Abstract
Intravital microscopy has emerged in the recent decade as an indispensible imaging modality for the study of the micro-dynamics of biological processes in live animals. Technical advancements in imaging techniques and hardware components, combined with the development of novel targeted probes and new mice models, have enabled us to address long-standing questions in several biology areas such as oncology, cell biology, immunology and neuroscience. As the instrument resolution has increased, physiological motion activities have become a major obstacle that prevents imaging live animals at resolutions analogue to the ones obtained in vitro. Motion compensation techniques aim at reducing this gap and can effectively increase the in vivo resolution. This paper provides a technical review of some of the latest developments in motion compensation methods, providing organ specific solutions.
Index Terms: Intravital microscopy, image stabilization, in vivo imaging, motion artifact and motion compensation
I. Introduction
Intravital optical imaging systems have been increasingly used for both experimental and clinical purposes. Intravital imaging microscopy in particular has obtained great consideration due to recent advancements in optical imaging and hardware, and the development of novel molecular tools such as new biological reporters, more efficient fluorochromes, and improved targeted and activatable contrast agents [1]-[4]. Its high spatial and temporal resolution, combined with its penetration depth and multi-reporter visualization capability, have all contributed in making it the perfect candidate for in vivo imaging studies enabling profound insight into in vivo biology. However, tissue movements caused by physiological processes such as respiratory and cardiac cycles critically limit the range of application of intravital microscopy. While at low resolution motion is not detrimental for image quality, as resolution increases the problem becomes more severe in particular for organs such as hearts and lungs. Therefore, intravital imaging microscopy applicability and its effective imaging resolution largely depends on motion compensation techniques. This technical review, which is focused on intravital microscopy for mouse imaging, summarizes motion compensation techniques at various levels of complexity and provides organ-specific motion compensation solutions.
II. Tissue Movements
Tissue movement imposes a practical limitation on the imaging resolution regardless of the physical limitation of the instrument [5]. This is particularly true for high resolution imaging modalities such as confocal and multiphoton microscopy, optical coherence tomography, and super-resolution microscopy. The most challenging situations generally occur in freely moving animals, whenever single cell imaging studies are concurrent with animal behavior. Single neural Ca2+ imaging for example has been recently demonstrated via implanted fiber optics multiphoton microscopy in freely behaving mice [6].
When no behavioral studies are conducted, sedation, analgesia and general anesthesia are normally used to reduce stress and pain and to provide adequate immobilization while greatly restricting physiological motion components [7]. The most common anesthetics used in mice are injected agents such as ketamine, avertin, pentobarbital in combination with other agents such as xylazine, or inhaled agents such as isofluorane or halothane [7]. Inhalation anesthesia is particularly indicated for prolonged imaging sessions over several hours, with less impact on liver and kidney functions. Injectable agents on the other side are more easy to administer and do not require any expensive equipment (e.g. vaporizers, flow meters, filtering units, etc) [7].
Even under deep anesthesia, tissue motion can still affect the quality of the recorded images depending on the targeted imaging organ and the imaging resolution. The two major sources of physiological movements are the respiratory and the cardiac cycle.
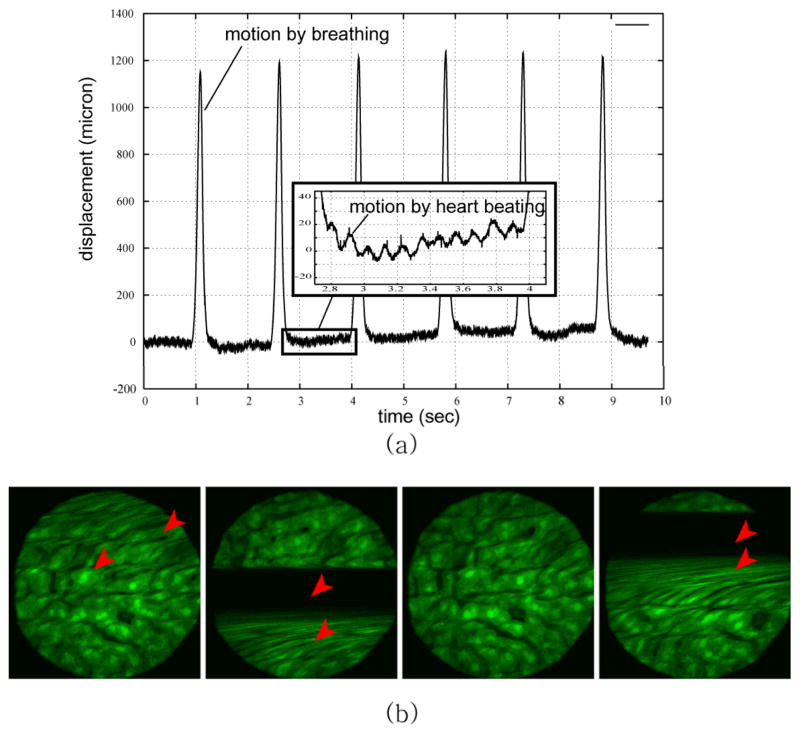
Fig. 1 shows the effect of respiration and heart beating during a mouse intravital imaging session. Movement of the mouse liver was measured with a laser displacement sensor without the presence of any motion restricting system [8]. As clearly emphasized breathing causes liver displacements at the mm-level with a frequency component of approximately 1 Hz, while heart beating generates shifts in position of approximately 10 microns with a frequency component in the range of 5–10 Hz [8].
Fig. 1.
Motion components during intravital imaging microscopy induce artifacts in the resulting images. (a) Vertical displacements of a mouse liver imaged in vivo, and measured with a laser displacement sensor coaxial with the imaging objective. Separate effects due to both respiratory and cardiac activities are shown. (b) Examples of the resulting motion artifacts in the mouse liver images. Arrows indicates the artifacts. Reprinted with permission from [8]. © 2008 IEEE
Of the two components, respiration is the most significant source of motion affecting all organs. This is particularly true for the lungs, but it extends to all the abdominal organs such as liver, kidney, pancreas and spleen. Its effect can be observed also at the brain level even with the skull tightly fixed with a stereotactic device [9]. Heart beating has also a broad effect on many organs, in particular on the heart itself. This has prevented until recently [10–11] to image in vivo the beating heart. Nevertheless, despite its much-reduced effect on all other organs when directly compared to respiration, its effect is still observable when high magnification studies are performed.
In addition to respiration and cardiac activity there are other sources of tissue movements such as peristalsis, muscle twitch, slow drift and so on. The effect of these movements is relatively local, rare or/and small compared to that of respiration and heart beating. Thus, those are not of great importance, but should be compensated too, for example, if repetitive long-time longitudinal imaging is required [12].
