Abstract
Zinc deficiency impairs the proliferation and differentiation of stem cells in the central nervous system that participate in neurogenesis. To examine the molecular mechanisms responsible for the role of this essential nutrient in neuronal precursor cells and neuronal differentiation, we identified zinc-dependent changes in the DNA-binding activity of zinc-finger proteins and other transcription factors in proliferating human Ntera-2 neuronal precursor cells undergoing retinoic acid-stimulated differentiation into a neuronal phenotype. We found that zinc deficiency altered binding activity of 28 transcription factors including retinoid X receptor (RXR) known to participate in neuronal differentiation. Alterations in zinc-finger transcription factor activity were not simply the result of removal of zinc from these proteins during zinc deficiency, as the activity of other zinc-binding transcription factors such as the glucocorticoid receptor was increased by as much as 2-fold over zinc adequate conditions and non-zinc binding transcription factors such as nuclear factor-1 and heat shock transcription factor-1 were increased by as much as 4-fold over control. Western analysis did not detect significant decreases in total RXR protein abundance in neuronal precursors, suggesting that the decrease in DNA-binding activity was not simply the result of a reduction in RXR levels in neuronal precursor cells. Rather, use of a reporter gene construct containing retinoic acid response elements upstream from a luciferase coding sequence revealed that zinc deficiency results in decreased transcriptional activity of RXR and reductions in retinoic acid-mediated gene transcription during neuronal differentiation. These results show that zinc deficiency has implications for both developmental and adult neurogenesis.
Keywords: NT2, retinoid X receptor, neurogenesis, zinc-finger, transcription
1. Introduction
Neurogenesis is the formation of mature neurons that are capable of integrating into neuronal circuits through the proliferation and differentiation of neural stem/progenitor cells. Not only is neurogenesis required during embryonic development of the central nervous system, but it also occurs in two discrete regions of the adult brain including the subventricular zone that surrounds the lateral ventricles1,2 and the dentate gyrus of the hippocampus.3,4 Retinoids with vitamin A activity, particularly retinoic acid, play a role in processes such as cell proliferation, cell differentiation, development, vision, and immunity. In addition to the well-studied role of retinoic acid during embryonic development,5 it appears that retinoic acid is also needed for adult hippocampal neurogenesis.6 Retinoic acid is not only required for the survival of proliferating cells in the dentate gyrus, but also for the early stages of neuronal differentiation.6 Thus, vitamin A deficiency in mice reduced overall neurogenesis in the dentate gyrus when compared to control animals.6
The biological functions of retinoic acid are largely mediated through the retinoid receptors. The retinoic acid receptors (RAR-α, -β, -γ) and the retinoid X receptors (RXR-α, -β, -γ) are ligand-activated transcription factors and are members of the steroid/thyroid nuclear receptor family. While RAR can bind to both all-trans-retinoic acid and 9-cis-retinoic acid, RXR can only bind to 9-cis-retinoic acid.7 The addition of all-trans-retinoic acid in an in vivo model results in the increase in transcriptionally active 9-cis-retinoic acid.8 Heterodimers of RAR and RXR bind to target genes at specific DNA sequences called RA-response elements to promote transcription, resulting in expression of target genes.7 RXR can also bind DNA as a homodimer or function as a heterodimer with other nuclear receptors such as peroxisome proliferator-activated receptor (PPAR), vitamin D receptor (VDR), and thyroid hormone receptor (TR), resulting in different signaling pathways.9 Nuclear hormone receptors are made up of many domains including the DNA-binding domain that is highly conserved. The DNA binding domain of nuclear hormone receptors contain zinc fingers that are formed through the coordination of one zinc ion to cysteine residues to form a compact structure.10 Another class of zinc fingers that are common in other transcription factors contain both cysteine and histidine residues in coordination with a zinc ion.11
