Abstract
The ancient fungus-growing termite (Mactrotermitinae) symbiosis involves the obligate association between a lineage of higher termites and basidiomycete Termitomyces cultivar fungi. Our investigation of the fungus-growing termite Macrotermes natalensis shows that Bacillus strains from M. natalensis colonies produce a single major antibiotic, bacillaene A (1), which selectively inhibits known and putatively antagonistic fungi of Termitomyces. Comparative analyses of the genomes of symbiotic Bacillus strains revealed that they are phylogenetically closely related to Bacillus subtilis, their genomes have high homology with more than 90% of ORFs being 100% identical, and the sequence identities across the biosynthetic gene cluster for bacillaene are higher between termite-associated strains than to the cluster previously reported in B. subtilis. Our findings suggest that this lineage of antibiotic-producing Bacillus may be a defensive symbiont involved in the protection of the fungus-growing termite cultivar.
Beneficial symbiotic associations between prokaryotes and eukaryotes are widespread in nature1. Such mutualistic relationships include defensive symbioses, which often involve selective antibiotics produced by prokaryotes against host antagonists2,3. Recent studies exploring chemical mediators of symbiotic interactions in beetles4,5, fungus-growing ants6,7,8, marine sponges9, and solitary wasps10 have provided insights into the fundamental functions of natural antibiotics, as well as the discovery of novel bioactive small molecules genetically coded by insect-associated microorganisms6,7,8,9,10,11,12.
Fungus-growing termites (Blattodea, Macrotermitinae) are major decomposers in the Old World tropics, where they form some of the most complex colony and mound structures known (Fig. 1a). The success of the Macrotermitinae is undoubtedly attributed to their engagement in a mutualistic symbiosis with Termitomyces fungi (Basidiomycota: Agaricales: Lyophyllaceae), which aid in the degradation of plant material13,14,15. The fungus is housed on a special substrate (fungus comb) in the nest, which is maintained by the termites through the continuous addition of partially digested plant material that has passed through the termite gut along with asexual Termitomyces spores16,17 (Fig. 1b). In return for continuous provisioning of a substrate for growth, Termitomyces serves as a nitrogen-rich food source for the termites. The association originated more than 35 million years ago and none of the more than 350 species of fungus-growing termites, or the fungus symbionts they maintain, have abandoned this long-term association18,19,20.
Figure 1.
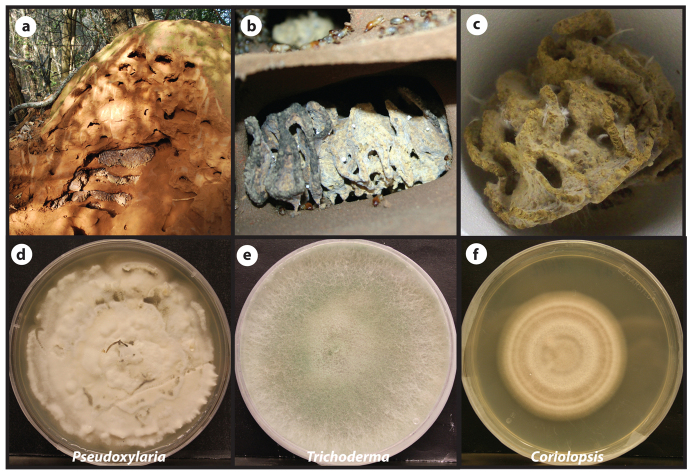
(a) Macrotermes natalensis fungus-growing termite colony. (b) A close up of the fungus comb (courtesy of Duur K. Aanen), generated by the termites through a mix of plant biomass and Termitomyces spores after termite gut passage, likely allowing for gut bacteria to control what enters the fungus comb. (c) In the absence of termites, the fungus comb is rapidly over-grown by Pseudoxylaria. (d–f) In vitro growth characteristics of three fungi isolated from M. natalensis fungus combs: (d) Pseudoxylaria, (e) Trichoderma and (f) Coriolopsis ((e) and (f), courtesy of Saria Otani).
The success of termite fungiculture is expected to rely on the termites effectively defending both themselves and their cultivar fungus from invading competitors and diseases. The maintenance of the cultivar fungus in monoculture within individual nests21,22,23 is predicted to make the fungus prone to exploitation in the absence of the termites24,25 (Fig. 1c), but only few candidate antagonists of the symbiosis have been identified. Ascomycete fungi in the subgenus Pseudoxylaria26,27,28 (Fig. 1d) are prevalent in fungus-growing termite nests27 and appear to compete with Termitomyces for the substrate provided by the termites29, and Trichoderma will rapidly overgrow the termite fungus when termite workers are absent30.