In general tissue motion, independently of its physiological nature, when combined with relatively slow scanning mechanism will introduce motion artifacts in the acquired images (Fig. 1(b)). The term “slow” here has to be put in context with the frequency of the motion cycle and its amplitude. As the resolution increases, the artifacts will become more prominent. Therefore, in order to fully exploit the high resolution offered by current microscopic techniques, motion should be properly compensated.
III. Physical Immobilization using Passive Mechanical Stabilizers
Physical immobilization can be considered as the first step in reducing organs’ motion components. Mechanical restriction has been widely used to limit and confine tissue motion. The idea is simple in the way that the imaged organ is typically being held firmly with the use of a rigid support. Dorsal skin window chambers, which were introduced about 70 years ago has been widely used for the study of subcutaneous tumor and angiogenesis [15], [16]. The window chamber tightly sandwiches the skin between two frames, providing long-term sequential imaging capabilities while creating a support frame for motion confinement and restriction. For imaging the brain, spinal cord, mammary gland, and abdominal organs, several other types of imaging window chambers haven been proposed by different authors [17]-[20].
Window chamber aside, a simple way to achieve physical immobilization is to cover the organ of interest with a glass cover slip. By applying a gentle pressure, a reduction in motion amplitude is easily obtained. Despite its simplicity, this approach is not always recommended because the pressure exerted in order to achieve stabilization can negatively impact physiological functions. Moreover, it’s not always possible to physically compress an organ within a mouse abdominal or chest cavity due to a lack of internal support.
As an alternative, in particular when using upright microscopes, exposed organs can be placed face down with the mouse body weight acting as pressure stabilizer [21]. Although this technique is easy to implement, it is suitable mainly for those organs that can be easily exteriorized, such as the small intestine, pancreas and the spleen [22], while it is not feasible for imaging carotids, arteries, liver, lymph nodes etc.
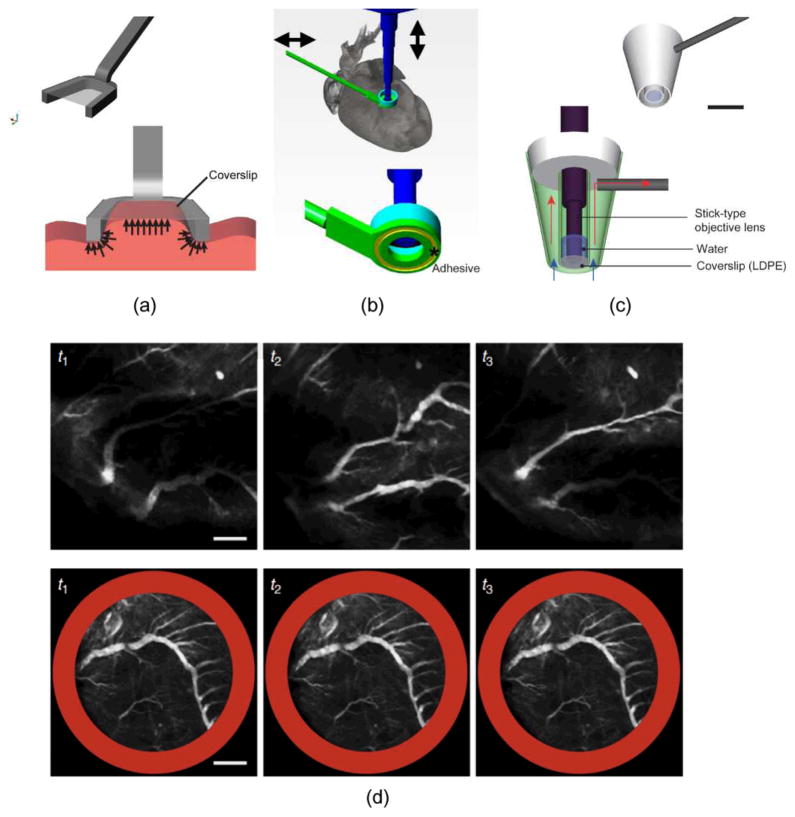
To overcome the limitations of both approaches, various mechanical stabilizers have been proposed. Small-sized mechanical holders, which are easy to be positioned inside the abdomen, have been demonstrated by [13], [22] (Fig. 2(a)). Another way of achieving stability consists in applying a thin layer of adhesives such as Dermabond, an FDA-approved clinical grade bonding, at the interface between a mechanical stabilizer and the organ of interest. Using this methodology Lee et al successfully stabilized the beating mouse heart [10] (Fig. 2(b)). Moreover, suctioning-based mechanical stabilizers have also been applied to hold soft tissue such as the lung [23]. This idea has been recently extended to heart imaging [11], [14] (Fig. 2(c)).
Fig. 2.
Examples of various types of mechanical stabilizers (a–c). (a) A compressive mechanical stabilizer for kidney and liver imaging. Reprinted with permission from [13]. © 2012 SPIE. (b) An adhesive based mechanical stabilizer for lung and heart imaging. Reprinted with permission from [10]. © 2012 Nature Publishing Group. (c) A suctioning mechanical stabilizer for lung and heart imaging. Scale bar: 5 mm. Reprinted with permission from [14]. © 2012 Landes Bioscience. (d) Image sequences of the vasculature in a mouse beating heart acquired without (upper row) and with (bottom row) stabilizer. Scale bar: 500 μm. Reprinted with permission from [10]. © 2012 Nature Publishing Group
In many instances, however, pure compressive mechanical stabilizers cannot completely suppress motion-induced artifacts because the necessary mechanical restrictions could cause severe physiological alterations. High pressure exerted on the tissues would not only have a negative effect on the organ physiological functions but would also cause harmful damage to the animal (e.g. mechanically restricting the motion of the heart would easily induce ischemia). It is therefore desirable to intentionally use a “soft” restriction approach allowing blood flow and minimal perturbation. As a result, to further compensate the residual motion components, these classes of stabilizers need to be used in combination with triggering/gating based acquisitions, image processing, or a combination of both.
IV. Active motion compensation stabilization
An alternative strategy for motion stabilization is to compensate tissue motion using an active tracking device. The principle is based on the fact that artifacts in the acquired images are due to tissue displacements relative to the imaging objective lens.
Once the relative motion existing between the imaged organ and the objective lens is removed, stabilized images with minimal motion artifacts can be acquired in real time. This idea can be implemented in two different ways: by moving the objective lens and keeping the animal at rest or alternatively by moving the subject itself while keeping the objective stationary [8], [12], [24]–[26]. For fast motion compensation, the best choice is to directly move the objective lens because high frequency components and amplitude of motion can cause undesirable effects on the animal. Moreover the presence of the heating plate, animal holders, and tubes for anesthesia could make it impractical for most users.