Previous work has shown that zinc regulates neuronal stem cell proliferation, survival, and differentiation.12,13 Three weeks of dietary zinc deficiency significantly decreased the number of proliferating cells labeled with the cell cycle marker Ki67 and significantly increased TUNEL labeled cells in the subgranular zone of the dentate gyrus, an indication that zinc deficiency impairs proliferation and survival in vivo. Cell proliferation measured by BrdU incorporation was also impaired in zinc deficient human Ntera-2 (NT2) neuronal precursor cells.12
In addition to proliferation and survival, it is also known that zinc is required for neuronal differentiation. The early marker of neuronal differentiation doublecortin (DCX) was reduced in mice fed a zinc deficient diet, suggesting that neuronal differentiation is also regulated by zinc.14 Using NT2 neuronal precursor cells that can differentiate into post-mitotic mature neurons after exposure to retinoic acid, it has been determined that zinc deficiency impairs neuronal differentiation and decreases the early neuronal markers TuJ1 and DCX. TGF-β signaling and expression of a variety of genes including RXRα were implicated as a mechanistic target for zinc regulation of neuronal differentiation.13 RXRα mRNA abundance was decreased by 40% in the hippocampus of rats fed a zinc deficient diet compared to control zinc adequate animals.13 Thus, we hypothesized that zinc deficiency impairs transcriptional activity during neuronal differentiation. This work not only focused on the role of zinc in the transcriptional activity of RXR and other zinc-finger proteins in neuronal precursor cells, but also examined DNA-binding activity of a variety of other non-zinc finger transcription factors that are expressed during neuronal differentiation.
2. Methods and Materials
2.1. Human neuronal precursor cell culture
Human NT2/D1 teratocarcinoma cells (Ntera2/D1, Stratgene, La Jolla, CA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma Aldrich, St. Louis, MO) and supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), 100 μg/ml of penicillin, 100 μg/ml of streptomycin, 0.25 μg/ml of amphotericin B (Sigma Chemicals, St. Louis, MO), and 0.5 μg/ml of gentamicin (GIBCO BRL, Rockville, MD). Cells were maintained in a 37°C humidified chamber in 5% CO2.
In order to induce zinc deficiency, NT2 cells were grown in zinc deficient media that was prepared by mixing fetal bovine serum with 10% Chelex-100 at 4°C overnight according to the method described in Ho et al.15 The Chelex-extracted serum was then added to DMEM for a final serum concentration of 10%. Zinc adequate media was prepared by the addition of 10% fetal bovine serum without Chelex treatment to DMEM.
2.2. Transcription factor profiling array
The Transcription Factor Activation Profiling Plate Array II (Signosis, Sunnyvale, CA) was used to analyze the activity of 96 different transcription factors in zinc adequate and zinc deficient NT2 cells according to manufacturer’s instructions. Nuclear proteins from NT2 cells were isolated using a Nuclear Extraction Kit (Signosis, Sunnyvale, CA). Biotin labeled probes based on the consensus sequences of transcription factor DNA-binding sites were mixed with 15 μg of nuclear protein extract to form transcription factor/probe complexes. The bound probes were separated from the complex and hybridized to a plate that was pre-coated with sequences complementary to the probes. The captured DNA probe was detected with streptavidin-HRP and signal intensity was measured with a microplate luminometer. Transcription factors were considered to be regulated by zinc deficiency if the fold change was ≤ -2 or ≥ 2 compared to zinc adequate control NT2 cells.
2.3. Dual luciferase reporter gene assay
An RXR dual luciferase reporter assay (SABiosciences, Frederick, MD) was used to quantify zinc regulation of RXR transcriptional activity. NT2 cells were seeded at a density of 5 × 103 cells per well in a 96 well plate. Cells were grown in either zinc adequate or zinc deficient media for 4 d. The RXR construct (200 ng/well) was transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions and cells were treated with 10 μM retinoic acid for 24 h after transfection. Firefly and renilla luciferase activity was measured with the Dual-Glo Luciferase Assay System (Promega, Fitchburg, WI) and a microplate luminometer. Negative and positive control constructs were used and supplied by the RXR dual luciferase reporter kit. RXR reporter expression was expressed as relative light units (RLUs) and the experimental reporter (firefly luciferase) was normalized with the control reporter (renilla luciferase).