Whether or not Pseudoxylaria and Trichoderma act as specialized disease-causing microbes in the fungus-growing termite symbiosis is not clear; however, their competitive and/or antagonistic potential against Termitomyces supports that it would be beneficial for the termite-fungus association to assure that they are suppressed. Obligate gut passage of the substrate prior to incorporation in the fungus comb may aid this, because this mode of substrate incorporation may allow for the selective inhibition of antagonists before entry to the fungus comb17.
Previous work in Odontotermes formosanus fungus-growing termites has suggested that gut- and fungus comb-residing Bacillus sp. may aid in the suppression of antagonistic fungi (Trichoderma) present in the substrate provided by the termites for Termitomyces growth. The study found that Trichoderma rapidly overgrows Termitomyces in vitro, that Bacillus sp. present in the termite gut and fungus comb produce a secretion that in vitro inhibits Trichoderma but not Termitomyces growth, but the compound responsible for this selective inhibition has remained unsolved30. Through bioassay-guided chemical analyses of Bacillus strains isolated from different colonies of Macrotermes natalensis, we show that termite-associated Bacillus strains produce a single major compound, bacillaene (1), which selectively inhibits known (Pseudoxylaria and Trichoderma) and potentially competitive or antagonistic (Coriolopsis, Umbelopsis and Fusarium) fungi that had been obtained in culture from M. natalensis colonies and, hence, may represent potential competitors or antagonists of Termitomyces. Whole-genome analyses of these strains revealed that they are phylogenetically close to Bacillus subtilis and have nearly identical genomes, including across the entire ca. 80 kb gene cluster coding for the production of bacillaene.
Results
Isolation and antifungal testing of symbiotic Bacillus from termites
Three Bacillus sp. strains (hereafter designated #9, #11 and #13) were isolated each from a different Macrotermes natalensis colony in Mookgophong, South Africa (S24°40′30.5″E28°47′50.4″). Isolates were obtained by the application of termite colony material (#9: fungus comb, #11: worker abdomen crushed in water, and #13: worker washed in water) on low-nutrient medium using standard isolation techniques. To explore the presence of antifungal activity, testing of the Bacillus strains was performed against Termitomyces, Pseudoxylaria (Fig. 1d) as well as four additional fungi isolated from the same termite species in addition to the beneficial Termitomyces fungus: Trichoderma sp. (Fig. 1e), Coriolopsis sp. (Fig. 1f), Umbelopsis sp. and Fusarium sp. Each bacterial strain was cultivated with each of the fungi on YEME agar plates. Seven days after inoculation, all three Bacillus strains inhibited the growth of all fungi except Termitomyces, suggesting the presence of a selective antifungal compound, which prompted us to make further efforts to identify the responsible compound.
Chemical identification of the antifungal compound
To find the compounds from the Bacillus strains responsible for the growth inhibition of the five fungi, we cultivated Bacillus strains in YEME liquid culture medium. An initial LC/MS (liquid chromatography and mass spectrometry) analysis of the ethyl acetate (EtOAc) extract of the cultures revealed a common major secondary metabolite in all three strains, and this compound displayed the typical polyene UV spectral feature (λmax at 346, 364, and 384 nm) and the low-resolution molecular ion [M + H]+ at m/z 581. Scaling up the culture conditions to 24 L of each of the Bacillus strains allowed for bioassay-guided fractionation to narrow down the active antifungal component in the extracts. The dried extract was fractionated under step gradient conditions using aqueous methanol (20, 40, 60, 80 and 100%) by open column reversed-phase chromatography on C18 resin. Each fraction was tested against Pseudoxylaria, Trichoderma, Coriolopsis, Umbelopsis and Fusarium using paper disk diffusion assays to trace active fractions.
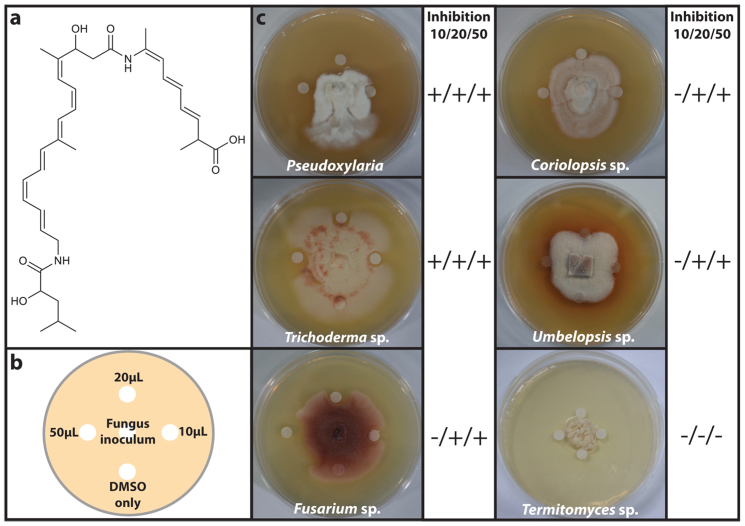
The antifungal assays demonstrated that the 80% aqueous MeOH fraction of each Bacillus culture was the most active, and LC/MS analysis of the fraction revealed the common major compound initially detected in the crude extract. Purification of the compound by preparative reversed-phase high performance liquid chromatography (HPLC) yielded the pure compound (1), which possessed the molecular formula C34H48N2O6 based on electrospray high-resolution mass spectrum ([M + H]+ at m/z 581.3585). Subsequently, we analyzed 1H and two-dimensional NMR spectra of compound 1, specifically 1H-1H correlation (COSY; Fig. S3), heteronuclear single quantum coherence (HSQC; Fig. S4), and heteronuclear multiple bond correlation (HMBC; Fig. S5). The spectroscopic analysis and literature search identified compound 1 as bacillaene A, a polyene polyketide secondary metabolite31 (Fig. 2a).
