Abstract
Stress granules (SGs) contain translationally-stalled mRNAs, associated preinitiation factors and specific RNA-binding proteins. In addition, many signaling proteins are recruited to SGs and/or influence their assembly, which is transient, lasting only until the cells adapt to stress or die. Beyond their role as mRNA triage centers, we posit that SGs constitute RNA-centric signaling hubs analogous to classical multiprotein signaling domains such as transmembrane receptor complexes. As signaling centers, SG formation communicates a “state of emergency”, and their transient existence alters multiple signaling pathways by intercepting and sequestering signaling components. SG assembly and downstream signaling functions may require a cytosolic phase transition facilitated by intrinsically disordered, aggregation-prone protein regions shared by RNA-binding and signaling proteins.
Keywords: Stress granules, Translation, Cell signaling, protein aggregation, intrinsically disordered
Stress granule (SG) assembly
Mammalian SGs were first described as cytoplasmic, non-membranous, phase dense structures assembled in response to the stress-induced phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) 1, the central trigger of the Integrated Stress Response (ISR) 2. Key features of the ISR are translational arrest, polysome disassembly, and SG assembly, which enable the cell to reprogram its translational repertoire via a process dubbed mRNA triage 2. Most SG components exhibit short residence times (seconds) whereas SGs themselves persist for minutes to hours, fusing with each other and with other RNA granules. A physical explanation for these properties has been lacking. Recent data suggest that dynamic RNA granules are maintained by fleeting, low-affinity interactions between disordered regions found in SG-nucleating RNA binding proteins. Once formed, SGs become hubs that intercept a subset of signaling molecules, thereby communicating a “state of emergency” to other signaling pathways, which modulate metabolism, growth and survival.
Different stresses activate one or more stress-sensing serine/threonine kinases that phosphorylate serine residue 51 of eIF2α 3–6 (Fig. 1, panel 1). These include: 1) HRI (heme-regulated initiation factor 2α kinase), which ensures the balanced synthesis of globin chains and heme during erythrocyte maturation 7, 8 and senses redox stress produced by sodium arsenite (arsenite) 9, a classical inducer of SGs 1; 2) PKR (Protein kinase RNA-activated), a double stranded RNA-dependent kinase activated by viral infection, heat and ultraviolet irradiation 5; 3) PERK (PKR-like endoplasmic reticulum (ER) kinase), an endoplasmic reticulum protein sensor activated by unfolded proteins in the ER lumen 3; and 4) GCN2 (general control nonderepressible 2), a protein that monitors amino acid levels and is activated by amino acid deprivation 6. Phosphorylation of eIF2α depletes the eIF2/tRNAiMet/GTP ternary complex that is required for translation initiation 10, 11. In the absence of the ternary complex, formation of the 48S preinitiation complex that normally assembles at the 5′-ends of capped mRNAs is disrupted, producing a translationally-stalled, non-canonical 48S complex unable to recruit the 60S ribosomal subunit 10. When elongation is unimpaired, translating ribosomes “run-off” polysomes, converting them into mRNPs (messenger ribonucleoprotein particles) that are eligible for assembly into SGs 12. Drugs or lipid mediators targeting eIF4A inhibit translation initiation independently of phospho-eIF2α 13, 14 (Fig. 1, panel 2) and initiate assembly of SGs containing eIF2/5 initiation factors, which are missing from the classical phospho-eIF2α-dependent SGs. Other proteins (e.g., G3BP, TIA-1) and post-translational modifications are required for translationally-stalled mRNPs to be assembled into SGs (Fig. 1, panel 5). Thus, SG assembly requires translationally-stalled mRNPs, but SG assembly is not necessary for translational arrest, and can in fact be uncoupled from it by knockdown of SG-assembly factors 15, 16 or by certain types of stress. Cells recovering from cold shock, for example, disassemble SGs long before translation is restored 17. The early concept that “SGs regulate translation” has been supplanted by the findings that “translational arrest regulates SGs”, leaving the question of “what then is the purpose of SGs?” Several excellent recent reviews have summarized how different viruses promote, prevent, or cause SG assembly to oscillate 18, 19; here, we consider how SG assembly interacts with cellular signaling cascades to influence an expanding list of cell activities, including proliferation, motility, reactive oxygen species (ROS) production and survival (Box 1).
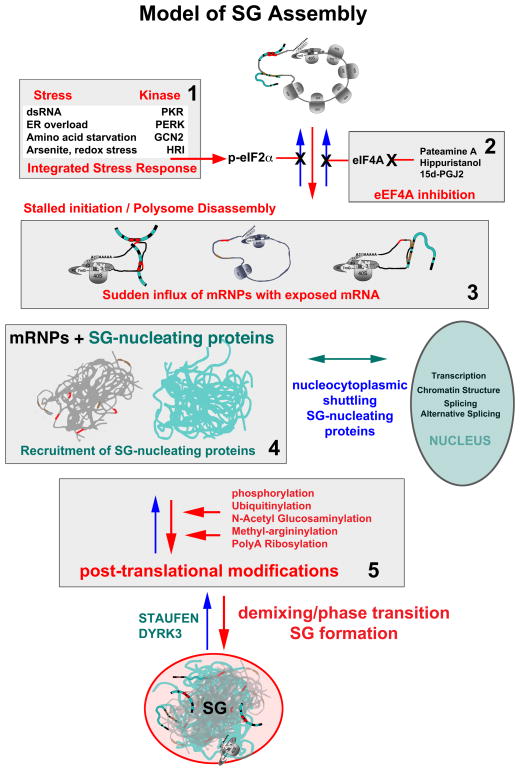
Fig. 1. Model of SG Assembly.
Stress induced phosphorylation of eIF2α (panel 1) or inactivation of eIF4A (panel 2) slows initiation rates and results in a sudden increase in non-polysomal mRNPs (panel 3), containing exposed mRNA regions previously masked by translating ribosomes. This transiently “naked” mRNA immediately binds any available mRNA binding proteins (panel 3: blue noodles), many of which are LC/ID rich (see Fig. 3), and many of which normally shuttle between the cytoplasm and the nucleus. This mRNA/protein mix is highly dynamic as the various mRNA binding proteins “trade up” to find their optimal binding sequences. The protein-mRNA mix “condenses” into immiscible droplets (panel 4), and the phase transition appears to be promoted by post-translational modifications of SG-associated proteins (panel 5). Once formed, SGs become hubs that intercept and interact with proteins involved in multiple signaling pathways, mRNA functions, and nuclear functions to influence global cell processes. Note that many of the steps shows here happen concurrently rather than in a linear sequence.
Box 1. SGs and cell survival.
SG assembly is a highly complex process common to all eukaryotic cells 2, 69. Genetic screens have established that more than one hundred proteins regulate mammalian SG assembly15 suggesting that SGs play important roles in helping cells respond to adverse environmental conditions, but the nature of these roles is obscure. Studies designed to compare the stress response in cells with or without SGs typically rely on knocking down proteins that are essential for SG assembly 70–73; however, the protein that is knocked down certainly has SG independent functions, confounding the interpretation of these results. Another approach has been to use stress in cells treated with drugs that block SG assembly (e.g., cycloheximide, emetine) 56; however, inhibition of protein synthesis is likely to have SG-independent effects on the stress response. Nevertheless, the finding that multiple interventions that prevent SG assembly render cells more susceptible to stress strongly suggests that SGs have cytoprotective effects 69.
Protein-interaction domains promote SG assembly
An early insight into the molecular mechanism controling the aggregation and localization of untranslated mRNPs into SGs came from studies of the RNA-binding protein TIA1, which contains a prion-related domain. This Q/N-rich motif of low amino acid complexity resembles that found in the aggregation domain of prion protein, mediates the reversible cytoplasmic aggregation of untranslated mRNPs 20, and can be functionally replaced with the aggregation domain from the yeast prion Sup35. Prion-related domains in Lsm proteins similarly promote the assembly of P-bodies, another RNA granule, indicating that reversible, low-affinityprotein-protein interactions are hallmarks of dynamic RNA granules 21, 22. A serendipitous discovery revealed that 5-aryl-isoxazole-3-carboxyamide, a compound that spontaneously assembles into microcrystals, selectively precipitates RNA-binding proteins 23, 24 containing prion-related or low complexity (LC) regions (glossary). Both LC and prion-related sequences are subtypes of intrinsically disordered (ID) proteins. Rather than assume fixed structural domains, ID regions can assume multiple conformations and mediate transient but specific interactions that are influenced by local environmental conditions, post-translational modification, and/or binding to other proteins 25, 26. LC and/or ID proteins commonly reside at hubs in protein:protein interaction networks 27. Algorithms are available that predict LC (SEG 28) and ID (IUPRED 29) regions in proteins of interest.