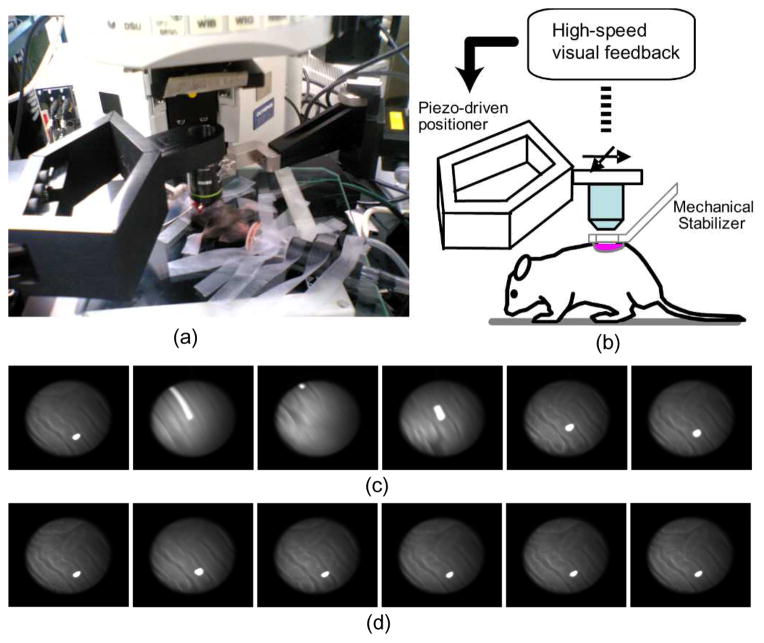
In most configurations, the moving objective lens is detached from the main microscope body and attached to a fast moving mechanism which is controlled with a feedback loop to follow the tissue motion as illustrated in Fig. 3. The organ’s absolute position can then be measured with a high speed visual information system [8], with a laser displacement sensor [24], or from a common-path optical coherence tomography distance sensor [26] while the objective is translated in real time keeping the relative distance between the two constant over time.
Fig. 3.
Active motion compensation by high-speed visual feedback control. (a) Stabilized confocal microscopy imaging setup. (b) System configuration: in vivo motion is calculated by a high-speed visual feedback system, and motion is compensated in real-time by moving the objective lens using a piezo-driven robotic closed arm with two degrees of freedom. (c) Unstable image sequence of a mouse kidney. (d) Stabilized image sequence acquired using the high-speed motion compensation system. Reprinted with permission from [8]. © 2008 IEEE
In a mouse under anesthesia, motion excursion can sometime reach large values in the order of a few millimeters, in particular when imaging areas in proximity of the chest cavity. Under such a condition, piezo-based active compensation systems are not practical due to the limited range of displacement they can provide. It is therefore recommended, whenever possible, to adopt passive mechanical stabilizers in combination with their use in a synergistic effort aimed at reducing the total amount of motion [8], [24].
Alternatively motion compensation along the plane perpendicular to the optical axis has been demonstrated by controlling an XY translational stage [12] while Z motion compensation has also been achieved using an objective focus motor [25].
V. Triggering/Gating based Acquisition
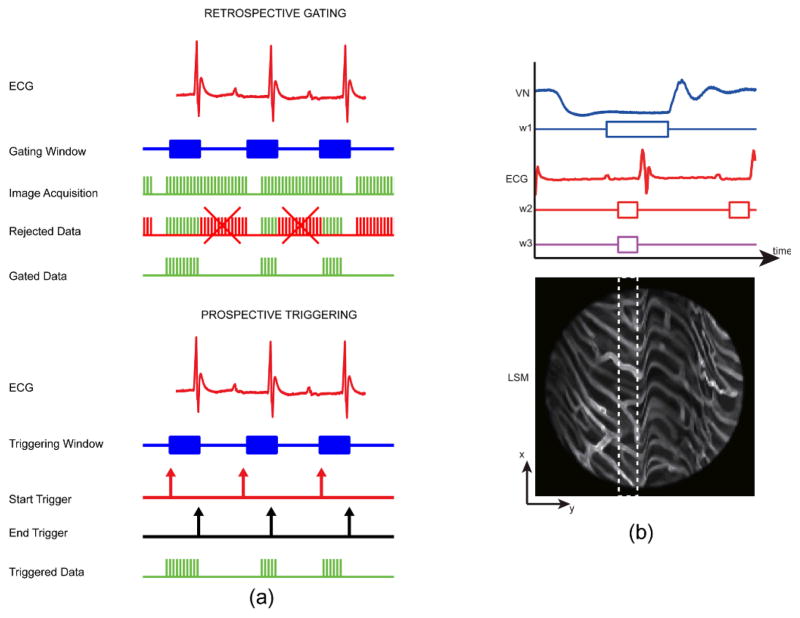
Throughout certain phases of the respiratory and cardiac cycles, the organs’ motion velocities reach a minimum. For respiratory activity, this can occur at both the end of inhalation and exhalation. For cardiac activity, it occurs within the PR segment of the electrocardiogram (ECG) signal, i.e. the time between the end of the P phase and the beginning of the QRS complex, when the heart is at rest. Acquisitions timed during these specific temporal intervals (Fig. 4a) will then guarantee images virtually free of any motion-induced artifact.
Fig. 4.
(a) Timing diagram of triggering and gating based acquisitions. Retrospective gated acquisition scheme (top): images are continuously acquired while the mouse ECG is simultaneously recorded. Following this non-selective acquisition, only part of the images (patches) that were acquired within the time of a specific gating window are representative of points belonging to the same physical plane. Prospective triggered acquisition scheme (down): images are triggered and acquired only during a specific triggering window which is determined in real time while monitoring cardiac and/or respiratory activity. (b) Double gating of the ECG and the ventilation (VN) signals, and the corresponding minimum artifact area (white dashed line box) within the raw acquired image. The ventilation gating window, w1 is chosen at the time of the end phase of expiration while the ECG gating window w2 is chosen at the time of the end diastole. Both gating windows thus correspond to the time of minimum motion for both lungs and heart, respectively. Reprinted with permission from [14]. © 2012 Landes Bioscience.
Retrospective gating methods continuously gather data while simultaneously recording motion information through physiological activity detection. Only images acquired during the time intervals of minimum motion will be considered. In contrast, prospective triggering methods trigger data acquisition by using physiological information recorded in real time. Again when images are triggered during the time intervals of minimum motion, artifact-free images will be collected. When multiple sources of motion are present, they will all contribute to image artifacts and multiple gating (AND of multiple movements) needs to be utilized. This is illustrated in Fig. 4, where both ventilation pressure signal (VN) and ECG traces are analyzed to define a new gating/triggering acquisition window.
Triggering/gating acquisitions are widely adopted in high resolution magnetic resonance imaging (MRI) [27], [28] and X-ray computed tomography (CT) imaging [29]-[32] and have been recently introduced for optical imaging [9], [10], [33], [34].