Figure 2.
(a) The chemical structure of bacillaene A (1). (b) Petri plate antifungal activity assay setup, showing the placement of three concentrations (10 μL, 20 μL, 50 μL) of 1 (10 mg/mL) dissolved in DMSO, the control (DMSO only, 10 μL) and the placement of the fungal inoculum. (c) Representative image examples of bacillaene A activity against Pseudoxylaria 10 days after inoculation, Trichoderma sp. 3 days after inoculation, Fusarium sp. 3 days after inoculation, Coriolopsis sp. 5 days after inoculation, Umbelopsis sp. 7 days after inoculation, and Termitomyces 20 days after inoculation in addition to qualitative indications of the presence/absence (+/−) of inhibition at concentrations 10 μL, 20 μL, and 50 μL of 1 (10 mg/mL) for the five contaminant fungi as well as Termitomyces.
We confirmed that bacillaene A is responsible for the antifungal activity observed using an antifungal assay (Fig. 2b). Petri dishes were observed daily for 30 days (Fig. 2c; Fig. S1). Bacillaene A (1) inhibited the growth of the fungi in a dose-dependent manner (Fig. 2c).
Genomic identification of the bacillaene biosynthetic gene cluster
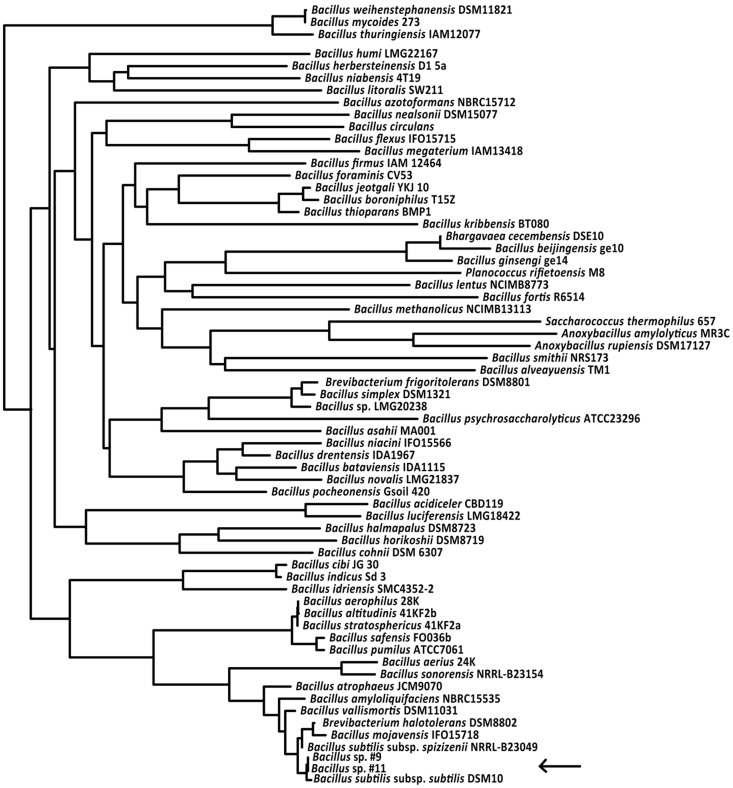
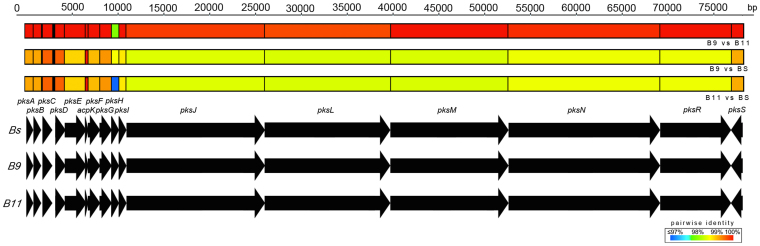
We obtained whole-genome data for two of the three Bacillus strains using mate-paired Illumina HiSeq sequencing, and genomes were assembled using Velvet32 and OSLay33 and annotated using BASYS34. The 16S rRNA genes were extracted from the genomes to obtain a phylogeny placement using the Ribosomal Database Project35 (RDP), which identified both strains as being indistinguishable from Bacillus subtilis (Fig. 3). Draft genome comparisons revealed that the two strains are almost identical with 4548 shared ORFs being 100% identical at the nucleotide level, 393 shared ORFs being less than 100% identical, but only 17 of these ORFs being less than 97% identical, despite the genomes being only at the draft level (Table 1; Table S1). However, when compared to B. subtillis 168, only 402 of the ORFs identified in Bacillus #9 are 100% identical to B. subtillis and only 455 of the ORFs identified in Bacillus #11 are 100% identical to B. subtillis (Table S1). We confirmed the presence of the genes necessary for bacillaene production in the two draft Bacillus genomes of strains #9 and #11, and compared the 16 bae genes coding for the pks complex coding for bacillaene A (1) to the published sequences obtained for Bacillus subtilis strain 16831,36,37,38,39. The ca. 80 kb gene cluster for the synthesis of bacillaene in both Bacillus #9 and #11 also has 16 genes, which are organized identically to those of B. subtilis (Fig. 4). Pairwise comparisons of individual pks genes between genomes indicated comparable percentage identities between the termite-associated strains and B. subtilis. However, the two termite-bacilli strains are more similar to each other across the entire gene cluster (Fig. 4; Table S3). In fact, Bacillus #9 and #11 are nearly 100% identical both at the nucleotide and amino acid level in 15 of the 16 genes, with the only exception (98% identical) being the pksH gene, which functions to install a methyl group, not part of the backbone of bacillaene (Fig. 4). This implies that termite-Bacillus produce the same chemical compound and are more genomically similar to each other, including with regard to the bacillaene gene cluster, than to bacillaene-producing species not associated with fungus-growing termites.