At high concentrations, LC/ID-containing proteins can spontaneously assemble hydrogels in vitro 23, 24. This in vitro”gelation” resembles the demixing phase transition that allows RNA germ cell granules in Caenorhabditis elegans 30, 31 to behave like liquid droplets, displaying surface tension and fusing with adjacent granules. These physical properties are attributed to reversible interactions between LC/ID regions contained within many highly flexible mRNPs that facilitate the demixing phase transition. Cytosolic liquid droplets tend to assume a spherical shape that minimizes surface tension, and germ cell granules and P-bodies are typically spherical. While SGs exhibit fusion and fission 32, their morphology is more amorphous and is not entirely explained by a phase transition model. Their non-spherical structure may arise from their complex and dynamic composition, as more than one hundred cellular and viral proteins are found in SGs 2. Most of those examined by photobleaching exhibit very short (seconds to minutes) residence times within SGs, much shorter than the lifetime of the SGs themselves (minutes to hours). A combination of RNA-RNA (for example microRNA-mRNA), RNA-protein, protein-protein, and phase transitions appear to mediate SG assembly, augmented and regulated by post-translational modifications.
In some well-characterized proteins, phosphorylation of serine residues within the LC/ID regions alters their association with SGs. G3BP is one of several proteins that nucleate SG assembly when overexpressed. Phosphorylation of G3BP at serine 149 within a LC/ID region (aa 142-205) impairs its SG nucleation ability 33. Similarly, the SG-nucleating protein tristetraprolin (TTP) can be phosphorylated at two serines (52 and 178) located within LC domains (Fig. 2), promoting its egress from SGs, without altering its targeting to PBs34; TTP:PB interactions are mediated through direct protein-protein interactions between TTP and several PB proteins 34, 35. Finally, phosphorylation of another SG/PB-associated protein, MEX3B, promotes binding of 14-3-3, and targeting to PBs without affecting its targeting to SGs 36. These findings are schematized in Fig. 2, which shows how phosphorylation and subsequent 14-3-3 binding regulate the trafficking of MEX3B (panel 1) and TTP (panel 2) to SGs and/or PBs. We posit that 14-3-3 binding stabilizes the LC/ID regions, restricting their ability to assume multiple conformations and thereby altering their tendency to partition with SGs or PBs. This example highlights how individual proteins are targeted to or excluded from SGs as directed by other signaling pathways.
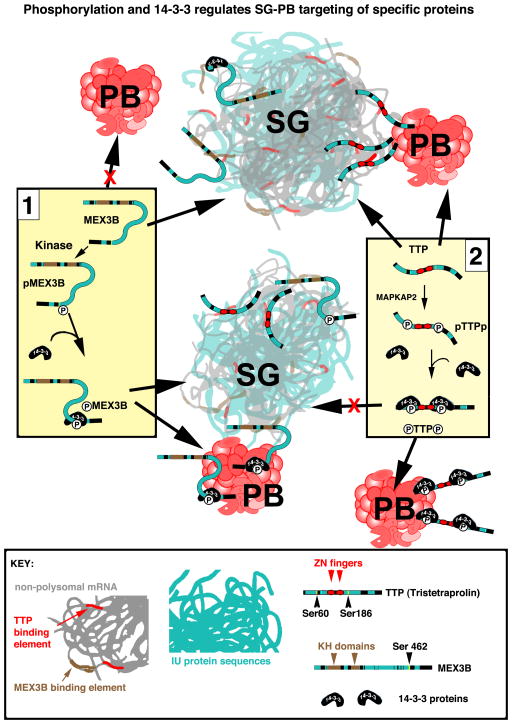
Fig. 2. Phosphorylation and 14-3-3 regulates SG-PB targeting of specific proteins.
MEX3B (Panel 1) possesses two KH domains (brown) and considerable ID sequence (aqua, also see Fig. 3). Non-phosphorylated MEX3B associates with SGs but not PBs. Phospho-MEX3B associates with both SGs and promotes SG-PB fusion 36. Its interaction with PBs requires phosphorylation of serine 462 and 14-3-3 binding, which are not required for its targeting to SGs 36. In contrast (Panel 2), non-phosphorylated TTP binds mRNA through its zinc fingers (red), associates with both SGs and PBs, and tethers SG and PB together 32, 34. MAPKAP2-induced phosphorylation of serines 60 and 186 promotes 14-3-3 binding, removing TTP from SGs while allowing its continued association with PBs 34, leading to the separation of SGs from PBs. The binding of 14-3-3 proteins to ID regions (proteins shown to scale) may stabilize the disordered regions, “freezing” them into a locked conformation (represented here by a rigid linear shape), thus taking TTP and perhaps other individual SG-associated proteins “out of phase” with the rapid and fleeting interactions within SGs.
In support of this concept, protein:protein interactions between Tob2, Pan3, Tnrc6c and the SG protein poly(A)-binding protein (PABP) are regulated via phosphorylation of Ser/Thr sites within ID regions flanking their common PAM2 (PABP-interacting motif 2) motifs 37. The PAM2 motif binds to the highly conserved MLLE domain in the C-terminal region of PABP 38 and is frequently located adjacent to clusters of phosphorylation sites within LC/ID regions. Reversible phosphorylation of these clusters modulates the strength of PABP/PAM2-containing protein interactions, thereby allowing PAM2-containing proteins to regulate mRNA stability, localization and translation. Since RNA-binding proteins are highly enriched in LC/ID sequences compared to the human proteome overall 39, the control of protein-protein interactions via reversible phosphorylation near or within LC/ID regions may contribute to post-transcriptional gene regulation. As shown with MEX3B and TTP, phosphorylation within LC/ID regions regulates the recruitment of individual proteins in or out of SGs. Since signaling proteins commonly possess LC/ID regions, their recruitment to SGs may be similarly regulated.
Recruitment of signaling proteins to SGs
The concentration-dependent aggregation of multivalent signaling proteins promotes a demixing phase transition from a soluble to an immiscible liquid state in vitro and in vivo 40. This process effectively segregates selected proteins from the cytosol, creating a circumscribed domain whose physical properties are distinct from that of the bulk cytosol. The LC/ID regions common to SG-associated RNA-binding proteins and signaling molecules suggest RNA granules may result from similar multivalent interaction mechanisms, first in granule assembly and later when recruiting different signaling molecules to mediate cross-talk with other signaling pathways. Several algorithms identified prion-related 41, LC 27, and ID regions 42 that are composed of a limited cohort of amino acids and do not fold into rigidly-defined tertiary structures such as α-helixes or β-sheets are commonly found in proteins whose overexpression nucleates SGs (Fig. 3) and other SG-associated proteins involved in cellular signaling (Fig. 4). Thus, the sequestration of signaling proteins in liquid phase RNA granules may integrate multiple stress signaling cascades to orchestrate the cellular response to stress.
