Abstract
Reaction of a 1:1 mixture of (L)AuCl [L = P(t-Bu)2o-biphenyl or IPr] and AgSbF6 with internal alkynes led to isolation of the corresponding cationic, two-coordinate gold π-alkyne complexes in ≥ 90% yield. Equilibrium binding studies show that the binding affinities of alkynes to gold(I) are strongly affected by the electron density of the alkyne and to a lesser extent on the steric bulk of the alkyne. These substituent effects on alkyne binding affinity are greater than are the differences between the inherent binding affinities of alkynes and alkenes to gold(I).
Keywords: Gold, Alkyne, Pi-complex, Binding affinity
1. Introduction
Over the past ten years, gold(I) complexes of the form (L)AuX [L = phosphine or N-heterocyclic carbene; X = weakly coordinating anionic ligand] have emerged as a soft, carbophilic Lewis acids for the activation of C–C multiple bonds toward nucleophilic addition [1]. These gold(I) complexes have shown particular utility as catalysts for the functionalization of alkynes and typically activate alkynes selectively in the presence of alkenes [1]. Although this selectivity has been attributed to kinetic rather than thermodynamic effects [2,3], little is know regarding the relative binding affinities of alkynes to the cationic, twelve-electron gold(I) fragment (L)Au+ or regarding the binding affinities of alkynes vis-a-vis alkenes toward gold(I). Computational analyses suggest that ethylene binds more strongly to cationic gold(I) than does acetylene [2,4] and Echavarren has shown that gold(I) binds preferentially to the C=C moiety of a terminally unsubstituted 1,6-enyne [2]. Although recent efforts have led to the synthesis of cationic, two-coordinate π-alkyne complexes that contain an N-heterocyclic carbene [3,5,6], a cyclic alkyl amino carbene [7], or a phosphine ligand [8], no information regarding the relative binding affinities of alkynes to gold(I) have emerged [9].
We have recently reported the syntheses and X-ray crystal structures of monomeric, cationic, two-coordinate gold(I) π-alkene complexes that contained the N-heterocyclic carbene ligand IPr [IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidine] or the sterically-hindered phosphine ligand P(t-Bu)2(o-biphenyl) [10–12]. An important component of these studies was the determination of the relative binding affinities of alkenes to the twelve-electron LAu+ [L = IPr, P(t-Bu)2(o-biphenyl)] fragments. These experiments revealed that the binding affinity of alkenes to gold(I) increases dramatically with the increasing electron density of the alkene and is attenuated by steric interactions between the alkene substituents and the (L)Au moiety [10,11]. We therefore considered that a similar approach might be suitable to generate data regarding the binding affinities of alkynes to gold(I). Here we report the synthesis of cationic, two-coordinate gold(I) π-alkyne complexes and an analysis of the binding affinities of substituted alkynes to gold(I). These studies reveal that the binding affinities of alkynes to gold(I) are strongly affected by alkyne substitution and these effects are greater than are the differences between the inherent binding affinities of alkynes and alkenes to gold(I).
2. Results and discussion
2.1. Synthesis of gold π-alkyne complexes
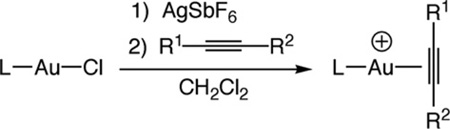
Cationic, two-coordinate gold(I) π-alkyne complexes were isolated employing a procedure analogous to that used to synthesize cationic gold(I) π-alkene complexes [10,11]. As an example, treatment of a methylene chloride suspension of [P(t-Bu)2o-biphenyl] AuCl and AgSbF6 with 3-hexyne (1.5 equiv.) at room temperature for 10 min led to isolation of {[P(t-Bu)2o-bipheny]Au[η2-EtC ≡ CEt]}+ SbF6− (1a) in 98% yield as an air and thermally stable white solid that was characterized by NMR spectroscopy and combustion analysis (Table 1). Complexation of the alkyne to gold in solution was established by spectroscopy. The 13C NMR spectrum of 1a displayed a resonance at δ 91.4 corresponding to the sp carbon atoms of the alkyne ligand, which was shifted downfield relative to that of free 3-hexyne (δ 81.0). Similarly, 1H NMR spectrum of 1a displayed resonances at δ 2.46 (q, J = 7.5 Hz) and 1.21 (t, J = 7.5 Hz) corresponding to the ethyl groups of the 3-hexyne ligand that were shifted downfield relative to those of free 3-hexyne (δ 2.15, 1.11).