Figure 3. A 16S rRNA gene phylogeny placing Bacillus #9 and #11 (indicated with an arrow) in a global Bacillus phylogeny, showing that Bacillus sp. associated with fungus-growing termites are indistinguishable from B. subtilis based on the full-length 16S rRNA gene.
Table 1. Genome characteristics of Bacillus associated with Macrotermes natalensis.
| Bacillus #9 | Bacillus #11 | |
|---|---|---|
| Median coverage depth | 157.1 | 155.9 |
| Number of nodes | 733 | 1051 |
| n50/max/total | 23077/114783/3958212 | 11138/62079/3932419 |
| Number of reads used | 11407671/11598752 | 11207443/11250000 |
| Number of supercontigs | 22 | 26 |
| Number of gaps in supercontigs | 265 | 509 |
| Percentage covered | 93% | 89% |
| ORFs identified and annotated | 4590 | 4879 |
| Genome draft length (bp) | 4101765 | 4244208 |
Figure 4. Comparative analyses of the bacillaene gene cluster between B. subtilis 168 (Bs), Bacillus #9 (B9) and Bacillus #11 (B11).
Sixteen genes code for the pks gene complex involved in bacillaene A (1) biosynthesis, and these are acpK, pksA, pksB, pksC, pksD, pksE, pksF, pksG, pksH, pksI, pksJ, pksL, pksM, pksN, pksR and pksS36. The gene cluster was identified on a single contig in both #9 and #11. The figure shows a bp-scale bar of the ca. 80 kb gene cluster (top) together with a diagram of the orientation of the genes (bottom) in each of the three genomes. Pairwise comparisons (coloured bars) indicate comparable identities between the termite-associated strains and B. subtilis, showing that the two termite strains are more similar (almost identical) to each other across the entire gene cluster.
Discussion
Our findings provide the first evidence of a specific role of bacillaene A (1) in a biological system. We obtained this result through optimization of the production and isolation of bacillaene A (1), combined with minimizing degradation of the compound by avoiding exposure to light. The compound was initially identified from Bacillus subtilis as an antibiotic agent inhibiting prokaryotic protein synthesis40. It was reported to display antibacterial activity against various gram-negative (Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, and Serratia marcescens) and gram-positive (Bacillus thuringiensis and Staphylococcus aureus) bacteria. However, it did not show antifungal activity against the yeasts Saccharomyces cerevisiae and Candida albicans40. Even though the gene cluster and the biosynthesis of bacillaene have been relatively well studied31,41,42, its biological role in nature has been poorly understood, possibly because of its notorious instability41. Comprehensive analysis of Bacillus amyloliquifaciens, which is a prolific bioactive secondary-metabolite producer, including bacillaene A (1), suggested a potential role of B. amyloliquifaciens as a defensive symbiont controlling plant pathogens43,44. Our findings suggest that bacillaene A (1) produced by the Bacillus obtained from three different colonies of M. natalensis could aid in the suppression of antagonistic fungi of the cultivar fungus Termitomyces. If so, our findings suggest that i) bacillaene inhibits phylogenetically diverse filamentous fungi, ii) Bacillus strains and their secondary metabolites could play a symbiotic role in nature, and iii) Bacillus could play a symbiotic role in an ancient mutualism between social insects and fungi.