VI. Planar Motion Compensation by Image Processing
When the amplitude of motion is small enough and there is enough correspondence between two consecutive images, image artifacts can be successfully removed through image processing.
Motion compensation by image processing has been extensively studied in computer vision and motion estimation and correction algorithms have been developed for applications such as video stabilization [35], motion tracking, and image mosaicking [36]. Because these imaging modalities are all based on CCD acquisition they cannot find a direct application in laser scanning microscopy imaging such as confocal and multiphoton microscopy, or optical coherence tomography. Instead of having all pixels acquired simultaneously as is the case for camera based modalities, here an excitation scanning point draws a continuous raster path with each image pixel corresponding to a different time point along the scanning trace (Fig. 5). This leads to the two most common sources of artifacts present in scanning based imaging modalities, due respectively to in-frame and interframe motion. In-frame motion artifacts arise when displacements occur within the image acquisition time. Interframe motion artifacts instead are due to motion events occurring between consecutive frames and within an image sequence (e.g. video-frame rate acquisitions).
Fig. 5.
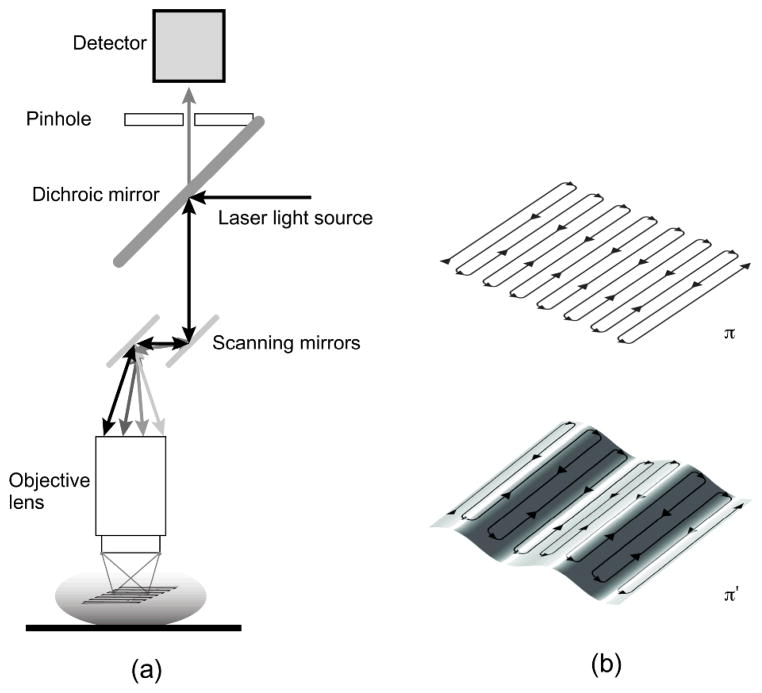
(a) Scheme of principle for laser scanning confocal microscopy. Two galvanometer mirrors oscillating on orthogonal axes scan the excitation laser beam along a raster path. Light is focused onto the sample and the emission light is descanned and detected through a dichroic mirror. (b) The raster scanning path lies on a horizontal imaging plane (top) perpendicular to the imaging objective. When the subject moves, the imaging plane in the organ’s reference frame will appear as a curved surface (bottom) modulated in time according to the motion periodicity. Reprinted with permission from [13]. © 2012 SPIE.
When the imaged organ shifts in position with a speed higher than that of the raster scanning, motion will translate into image blurring. Streaking artifacts are the most common example and are produced by rapid movements of sample or by prolonged scanning times. Reducing the integration time will decrease their impact in the acquired images, but it will go at the expenses of the image quality since the faster the acquisition rate the higher the noise. Generally streaking artifacts can be removed using image deconvolution. In LSM in particular, the induced blurring can be modeled as a geometric distortion, which is the major in-frame motion artifact occurring in scanning imaging techniques,
Different authors have systematically modeled these motion-induced distortions by considering the scanning acquisition paths used during the image acquisition and several image processing algorithms have been proposed. Vercauteren et al. employed a motion distortion model based on constant velocity assumption within a time frame [37]. Greenberg et al. have implemented a Lucas-Kanade framework which is a general image registration modeling commonly used in image processing [38]. Dombeck et al. have also demonstrated motion compensation using a Hidden Markov Model (HMM) to correct for motion distortions [39].
Inter-frame artifacts can be corrected by image registration with realignment of the video-frame sequence and/or removal of unmatched frames, leading to complete automatic removal of inter-frame motion [40]. In order to work under such conditions distortion-free images need to be acquired with high scanning speed such as in the multi-points high speed scanning system [41]–[43]. Using super-high speed imaging and exploiting repeatability of heart motion, Liebling et al. demonstrated tomographic imaging of the beating heart in a zebrafish [41]. Motion compensation by image processing, when feasible, provides an attractive solution because it requires minimal resources.
VII. Motion Compensation via Post-Processed Gated Imaging
Despite the use of different stabilizers, motion in living animals is not confined along two dimensions but it extends also along the axis of the imaging objective perpendicular to the stabilizer plane. While synchronization of image acquisition with the tissue motion components such as the heart beat and the respiration leads to reproducible observations, images present deformation modulated by the two physiological components. Points belonging to the microscope imaging plane during a raster scanning acquisition will lie at different depths within the moving tissue due to the temporal motion components induced by the animals breathing or heart activity (Figs. 5 and 6). The acquired image will therefore not be representative of a physical horizontal plane intersecting the organ in motion but will instead resemble a curved surface due to the relative motion of the imaged organ with respect to the microscope scanning trajectory (Fig. 5b).
Fig. 6.
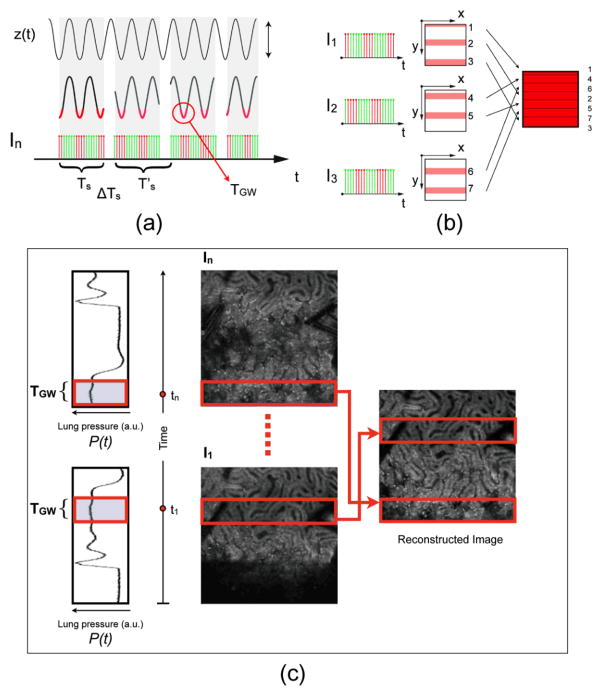
Scheme of retrospective breathing-gated image reconstruction. (a) Having the knowledge of the motion function z(t), here modeled for simplicity as a sinusoidal function, it is possible to set a specific time gating window TGW triggered on a particular phase of the motion. (b) By collecting several images (I1, I2, I3, …), and selecting the corresponding “patches” we can reconstruct an image where each point has the same height within in the imaged volume. (c) A reconstructed motion-free image obtained starting from raw images, using the retrospective breathing-gated image reconstruction technique. Reprinted with permission from [13]. © 2012 SPIE.