Table 1.
Synthesis and alkyne C(sp) 13C NMR chemical shifts of gold π-alkyne complexes 1a–1e and 2a–2c.
 | |||||
|---|---|---|---|---|---|
| entry | L | alkyne | complex | yield (%)a | δ C=C |
| 1 | P(t-Bu)2(o-biphenyl) | 1a | 98 | 91.4 | |
| 2 | P(t-Bu)2(o-biphenyl) | 1b | 96 | 88.7, 75.4 | |
| 3 | P(t-Bu)2(o-biphenyl) | 1c | 90 | 98.3, 87.0 | |
| 4 | P(t-Bu)2(o-biphenyl) | 1d | 91 | 84.9 | |
| 5 | P(t-Bu)2(o-biphenyl) | 1e | (92) | 90.6, 86.0 | |
| 6 | IPr | 2a | 94 | 87.2 | |
| 7 | IPr | 2b | 99 | 86.9, 83.5 | |
| 8 | IPr | 2c | (97) | 81.5 | |
Isolated yield. Values in parentheses refers to 1H NMR yield for in situ generated complexes.
In addition to complex 1a, gold π-alkyne complexes {[P (t-Bu)2(o-biphenyl)]Au[η2-alkyne]}+ SbF6− [alkyne = 2-hexyne (1b), 4,4-dimethyl-2-pentyne (1c), 2-butyne (1d)] and {(IPr)Au [η2-alkyne]}+ SbF6− [alkyne = 3-hexyne (2a), 1-phenylpropyne (2b)] were isolated in ≥ 90% yield and were fully characterized (Table 1). In addition, the π-alkyne complexes {[P(t-Bu)2(o-biphenyl)] Au[η2-PhC≡CMe]}+ SbF6− (1e) and {(IPr)Au[η2-MeC≡CMe]}+ SbF6− (2c) were generated cleanly in solution and were characterized spectroscopically without isolation (Table 1). Worth noting, the 13C NMR chemical shifts of the alkyne sp carbon atoms of the phosphine complexes 1a1dand 1e were, in each case, shifted downfield relative to the alkyne sp carbon resonances of the corresponding IPr complexes 2a–2c. This observation points to greater positive charge on the alkyne carbon atoms of the phosphine complexes relative to the IPr complexes owing to the greater donor properties of the IPr ligand relative to the phosphine, as has been previously noted [13].
2.2. Determination of alkyne binding constants
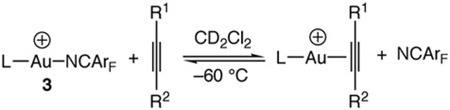
We have previously determined the relative binding affinities of alkenes to the twelve-electron gold fragments [(L)Au+ [L = P (t-Bu)2o-biphenyl, IPr] by measuring the equilibrium constants for displacement of NCArF [NCArF–N≡C-3,5-C6H3(CF3)2] from [LAu (NCArF)+ SbF6− [L = P(t-Bu)2o-biphenyl (3a), IPr (3b)] with alkenes in CD2Cl2 at − 60 °C employing 1H NMR analysis [10,11]. Employing a similar approach, we determined equilibrium constants for displacement of NCArF from 3a with 2-hexyne (Keq = 86 ± 7), 4,4-dimethyl-2-propyne (Keq = 58 ± 4), 2-butyne (Keq = 25 ± 2), and 1-phenylpropyne (Keq = 0.43 ± 0.02) and for displacement of NCArF from 3b with 2-butyne (Keq = 24 ± 1) and 1-phenylpropyne (Keq = 8.6 ± 0.6) (Table 2).