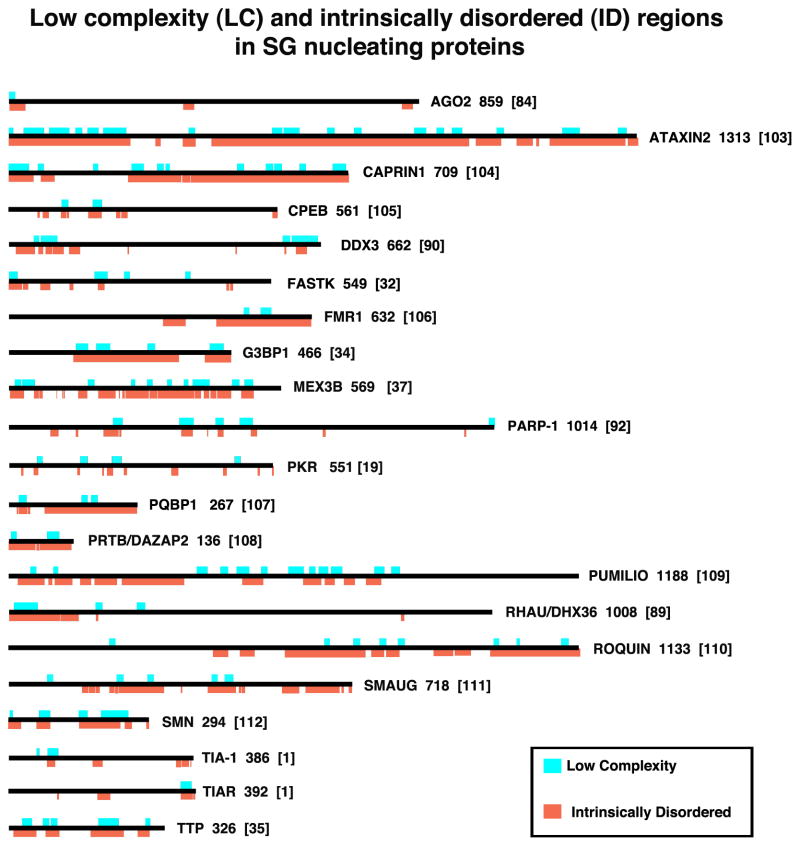
Figure 3. Low complexity (LC) and intrinsically disordered (ID) regions in SG nucleating proteins.
SG nucleating proteins are shown to scale and their LC (aqua) and ID (orange) regions are indicated. The numbers to the right of each schematic indicates the number of amino acids in the isoform shown (usually the largest one); numbers in brackets indicate the reference. LC (aqua) regions were obtained from the NCBI “conserved domains” graphic, which calculates low complexity regions using the SEG program 28. Intrinsically Disordered regions were determined using the programs 29 on the ANCHOR website (http://anchor.enzim.hu/), in which regions of disorder exceeding 50% on the Intrinsically Unordered histograms were graphically rendered (orange). Note that all SG-nucleating proteins contain at least some LC/ID regions, and that some SG-nucleating signaling proteins (OGFOD1, DYRK3) are shown in Fig. 4 rather than here.
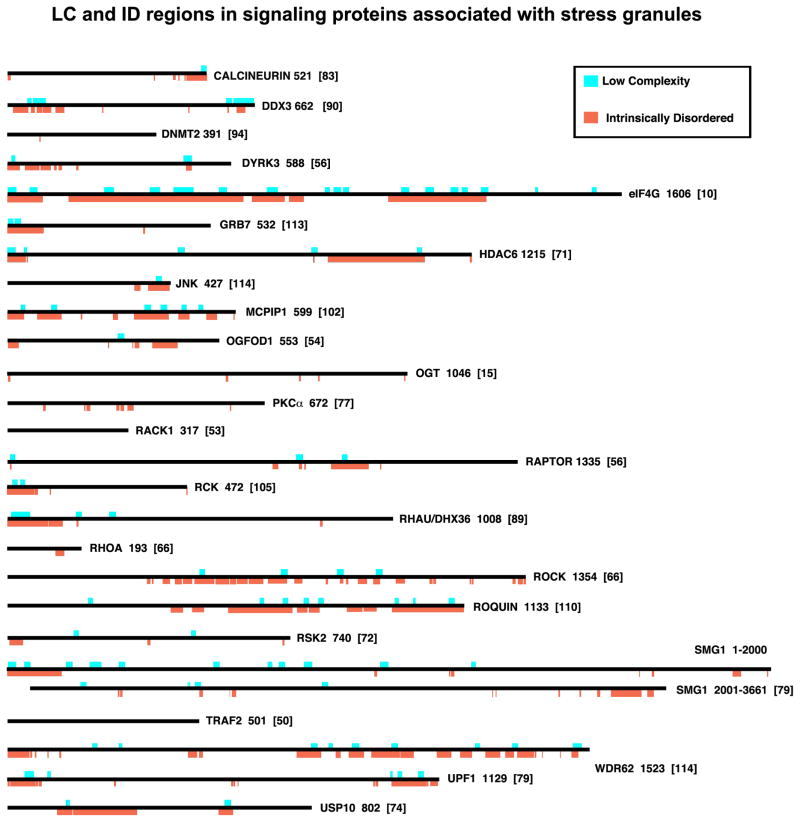
Figure 4. LC and ID regions in signaling proteins associated with stress granules.
Proteins are shown to scale, with LC (aqua) and ID (orange) regions indicated. Note that several SG-associated signaling proteins (RACK1, TRAF2) are highly structured and devoid of LC/ID regions; these proteins bind to SG-integral factors (eIF3 and 40S ribosomal subunits) and proteins (eIF4G) that presumably regulates their recruitment to SGs via more canonical binding of rigid domains. Note that some proteins here (DYRK3, OGFOD1) are also SG-nucleating proteins. The numbers to the right of each schematic indicates the number of amino acids in the isoform shown (usually the largest one); numbers in brackets indicate the reference.
Signaling proteins and enzymes recruited to SGs are legion, including adaptor/scaffold proteins, protein and lipid kinases, phosphatases, ribonucleases, helicases, ribosyltransferases, glucosyltransferases, GTPases, methyltransferases, and ubiquitin modifying enzymes (Table I). Some of these proteins (e.g., DDX3/Ded1, roquin) nucleate SG assembly, and their reduced expression inhibits SG assembly—hence their ability to promote SG assembly can influence any signaling pathways whose components partition into SGs. In some cases, enzymatic activity of these proteins (e.g., SMG-1) promotes SG assembly, but whether this occurs prior to SG assembly or within the SG is not known. The recruitment of some scaffolding proteins (e.g., TRAF2, RACK1) to SGs inhibits the signaling pathways in which these proteins participate, without affecting SG assembly, hence acting downstream. Many SG-associated signaling proteins possess LC/ID regions (Fig. 4) that may regulate their interactions with SGs. Typically, studies defining specific proteins as SG-associated use only one or two cell lines and one type of stress, hence the generalizability and cellular specificity of these phenomena is not established, yet the extensive list indicates that the recruitment of signaling proteins to SGs is common.
Table I.