2.4. Western blot analysis
NT2 cells were grown in zinc adequate or zinc deficient media for 4 d and were incubated with 10 μM retinoic acid for 24 h starting on day 3. Cell samples were harvested in Triton X-100 lysis buffer with a protease inhibitor cocktail. Samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using a 10% polyacrylamide gel and then transferred to a 0.2 μm nitrocellulose membrane on ice. The membrane was blocked with Superblock blocking buffer in TBS for 1 h at room temperature followed by overnight incubation at 4°C with rabbit polyclonal antibodies for RXRα at 1:5000 (D-20: sc-553; Santa Cruz Biotechnology, Santa Cruz, CA) and CRABP2 at 1:1000 (ab74365; Abcam, Cambridge, MA). A mouse monoclonal antibody for β-actin at 1:1000 (A2228; Sigma Aldrich, St. Louis, MO) was used as a loading control. Membranes were then washed and incubated with their corresponding IRDye infrared dyes at 1:20,000. Immunoreactive bands were visualized using the Odyssey infrared detection method with corresponding software (LI-COR Biosciences, NE).
2.5. Cellular zinc measurements
Cell samples were digested in concentrated trace metal free nitric acid and diluted in 4% trace metal free nitric acid. Samples were diluted and total zinc concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS) on a Finnegan Element-1 ICP-MS at the National High Magnetic Field Laboratory. NT2 cell zinc concentrations are reported as μg zinc/mg cell protein. All glassware and plasticware were acid washed to ensure that all supplies were zinc free.
2.6. Statistical analysis
Data were analyzed using a one-way ANOVA followed by a by Newman–Keuls Multiple-comparison post hoc test using GraphPad Prism 5.0 software (Prism; GraphPad, San Diego, CA) and were considered significant at p<0.05.
3. Results
3.1. Media and cellular zinc
Chelex-treatment of serum resulted in a 62% reduction in media zinc (14.15±0.89 μg/L vs 37.75±0.92 μg/l) as previously described.13 One day of zinc deficiency resulted in a 25% reduction in cellular zinc compared to cells exposed to zinc adequate media (0.18±0.027 vs 0.24±0.047 μg zinc/mg protein). Continuation of the deficiency for 4 d resulted in a further reduction in cellular zinc to approximately 40% of control values (0.10±0.015 vs 0.24±0.047 μg zinc/mg protein; p<0.01).
3.2. Transcription factor DNA binding activity
Nuclear proteins, isolated from NT2 cells and used in a DNA binding assay, showed that 4 d of zinc deficiency differentially affected the binding activities of a number of zinc finger transcription factors. Of the total 27 zinc finger transcription factors, 7 were down-regulated, 18 were not regulated, and 2 were up-regulated by zinc deficiency (Table 1). Specifically, zinc deficiency reduced the binding of RXR to DNA response elements by 50% (Table 1). In addition to zinc finger transcription factors, there are a number of transcription factors that do not have zinc as part of their protein structure but are otherwise regulated by zinc deficiency (Table 2). Zinc deficiency regulated the DNA binding of 19 non-zinc finger transcription factors, of which 7 were reduced and 12 were enhanced.
Table 1.