Table 2.
Equilibrium constants for displacement of NCArF from 3a [L = P(t-Bu)2o-biphenyl] or 3b (L = IPr) with alkynes in CD2Cl2 at −60°C.
 | |||
|---|---|---|---|
| entry | L | alkyne | Keq |
| 1 | P(t-Bu)2(o-biphenyl) | 86 ± 7 | |
| 2 | P(t-Bu)2(o-biphenyl) | 58 ± 4 | |
| 3 | P(t-Bu)2(o-biphenyl) | 25 ± 2 | |
| 4 | P(t-Bu)2(o-biphenyl) | 0.43 ± 0.02 | |
| 5 | IPr | 24 ± 1 | |
| 6 | IPr | 8.6 ± 0.6 | |
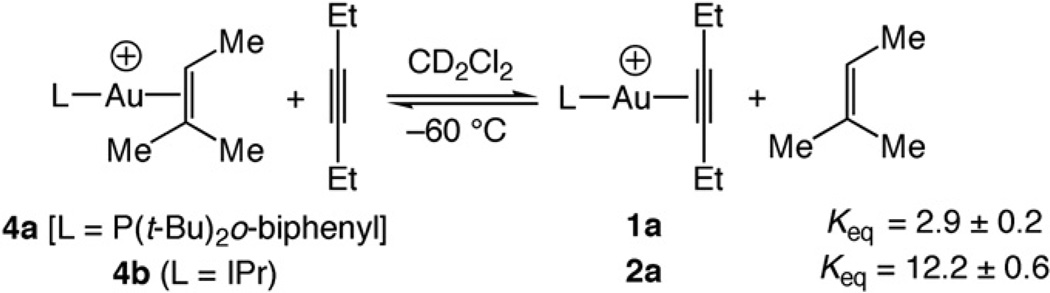
Attempts to determine the equilibrium constants for displacement of NCArF from either 3a or 3b with 3-hexynewere unsuccessful due to the near quantitative displacement of NCArF in each case. To circumvent this problem, we determined the equilibrium constants for displacement of the more tightly binding 2-methyl-2-butene from {(L)Au[η2-Me(H)C=CMe2]}+ SbF6− [L = P(t-Bu)2o-biphenyl (4a), IPr (4b)] with 3-hexyne (Keq=2.9 ± 0.2 for 4a and 12.2 ± 0.6 for 4b) (Scheme 1). From these values and from the equilibrium constants previously determined for the displacement of NCArF from 3a and 3b with 2-methyl-2-butene (Keq = 156 ± 8 for 3a and 64 ± 4 for 3b), we estimate equilibrium constants for the displacement of NCArF from 3a and 3b with 3-hexyne of Keq = 450 ± 39 and 780 ± 62, respectively.
Scheme 1.
Taken together, the equilibrium binding affinities of alkynes to the gold fragment [P(t-Bu)2o-biphenyl]Au+ decrease by a factor of ~1000 in the order 3-hexyne > 2-hexyne > 4,4-dimethyl-2-pentyne > 2-butyne > 1-phenylpropyne. Likewise, the equilibrium binding affinities of alkynes to the gold fragment (IPr)Au+ decrease by a factor of ~90 in the order 3-hexyne > 2-butyne > 1-phenylpropyne. Several points are worth noting. The strong sensitivity of alkyne binding affinity to the electron density of the alkyne is revealed both by the precipitous drop in the binding affinity of 2-hexyne relative to 3-hexyne and in the binding affinity of 1-phenylpropyne relative to 4,4-dimethyl-2-pentyne in the phosphine series. In comparison, the modest decrease in the binding affinity of 4,4-dimethyl-2-pentyne relative to 2-hexyne suggests that steric factors play only a minor role in the determination of alkyne binding affinity. The greater sensitivity of alkyne binding affinity to the electron density of the alkyne in the phosphine series relative to the carbene series is consistent with the greater L→M σ-component of the gold–alkyne binding interaction in the former series relative to the latter [13], which was also suggested by the 13C NMR data (see above). In a similar manner, the binding affinities of alkenes to [P(t-Bu)2o-biphenyl]Au+ were more strongly dependent on alkene electron density than were binding affinities of alkenes to (IPr)Au+ [10,11].