Macrotermes natalensis workers continuously bring in partly degraded plant material (mainly decaying wood) to their colony, and this substrate inevitably harbours microbes that have the potential to compete with or antagonize the termites' mutualistic fungus. The substrate for Termitomyces is not directly incorporated into the fungus comb, but experiences obligate gut passage prior to incorporation17. The maintenance of defensive gut microbes that can aid in selective inhibition of antagonistic fungi consequently allows for the termites to control the characteristics of the comb substrate to avoid entry of harmful fungi. Our findings suggest that a lineage of Bacillus serves a defensive role through the production of a major compound, bacillaene A (1) that does not harm the termites' mutualistic fungus, but suppresses the growth of known (Pseudoxylaria and Trichoderma) and putative antagonistic fungi of the symbiosis. The Bacillus lineage has so far been identified in Odontotermes formosanus30 and M. natalensisthis study, two of the most ecologically important and phylogenetically diverse fungus-growing termite genera18. Phylogenetic comparison of the 16S rRNA gene between the Bacillus strains identified in this study with those identified by Mathew et al.30 (Fig. S6), showed that M. natalensis strains were 98.2 ± 0.03% (mean ± SE) similar across the 437 bp fragment to those obtained from O. formosanus guts, while they were more distant from isolates from fungus comb (94.1 ± 0.096%). This suggests that the same operational taxonomic unit likely is present in the two termite species and that the Bacillus strains we isolated in this study likely originated from the termite gut; however, whether a specific lineage of Bacillus associates with the entire Macrotermitinae sub-family remains to be explored.
Bacillaene-producing Bacillus has so far been identified in both fungus-growing termite guts and within the fungus comb30, this study. This suggests that Bacillus suppression can take place both during the passage of crude forage through the termite gut, which may allow for partial or complete suppression of incoming fungi, and also later within the fungus comb when Termitomyces hyphae decompose the comb substrate. This is possible because Termitomyces itself is not adversely affected by bacillaene, which provides an interesting contrast to the utilization of bacteria-derived antifungals in the other major fungus-farming symbiosis: the Neotropical fungus-growing ants (tribe attini). Fungus-growing ants associate with antibiotic-producing Actinobacteria for the suppression of ascomycete Escovopsis spp. parasites of the ants' mutualistic fungus6,7,8. The ants maintain the bacteria on specific locations on the ant body45, consistent with active use of bacteria-derived compounds during fungus garden hygienic behaviours. In the ants, such tight control of the location and distribution of antifungal compounds may be necessary, because the ants' mutualistic fungus can be inhibited by the bacteria-derived antifungals46. In fungus-growing termites, this potential for conflict between fungal and bacterial mutualists of the insect host appears to be avoided, making it possible to maintain the bacterium in both the insect gut and in the fungus comb, potentially allowing for efficient suppression of unwanted microbial contaminants both during preparation and degradation of the fungus garden substrate.
Methods
Collections
Three isolates of Bacillus (#9, #11 and #13) were obtained from three different Macrotermes natalensis colonies collected in Mookgophong (previously Naboomspruit, S24°40′30.5″E28°47′50.4″, elevation 1,045 m), South Africa on the 15th of January 2010. Isolates were obtained by crushing workers in PBS and plating on Chitin (per liter: 4 g chitin, 0.7 g K2HPO4, 0.3 g KH2PO4, 0.5 g MgSO4·5H2O, 0.01 g FeSO4·7H2O, 0.001 g ZnSO4, 0.001 g MnCl2 and 20 g agar) or microcrystalline (per liter: 5 g microcrystalline and 20 g of agar) medium. After ca. 14 days of growth on these low-nutrient media, Bacillus-like CFUs were transferred to Yeast Malt Extract Medium (per liter: 10 g malt extract, 4 g yeast extract, 4 g glucose, 15 g agar).
Chemical analyses
Bacillus strains #9, #11 and #13 were cultivated in 25 mL YEME liquid medium (per liter: 10 g malt extract, 4 g yeast extract, 4 g glucose) of a 100 mL Erlenmeyer flask with shaking at 200 rpm at 30°C for 2 days. Then 10 mL of culture was inoculated to 1 L of YEME medium in 2.8 L Fernbach flask and cultured at 180 rpm at 30°C for 2 days. 24 L of each (total 72 L for three strains) were prepared and cultured. The liquid cultures were extracted with a total of 72 L of EtOAc. The EtOAc layer was concentrated with a rotary evaporator to yield 3 g of dry extract material. The dry crude extract was re-suspended in MeOH and dried with celite. The celite-adsorbed material was fractionated by column chromatography on C18 resin with combinations of MeOH and water (2:8, 4:6, 6:4, 8:2, and 10:0 MeOH to water). Because the 80% fraction was the most active in the antifungal assay, the 80% fraction was further purified through preparative reversed-phase HPLC (Phenomenex Luna column C18 (2), 250 × 21.20 mm, UV detection 360 nm, flow rate 10 mL/min). Bacillaene (1) eluted at 25 min using isocratic 70% aqueous MeOH with 0.1 formic acid and overall 9 mg of pure bacillaene (1) were obtained.