SG-associated signaling molecules
| Stress granule protein | Enzymatic activity | Role in SG assembly/signalling |
|---|---|---|
| RSK2 (p90 ribosomal S6 kinase) | Kinase | Modulates both SG assembly and cell survival during arsenite-induced stress 71. Reduced expression of RSK2 inhibits SG assembly and survival of MCF7 cells exposed to arsenite. The N-terminal kinase domain of RSK2 (amino acids 1-389 containing an LC/ID region at 174-187) directly binds to the prion-related C-terminus of TIA-1, which is required for RSK2 recruitment to SGs. |
| PKCα (protein kinase Cα) | Kinase | Enhances SG assembly in cells subjected to heat shock or arsenite-induced stress 76, possibly by binding to G3BP2, a key regulator of SG assembly 77. |
| SMG-1 | Kinase | Phosphoinositide 3 kinase-like kinase essential for nonsense mediated decay (NMD) that promotes arsenite- or H2O2- (but not heat-) induced SG assembly 78. The SMG-1 substrate Upf1 is also recruited to arsenite-induced SGs 78. Both SMG-1 and Upf1 possess multiple LC/ID regions. |
| MK-STYX (mitogen-activated protein kinase phosphoserine/threonin e/tyrosine-binding protein) | Pseudo-phosphatase | Structurally related to a family of dual specificity protein tyrosine phosphatases 79, 80. Interacts with the SG-nucleating protein G3BP 81 and inhibits arsenite-induced SG assembly. |
| Calcineurin | Phosphatase | In cold-shocked Cryptococcus neoformans, the calcineurin catalytic subunit Cna1 is recruited to RNA granules that contain both P-body (Dcp1) and SG (Pub1) markers 82. |
| AGO-2 (argonaute-2) | Ribonuclease | Recruitment of AGO-2 to SGs 83, 84 correlates with stress-induced reductions in RNA interference by both siRNAs and miRNAs 85, suggesting that AGO-2/RISC sequestration in SGs inhibits or stalls its cleavage of miRNA-targeted transcripts. |
| Rck/p54 | Helicase | Rck/p54 may bind mRNAs released from polysomes due to blocked translation initiation 86 thereby facilitating the repackaging of remodeled mRNPs into RNA granules. Consistent with this model, TTP cooperates with Rck/p54 to inhibit mRNA translation 87. |
| RHAU | Helicase | The recruitment of RHAU to SGs requires its N-terminal RNA-binding domain 88; targeted knockdown of RHAU does not inhibit SG assembly 88. Possibly RHAU, like Rck/p54, prepares selected mRNAs for movement to SGs. |
| DDX3/Ded1 | Helicase | Overexpression of DDX3 nucleates SG assembly, whereas its knockdown inhibits SG assembly. Its helicase activity is dispensable for SG nucleation, whereas its eIF4E binding is required 89 suggesting that DDX3 may inhibit translation initiation. The helicase activity of DDX3/Ded1 may facilitate a transition from translational repression to active translation, thus allowing mRNA to escape from RNA granules and resume translation at polysomes 90. |
| Poly(ADP)ribosyltransf erase | ADP ribose polymerase | Individual overexpression of any of six poly (ADP) ribose polymerases nucleates SGs without increasing the phosphorylation of eIF2α, whereas overexpression of two distinct poly (ADP) ribose glycohydrolases inhibits arsenite-induced SG assembly 91. These findings implicate the poly (ADP) ribose modification of SG proteins in SG assembly; but the mechanism remains unclear. |
| PRMT3 (protein arginine methytransferase 3) | Methyltransfer ase | Responsible for asymmetric dimethylarginine modifications. The methylated tudor domain of TDRD3 promotes its recruitment to SGs 92. |
| Dnmt2 | Methyltransfer ase | Methylates a conserved cytosine residue in the anti-codon loops of tRNAAsp, tRNAVal, and tRNAGly 93. The viability of Drosophila Dnmt2 mutants exposed to heat, paraquat or H2O2 is significantly reduced relative to wild type control flies, implicating this methylation event in the stress response program. Anigogenin-induced tRNA cleavage is also involved in a stress response program in mammalian cells 94–96, therefore Dnmt2-induced methylation of tRNA may prevent angiogenin-induced tRNA cleavage. Indeed, Dnmt2 inhibits angiogenin-induced cleavage of tRNAAsp and tRNAGly, but not tRNAMet. Possibly sequestration of Dnmt2 at SGs prevents tRNA methylation under stress conditions to regulate this stress-response program. |
| Roquin | Ubiquitin modifying enzyme | Overexpression of full length Roquin or its isolated Roquin domain nucleates SG assembly 97. Recruitment to SGs has been implicated in the activity of the Roquin 1 and Roquin 2 paralogs that promote the degradation of mRNA encoding the co-stimulatory molecules Icos and OX-40 98, 99 to promote autoimmunity. Roquin also binds a stem loop structure to destabilize selected mRNAs 100. |
| MCPIP1 | Ubiquitin modifying enzyme | Possesses both RNAse and deubiquitinating activities 101. Overexpressed MCPIP1 forms cytoplasmic granules that include components of both SGs and P-bodies, but paradoxically block arsenite-induced SG assembly. Splenocytes lacking MCPIP1 exhibit spontaneous SGs without stress, and form more SGs than control splenocytes in response to arsenite, suggesting that MCPIP1 inhibits SG assembly. Point mutants lacking either RNAse or deubiquitinating activities reveal that MCPIP1-induced granule assembly requires its deubiquitinating activity, whereas MCPIP1- induced inhibition of SG assembly requires its RNAse activity. |
| USP10 | Ubiquitin protease | Interacts with G3BP and PABP 73. Knockdown dampens SG assembly in and correlates with increased production of reactive oxygen species (ROS) and increased apoptosis, both of which are reversed by overexpression of WT or deubiquitinase-inactive USP10. |
SGs comprise dynamic cytoplasmic scaffolds created from mRNPs released from polysomes during stress 43, analogous to multiprotein complexes assembled at the immunological synapse to mediate signaling through the T cell antigen receptor 44, and to lipid raft recruitment of signaling proteins to alter cellular signaling events 45. A similar protein-nucleated, mRNA-containing signaling center mediates the endoplasmic reticulum (ER) stress response, in which oliomerization of IRE1, a transmembrane nuclease/kinase, nucleates the assembly of the “UPRosome” (Unfolded Protein Response-osome, reviewed in 46). IRE1 oligomerization and autophosphorylation trigger the recruitment of translationally-stalled HAC1 mRNA (XBP1 in mammals) to the cytoplasmic face of the IRE1 foci, the assembly of which is required for both functional splicing and downstream signaling events 47. Recruitment of the HAC1/XBP1 mRNA requires both the inhibitory 5′UTR stem loop that stalls ribosomes, and other sequences within the mRNA 3′ untranslated region. IRE1 cleaves off the HAC1 mRNA inhibitory stem loop and the mRNA is ligated by tRNA ligase, thus activating the translation of HAC1/XBP1 protein, an active transcription factor for proteins that resolve ER stress. Interestingly, IRE1 also cleaves and degrades other specific mRNAs through a process termed IRE1-dependent decay. In mammals, phosphorylated IRE1 oligomers also recruit two signaling proteins common to SGs, RACK1 (see below) and tumor necrosis factor receptor associated factor-2 (TRAF2) 47, 48. TRAF2 is an adaptor protein that links the TNF receptor to a signaling cascade that activates NF-kB 49. In cells subjected to heat shock, eIF4G recruits TRAF2 to SGs to disable a TNF-triggered signaling cascade that activates the NF-kB transcription factor. Although TRAF2 does not possess LC/ID regions, its interacting partner eIF4G possesses extensive LC/ID regions that are evolutionarily conserved (Fig. 4). The stress-activated molecular event(s) that promote interactions between eIF4G and TRAF2 have not been identified. In contrast, recruitment of TRAF2 to IRE1-UPRosomes leads to JNK activation and promotes cell death 50.
RACK1/p38/JNK signaling
RACK1 is a pleiotropic adaptor protein that integrates cell adhesion, polarity and motility 51. Although it lacks LC/ID regions, it is an integral part of the small 40S ribosomal subunit and binds the multi-subunit eIF3 complex, both of which are core SG constituents. The sequestration of RACK1 at SGs inhibits the stress-induced activation of the p38/c-Jun N-terminal kinase (JNK) signaling cascade that triggers apoptotic death 52. RACK1 serves as a scaffold that multimerizes MTK1, a mitogen activated protein kinase that acts upstream of p38 and JNK to trigger apoptotic cell death; this process is inhibited when RACK1 is sequestered at SGs. RACK1/PP2A are also recruited to the UPRosome, wherein RACK1/PP2A mediate the dephosphorylation of IRE1 in pancreatic beta cells 48. RACK1 is also one of several SG proteins that are modified by O-linked N-Acetylglucosamine (GlcNAc) in response to arsenite-induced stress 15, but whether GlcNAC modification of RACK1 alters its recruitment to SGs is unknown. Knockdown of O-GlcNAc transferase (OGT) strongly inhibits arsenite-induced GlcNAc modifications and SG assembly, but allows phosphorylation of eIF2α and disassembly of polysomes15. While GlcNAc modifications promote aggregation of untranslated mRNPs into SGs, the mechanism is unknown. One possibility is that GlcNAc modifications antagonize selective “reordering” of LC/ID regions via phosphorylation and 14-3-3 binding (Fig. 2).
Integrated Stress Response/Phospho-eIF2α signaling
In mammalian cells, SG assembly is predominantly initiated by stress-induced phosphorylation of eIF2α, especially in response to viral infection 18. OGFOD1 (2-oxoglutarate and Fe(II)-dependent oxygenase domain containing) is a SG-nucleating protein that interacts (directly or indirectly) with the SG proteins G3BP, USP10, caprin1, YB-1, HRI kinase, and its substrate eIF2α 53. OGFOD1 expression levels correlate with both phosphorylation of eIF2α and SG assembly, suggesting that OGFOD1 acts as a scaffold to facilitate HRI-induced phosphorylation of eIF2α. OGFOD1 contains LC/ID regions, as do its binding partners (Fig. 4), consistent with their ability to promote/nucleate SG assembly.