Interestingly, 3-hexyne binds more tightly to the gold(I) fragments (L)Au+ [L=P(t-Bu)2o-biphenyl, IPr] than do any of the alkenes we have investigated. Perhaps the most direct comparison between the inherent binding affinities of alkynes and alkenes to cationic gold(I) is between 2-butyne and cis- and trans-2-butene. The equilibrium constant for displacement of NCArF from 3a with 2-butyne (Keq=25±2) falls between the values determined for displacement of NCArF from 3a with cis-2-butene (Keq = 126 ± 9) and trans-2-butene (Keq = 14.1 ± 0.1). Similarly, the equilibrium constant for displacement of NCArF from 3b with 2-butyne (Keq = 24 ± 1) falls between the values determined for displacement of NCArF from 3b with cis-2-butene (Keq = 38 ± 2) and trans-2-butene (Keq = 12.5 ± 0.5). These data suggest that there is little inherent difference in the binding affinities of alkenes and alkynes to gold(I).
3. Conclusions
We have synthesized and fully characterized a family of cationic, linear gold π-alkyne complexes that contain either the sterically-hindered phosphine P(t-Bu)2(o-biphenyl) ligand or the N-hetero-cyclic carbene ligand IPr. Equilibrium binding studies reveal that the binding affinities of alkynes to gold(I) are strongly affected by the electron density of the alkyne and to a lesser extent on the steric bulk of the alkyne. The effect of the electron density of the alkyne on binding affinity was more pronounced for the phosphine series than for the NHC series. The safest conclusion we can draw from the data we have collected regarding the binding affinities of alkynes and alkenes [10,11] to gold(I) is that the variability in binding affinity as a function of substitution of the alkene or alkyne is greater than is the inherent difference between the binding affinities of alkenes and alkynes to gold(I).
4. Experimental
4.1. General methods
Reactions were performed under a nitrogen atmosphere employing standard Schlenk and glovebox techniques unless specified otherwise. NMR spectra were obtained on a Varian spectrometer operating at 500 MHz for 1H NMR, 101 MHz for 13C NMR, and 202 MHz for 31P NMR in CD2Cl2 at 25 °C unless noted otherwise. IR spectra were obtained on a Nicolet Avatar 360-FT IR spectrometer. Elemental analyses were performed by Complete Analysis Laboratories (Parsippany, NJ). Mass spectra were obtained on an Applied Biosystems Voyager-DE Pro MALDI mass spectrometer operating at a mass range of 500–4000u with a dihydroxyacetophenone matrix (10 mg/1 mL DCM) and was calibrated with PEG1000.
Methylene chloride was purified by passage through columns of activated alumina under nitrogen. CDCl3 and CD2Cl2 were dried over CaH2 and distilled under nitrogen prior to use. (IPr)AuCl, hexanes, AgSbF6, 3-hexyne, 2-butyne, 1-phenylpropyne, 2-hexyne, and 4,4-dimethyl-2-pentyne were purchased from major chemical suppliers and used as received. [P(t-Bu)2o-bipheny]AuCl [14], {[P(t-Bu)2o-bipheny]Au(NCArF)}+ SbF6− (3a) [11], {(IPr)Au(NCArF)}+SbF6− (3b) [10], {[P(t-Bu)2o-bipheny]Au[η2-Me(H)C=CMe2]}+ SbF6− (4a) [11], and {(IPr)Au[η2-Me(H)C=CMe2]}+ SbF6− (4b) [10] were prepared employing published procedures.