Antifungal bioassay
For the paper disk diffusion assay on agar plates against Coriolopsis (Fungus #8), Umbelopsis (Fungus #14), Fusarium (Fungus #18), Trichoderma (Fungus #22) and Pseudoxylaria (Fungus 802-2), 9 cm diameter Petri dishes containing 20 mL of YEME agar medium were used. First, colonies of the fungal strains were inoculated in the center of the YEME agar plate and incubated at 30°C. After 3 days, four 6 mm diameter sterile paper disks were placed on the surface of each petri dish and then imbued with the crude extracts of bacillus strains #9, 11 and 13, the fractions of the extracts, and pure bacillaene (1) dissolved in DMSO with various concentrations. To set up a negative control, 10 μL DMSO was also added to every petri dish. Every petri dish was monitored daily for 30 days. As bacillaene (1) is very unstable under light, the experiment was performed in the dark.
Genome sequencing and assembly
DNA was extracted from 50 mL LB (tryptone: 10.0 g, yeast extract: 5.0 g, NaCl: 10.0 g) liquid broth culture of each Bacillus strain. 1 mL of culture was spun for 20 minutes at 13000 rpm, after which the supernatant was removed. 500 μL of CTAB buffer (10 mL 1 M Tris (pH 8.4), 5 mL 0.5 M EDTA (pH 8), 28 mL 5 M 5NaCl, 2 g cetyltrimethylammonium bromide, 57 mL ddH2O) was then added to each tube. Cells were subjected to two repeated cycles of freezing in −80°C and thawing at 65°C in a heat block. One volume of phenol-choloroform was added to samples, before vortexing and centrifugation for 10 min (13.000 rpm). Supernatants were transferred to clean 1.5 mL eppendorf tubes. 400 μL of cold 100% isopropanol were used for precipitation. After another round of centrifugation (20 min at 13,000 rpm), the pellet of DNA was washed with 70% ethanol and re-suspended in 50 μL ddH2O. Whole-genome sequences were achieved using mate-paired Illumina HiSeq at Beijing Genomics Institute (www.genomics.cn) and genomes were assembled using Velvet32, checked using Hawkey47 (MUMmer 3 package) and following the assembly, contigs were oriented and assembled to supercontigs using the OSLay software33 with the B. subtilis genome sequence (ATCC 7003, AP012496) as a reference genome. Gene annotations and comparisons were performed using the BASYS software34 (for full results, see Table S1). Contigs for draft genomes of Bacillus #9 and #11 are deposited in GenBank under the accession numbers APMX00000000 and APMW00000000 (the versions described here are the first, APMX01000000 and APMW01000000).
Comparative bacillaene gene cluster analyses
We confirmed the presence of the genes necessary for bacillaene production in two Bacillus strains. The sixteen genes coding for the bae gene complex involved in bacillaene biosynthesis (acpK, pksA, pksB, pksC, pksD, pksE, pksF, pksG, pksH, pksI, pksJ, pksL, pksM, pksN, pksR and pksS36) were compared to published bae sequences from B. subtilis (str. 168 AL009126). Gaps in the cluster sequences were closed using Sanger sequencing. DNA was amplified using a combination of primers provided in Table S2. PCR was performed in a final volume of 20 μL using the VWR ready-to-use mix and 1 μL of each primer (10 μM) at the conditions: 94°C for 30 s, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 60 s, and final extension at 72°C for 5 min. 5 μL of each PCR product was run on a 1% agarose gel containing 1x GELRED for 30 min. PCR product (15 μL) was purified using the Invitek kit, reeluted in sterilized milliQ water and sent to MWG for sequencing. Comparisons of bacillaene gene similarities between strains at the nucleotide level were made using nucmer47 (implemented in MUMmer 3 package) and blastn48 (Blast 2.2.27+) (Fig. 4; Table S3). All sequences have been deposited to GenBank (Accession numbers KC832420-KC832451).
Author Contributions
S.U., A.F., P.S., D.C.O. and M.P. designed the experiments; M.P. collected samples; S.U., A.F. and P.S. performed the experiments; D.C.O. and M.P. performed the general supervision of the project. D.C.O. and M.P. organized and drafted the paper with all authors contributing to the discussion of the data and to the writing.