Effect of zinc deficiency on zinc-finger transcription factors
| Transcription Factor | Description | ZD/ZA* |
|---|---|---|
| TR | Thyroid hormone receptor | 0.3 |
| COUP-TF | Chicken ovalbumin upstream promoter transcription factor | 0.4 |
| Snail | Snail 1 zinc finger protein | 0.4 |
| RXR | Retinoid X receptor | 0.5 |
| SF1 | Steroidogenic factor 1 | 0.5 |
| MZF | Myeloid zinc finger | 0.5 |
| ATF2 | Activating transcription factor 2 | 0.5 |
| PLAG1 | Pleiomorphic adenoma gene 1 | 0.6 |
| p53 | Tumor protein 53 | 0.6 |
| VDR | Vitamin D receptor | 0.6 |
| YY1 | Yin yang 1 | 0.6 |
| Gfi-1 | Growth factor independent protein 1 | 0.7 |
| SMUC | Snail-related transcription factor | 0.7 |
| AR | Androgen receptor | 0.9 |
| Gli1 | Gli zinc finger transcription factor | 1 |
| Sp1 | Specificity Protein 1 | 1 |
| ROR | Retinoic acid receptor-related orphan receptor | 1 |
| KLF4 | Kruppel-like factor 4 | 1.2 |
| PXR | Pregnane X receptor | 1.2 |
| ER | Estrogen receptor | 1.4 |
| EGR | Early growth response | 1.5 |
| PPAR | Peroxisome proliferator-activated receptor | 1.6 |
| WT1 | Wilms tumor protein 1 | 1.6 |
| CAR | Constitutive androstane receptor | 1.8 |
| HNF4 | Hepatocyte nuclear factor 4 | 1.8 |
| GR/PR | Glucocorticoid receptor/Progesterone receptor | 1.9 |
| GATA | GATA transcription factor | 2.6 |
Luminescence of zinc deficient (ZD) cells relative to zinc adequate (ZA) cells
Table 2.
Effect of zinc deficiency on non-zinc finger transcription factors
| Transcription Factor | Description | ZD/ZA* |
|---|---|---|
| Ets | E-twenty six | 0.4 |
| SATB1 | Special AT-rich sequence binding protein 1 | 0.4 |
| FOXA1 | Forkhead box protein A1 | 0.5 |
| Pax3 | Paired box protein 3 | 0.5 |
| Pax5 | Paired box protein 5 | 0.5 |
| Stat1 | Signal transducer and activator of transcription 1 | 0.5 |
| TFIID | TATA box binding protein | 0.5 |
| Pit | Pituitary specific transcription factor 1 | 2.0 |
| C/EBP | CCAAT/enhancer binding protein, alpha | 2.1 |
| Stat3 | Signal transducer and activator of transcription 3 | 2.4 |
| SOX9 | SOX protein 9 | 2.6 |
| AP4 | Activator protein 4 | 2.7 |
| NRF1 | Nuclear respiratory factor 1 | 2.8 |
| NF-E2 | Nuclear factor (erythroid-derived 2) | 2.9 |
| FOXO1 | Forkhead box protein O1 | 3.0 |
| HSF | Heat shock transcription factor 1 | 3.0 |
| Prox1 | Prospero homeobox protein 1 | 3.8 |
| NF1 | Nuclear factor 1 | 3.9 |
| CDP | CCAAT displacement protein | 4.6 |
Luminescence of zinc deficient (ZD) cells relative to zinc adequate (ZA) cells
3.3. RXR-mediated transcriptional activity
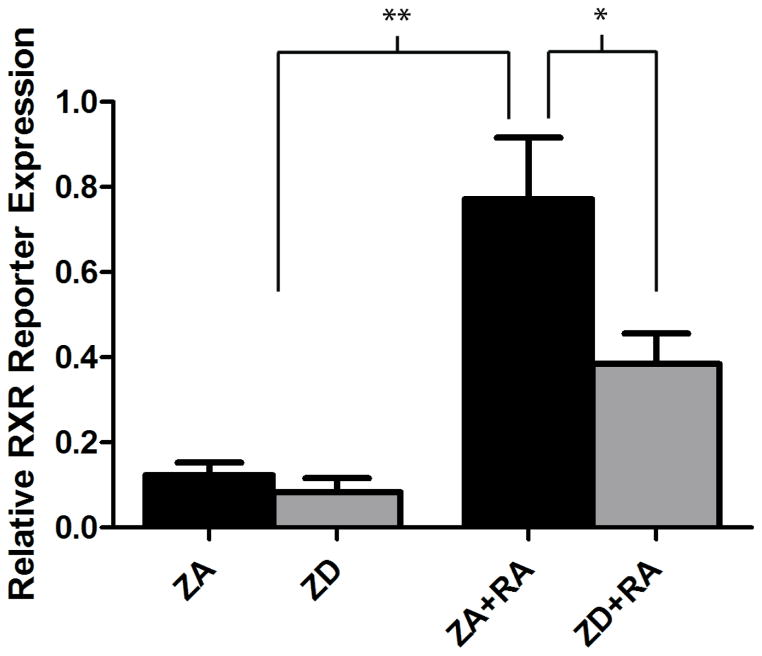
The dual luciferase RXR reporter assay showed very low expression of the RXR reporter in zinc adequate and zinc deficient NT2 cells. There was a 6.4-fold increase in reporter expression when NT2 cells were treated with retinoic acid (p<0.001). The expression of the RXR reporter was significantly decreased by 50% in zinc deficient cells treated with retinoic acid compared to zinc adequate cells treated with retinoic acid (p<0.05) (Fig. 1).