As a result it will be affected by distortions, which will change the geometry of the imaged organ. Due to their long excursions, breathing-induced artifacts are the major cause of movement in living animals and are therefore particularly detrimental even under condition of high speed acquisition. One possible way to remove them is to hyper-ventilate the animal within the few moments preceding the image acquisition and bringing it to a stop while collecting the data. But obviously a breathing suppression methodology is not feasible for prolonged in vivo imaging sessions. A possible alternative approach is a technique known as sequential cardiorespiratory gating (SCG) segmented microscopy, a stabilization method that is commonly used for cardiac MRI [10]. During imaging, the breathing activity is recorded and a time window coincident with the end phase of the respiration is chosen. Portion of the acquired images (patches) that temporally coincide with the selected time windows are then extracted among all collected images and combined together to produce a final reconstructed image.
By choosing appropriately both acquisition and motion periods (unsynchronized acquisition), this temporal portion area of the original image (“patch”) will span over time the entire objective’s field of view (Fig. 6). Using direct knowledge of the motion function z(t) and choosing a gating window centered around time points with the same vertical coordinates, it is possible in principle to combine all the patches selectively extracted from each image and to reconstruct a final one which is representative of an actual horizontal optical sectioning plane within the organ. [13]
The same approach can be extended to cardiac motion compensation. In this case a second time window coincident with the end diastole is selected and temporal portion areas corresponding to this window are selected to give rise in a similar fashion to reconstructed images representative of horizontal planes of the resting heart. This approach has permitted recently to image for the first time the beating heart in vivo at the subcellular level [10].
We have to emphasize that, in order to enable assisted motion-synchronized scanning, it is crucial to introduce reproducibility in the position of the moving organs at the selected gating time window within the cardiac and respiratory cycles. Only under this assumption, all patches will then be representative of the same horizontal plane and can be merged without introducing any patch discontinuity. Motion reproducibility can be highly enhanced by introducing mechanical stabilizers (Fig. 2), which in addition contributes to the reduction of the motion amplitude. Reproducibility in the axial motion of around 7 microns has been demonstrated to enable heart imaging in vivo in the beating heart [10].
VIII. Specific solutions for Organ Motion Compensation
The compensation schemes described above cannot be always practically implemented for all possible imaging conditions. Depending on the organ of interest, different methodologies need to be implemented and adapted (Table 1).
A. Brain
Since skull represents a natural constraint for the brain it is generally used as a convenient support to achieve physical stabilization. In anesthetized mice, the skull is often immobilized by stereotactic holders or alternatively by head plates firmly held with dental cement [17]. For all those cases where imaging needs to be conducted in awake and freely behaving mice, special wearable miniaturized microscopes can be alternatively employed [39]. In addition to these hardware approaches, image processing [38], [40] and triggering based acquisitions [9] can be implemented in order to image the brain at high resolution. A different interesting approach recently proposed by Matsumoto et al. exploited additional thoracotomy in order to reduce motion artifacts for brain imaging by lowering the internal pressure in an effort to reduce the physiological motion [44].
B. Spinal cord
Hardware stabilizers for vertebral column immobilization are commonly utilized for spinal cord imaging. Both commercial and custom-made vertebral clamp holders have been employed to this end. The detrimental effects of the respiratory cycle can be avoided by using mechanical ventilation control [45], by elevating the mouse off the surgical table [46], or by dampening the movements with agarose [47]. The major limitation of these methods lies in the impossibility of running longitudinal studies. To overcome this downside, a custom-made imaging chamber has been proposed [18].
C. Kidney, Liver, and Spleen
Custom-made small-sized mechanical stabilizers are usually exploited to immobilize these organs. Various types of stabilizers can be fabricated [13], [22], [48]. Toiyama et al. have proposed an organ stabilizing device which applies pressure on the imaged organ’s surface [48]. Lee et al. also proposed a small restraining stabilizer but instead of applying high pressure on the organ, a certain degree of freedom in the motion is guaranteed while introducing reproducibility in the organs’ excursions [13]. The remaining artifacts are further removed by either synchronization or image processing (Fig. 7a,b). Cao et al. designed a scooping micro-device to separate the imaged organ from the moving body [22]. Swirski et al. imaged the mouse spleen using a pressure mechanical stabilizer that is attached to the objective and finely adjustable using a micrometer [49]. For kidney, which can be partially externalized, motion restriction is possible without the use of any stabilizer. Using an inverted microscope Dunn et al. successfully imaged the externalized mouse kidney as compressed by its own body weight on an imaging cover slip [21].
Fig. 7.
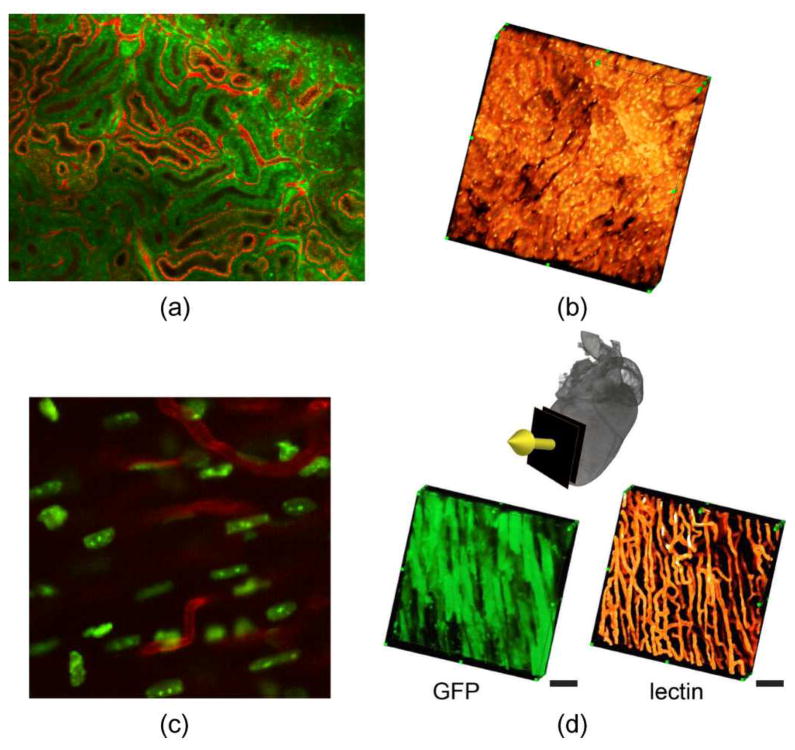
Examples of motion compensated microscopy for in vivo mouse imaging. Planar (a) and tomographic (b) reconstruction of a mouse kidney imaged in vivo [13]. Planar (c) and tomographic reconstructions (d) of the in vivo beating heart [10]. Scale bars: 50 μm. Reprinted with permission from [10]. © 2012 Nature Publishing Group. Reprinted with permission from [13]. © 2012 SPIE.
