effects of estrogens on blood clotting time. Proc West Pharmacol Soc. 1983;26:45–48. [PubMed] [Google Scholar]
- Miller V. M., Vanhoutte P. M. Progesterone and modulation of endothelium-dependent responses in canine coronary arteries. Am J Physiol. 1991 Oct;261(4 Pt 2):R1022–R1027. doi: 10.1152/ajpregu.1991.261.4.R1022. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994 Jan;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Paigen B., Holmes P. A., Mitchell D., Albee D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis. 1987 Apr;64(2-3):215–221. doi: 10.1016/0021-9150(87)90249-8. [DOI] [PubMed] [Google Scholar]
- Paigen B., Ishida B. Y., Verstuyft J., Winters R. B., Albee D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis. 1990 Mar-Apr;10(2):316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- Paigen B., Morrow A., Holmes P. A., Mitchell D., Williams R. A. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987 Dec;68(3):231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- Plump A. S., Breslow J. L. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr. 1995;15:495–518. doi: 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- Pászty C., Maeda N., Verstuyft J., Rubin E. M. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994 Aug;94(2):899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pászty C., Maeda N., Verstuyft J., Rubin E. M. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994 Aug;94(2):899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J. H., Xie P. Z., Fishbein M. C., Kreuzer J., Drake T. A., Demer L. L., Lusis A. J. Pathology of atheromatous lesions in inbred and genetically engineered mice. Genetic determination of arterial calcification. Arterioscler Thromb. 1994 Sep;14(9):1480–1497. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- Ralston S. H., Russell R. G., Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990 Sep;5(9):983–988. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- Reddick R. L., Zhang S. H., Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994 Jan;14(1):141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I., el-Sabban F., Allen A. D., Nelson G. H., Bhatnagar A. S., Choi S. Effects of estradiol on platelet aggregation in mouse mesenteric arterioles and ex vivo. Thromb Res. 1985 Aug 1;39(3):253–262. doi: 10.1016/0049-3848(85)90220-8. [DOI] [PubMed] [Google Scholar]
- Sacks F. M., McPherson R., Walsh B. W. Effect of postmenopausal estrogen replacement on plasma Lp(a) lipoprotein concentrations. Arch Intern Med. 1994 May 23;154(10):1106–1110. [PubMed] [Google Scholar]
- Sugioka K., Shimosegawa Y., Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987 Jan 1;210(1):37–39. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- Wade G. N., Powers J. B. Tamoxifen antagonizes the effects of estradiol on energy balance and estrous behavior in Syrian hamsters. Am J Physiol. 1993 Sep;265(3 Pt 2):R559–R562. doi: 10.1152/ajpregu.1993.265.3.R559. [DOI] [PubMed] [Google Scholar]
- Wade G. N., Schneider J. E. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992 Summer;16(2):235–272. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- Walsh B. W., Li H., Sacks F. M. Effects of postmenopausal hormone replacement with oral and transdermal estrogen on high density lipoprotein metabolism. J Lipid Res. 1994 Nov;35(11):2083–2093. [PubMed] [Google Scholar]
- Walsh B. W., Schiff I., Rosner B., Greenberg L., Ravnikar V., Sacks F. M. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991 Oct 24;325(17):1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- Walsh B. W., Schiff I., Rosner B., Greenberg L., Ravnikar V., Sacks F. M. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991 Oct 24;325(17):1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- Weinstein I., Turner F. C., Soler-Argilaga C., Heimberg M. Effects of ethynyl estradiol on serum lipoprotein lipids in male and female rats. Biochim Biophys Acta. 1978 Sep 28;530(3):394–401. doi: 10.1016/0005-2760(78)90159-5. [DOI] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]