Target of rapamycin (TOR) signaling
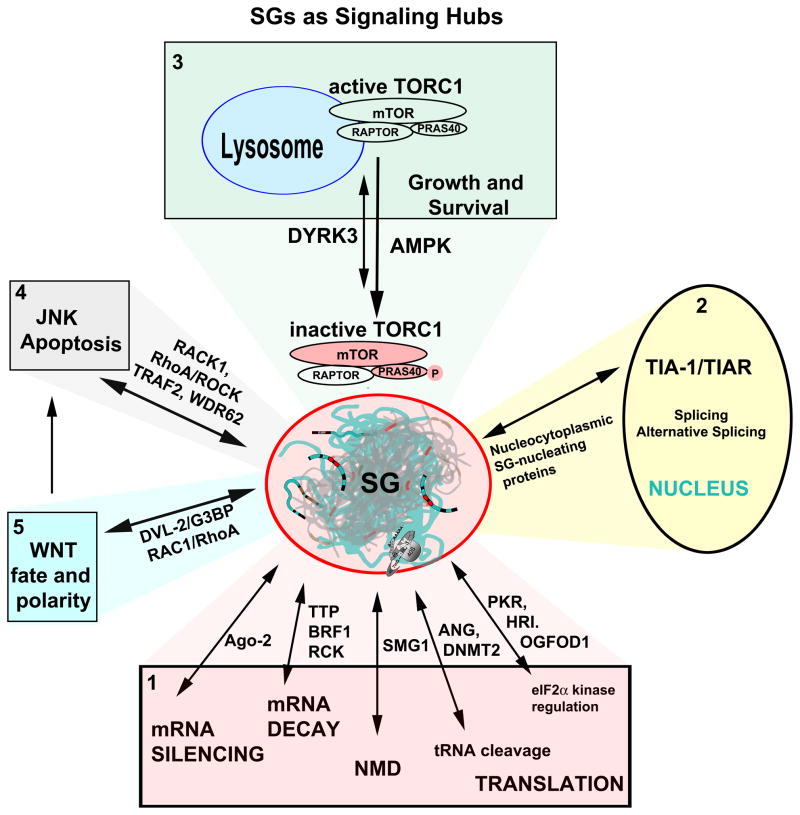
The conserved kinase TOR assembles two distinct complexes (TORC1 and TORC2) that control cellular growth and metabolism 54. SG assembly in both yeast and human cells alters TORC1 signaling by sequestering both TORC1 and downstream kinases to alter signaling during stress 55, 56. Under optimal growth conditions, signals from growth factor receptors and environmental nutrients conspire to keep TORC1 active at vacuolar or lysosomal membranes 54, allowing TORC1 to promote protein synthesis and inhibit autophagy. Amino acid deprivation inactivates TORC1, and it is released from the lysosomal membrane (Fig. 5, panel 3) 57. Inactivated TORC1 accumulates in SGs assembled in response to stress-induced phosphorylation of eIF2α. During recovery from stress, re-activation of TORC1 correlates with its release from disassembled SGs 55, 56 suggesting that SG association regulates the timing of TORC1 inactivation and reactivation in stressed cells (Fig. 5).
Figure 5. SGs as Signaling Hubs.
SG (center) assembly and disassembly are in dynamic equilibrium with protein translation and mRNA metabolism (Panel 1); their formation is nucleated by protein effectors of mRNA silencing, decay, quality control (nonsense-mediated decay, NMD), tRNA cleavage, or translation). Nucleocytoplasmic shuttling proteins (panel 2) comprise a major class of SG-nucleating proteins such as TIA-1/TIAR, whose nuclear functions involve alternative splicing; this process may be altered when TIA-1/R are sequestered in SGs. TORC1 signaling (panel 3) both regulates and is regulated by SG assembly through DYRK355 (see text); regulation of TORC1 by AMPK may occur during cold shock 17. Multiple signaling proteins sequestered in SGs are effectors of JNK (panel 4) and WNT (panel 5) signaling. Double arrows signify reversible changes in location (nucleus, cytoplasm, lysosome) or state (soluble versus partitioned into SGs via a phase transition).
In mammalian cells, another connection between SGs and TORC1 was discovered when a chemical inhibitor of the dual specificity tyrosine-phosphorylation regulated kinase 3 (DYRK3) was identified as a compound that delays SG disassembly in cells recovering from arsenite-induced stress 55. Remarkably, overexpressed kinase-inactive DYRK3, or an isolated N-terminal domain encoding an LC/ID region (Fig. 4), can nucleate SG assembly and drive TORC1 from lysosomes into SGs concurrent with TORC1 inactivation (as assessed by phosphorylation of the TORC1 substrate ribosomal S6 kinase). Both kinase-dead or truncated DYRK3 nucleate SGs that both sequester and inactivate TORC1. In addition, the kinase activity of DYRK3 promotes mTOR activation by two other mechanisms: first, it phosphorylates and inactivates the TORC1 inhibitory subunit PRAS40 (Proline-rich AKT substrate), a protein composed almost entirely of LC/ID regions (Fig. 4). Second, DYRK3 phosphorylates an unknown substrate (possibly DYRK3 itself) to inhibit the DRYK3 N-terminus from nucleating SGs. In combination, these mechanisms allow DRYK3 to regulate TORC1 activation by both LC/ID-mediated compartmentalization to SGs and via kinase-dependent phosphorylation of regulatory PRAS40. Interestingly, phosphorylation of both RAPTOR 58 and PRAS40 59, 60 results in 14-3-3 binding and inactivation of TORC1—whether 14-3-3 binding regulates the sequestration of RAPTOR or PRAS40 in SGs remains unknown.
The PABP-interacting protein Pbp1 was identified as a repressor of yeast TORC1 using an overexpression screen 56. Both overexpressed Pbp1 and heat shock induce the assembly of cycloheximide (a translation elongation inhibitor)-reversible yeast SGs (Box 2) that contain TOR and KOG1/RAPTOR. This correlates with the inactivation of TORC1, as quantified by phosphorylation of the downstream kinase Sch9/RPS6. Cycloheximide-mediated dissolution of heat shock-induced SG assembly accelerated the reactivation of TORC1. Thus, as in the mammalian system, the sequestration of yeast TORC1 at SGs regulates TORC1 activation/inactivation. As with mammalian TORC1, the yeast scaffolding subunit KOG1/RAPTOR contains limited LC/ID regions. A yeast-specific TORC1 subunit TCO89 is largely composed of LC/ID regions (not shown), as is metazoan-specific PRAS40. Whether TCO89 associates with yeast SGs (as PRAS40 does with mammalian SGs) is unknown.
Box 2. Mammalian SGs versus Yeast SGs/EGPBs.
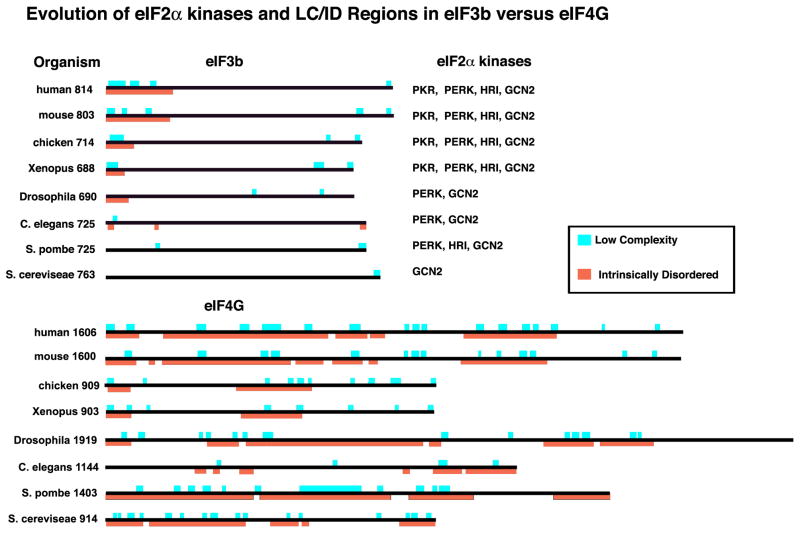
Mammalian SGs and yeast SGs/EGPBs share a requirement for non-polysomal mRNA, but appear to have distinct triggering signals and compositions. Yeast SGs are not dependent on p-eIF2α phosphorylation, and yeast SGs triggered by glucose starvation lack 40S ribosomal subunits and eIF3, which is both a signature component of mammalian SGs and required for mammalian SG assembly15. Yeast possess fewer eIF3 subunits than mammals, so one possibility is that the additional metazoan eIF3 subunits provide expanded regulation and integration with more complex signaling pathways. In addition, mammalian eIF4G possesses a discrete eIF3-binding domain not seen in yeast, hence eIF4G:eIF3 interactions may be more robust in mammalian cells 74. Third, metazoans have multiple eIF2α kinases, whereas budding yeast have only one (Fig I): evolution provides an increasingly dominant role for phospho-eIF2α that correlates with the eIF2α dependence on SG formation. Finally, the major scaffolding subunit of eIF3 75 and SG marker, eIF3b, exhibits an evolutionary LC/ID region in its N-terminal region that is progressively expanded in organisms containing multiple eIF2α kinases, especially PKR (Fig I). In contrast, the scaffolding protein eIF4G retains a high LC/ID content throughout evolution, consistent with its inclusion in SGs of all species. We speculate that SG nucleation may be an as-yet unrecognized intrinsic function of eIF4G.