D. Lung
The lungs present very peculiar characteristics in terms of stiffness and handling. Tight motion restriction can lead to perturbation of the normal physiology and even delicate handling can induce damage and superficial bleeding. A suction based motion stabilizer has been proposed for lung imaging [23], [50], [51]. Wagner et al. have also successfully used suctioning stabilizer observing the dog lung through a thoracic window [50]. Recently Looney et al. observed the mouse lung immune system by designing a miniaturized suctioning device [23]. Fabrication of this stabilizer is a little complicate but details of the design are given in [51].
E. Heart
Heart imaging at high resolution is particularly challenging. High frequency components are present along the cardiac cycle and the heart itself is the source of motion, due to individual myocytes contractions. Moreover its proximity to the lungs, another major source of motion extends further its range of motion. As for the hearts, physical constraints are not suitable if high forces are applied since motion restriction can cause severe damage to the myocytes and decreased blood perfusion leading to ischemia. Only recently Lee et al. successfully imaged the beating heart in vivo at subcellular resolution (Fig. 7c,d) using a methodology based on a mechanical stabilizer in combination with gated imaging [10]. Other approaches exploiting suction-based heart stabilizers have been also proposed by the same group [14] and other [11] for mouse heart imaging.
F. Other organs
If the organ to be imaged can be exteriorized such is the case for the small intestine, pancreas and spleen, a compressive coverslip with an underneath support can be simply utilized to immobilize it. Mouse small intestine [52], submandibular gland [53], sternomastoid muscle [54], pancreas [22], and ear [55] have all been imaged using custom-made devices based on this idea.
IX. Conclusion
As illustrated, recent advances in motion compensation technologies have been capable of overcoming the motion related limitations currently affecting intravital microscopy, eventually leading to the full exploitation of temporal and spatial resolution potentially offered by both confocal and multiphoton microscopy. In the next years to come, it is expected that some of the most advanced motion compensation techniques will be standardized and many novel sophisticated developments will follow further improving the performances of current imaging technologies. These will likely allow researchers to address unresolved long-standing questions and will open a new window into in vivo single cell physiology and biochemistry.
TABLE I.
Organ specific Physical immobilization techniques
| Imaging Organ | Techniques | References |
|---|---|---|
| Brain | Stereotaxic with cranial window | [17] |
| Miniaturized wearable microscope | [6] | |
| Spinal Cord | Spinal clamps with mechanical ventilation, elevation of the mouse, agarose dampening | [45],[46], [47] |
| Spinal imaging chamber | [18] | |
| Skin | Dorsal skin fold chamber | [15],[16] |
| Kidney | Externalization and compression with body weight via inverted microscopy | [21] |
| Custom-made holding device | [13] | |
| Externalization with a custom-made supporting device | [22] | |
| Spleen | Holder produced by microscope vendors | [49] |
| Lung | Custom-made suctioning device | [23],[50] |
| Heart | Custom-made gluing device | [10] |
| Custom-made suctioning device | [11],[14] | |
| Submandibular gland | Externalization with a custom-made supporting device | [53] |
| Sternomastoid muscle | Custom-made supporting device in combination with a coverslip | [54] |
| Small intestine | Custom-made supporting device in combination with a coverslip | [52] |
| Tibialis Anterior muscle | Custom-made fixing device | [56] |
| Ear | Custom-made supporting device in combination with a coverslip | [55] |
Acknowledgments
This project was funded in part by Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (under Contract No. HHSN26820100004xC), and from the Institute of Biomedical Engineering (under R01EB006432)..
Biographies

Claudio Vinegoni (PhD 2002) is currently Asst. Professor at Harvard Medical School, and works at the Center for Systems Biology at Massachusetts General Hospital (MGH). He has published over 70 original publications in peer reviewed journals. His research activity involves the development of novel optical imaging instruments and techniques with applications in the clinical and biomedical arena.

Sungon Lee received the BS degree from Seoul National University, Rep. of Korea in 1997, the MS degree from POSTECH, Rep. of Korea in 1999, and Ph.D. degree from University of Tokyo, Japan in 2008. He was a postdoctoral fellow at the MGH Center for Systems Biology, Harvard Medical School, MA from Dec. 2010 to Mar. 2012. Since 1999, he has been with Interaction and Robot Research Center, KIST, Rep. of Korea, and is currently a senior research scientist. His research interests include biomedical robotics, medical image processing, motion compensation systems.

Paolo Fumene Feruglio (PhD 2010) is Visiting Research Fellow at the Center for Systems Biology, Harvard Medical School and Res. Assist. at the University of Verona. He graduated in Electrical Engineering at the University of Padova and received his Ph.D in Multimodal Imaging in Biomedicine at the University of Verona. His research involves the developing of models and algorithms to process and analyze data with application to in vivo and ex vivo molecular imaging techniques at macro and microscopic level.

Ralph Weissleder (PhD 1985) is a Professor at Harvard Medical School, Director of the Center for Systems Biology at Massachusetts General Hospital (MGH), and Attending Clinician (Interventional Radiology) at MGH. Dr. Weissleder is also a member of the Dana Farber Harvard Cancer Center, an Associate Member of the Broad Institute (Chemical Biology Program) and a member of the Harvard Stem Cell Institute (HSCI) leading its Imaging Program. He has published over 500 original publications in peer-reviewed journals and has authored several textbooks.
Contributor Information
Claudio Vinegoni, Email: cvinegoni@mgh.harvard.edu, Center for System Biology, Massachusetts General Hospital and Harvard Medical School, Richard B. Simches Research Center, 185 Cambridge Street, Boston 02114, USA.
Sungon Lee, Email: solee@kist.re.kr, Center for System Biology, Massachusetts General Hospital and Harvard Medical School, Richard B. Simches Research Center, 185 Cambridge Street, Boston 02114, USA. He is now with Interaction and Robotics Research Center, Korea Institute of Science and Technology, Hwarangno 14-gil 5, Seoul 136-791 Korea.