4.2. Synthesis of gold(I) π-alkyne complexes
4.2.1. {[P(t-Bu)2o-bipheny]Au[η2-EtC≡CEt]}+ SbF6− (1a)
A suspension of [P(t-Bu)2o-bipheny]AuCl (35 mg, 0.066 mmol), AgSbF6 (22.7 mg, 0.066 mmol), and 3-hexyne (8.2 mg, 0.10 mmol) in CH2Cl2 (1.5 mL) was stirred in a sealed flask at room temperature for 10 min. The resulting suspension was filtered through a pad of Celite, which was flushed with additional CH2Cl2. The filtrate was concentrated to ~2 mL, diluted with two volumes of hexanes, and cooled at 4 °C for 24 h to give 1a (52.5 mg, 98%) as a white solid. 1H NMR: δ 7.95–7.90 (m, 1 H), 7.70–7.60 (m, 2 H), 7.57–7.52 (m, 1 H), 7.48 (t, J = 7.5 Hz, 2 H), 7.35–7.31 (m, 1 H), 7.24–7.20 (m, 2 H), 2.46 (q, J = 7.5 Hz, 4 H),1.45 (d, J = 16 Hz,18 H),1.21 (t, J = 7.5 Hz, 6 H). 13C {1H} NMR: δ 149.1 (d, J = 12.3 Hz), 143.4 (d, J = 6.9 Hz), 134.1, 133.7 (d, J = 7.8 Hz), 132.1 (d, J = 3.1 Hz), 130.0, 129.1, 128.6, 128.3 (d, J = 7.0 Hz), 123.7 (d, J = 49.3 Hz), 91.4, 39.0 (d, J = 24.6 Hz), 31.2 (d, J = 6.2 Hz), 16.4, 14.1. 31P{1H} NMR: δ 65.5. Anal. calcd (found) for C26H37PF6AuSb: H, 4.59 (4.51); C, 38.40 (38.49).
All remaining gold(I) π-alkyne complexes were synthesized employing similar procedures unless noted otherwise.
4.2.2. {[P(t-Bu)2o-bipheny]Au[η2-MeC≡CCH2CH2CH3]}+ SbF6− (1b)
White solid, 96%. 1H NMR: δ 7.95–7.90 (m, 1 H), 7.70–7.60 (m, 2 H), 7.58–7.52 (m, 1 H), 7.49 (t, J = 7.5 Hz, 2 H), 7.36–7.30 (m, 1 H), 7.23 (d, J = 7.0 Hz, 2 H), 2.37 (qt, J = 2.5, 7.0 Hz, 2 H), 2.16 (t, J = 2.0 Hz, 3 H), 1.61 (sextet, J = 7.5 Hz, 2 H), 1.45 (d, J = 16.5 Hz, 18 H), 1.00 (t, J = 7.5 Hz, 3 H). 13C{1H} NMR: δ 149.8 (d, J = 12.8 Hz), 143.1 (d, J = 6.7 Hz), 133.8, 133.3 (d, J = 7.7 Hz), 131.8, 129.6, 128.7, 128.3, 128.0 (d, J = 7.7 Hz), 123.4 (d, J = 48.0 Hz), 88.7 (d, J = 7.7 Hz), 86.3 (d, J = 6.7 Hz), 38.5 (d, J = 25.1 Hz), 30.8 (d, J = 6.8 Hz), 24.0, 22.4, 13.2, 7.2. 31P{1H} NMR: δ 65.3. Anal. calcd (found) for C26H37PF6AuSb: H, 4.59 (4.36); C, 38.40 (38.35).