Supplementary Material
Supplementary information
Table S1
Table S2
Table S3
Acknowledgments
We thank ZW de Beer, M Wingfield and the staff and students at the Forestry and Agricultural Biotechnology Institute, University of Pretoria, for assistance during collections, the Oerlemans family (Mookgophong) for permission to sample colonies at their farm, H Song and J Sun at BGI Europe for assistance with genome sequencing, and JJ Boomsma, JM Thomas and two anonymous reviewers for helpful comments on an earlier version of the manuscript. This work was supported by the National Research Foundation of Korea (NRF) from the Korean government (MEST) (No. 2009-0083533) to DCO, an International Early Career Scientist grant from the Howard Hughes Medical Institute to DCO, the EU Erasmus program to AF, a Marie Curie postdoc fellowship to PS, and a STENO grant from the Danish Agency for Science, Technology and Innovation to MP.
References
- Moran N. A. Symbiosis. Curr. Biol. 16, R866–R871 (2006). [DOI] [PubMed] [Google Scholar]
- Schmidt E. W. Trading molecules and tracking targets in symbiotic interactions. Nat. Chem. Biol. 8, 466–473 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. F. & Torres M. S. Defensive mutualism in microbial symbiosis [White J. F., & Torres M. S. (eds)] (CRC Press, Taylor & Francis Group, Boca Raton, London, New York, 2009). [Google Scholar]
- Scott J. J. et al. Bacterial protection of beetle-fungus mutualism. Science 322, 63 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.-C., Scott J. J., Currie C. R. & Clardy J. Mycangimycin, a polyene peroxide from a mutualist Streptomyces sp. Org. Lett. 11, 633–636 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C. R., Scott J. A., Summerbell R. C. & Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398, 701–704 (1999). [Google Scholar]
- Currie C. R. et al. Ancient Tripartite Coevolution in the Attine Ant-Microbe Symbiosis. Science 299, 386–388 (2003). [DOI] [PubMed] [Google Scholar]
- Oh D.-C., Poulsen M., Currie C. R. & Clardy J. Dentigerumycin: a bacterial mediator of an ant-fungus symbiosis. Nat. Chem. Biol. 5, 391–393 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J. et al. Exploring the chemistry of uncultivated bacterial symbionts: Antitumor polyketides of the pederin family. J. Nat. Prod. 68, 472–479 (2005). [DOI] [PubMed] [Google Scholar]
- Kroiss J. et al. Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 6, 261–263 (2010). [DOI] [PubMed] [Google Scholar]
- Poulsen M., Oh D.-C., Clardy J. & Currie C. R. Chemical analyses of wasp-associated Streptomyces bacteria reveal a prolific potential for natural products discovery. PLoS ONE 6, e16763 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.-C., Poulsen M., Currie C. R. & Clardy J. Sceliphrolactam, a polyene macrocyclic lactam from a wasp-associated Streptomyces sp. Org. Lett. 13, 752–755 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo F., Inoue T., Azuma J. I., Tayasu I. & Abe T. Role of the mutualistic fungus in lignin degradation in the fungus-growing termite Macrotermes gilvus (Isoptera; Macrotermitinae). Soil Biol. Biochem. 32, 653–658 (2000). [Google Scholar]
- Hyodo F. et al. Differential role of symbiotic fungi in lignin degradation and food provision for fungus-growing termites (Macrotermitinae: Isoptera). Funct. Ecol. 17, 186–193 (2003). [Google Scholar]
- Rohrmann G. The origin, structure, and nutritional importance of the comb in two species of Macrotermitinae. Pedobiologia 18, 89–98 (1978). [Google Scholar]
- Rouland-Lefèvre C. [Symbiosis with fungi.] Termites: evolution, sociality, symbioses, ecology [Abe T., Bignell D. E., Higashi M. (eds.)] [289–306] (Kluwer Academic Publishers, Dordrecht, 2000). [Google Scholar]
- Nobre T., Rouland-Lefèvre C. & Aanen D. K. [Comparative biology of fungus cultivation in termites and ants.] Biology of Termites: a modern synthesis [Bignell D. E., Roisin Y., Lo N. (eds.)] [193–210] (Springer, Heidelberg, 2011). [Google Scholar]
- Aanen D. K. et al. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc. Natl. Acad. Sci. USA 99, 14887–14892 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aanen D. K. & Boomsma J. J. [Evolutionary dynamics of the mutualistic symbiosis between fungus-growing termites and Termitomyces fungi.] Insect-Fungal Associations: Ecology and Evolution [Vega F., Blackwell M. (eds.)] [191–210] (Oxford University Press, 2005). [Google Scholar]
- Frøslev T., Aanen D. K., Læssøe T. & Rosendahl S. Phylogenetic relationships in Termitomyces and related genera. Myc. Res. 107, 1277–1286 (2003). [DOI] [PubMed] [Google Scholar]
- Katoh H., Miura T., Maekawa K., Shinzato N. & Matsumoto T. Genetic variation of symbiotic fungi cultivated by the macrotermitine termite Odontotermes formosanus (Isoptera: Termitidae) in the Ryukyu Archipelago. Mol. Ecol. 11, 1565–1572 (2002). [DOI] [PubMed] [Google Scholar]