Paolo Fumene Feruglio, Center for System Biology, Massachusetts General Hospital and Harvard Medical School, Richard B. Simches Research Center, 185 Cambridge Street, Boston 02114, USA and with the Department of Neurological and Movement Sciences, University of Verona, Strada Le Grazie 8, 37134 Verona, Italy.
Ralph Weissleder, Center for System Biology, Massachusetts General Hospital and Harvard Medical School, Richard B. Simches Research Center, 185 Cambridge Street, Boston 02114, USA.
References
- 1.Weigert R, Sramkova M, Parente L, Amornphimoltham P, Masedunskas A. Intravital microscopy: a novel tool to study cell biology in living animals. Histochem Cell Biol. 2010 May;133:481–91. doi: 10.1007/s00418-010-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittet MJ, Weissleder R. Intravital imaging. Cell. 2011 Nov 23;147:983–91. doi: 10.1016/j.cell.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousso P, Moreau HD. Functional immunoimaging: the revolution continues. Nat Rev Immunol. 2012 Dec;12:858–64. doi: 10.1038/nri3342. [DOI] [PubMed] [Google Scholar]
- 4.Ritsma L, Ponsioen B, van Rheenen J. Intravital imaging of cell signaling in mice. IntraVital. 2012;1:0–8. [Google Scholar]
- 5.Mai W, Badea CT, Wheeler CT, Hedlund LW, Johnson GA. Effects of breathing and cardiac motion on spatial resolution in the microscopic imaging of rodents. Magn Reson Med. 2005 Apr;53:858–65. doi: 10.1002/mrm.20400. [DOI] [PubMed] [Google Scholar]
- 6.Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RP, Ko TH, Burns LD, Jung JC, Schnitzer MJ. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat Methods. 2008 Nov;5:935–8. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. Mice anesthesia, analgesia, and care, Part II: anesthetic considerations in preclinical imaging studies. ILAR J. 2012;53:E70–81. doi: 10.1093/ilar.53.1.70. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Nakamura Y, Yamane K, Toujo T, Takahashi S, Tanikawa Y, Takahashi H. Image Stabilization for In Vivo Microscopy by High-Speed Visual Feedback Control. IEEE Trans on Robotics. 2008 Feb 25;24:45–54. [Google Scholar]
- 9.Paukert M, Bergles DE. Reduction of motion artifacts during in vivo two-photon imaging of brain through heartbeat triggered scanning. Journal of Physiology-London. 2012 Jul;590:2955–2963. doi: 10.1113/jphysiol.2012.228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Vinegoni C, Feruglio PF, Fexon L, Gorbatov R, Pivoravov M, Sbarbati A, Nahrendorf M, Weissleder R. Real-time in vivo imaging of the beating mouse heart at microscopic resolution. Nat Commun. 2012 Sep 11;3:1054. doi: 10.1038/ncomms2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung K, Kim P, Leuschner F, Gorbatov R, Kim JK, Ueno T, Nahrendorf M, Yun SH. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ Res. 2013 Mar 15;112:891–9. doi: 10.1161/CIRCRESAHA.111.300484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder JL, Luger-Hamer M, Pursley R, Pohida T, Chefd’Hotel C, Kellman P, Balaban RS. Short Communication: Subcellular Motion Compensation for Minimally Invasive Microscopy, In Vivo: Evidence for Oxygen Gradients in Resting Muscle. Circulation Research. 2010;106:1129–1133. doi: 10.1161/CIRCRESAHA.109.211946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Vinegoni C, Feruglio PF, Weissleder R. Improved intravital microscopy via synchronization of respiration and holder stabilization. J Biomed Opt. 2012 Sep;17:96018–1. doi: 10.1117/1.JBO.17.9.096018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinegoni C, Lee S, Gorbatov R, Weissleder R. Motion compensation using a suctioning stabilizer for intravital microscopy. IntraVital. 2012;1:115–121. doi: 10.4161/intv.23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algire GH. An Adaptation of the Transparent-Chamber Technique to the Mouse. Journal of the National Cancer Institute. 1943 Aug 1;4:1–11. [PubMed] [Google Scholar]
- 16.Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993 Oct;143:1055–62. [PMC free article] [PubMed] [Google Scholar]
- 17.Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hubener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4:1128–44. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrar MJ, Bernstein IM, Schlafer DH, Cleland TA, Fetcho JR, Schaffer CB. Chronic” in vivo imaging in the mouse spinal cord using an implanted chamber. Nat Methods. 2012 Mar;9:297–302. doi: 10.1038/nmeth.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008 Dec;5:1019–21. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritsma L, Steller EJ, Ellenbroek SI, Kranenburg O, Borel Rinkes IH, van Rheenen J. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat Protoc. 2013 Mar;8:583–94. doi: 10.1038/nprot.2013.026. [DOI] [PubMed] [Google Scholar]
- 21.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002 Sep;283:C905–16. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 22.Cao L, Kobayakawa S, Yoshiki A, Abe K. High resolution intravital imaging of subcellular structures of mouse abdominal organs using a microstage device. PLoS One. 2012;7:e33876. doi: 10.1371/journal.pone.0033876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. Stabilized imaging of immune surveillance in the mouse lung. Nat Methods. 2011 Jan;8:91–6. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laffray S, Pages S, Dufour H, De Koninck P, De Koninck Y, Cote D. Adaptive movement compensation for in vivo imaging of fast cellular dynamics within a moving tissue. PLoS One. 2011;6:e19928. doi: 10.1371/journal.pone.0019928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakalar M, Schroeder JL, Pursley R, Pohida TJ, Glancy B, Taylor J, Chess D, Kellman P, Xue H, Balaban RS. Three-dimensional motion tracking for high-resolution optical microscopy, in vivo. J Microsc. 2012 Jun;246:237–47. doi: 10.1111/j.1365-2818.2012.03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Zhang K, Lin C, Kang JU. Motion compensated fiber-optic confocal microscope based on a common-path optical coherence tomography distance sensor. Optical Engineering. 2011;50:083201-083201–7. [Google Scholar]
- 27.Hedlund LW, Johnson GA. Mechanical ventilation for imaging the small animal lung. ILAR J. 2002;43:159–74. doi: 10.1093/ilar.43.3.159. [DOI] [PubMed] [Google Scholar]
- 28.Wiesmann F, Szimtenings M, Frydrychowicz A, Illinger R, Hunecke A, Rommel E, Neubauer S, Haase A. High-resolution MRI with cardiac and respiratory gating allows for accurate in vivo atherosclerotic plaque visualization in the murine aortic arch. Magn Reson Med. 2003 Jul;50:69–74. doi: 10.1002/mrm.10500. [DOI] [PubMed] [Google Scholar]