Figure 1.
Effect of zinc deficiency on RXR-mediated transcriptional activity in retinoic acid-treated NT2 cells. Cells were grown in zinc adequate (ZA) or zinc deficient (ZD) media for 4 d before transient transfection of an inducible RXR-responsive firefly luciferase reporter and a constitutively expressing renilla luciferase reporter to control for transfection efficiency and then treated with or without retinoic acid (RA) for 24 h. Bars represent mean±SEM (n=3) and represent firefly reporter activity normalized to renilla reporter activity. *ZD+RA is significantly different than ZA+RA at p<0.05. **ZA and ZD are significantly different than ZA+RA at p<0.001 (one-way ANOVA followed by Newman–Keuls Multiple-comparison post hoc test).
3.4. RXRα protein abundance
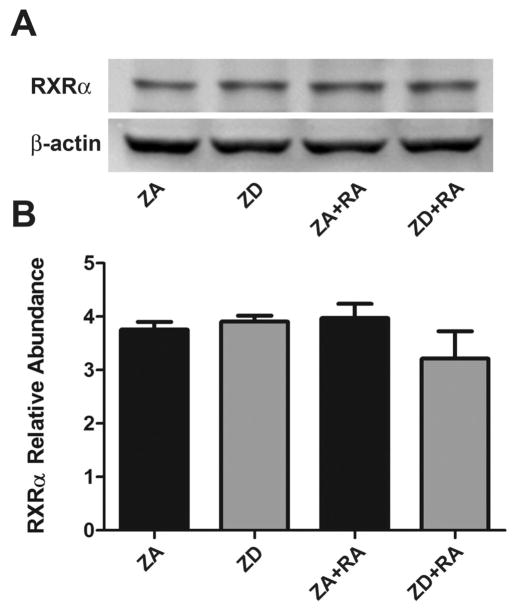
Western blot analysis revealed that RXRα is expressed by NT2 cells treated with or without retinoic acid (Fig. 2A). There were no significant differences in RXRα protein levels across treatment groups. Zinc deficiency and retinoic acid treatment did not alter protein expression levels (Fig. 2B).
Figure 2.
Effect of zinc deficiency on RXRα abundance in retinoic acid-treated NT2 cells. (A) Representative western blot of retinoid X receptor α (RXRα) expression in cell lysates grown in zinc adequate (ZA) or zinc deficient (ZD) media for 4 d and treated with or without retinoic acid (RA) for 24 h. (B) Western blot analysis of relative RXRα abundance in ZA and ZD cells alone and cells in the same conditions treated with RA. Bars represent mean±SEM (n=3). No statistical differences were found between groups.
3.5 RXR target CRABP
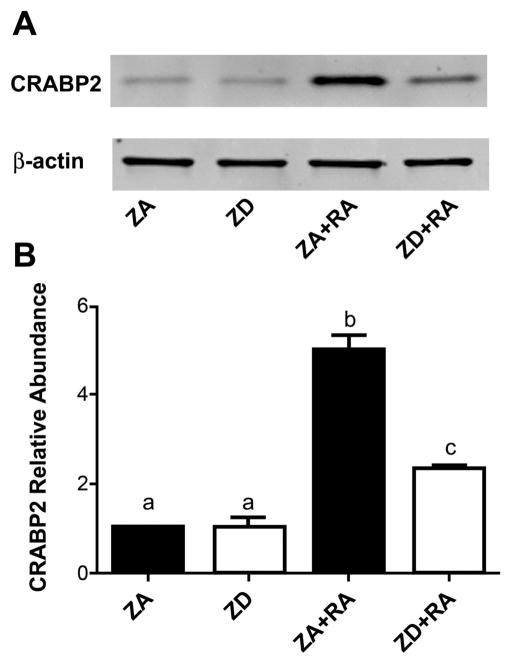
Expression of CRABP2, which has a known RXR binding site in the 5′flanking region of the gene, was low in NT2 cells grown in ZA and ZD media alone (Fig 3A). However, CRABP2 expression was significantly increased (approximately 5-fold) when cells were treated with RA (p<0.001). Zinc deficiency reduced RA-stimulated increases in CRABP2 expression by 53% (p<0.001; Fig 3B).