Figure I. Evolution of eIF2α kinases and LC/ID regions in eIF3b versus eIF4G.
Proteins are shown to scale, with LC (aqua) and ID (orange) regions and total number of amino acids as indicated. The evolution of an increasingly large LC/ID region at the N-terminus of eIF3b parallels the appearance of PKR, the importance of phospho-eIF2α in SG assembly, and of the inclusion of eIF3 in SGs.
Rho GTPase signaling
Rho GTPases modulate various aspects of vesicle trafficking, cell cycle progression and cytoskeletal rearrangement 61–63. Upon activation, Ras homolog gene family member A (RhoA) binds and activates its downstream kinase, Rho-associated, coiled-coil containing protein kinase 1 (ROCK1). ROCK1 in turn phosphorylates JNK-interacting protein 3 (JIP-3) resulting in activation of JNK and induction of apoptosis 64. Just as sequestration of RACK1 at SGs prevents JNK-induced apoptosis, sequestration of active ROCK1 at SGs prevents phosphorylation of JIP-1, activation of JNK, and apoptosis 65 (Fig. 5, panels 4 and 5). Thus, assembly of SGs signals a state of emergency that alters the RhoA/ROCK1 signaling program to preserve cell survival.
Rho GTPases are also downstream effectors of the Wnt signaling pathway that regulates cell fate determination, proliferation and polarization events in multicellular organisms 66. Wnt binds to cellular proteins that trigger Dishevelled (Dvl)-dependent stabilization of β-catenin and/or modulation of Rac1 and RhoA GTPase activation. In NIH3T3 cells, Wnt signaling triggers Dvl-dependent activation of the Rac1 GTPase. Activated Rac1, in turn, inactivates the RhoA GTPase to inhibit SG assembly 67. Thus, Wnt signaling antagonizes SG assembly. In addition to activating Rac1, Dvl2, an isoform of Dvl, directly binds to the SG nucleating protein G3BP and inhibits SG assembly 67. As a point mutation in the Dvl/Egl-10/Pleckstrin (DEP) domain (K446M) eliminates Dvl2-induced inhibition of SG assembly without disrupting G3BP binding, it is possible that wild type, but not mutant, Dvl2 organizes an LC/ID region in G3BP in a way that prevents its recruitment to SGs. These findings implicate key components of the Wnt signaling cascade in SG assembly and signaling (Fig. 5, panel 5).
Concluding remarks
The evolving “phase transition” model of RNA granules describes a fluid-like conglomeration of RNA and disordered RNA-binding proteins that comprise the “dark matter” of RNA granules; that is, the missing component that gives both stable (germ cell granules) and dynamic (SGs, PBs) RNA granules their form and physical properties. This model proposes that RNA granules form when fleeting and multiple low-affinity interactions between ID/LC-containing proteins promote a demixing phase transition that partitions mRNPs into physically discrete cytoplasmic entities. We propose that SG assembly creates cytoplasmic, mRNP-nucleated signaling centers analogous to classical receptor-mediated signaling complexes, wherein catalytic and structural/scaffolding molecules are concentrated to coordinate signal transduction events. SG formation occurs downstream of stress-induced translational arrest, but the subsequent assembly of translationally-stalled mRNPs into SGs serves to create hubs which compete for components of classical signaling pathways and thus alter their outcome. SGs may inhibit growth signaling by diverting TORC1 from its active location at lysosomes, may act as a “dead man’s switch” to delay apoptosis by sequestration of RACK1 from JNK, and may influence polarity via disruption of Wnt. Although direct data are lacking, SG sequestration of proteins that regulate alternative splicing (Fig. 5, panel 2) may also alter splicing and thus modulate gene expression.
Phase transitions are concentration-dependent, and SGs contain both mRNA and LC/ID proteins. Increased concentration of either component seems able to induce SG—formation stress causes a sudden “nucleating” excess of mRNA released from polysomes, while overexpression of SG-nucleating proteins promotes SG assembly via “supersaturating” concentrations of LC/ID RNA binding proteins. This model is supported by the finding that macromolecular crowding promotes SG assembly in cells (and in vitro) exposed to osmotic stress by increasing the concentration of both RNA and LC/ID proteins 68. Elucidation of the specific molecular events that drive LC/ID-containing proteins to “demix” or “phase out” of the cytosol and assume a cytosol-insoluble liquid state is an important area for future research. Do specific RNA sequence motifs contribute, or does RNA play a more general role analogous to that of lipids in membranes? How do SG-associated post-translational modifications such as GlcNAc addition, ubiquitinylation, polyribosylation, and arginine methylation contribute to this process? Are 14-3-3 interactions a general way to regulate traffic of specific proteins to existing SGs? Our current understanding is very far from complete, but the evolving concept of SGs as transient, RNA-centric signaling modules that recruit other diverse signaling proteins expands the repertoire of SG functions beyond the realm of translational control--one can no longer assume that a protein recruited to SGs has a role in mRNA metabolism. “Nothing is certain except change” is a good motto for this stressful field.
Highlights.
Stress granule assembly may involve a demixing phase transition
Intrinsically disordered motifs may promote recruitment to stress granules
Stress granules may function as RNA-centric signaling hubs
Glossary
- 14-3-3 proteins
A family of ~30 kDa adaptor proteins that bind to phospho-serine or phosho-threonine residues on diverse signaling proteins resulting in altered subcellular localization and/or function
- Demixing phase transition
the transformation of a material from one physical state to another. LC/ID region-containing proteins are proposed to undergo phase transitions in the cell, whereby they condense from a diffuse, soluble state into a concentrated liquid droplet phase (or aggregate into insoluble fibrils)
- Intrinsically disordered region
A protein sequence that does not assume a defined structural motif such as a β-pleated sheet or an α-helix in isolation, but may assume many conformations in association with other proteins or factors
- Low complexity region
A protein sequence containing limited diversity in amino acid composition
- Prion-related domain
a protein sequence rich that is rich in uncharged polar amino acids such as glutamine, asparagine, glycine, proline, serine, and tyrosine, and is capable of assuming two stable conformations, one soluble and one insoluble. The insoluble conformers self-aggregate and are associated with various pathologies
- Processing Bodies (P-bodies)
cytoplasmic structures containing mRNA decay enzymes, eIF4E, and mRNA but lacking eIF4G and PABP
- Stress
A rapid change in conditions. In this review, we refer to environmental stresses such as heat shock, cold shock, redox stress, unfolded proteins or double-stranded RNA resulting from viral infection
- Stress granules
transient, dynamic cytoplasmic structures containing aggregates of non-polysomal mRNA bound to a subset of 48S preinitiation factors, PABP, and specific RNA binding proteins
- Stress granule-nucleating protein
A protein that is recruited to SGs upon stress, and whose overexpression results in SG formation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–41. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends in biochemical sciences. 2008;33:141–50. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Harding H, Novoa Y, Zhang H, Zeng R, Wek M, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 4.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA- dependent protein kinase. The Journal of biological chemistry. 1998;273:2416–23. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 6.Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol. 1995;15:4497–506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21:7971–80. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghisolfi L, Dutt S, McConkey ME, Ebert BL, Anderson P. Stress granules contribute to alpha-globin homeostasis in differentiating erythroid cells. Biochemical and biophysical research communications. 2012;420:768–74. doi: 10.1016/j.bbrc.2012.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen E, Kedersha N, Song B, Scheuner D, Gilks N, Han A, et al. Heme-regulated inhibitor (HRI) kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 (eIF2) inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. The Journal of biological chemistry. 2005;280:16925–33. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- 10.Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, et al. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Molecular biology of the cell. 2002;13:195–210. doi: 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol. 2003;284:C273–84. doi: 10.1152/ajpcell.00314.2002. [DOI] [PubMed] [Google Scholar]