4.2.3. {[P(t-Bu)2o-bipheny]Au[η2-MeC≡CC(CH3)3]}+ SbF6− (1c)
White solid, 90%. 1H NMR: δ 7.96–7.90 (m, 1 H), 7.70–7.60 (m, 2 H), 7.59–7.52 (m, 1 H), 7.49 (t, J = 7.5 Hz, 2 H), 7.35–7.28 (m, 1 H), 7.22 (d, J = 7.5 Hz, 2 H), 2.19 (s, 3 H), 1.47 (d, J = 16.5 Hz, 18 H), 1.27 (s, 9 H). 13C{1H} NMR: δ 149.2 (d, J = 12.6 Hz), 143.4 (d, J = 6.8 Hz), 134.2, 133.9 (d, J = 7.7 Hz), 132.2, 130.0, 129.4, 128.5, 128.3 (d, J = 7.7 Hz), 123.5 (d, J = 47.9 Hz), 98.3, 87.0, 39.2 (d, J = 24.9 Hz), 31.9, 31.2 (d, J = 5.8 Hz), 8.4. 31P{1H} NMR: δ 65.9. Anal. calcd (found) for C27H39PF6AuSb: H, 4.75 (4.66); C, 39.20 (39.14).
4.2.4. {[P(t-Bu)2o-bipheny]Au[η2-MeC≡CMe]}+ SbF6− (1d)
White solid, 91%. 1H NMR: δ 8.00–7.84 (m, 1 H), 7.72–7.44 (m, 5 H), 7.38–7.30 (m, 1 H), 7.30–7.16 (m, 2 H), 2.11 (s, 6 H), 1.44 (d, J = 16.5 Hz, 18 H). 13C{1H} NMR: δ 149.1 (d, J = 13.0 Hz), 143.5 (d, J = 7.0 Hz), 134.0, 133.6 (d, J = 6.9 Hz), 132.1, 129.9, 128.9, 128.7, 128.3 (d, J = 7.8 Hz),123.8 (d, J = 48.6 Hz), 84.9, 39.7 (d, J = 24.6 Hz), 31.2 (d, J = 6.2 Hz), 7.4. 31P{1H} NMR: δ 65.2. Anal. calcd (found) for C24H33PF6AuSb: H, 4.24 (4.23); C, 36.71 (36.62).
4.2.5. {[P(t-Bu)2o-bipheny]Au[η2-PhC≡CMe]}+ SbF6− (1e)
An NMR tube containing a suspension of [P(t-Bu)2o-bipheny] AuCl (30 mg, 0.057 mmol), AgSbF6 (19.4 mg, 0.057 mmol), 2-butyne (6.6 mg, 0.057 mmol), and 1,3-dimethoxybenzene (1.4 mg, internal standard) in CD2Cl2 (0.5 mL) was shaken briefly and allowed to stand at room temperature. Following precipitation of AgCl, the sample was analyzed by 1H and 13C NMR spectroscopy at 25 °C, which revealed formation of 1e in 92 ± 5% yield. 1H NMR: δ 7.93–7.81 (m, 1H), 7.70–7.50 (m, 4H), 7.46 (t, J = 7.5 Hz, 2H), 7.43–7.32 (m, 4H), 7.32–7.24 (m, 1H), 7.16 (d, J = 7.0 Hz, 2H), 2.35 (s, 3H), 1.34 (d, J = 16.5 Hz, 18H), 1.00. 13C{1H} NMR: δ 147.9 (d, J = 12.5 Hz), 142.9 (d, J = 6.7 Hz), 133.4, 132.7 (d, J = 7.2 Hz), 132.1, 131.4, 131.1, 129.2, 129.0, 128.2, 127.8 (d, J = 7.7 Hz), 127.1, 122.6 (d, J = 49.6 Hz), 117.9, 90.6 (d, J = 8.6 Hz), 86.0 (d, J = 7.2 Hz), 37.8 (d, J = 24.9 Hz), 30.3 (d, J = 5.8 Hz), 8.4. 31P{1H} NMR: δ 65.7.
4.2.6. {(IPr)Au(η2-EtC≡CEt)}+ SbF6− (2a)
White solid, 94%. 1H NMR (400 MHz, CDCl3): δ 7.52 (t, J = 8 Hz, 2H), 7.49 (s, 2 H), 7.30 (d, J = 7.6 Hz, 4H), 2.48 (sept, J = 7.2 Hz, 4H), 2.20 (q, J = 7.6 Hz, 4H), 1.23 (d, J = 6.8 Hz, 24H), 0.57 (t, J = 7.6 Hz, 6H). 13C{1H} NMR (CDCl3): δ 177.2, 145.7, 133.0, 131.4, 125.1, 124.5, 87.2, 28.8, 24.6, 23.9, 14.7, 13.1. Anal. calcd (found) for C33H46AuF6N2Sb: H, 5.13 (5.05); C, 43.87 (43.76); N, 3.10 (3.37).