Figure 3.
Effect of zinc deficiency on a downstream target of the retinoic acid pathway in retinoic acid-treated NT2 cells. (A) Representative western blot of cellular retinoic acid-binding protein 2 (CRABP2) expression in cell lysates grown in zinc adequate (ZA) or zinc deficient (ZD) media for 4 d and treated with or without retinoic acid (RA) for 24 h. (B) Western blot analysis of relative CRABP2 abundance in ZA and ZD cells alone and cells in the same conditions treated with RA. Bars represent mean±SEM (n=3). Bars with different letters are statistically different at p<0.001 except for a and c which are statistically different at p<0.01 (one-way ANOVA followed by Newman Keuls Multiple-comparison post hoc test).
4. Discussion
Previous work in our lab has shown that zinc is essential for neuronal stem cell proliferation and differentiation. Thus, the current work was designed to identify zinc-regulated transcriptional mechanisms responsible for these observations. This includes zinc finger transcription factors and nuclear receptors as well other transcription factors that do not bind zinc. Because our previous work identified the zinc regulation of hippocampal RXRα mRNA, that encodes a nuclear receptor that plays a role in neuronal differentiation,13 we also wanted to explore the effect of zinc restriction on this RXR isoform. Thus, this work directly tested the hypothesis that zinc deficiency represses neuronal differentiation via non-zinc finger and zinc finger transcription factors including RXR mediated gene expression in human neuronal precursor cells.
Reductions in cellular zinc down-regulated the DNA-binding activity of a number of zinc finger transcription factors including COUP-TF, Snail, steroidogenic factor 1, myeloid zinc finger protein, activating transcription factor 2, and thyroid hormone receptor. While future work will be needed to confirm the role of zinc in the binding activity reported here, it is clear that changes in the transcriptional activity of these proteins could lead to significant molecular alterations. For example, the thyroid hormone receptor, which can form a heterodimer with RXR, was the most robustly down-regulated transcription factor. Thyroid hormone is essential for development of the mammalian brain as well as adult brain function. These hormones act via the thyroid hormone nuclear receptor, the most common of which is the TRα1 isoform in the brain.16 Previous work has shown that when the human TRα1 was expressed in a bacterial expression system and was depleted of zinc through chelation, the thyroid hormone receptor was not able to bind the target DNA.17 These findings are consistent with the data reported here in human neuronal precursor cells.
In zinc adequate cells, we showed that the nuclear receptor for RA, RXR, binds to DNA elements and that this binding was impaired by zinc deficiency. To determine if this leads to a functional change in RXR transcriptional activity, we employed a reporter gene assay. The addition of RA to zinc adequate NT2 cells enhanced RXR-stimulated gene expression in the reporter system. Consistent with reduced DNA binding, downstream gene expression was significantly reduced in zinc deficient cells. The decreased ability of RXR to stimulate gene expression occurred in the absence of a decrease in total RXR protein abundance, confirming zinc deficiency-mediated changes in DNA binding activity.