- 12.Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, et al. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151:1257–68. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordeleau ME, Matthews J, Wojnar JM, Lindqvist L, Novac O, Jankowsky E, et al. Stimulation of mammalian translation initiation factor eIF4A activity by a small molecule inhibitor of eukaryotic translation. Proc Natl Acad Sci U S A. 2005;102:10460–5. doi: 10.1073/pnas.0504249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang Y, Kedersha N, Low WK, Romo D, Gorospe M, Kaufman R, et al. Eukaryotic initiation factor 2alpha-independent pathway of stress granule induction by the natural product pateamine A. The Journal of biological chemistry. 2006;281:32870–8. doi: 10.1074/jbc.M606149200. [DOI] [PubMed] [Google Scholar]
- 15.Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nature cell biology. 2008;10:1224–31. doi: 10.1038/ncb1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchan JR, Muhlrad D, Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J Cell Biol. 2008;183:441–55. doi: 10.1083/jcb.200807043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann S, Cherkasova V, Bankhead P, Bukau B, Stoecklin G. Translation suppression promotes stress granule formation and cell survival in response to cold shock. Molecular biology of the cell. 2012;23:3786–800. doi: 10.1091/mbc.E12-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd RE. Regulation of stress granules and P-bodies during RNA virus infection. Wiley interdisciplinary reviews RNA. 2013;4:317–31. doi: 10.1002/wrna.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White JP, Lloyd RE. Regulation of stress granules in virus systems. Trends in microbiology. 2012;20:175–83. doi: 10.1016/j.tim.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Molecular biology of the cell. 2004;15:5383–98. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decker CJ, Teixeira D, Parker R. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J Cell Biol. 2007;179:437–49. doi: 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reijns MA, Alexander RD, Spiller MP, Beggs JD. A role for Q/N-rich aggregation-prone regions in P-body localization. Journal of cell science. 2008;121:2463–72. doi: 10.1242/jcs.024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, et al. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell. 2012;149:768–79. doi: 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–67. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malinovska L, Kroschwald S, Alberti S. Protein disorder, prion propensities, and self-organizing macromolecular collectives. Biochimica et biophysica acta. 2013;1834:918–31. doi: 10.1016/j.bbapap.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Uversky VN. Unusual biophysics of intrinsically disordered proteins. Biochimica et biophysica acta. 2013;1834:932–51. doi: 10.1016/j.bbapap.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Coletta A, Pinney JW, Solis DY, Marsh J, Pettifer SR, Attwood TK. Low-complexity regions within protein sequences have position-dependent roles. BMC systems biology. 2010;4:43. doi: 10.1186/1752-0509-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wootton JC, Federhen S. Analysis of compositionally biased regions in sequence databases. Methods in enzymology. 1996;266:554–71. doi: 10.1016/s0076-6879(96)66035-2. [DOI] [PubMed] [Google Scholar]
- 29.Dosztanyi Z, Csizmok V, Tompa P, Simon I. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics. 2005;21:3433–4. doi: 10.1093/bioinformatics/bti541. [DOI] [PubMed] [Google Scholar]
- 30.Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–32. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 31.Weber SC, Brangwynne CP. Getting RNA and protein in phase. Cell. 2012;149:1188–91. doi: 10.1016/j.cell.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fitzler M, et al. Stress granules and processing bodies are dynamically liked sites of mRNP remodeling. J Cell Biol. 2005;169:871–84. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, et al. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160:823–31. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Stoecklin G, Stubbs T, Kedersha N, Blackwell TK, Anderson P. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. Embo J. 2004;23:1313–24. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franks TM, Lykke-Andersen J. TTP and BRF proteins nucleate processing body formation to silence mRNAs with AU-rich elements. Genes Dev. 2007;21:719–35. doi: 10.1101/gad.1494707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courchet J, Buchet-Poyau K, Potemski A, Bres A, Jariel-Encontre I, Billaud M. Interaction with 14-3-3 adaptors regulates the sorting of hMex-3B RNA-binding protein to distinct classes of RNA granules. The Journal of biological chemistry. 2008;283:32131–42. doi: 10.1074/jbc.M802927200. [DOI] [PubMed] [Google Scholar]
- 37.Huang KL, Chadee AB, Chen CY, Zhang Y, Shyu AB. Phosphorylation at intrinsically disordered regions of PAM2 motif-containing proteins modulates their interactions with PABPC1 and influences mRNA fate. RNA. 2013;19:295–305. doi: 10.1261/rna.037317.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozlov G, De Crescenzo G, Lim NS, Siddiqui N, Fantus D, Kahvejian A, et al. Structural basis of ligand recognition by PABC, a highly specific peptide-binding domain found in poly(A)-binding protein and a HECT ubiquitin ligase. EMBO J. 2004;23:272–81. doi: 10.1038/sj.emboj.7600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–40. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michelitsch MD, Weissman JS. A census of glutamine/asparagine-rich regions: implications for their conserved function and the prediction of novel prions. Proc Natl Acad Sci U S A. 2000;97:11910–5. doi: 10.1073/pnas.97.22.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. Journal of molecular biology. 2004;337:635–45. doi: 10.1016/j.jmb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Lewitzky M, Simister PC, Feller SM. Beyond ‘furballs’ and ‘dumpling soups’ - towards a molecular architecture of signaling complexes and networks. FEBS letters. 2012;586:2740–50. doi: 10.1016/j.febslet.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Dustin ML, Groves JT. Receptor signaling clusters in the immune synapse. Annual review of biophysics. 2012;41:543–56. doi: 10.1146/annurev-biophys-042910-155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonnino S, Prinetti A. Membrane domains and the “lipid raft” concept. Current medicinal chemistry. 2013;20:4–21. [PubMed] [Google Scholar]
- 46.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature reviews Molecular cell biology. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 47.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature reviews Molecular cell biology. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 48.Qiu Y, Mao T, Zhang Y, Shao M, You J, Ding Q, et al. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells. Science signaling. 2010;3:ra7. doi: 10.1126/scisignal.2000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim WJ, Back SH, Kim V, Ryu I, Jang SK. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol Cell Biol. 2005;25:2450–62. doi: 10.1128/MCB.25.6.2450-2462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 51.Adams DR, Ron D, Kiely PA. RACK1, A multifaceted scaffolding protein: Structure and function. Cell communication and signaling: CCS. 2011;9:22. doi: 10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nature cell biology. 2008;10:1324–32. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- 53.Wehner KA, Schutz S, Sarnow P. OGFOD1, a novel modulator of eukaryotic translation initiation factor 2alpha phosphorylation and the cellular response to stress. Mol Cell Biol. 2010;30:2006–16. doi: 10.1128/MCB.01350-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends in molecular medicine. 2012;18:524–33. doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, Pelkmans L. Dual Specificity Kinase DYRK3 Couples Stress Granule Condensation/Dissolution to mTORC1 Signaling. Cell. 2013;152:791–805. doi: 10.1016/j.cell.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 56.Takahara T, Maeda T. Transient sequestration of TORC1 into stress granules during heat stress. Mol Cell. 2012;47:242–52. doi: 10.1016/j.molcel.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Yan G, Lai Y, Jiang Y. The TOR complex 1 is a direct target of Rho1 GTPase. Mol Cell. 2012;45:743–53. doi: 10.1016/j.molcel.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nature cell biology. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 61.Chi X, Wang S, Huang Y, Stamnes M, Chen JL. Roles of rho GTPases in intracellular transport and cellular transformation. International journal of molecular sciences. 2013;14:7089–108. doi: 10.3390/ijms14047089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanna S, El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cellular signalling. 2013 doi: 10.1016/j.cellsig.