4.2.7. {(IPr)Au(η2-PhC≡CMe)}+ SbF6− (2b)
Pale green solid, 99%. 1H NMR (CDCl3): δ 7.58 (t, J = 8 Hz, 2H), 7.51 (s, 2 H), 7.36 (d, J = 8.0 Hz, 1H), 7.31 (d, J = 8 Hz, 4H), 7.15 (t, J = 7.5 Hz, 2H), 6.81 (d, J = 7.5 Hz, 2H), 2.45 (sept, J = 7 Hz, 4 H), 2.05 (s, 3 H), 1.22 (d, J = 7 Hz, 12 H), 1.14 (d, J = 7 Hz, 12 H). 13C{1H} NMR (CDCl3): δ 175.7, 145.8, 132.9, 131.8, 131.4, 129.1, 125.1, 124.5, 117.1, 86.9, 83.5, 28.7, 24.5, 23.9, 6.6. MALDI-MS calcd (found) for [C36H44N2Au+ (M+): 701.3 (700.9).
4.2.8. {(IPr)Au(η2-MeC≡CMe)}+ SbF6− (2c)
An NMR tube containing a suspension of (IPr)AuCl (35 mg, 0.066 mmol), AgSbF6 (22.7 mg, 0.066 mmol), 2-butyne (3.6 mg, 0.066 mmol), and toluene (1.2 mg, internal standard) in CD2Cl2 (0.5 mL) was shaken briefly and allowed to stand at room temperature. Following precipitation of AgCl, the sample was analyzed by 1H and 13C NMR spectroscopy at 25 °C, which revealed formation of 2c in 97 ± 5% yield. 1H NMR (300 MHz): δ 7.60 (t, J = 8.1 Hz, 2H), 7.48 (s, 2H), 7.39 (d, J = 7.8 Hz, 4H), 2.51 (septet, J = 6.9 Hz, 4H), 1.88 (s, 6H), 1.29 (d, J = 6.9 Hz, 12H), 1.27 (d, J = 6.9 Hz, 12H). 13C{1H} NMR: δ 177.1, 146.1, 133.3, 131.8, 125.2, 124.9, 81.5, 29.2, 24.8, 24.1, 6.1.
4.3. Determination of alkyne binding constants
4.3.1. Reaction of 2-butyne with {[P(t-Bu)2o-bipheny]Au(NCArF)}+SbF6− (3a)
2-Butyne (1.03 mg, 0.019 mmol) was added via gas tight syringe to an NMR tube sealed with a rubber septum that contained a CD2Cl2 solution of 3a (20 mg, 0.021 mmol) at −60°C. The tube was shaken, placed in the probe of an NMR spectrometer cooled at −60°C and allowed to equilibrate for 10 min. The relative concentrations of 3a, 1d, NCArFand 2-butyne were determined by integrating the resonances corresponding to the aromatic protons of bound [δ 8.41, 8.38 (2:1)] and free [δ 8.15, 8.14 (2:1)] NCArF and the resonances corresponding to the methyl protons of bound (δ 2.06) and free (δ 1.70) 2-butyne. An equilibrium constant of Keq = [1d][NCArF]/[3a][2-butyne] = 25 ± 2 was determined (Table 2).