We next wanted to confirm that decreased RXR binding activity and resulting decreases in mRNA results in physiologically meaningful down-regulation of downstream gene expression and changes in retinoic acid-regulated protein expression. To do this we chose a downstream gene target, CRABP2, that is known to be induced by retinoic acid by the binding of RAR/RXR heterodimers on its retinoic acid response element (RARE).18,19 The CRABP2 gene encodes a protein that moves from the nucleus to the cytoplasm after RA binding.20 Consistent with the results from the reporter gene assay, the protein that is encoded by the CRABP2 gene is induced by RA under normal zinc conditions. Protein abundance is significantly reduced in zinc deficient conditions in the presence of RA. This reduction confirms our findings that zinc deficiency reduces RA-mediated gene expression and subsequent protein abundance during neuronal differentiation, likely through the impairment RXR binding to the response element.
In addition to changes in zinc finger transcription factors and nuclear receptors, zinc deficiency resulted in alterations to the activity of a variety of important transcription factors that do not require zinc for DNA-binding. For example, the heat shock response, which is induced under stressful conditions, appears to be induced in zinc deficient NT2 cells. DNA binding of the heat shock transcription factor 1, HSF1, which induces the transcription of heat shock protein expression21 was increased by 3-fold in zinc deficient neuronal precursor cells. Furthermore, SATB1, which is required for neuronal differentiation of at least some sub-types of neurons,22 was reduced to 50% of control by zinc deficiency. Together, these data suggest that zinc regulated neuronal precursors via a variety of transcription factors that include both zinc finger proteins as well as other transcription factors that control cell proliferation and differentiation.
Zinc deficiency did not simply result in a global reduction in the activity of zinc proteins through removal of zinc from the zinc finger complex, because DNA binding of a number of transcription factors including the pregnane x receptor and the housekeeping protein Sp1 were not reduced in zinc deficient NT2 cells. Interestingly, one zinc finger transcription factor, the glucocorticoid receptor, had increased DNA binding activity in zinc deficient cells when compared to zinc adequate control cells. Glucocorticoid receptors, located in both neurons and glial cells, that are activated by glucocorticoids have been shown to inhibit proliferation in neural cells23 and induce cell death in neural progenitors and mature neurons.24
Clearly the question that emerges from this work is the mechanism responsible for the observed regulation of transcriptional activity by zinc. We observed that zinc deficiency impairs the DNA binding activity of a number of transcription factors, while the binding activity of other transcription factors is increased. This is true for both zinc finger proteins and a number of transcription factors that do not use the zinc finger motif. Clearly, increases or decreases in the abundance of a transcription factor would alter its total DNA binding capacity. Additionally, in a zinc finger protein, removal of zinc results in a change in protein conformation that alters DNA binding ability. This mechanism could be independent of protein abundance, as we show for the case of RXR, that has previously been shown to regulate a large variety of downstream gene targets involved in retinoic acid-induced reductions in proliferation and increases in neuronal differentiation including Meis2,25 the homeobox box protein HoxD1,26 Neurogranin27, and insulin-like growth factor binding protein-3 (IGFBP-3).28
This work has shown that the essential nutrient zinc plays a key role in the molecular mechanisms at work in the differentiation of neuronal precursor cells into functional neurons. Reductions in zinc availability impair the ability of RXR to bind DNA response elements, altering the transcription of target genes which would reduce the ability of retinoic acid to promote neuronal differentiation that is needed for both developmental and adult neurogenesis.