- Moriya S. et al. Fungal community analysis of fungus gardens in termite nests. Micr. Environ. 20, 243–252 (2005). [Google Scholar]
- Aanen D. K. et al. High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science 326, 1103–1106 (2009). [DOI] [PubMed] [Google Scholar]
- Zhu Y. et al. Genetic diversity and disease control in rice. Nature 406, 718–722 (2000). [DOI] [PubMed] [Google Scholar]
- King K. C. & Lively C. M. Does genetic diversity limit disease spread in natural host populations. Heredity 109, 199–203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedegbe H. J., Miambi E., Pando A., Houngnandan P. & Rouland-Lefevre C. Molecular diversity and host specificity of termite-associated Xylaria. Mycologia 101, 686–691 (2009). [DOI] [PubMed] [Google Scholar]
- Visser A. A. et al. Levels of specificity of Xylaria species associated with fungus-growing termites: a phylogenetic approach. Mol. Ecol. 18, 553–567 (2009). [DOI] [PubMed] [Google Scholar]
- Hsieh H. M. et al. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phyl. Evol. 54, 957–969 (2010). [DOI] [PubMed] [Google Scholar]
- Visser A. A., Kooij P. W., Debets A. J. M., Kuyper T. W. & Aanen D. K. Pseudoxylaria as stowaway of the fungus-growing termite nest: Interaction asymmetry between Pseudoxylaria, Termitomyces and free-living relatives. Fung. Ecol. 4, 322–332 (2011). [Google Scholar]
- Mathew G. M., Ju Y.-M., Lai C.-Y., Mathew D. C. & Huang C. C. Microbial community analysis in the termite gut and fungus comb of Odontotermes formosanus: the implication of Bacillus as mutualists. FEMS Microbiol. Ecol. 79, 504–517 (2011). [DOI] [PubMed] [Google Scholar]
- Butcher R. A. et al. The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 104, 1506–1509 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino D. R. & Birney E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18, 821–829 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. C., Schuster S. C. & Huson D. H. OSLay: optimal syntenic layout of unfinished assemblies. Bioinformatics 23, 1573–1579 (2007). [DOI] [PubMed] [Google Scholar]
- Van Domselaar G. H. et al. BASys: a web server for automated bacterial genome annotation. Nucl. Acids. Res. 33, W455–459 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. R. et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucl. Acids. Res. 37, D141–145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. H. et al. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J. Bact. 188, 4024–4036 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder P. R. & Giles N. H. Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 34, 345–348 (1947). [PubMed] [Google Scholar]
- Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA 44, 1072–1078 (1958). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. R. & Wipat A. Sequencing and functional analysis of the genome of Bacillus subtilis strain 168. FEBS Lett. 389, 84–87 (1996). [DOI] [PubMed] [Google Scholar]
- Patel P. S. et al. Bacillaene, a novel inhibitor of prokaryotic protein synthesis produced by Bacillus subtilis. J. Antibiot. 48, 997–1003 (1995). [DOI] [PubMed] [Google Scholar]
- Moldenhauer J., Chen X. H., Borriss R. & Piel J. Biosynthesis of the antibiotic bacillene, the product of a giant polyketide synthase complex of the trans-AT family. Angew. Chem. Int. Ed. 46, 8195–8197 (2007). [DOI] [PubMed] [Google Scholar]
- Moldenhauer J. et al. The final steps of bacillaene biosynthesis in Bacillus amyloliquefaciens FZB42: direct evidence for β, γ dehydration by a trans-acyltransferase polyketide syntheas. Angew. Chem. Int. Ed. 49, 1465–1467 (2010). [DOI] [PubMed] [Google Scholar]
- Chen X. H. et al. Comparative analysis of the complete genome sequences of the plant growth promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotech. 25, 1007–1014 (2007). [DOI] [PubMed] [Google Scholar]
- Chen X. H. et al. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechn. 140, 27–37 (2009). [DOI] [PubMed] [Google Scholar]
- Currie C. R., Poulsen M., Mendenhall J., Boomsma J. J. & Billen J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311, 81–83 (2006). [DOI] [PubMed] [Google Scholar]
- Poulsen M. & Currie C. R. Symbiont interactions in a tripartite mutualism: Exploring the presence and impact of antagonism between two fungus-growing ant mutualists. PLoS ONE 5, e8748 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S. et al. Versatile and open software for comparing large genomes. Genome Biol. 5, R12 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S., Gish W., Miller W., Myers E. & Lipman D. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Table S1
Table S2
Table S3