- 29.Bartling SH, Dinkel J, Stiller W, Grasruck M, Madisch I, Kauczor HU, Semmler W, Gupta R, Kiessling F. Intrinsic respiratory gating in small-animal CT. Eur Radiol. 2008 Jul;18:1375–84. doi: 10.1007/s00330-008-0903-3. [DOI] [PubMed] [Google Scholar]
- 30.Dinkel J, Bartling SH, Kuntz J, Grasruck M, Kopp-Schneider A, Iwasaki M, Dimmeler S, Gupta R, Semmler W, Kauczor HU, Kiessling F. Intrinsic gating for small-animal computed tomography: a robust ECG-less paradigm for deriving cardiac phase information and functional imaging. Circ Cardiovasc Imaging. 2008 Nov;1:235–43. doi: 10.1161/CIRCIMAGING.108.784702. [DOI] [PubMed] [Google Scholar]
- 31.Ertel D, Kyriakou Y, Lapp RM, Kalender WA. Respiratory phase-correlated micro-CT imaging of free-breathing rodents. Phys Med Biol. 2009 Jun 21;54:3837–46. doi: 10.1088/0031-9155/54/12/015. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z. Multislice CT angiography in cardiac imaging: prospective ECG-gating or retrospective ECG-gating? Biomed Imaging Interv J. 2010 Jan-Mar;6:e4. doi: 10.2349/biij.6.1.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gioux S, Ashitate Y, Hutteman M, Frangioni JV. Motion-gated acquisition for in vivo optical imaging. Journal of Biomedical Optics. 2009;14:064038. doi: 10.1117/1.3275473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaughlin RA, Armstrong JJ, Becker S, Walsh JH, Jain A, Hillman DR, Eastwood PR, Sampson DD. Respiratory gating of anatomical optical coherence tomography images of the human airway. Opt Express. 2009 Apr 13;17:6568–77. doi: 10.1364/oe.17.006568. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita Y, Ofek E, Ge W, Tang X, Shum HY. Full-Frame Video Stabilization with Motion Inpainting. IEEE Trans Pattern Anal Mach Intell. 2006;28:1150–1163. doi: 10.1109/TPAMI.2006.141. [DOI] [PubMed] [Google Scholar]
- 36.Davis J. Mosaics of Scenes with Moving Objects. Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition; 1998. [Google Scholar]
- 37.Vercauteren T, Perchant A, Malandain G, Pennec X, Ayache N. Robust mosaicing with correction of motion distortions and tissue deformations for in vivo fibered microscopy. Med Image Anal. 2006 Oct;10:673–92. doi: 10.1016/j.media.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg D, Kerr J. Automated correction of fast motion artifacts for two-photon imaging of awake animals. Journal of Neuroscience Methods. 2009;176:1–15. doi: 10.1016/j.jneumeth.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007 Oct 4;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soulet D, Paré A, Coste J, Lacroix S. Automated Filtering of Intrinsic Movement Artifacts during Two-Photon Intravital Microscopy. PLoS One. 2013;8:e53942. doi: 10.1371/journal.pone.0053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liebling M, Forouhar AS, Gharib M, Fraser SE, Dickinson ME. Four-dimensional cardiac imaging in living embryos via postacquisition synchronization of nongated slice sequences. Journal of Biomedical Optics. 2005;10:054001. doi: 10.1117/1.2061567. [DOI] [PubMed] [Google Scholar]
- 42.Vermot J, Fraser SE, Liebling M. Fast fluorescence microscopy for imaging the dynamics of embryonic development. HFSP J. 2008 Jun;2:143–55. doi: 10.2976/1.2907579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohn J, Yang J, Fraser SE, Lansford R, Liebling M. High-speed multicolor microscopy of repeating dynamic processes. Genesis. 2011 Jul;49:514–21. doi: 10.1002/dvg.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto N, Takahara Y, Matsuki N, Ikegaya Y. Thoracotomy reduces intrinsic brain movement caused by heartbeat and respiration: A simple method to prevent motion artifact for in vivo experiments. Neuroscience Research. 2011 Oct;71:188–191. doi: 10.1016/j.neures.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005 May;11:572–7. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 46.Davalos D, Lee JK, Smith WB, Brinkman B, Ellisman MH, Zheng B, Akassoglou K. Stable in vivo imaging of densely populated glia, axons and blood vessels in the mouse spinal cord using two-photon microscopy. J Neurosci Methods. 2008 Mar 30;169:1–7. doi: 10.1016/j.jneumeth.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johannssen HC, Helmchen F. In vivo Ca2+ imaging of dorsal horn neuronal populations in mouse spinal cord. J Physiol. 2010 Sep 15;588:3397–402. doi: 10.1113/jphysiol.2010.191833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toiyama Y, Mizoguchi A, Okugawa Y, Koike Y, Morimoto Y, Araki T, Uchida K, Tanaka K, Nakashima H, Hibi M, Kimura K, Inoue Y, Miki C, Kusunoki M. Intravital imaging of DSS-induced cecal mucosal damage in GFP-transgenic mice using two-photon microscopy. J Gastroenterol. 2010 May;45:544–53. doi: 10.1007/s00535-009-0187-7. [DOI] [PubMed] [Google Scholar]
- 49.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009 Jul 31;325:612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner WW., Jr Pulmonary microcirculatory observations in vivo under physiological conditions. J Appl Physiol. 1969 Mar;26:375–7. doi: 10.1152/jappl.1969.26.3.375. [DOI] [PubMed] [Google Scholar]
- 51.Lamm WJ, Bernard SL, Wagner WW, Jr, Glenny RW. Intravital microscopic observations of 15-microm microspheres lodging in the pulmonary microcirculation. J Appl Physiol. 2005 Jun;98:2242–8. doi: 10.1152/japplphysiol.01199.2004. [DOI] [PubMed] [Google Scholar]
- 52.Klinger A, Orzekowsky-Schroeder R, von Smolinski D, Blessenohl M, Schueth A, Koop N, Huettmann G, Gebert A. Complex morphology and functional dynamics of vital murine intestinal mucosa revealed by autofluorescence 2-photon microscopy. Histochem Cell Biol. 2012 Mar;137:269–78. doi: 10.1007/s00418-011-0905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008 Sep;9:1801–10. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lichtman JW, Magrassi L, Purves D. Visualization of neuromuscular junctions over periods of several months in living mice. J Neurosci. 1987 Apr;7:1215–22. doi: 10.1523/JNEUROSCI.07-04-01215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li JL, Goh CC, Keeble JL, Qin JS, Roediger B, Jain R, Wang Y, Chew WK, Weninger W, Ng LG. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat Protoc. 2012 Feb;7:221–34. doi: 10.1038/nprot.2011.438. [DOI] [PubMed] [Google Scholar]
- 56.Rothstein EC, Nauman M, Chesnick S, Balaban RS. Multi-photon excitation microscopy in intact animals. J Microsc. 2006 Apr;222:58–64. doi: 10.1111/j.1365-2818.2006.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]