Acknowledgments
NIH R01 GM081382 “Development of sensitive fluorescent probes for physiological Zn2+ over large concentration ranges”
The authors would like to thank Charles Badland for his help in creating figures for this manuscript, Dr. Vincent J. Salters at the National High Magnetic Field Laboratory (Tallahassee, FL) for measuring zinc levels in cells, and Shannon Gower-Winter for her technical and editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–8. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 2.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci U S A. 2001;98:4752–7. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–44. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 4.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–73. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kam RK, Deng Y, Chen Y, Zhao H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012;2:11. doi: 10.1186/2045-3701-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:3902–7. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 8.Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl cad Sci U S A. 2003;100:7135–40. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 10.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe SA, Nekludova L, Pabo CO. DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct. 2000;29:183–212. doi: 10.1146/annurev.biophys.29.1.183. [DOI] [PubMed] [Google Scholar]
- 12.Corniola RS, Tassabehji NM, Hare J, Sharma G, Levenson CW. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res. 2008;1237:52–61. doi: 10.1016/j.brainres.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Gower-Winter SD, Corniola RS, Morgan TJ, Jr, Levenson CW. Zinc deficiency regulates hippocampal gene expression and impairs neuronal differentiation. Nutr Neurosci. 2013 doi: 10.1179/1476830512Y.0000000043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao HL, Zheng W, Xin N, Chi ZH, Wang ZY, Chen J, Wang ZY. Zinc deficiency reduces neurogenesis accompanied by neuronal apoptosis through caspase-dependent and -independent signaling pathways. Neurotox Res. 2009;16:416–25. doi: 10.1007/s12640-009-9072-7. [DOI] [PubMed] [Google Scholar]
- 15.Ho E, Courtemanche C, Ames B. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr. 2003;133:2543–8. doi: 10.1093/jn/133.8.2543. [DOI] [PubMed] [Google Scholar]
- 16.Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–59. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto T, Sakurai A, DeGroot LJ. Effects of zinc and other divalent metals on deoxyribonucleic acid binding and hormone-binding activity of human 1 thyroid hormone receptor expressed in Escherichia coli. Endocrinology. 1991;129:3027–33. doi: 10.1210/endo-129-6-3027. [DOI] [PubMed] [Google Scholar]
- 18.Aström A, Pettersson U, Chambon P, Voorhees JJ. Retinoic acid induction of human cellular retinoic acid-binding protein-II gene transcription is mediated by retinoic acid receptor-retinoid X receptor heterodimers bound to one far upstream retinoic acid-responsive element with 5-base pair spacing. J Biol Chem. 1994;269:22334–9. [PubMed] [Google Scholar]
- 19.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 20.Sessler RJ, Noy N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Mol Cell. 2005;18:343–53. doi: 10.1016/j.molcel.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Hong Y, Rogers R, Matunis MJ, Mayhew CN, Goodson ML, Park-Sarge OK, Sarge KD. Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification. J Biol Chem. 2001;276:40263–7. doi: 10.1074/jbc.M104714200. [DOI] [PubMed] [Google Scholar]
- 22.Close J, Xu H, De Marco García N, Batista-Brito R, Rossignol E, Rudy B, Fishell G. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci. 2012;32:17690–705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crochemore C, Michaelidis TM, Fischer D, Loeffler JP, Almeida OF. Enhancement of p53 activity and inhibition of neural cell proliferation by glucocorticoid receptor activation. FASEB J. 2002;16:761–70. doi: 10.1096/fj.01-0577com. [DOI] [PubMed] [Google Scholar]
- 24.Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 2005 Aug;10(8):790–8. doi: 10.1038/sj.mp.4001679. [DOI] [PubMed] [Google Scholar]
- 25.Oulad-Abdelghani M, Chazaud C, Bouillet P, Sapin V, Chambon P, Dollé P. Meis2, a novel mouse Pbx-related homeobox gene induced by retinoic acid during differentiation of P19 embryonal carcinoma cells. Dev Dyn. 1997;210:173–83. doi: 10.1002/(SICI)1097-0177(199710)210:2<173::AID-AJA9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Manohar CF, Salwen HR, Furtado MR, Cohn SL. Up-regulation of HOXC6, HOXD1, and HOXD8 homeobox gene expression in human neuroblastoma cells following chemical induction of differentiation. Tumour Biol. 1996;17:34–47. doi: 10.1159/000217965. [DOI] [PubMed] [Google Scholar]
- 27.Han NL, Wen J, Lin Q, Tan PL, Liou YC, Sheu FS. Proteomics analysis of the expression of neurogranin in murine neuroblastoma (Neuro-2a) cells reveals its involvement for cell differentiation. Int J Biol Sci. 2007;3:263–73. doi: 10.7150/ijbs.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalluri HS, Dempsey RJ. IGFBP-3 inhibits the proliferation of neural progenitor cells. Neurochem Res. 2011;36:406–11. doi: 10.1007/s11064-010-0349-2. [DOI] [PubMed] [Google Scholar]