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Wilson KF, Erickson JW, Antonyak MA, Cerione RA. Rho GTPases and their roles in cancer metabolism. Trends in molecular medicine. 2013;19:74–82. doi: 10.1016/j.molmed.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ongusaha PP, Qi HH, Raj L, Kim YB, Aaronson SA, Davis RJ, et al. Identification of ROCK1 as an upstream activator of the JIP-3 to JNK signaling axis in response to UVB damage. Science signaling. 2008;1:ra14. doi: 10.1126/scisignal.1161938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsai N-P, Wei LN. RhoA/ROCK1 signaling regulates stress granule formation and apoptosis. Cellular Signaling. 2009 doi: 10.1016/j.cellsig.2009.12.001. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cellular signalling. 2010;22:717–27. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 67.Sahoo PK, Murawala P, Sawale PT, Sahoo MR, Tripathi MM, Gaikwad SR, et al. Wnt signalling antagonizes stress granule assembly through a Dishevelled-dependent mechanism. Biology open. 2012;1:109–19. doi: 10.1242/bio.2011023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bounedjah O, Hamon L, Savarin P, Desforges B, Curmi PA, Pastre D. Macromolecular crowding regulates assembly of mRNA stress granules after osmotic stress: new role for compatible osmolytes. The Journal of biological chemistry. 2012;287:2446–58. doi: 10.1074/jbc.M111.292748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buchan JR, Parker R. Eukaryotic stress granules: The ins and outs of translation. Mol Cell. 2009;36:932–41. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007;21:3381–94. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisinger-Mathason TS, Andrade J, Groehler AL, Clark DE, Muratore-Schroeder TL, Pasic L, et al. Codependent functions of RSK2 and the apoptosis-promoting factor TIA-1 in stress granule assembly and cell survival. Mol Cell. 2008;31:722–36. doi: 10.1016/j.molcel.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim B, Cooke HJ, Rhee K. DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress. Development. 2012;139:568–78. doi: 10.1242/dev.075846. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi M, Higuchi M, Matsuki H, Yoshita M, Ohsawa T, Oie M, et al. Stress granules inhibit apoptosis by reducing reactive oxygen species production. Mol Cell Biol. 2013;33:815–29. doi: 10.1128/MCB.00763-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchell S, Walker S, Rajogopal V, Echeverria Aitken C, Lorsch J. Recruiting knotty partners: The rolees of translation initiation factors in mRNA recruitment to the eukaryotic ribosome. 2011. [Google Scholar]
- 75.Zhou M, Sandercock AM, Fraser CS, Ridlova G, Stephens E, Schenauer MR, et al. Mass spectrometry reveals modularity and a complete subunit interaction map of the eukaryotic translation factor eIF3. Proc Natl Acad Sci U S A. 2008;105:18139–44. doi: 10.1073/pnas.0801313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobayashi T, Winslow S, Sunesson L, Hellman U, Larsson C. PKCalpha binds G3BP2 and regulates stress granule formation following cellular stress. PloS one. 2012;7:e35820. doi: 10.1371/journal.pone.0035820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M. Both G3BP1 and G3BP2 contribute to stress granule formation. Genes to cells: devoted to molecular & cellular mechanisms. 2013;18:135–46. doi: 10.1111/gtc.12023. [DOI] [PubMed] [Google Scholar]
- 78.Brown JA, Roberts TL, Richards R, Woods R, Birrell G, Lim YC, et al. A novel role for hSMG-1 in stress granule formation. Mol Cell Biol. 2011;31:4417–29. doi: 10.1128/MCB.05987-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wishart MJ, Denu JM, Williams JA, Dixon JE. A single mutation converts a novel phosphotyrosine binding domain into a dual-specificity phosphatase. The Journal of biological chemistry. 1995;270:26782–5. doi: 10.1074/jbc.270.45.26782. [DOI] [PubMed] [Google Scholar]
- 80.Wishart MJ, Dixon JE. Gathering STYX: phosphatase-like form predicts functions for unique protein-interaction domains. Trends in biochemical sciences. 1998;23:301–6. doi: 10.1016/s0968-0004(98)01241-9. [DOI] [PubMed] [Google Scholar]
- 81.Barr JE, Munyikwa MR, Frazier EA, Hinton SD. The pseudophosphatase MK-STYX inhibits stress granule assembly independently of Ser149 phosphorylation of G3BP-1. FEBS J. 2013;280:273–84. doi: 10.1111/febs.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kozubowski L, Aboobakar EF, Cardenas ME, Heitman J. Calcineurin colocalizes with P-bodies and stress granules during thermal stress in Cryptococcus neoformans. Eukaryotic cell. 2011;10:1396–402. doi: 10.1128/EC.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leung AK, Calabrese JM, Sharp PA. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci U S A. 2006;103:18125–30. doi: 10.1073/pnas.0608845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leung AK, Sharp PA. Quantifying Argonaute Proteins In and Out of GW/P-Bodies: Implications in microRNA Activities. Advances in experimental medicine and biology. 2013;768:165–82. doi: 10.1007/978-1-4614-5107-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Detzer A, Engel C, Wunsche W, Sczakiel G. Cell stress is related to relocalization of Argonaute 2 and to decreased RNA interference in human cells. Nucleic acids research. 2011;39:2727–41. doi: 10.1093/nar/gkq1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ernoult-Lange M, Baconnais S, Harper M, Minshall N, Souquere S, Boudier T, et al. Multiple binding of repressed mRNAs by the P-body protein Rck/p54. RNA. 2012;18:1702–15. doi: 10.1261/rna.034314.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qi MY, Wang ZZ, Zhang Z, Shao Q, Zeng A, Li XQ, et al. AU-rich-element-dependent translation repression requires the cooperation of tristetraprolin and RCK/P54. Mol Cell Biol. 2012;32:913–28. doi: 10.1128/MCB.05340-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chalupnikova K, Lattmann S, Selak N, Iwamoto F, Fujiki Y, Nagamine Y. Recruitment of the RNA helicase RHAU to stress granules via a unique RNA-binding domain. The Journal of biological chemistry. 2008;283:35186–98. doi: 10.1074/jbc.M804857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shih JW, Wang WT, Tsai TY, Kuo CY, Li HK, Wu Lee YH. Critical roles of RNA helicase DDX3 and its interactions with eIF4E/PABP1 in stress granule assembly and stress response. The Biochemical journal. 2012 doi: 10.1042/BJ20110739. [DOI] [PubMed] [Google Scholar]
- 90.Hilliker A, Gao Z, Jankowsky E, Parker R. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol Cell. 2011;43:962–72. doi: 10.1016/j.molcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell. 2011;42:489–99. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goulet I, Boisvenue S, Mokas S, Mazroui R, Cote J. TDRD3, a novel Tudor domain-containing protein, localizes to cytoplasmic stress granules. Human molecular genetics. 2008;17:3055–74. doi: 10.1093/hmg/ddn203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, et al. RNA methylation by Dnmt2 protects tRNAs agains stress-induced cleavage. Genes Dev. 2010 doi: 10.1101/gad.586710. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Emara M, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, et al. Angiogenin-induced tiRNAs promote stress-induced stress granule assembly. The Journal of biological chemistry. 2010;285:10959–68. doi: 10.1074/jbc.M109.077560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-Induced tRNA Fragments Inhibit Translation Initiation. Mol Cell. 2011;43:613–23. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Athanasopoulos V, Barker A, Yu D, Tan AH, Srivastava M, Contreras N, et al. The ROQUIN family of proteins localizes to stress granules via the ROQ domain and binds target mRNAs. FEBS J. 2010;277:2109–27. doi: 10.1111/j.1742-4658.2010.07628.x. [DOI] [PubMed] [Google Scholar]
- 98.Vogel KU, Edelmann SL, Jeltsch KM, Bertossi A, Heger K, Heinz GA, et al. Roquin Paralogs 1 and 2 Redundantly Repress the Icos and Ox40 Costimulator mRNAs and Control Follicular Helper T Cell Differentiation. Immunity. 2013;38:655–68. doi: 10.1016/j.immuni.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Pratama A, Ramiscal RR, Silva DG, Das SK, Athanasopoulos V, Fitch J, et al. Roquin-2 Shares Functions with Its Paralog Roquin-1 in the Repression of mRNAs Controlling T Follicular Helper Cells and Systemic Inflammation. Immunity. 2013;38:669–80. doi: 10.1016/j.immuni.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Leppek K, Schott J, Reitter S, Poetz F, Hammond MC, Stoecklin G. Roquin promotes constitutive mRNA decay via a conserved class of stem-loop recognition motifs. Cell. 2013;153:869–81. doi: 10.1016/j.cell.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 101.Qi D, Huang S, Miao R, She ZG, Quinn T, Chang Y, et al. Monocyte chemotactic protein-induced protein 1 (MCPIP1) suppresses stress granule formation and determines apoptosis under stress. The Journal of biological chemistry. 2011;286:41692–700. doi: 10.1074/jbc.M111.276006. [DOI] [PMC free article] [PubMed] [Google Scholar]