To ensure that equilibrium was achieved under these conditions, the following control experiment was performed. NCArF (4.9 mg, 0.02 mmol) was added via syringe to an NMR tube that contained a CD2Cl2 solution of 2-butyne complex 1d (16 mg, 0.02 mmol) at − 60 °C. The tube was shaken, placed in the probe of an NMR spectrometer cooled at −60 °C and allowed to equilibrate for 10 min. The relative concentrations of 3a, 1d, NCArFand 2-butyne were determined as was described in the preceding paragraph. The equilibrium constant determined from this experiment {Keq = [1d] [NCArF]/[3a][2-butyne] = 24 ± 1} was not significantly different from that obtained from treatment of 3a with 2-butyne.
Similar procedures were employed to determine the equilibrium constants for the displacement of NCArF from 3a with 2-hexyne, 4,4-dimethyl-2-pentyne, and 1-phenylpropyne and for displacement of NCArF from 3b with 2-butyne and 1-phenylpropyne. Error limits refer to the standard deviation in Keq determined from three independent experiments.
4.3.2. Reaction of 3-hexyne with {[P(t-Bu)2o-biphenyl]Au[η2-Me(H) C=CMe2]}+ SbF6− (4a)
3-Hexyne (1.6 mg, 0.02 mmol)was added via gas tight syringe to an NMR tube sealed with a rubber septum that contained a CD2Cl2 solution of 4a (16 mg, 0.02 mmol) at −60°C. The tube was shaken, placed in the probe of an NMR spectrometer cooled at −60°C and allowed to equilibrate for 10 min. The relative concentrations of 4a, 1a, 2-methyl-2-butene, and 3-hexyne were determined by integrating the resonances corresponding to the olefinic proton of bound (δ 3.99) and free (δ 5.12) 2-methyl-2-butene and the resonances corresponding to the methylene protons of bound (δ 2.40) and free (δ 2.10) 3-hexyne. An equilibrium constant of Keq = [1a][2-methyl-2-butene]/[4a][3-hexyne] = 2.9 ± 0.2 was determined (Scheme 1). Multiplication of this result by the equilibrium constant for displxacement of NCArF from 3a with 2-methyl-2-butene (Keq = 156 ± 8) [10] provided an equilibrium constant for the displacement of NCArF from 3a with 3-hexyne of Keq = [1a] [NCArF]/[3a][3-hexyne] = 450 ± 45.
A similar procedure was employed to determine the equilibrium constant for the displacement of 2-methyl-2-butene from 4b with 3-hexyne (Keq = 12.2 ± 0.6) and to calculate the equilibrium constant for the displacement of NCArF from 3b with 3-hexyne (Keq = 780 ± 62). Error limits for the reactions of 3-hexyne with complexes 4a and 4b refer to the standard deviation in Keq determined from three independent experiments. Error limits for equilibrium binding affinities of 3-hexyne relative to NCArF were estimated from propagation of the errors of the associated Keq measurements.
Acknowledgements
The authors thank the NSF (CHE-0555425) for support of this research and the NCBC (2008-IDG-1010) for support of the Duke University NMR facility. TJB thanks Duke University for a Zeilik Fellowship.
References
- 1.a Sengupta S, Shi X. Chem Cat Chem. 2010;2:609–619. [Google Scholar]; b Li Z, Brouwer C, He C. Chem. Rev. 2008;108:3239–3265. doi: 10.1021/cr068434l. [DOI] [PubMed] [Google Scholar]; c Widenhoefer RA. Chem. Eur. J. 2008;14:5382–5391. doi: 10.1002/chem.200800219. [DOI] [PubMed] [Google Scholar]; d Gorin DJ, Sherry BD, Toste FD. Chem. Rev. 2008;108:3351–3378. doi: 10.1021/cr068430g. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Arcadi A. Chem. Rev. 2008;108:3266–3325. doi: 10.1021/cr068435d. [DOI] [PubMed] [Google Scholar]; f Jiménez-Núñez E, Echavarren AM. Chem. Rev. . 2008;108:3326–3350. doi: 10.1021/cr0684319. [DOI] [PubMed] [Google Scholar]; g Bongers N, Krause N. Angew. Chem. Int. Ed. 2008;47:2178–2181. doi: 10.1002/anie.200704729. [DOI] [PubMed] [Google Scholar]
- 2.Garcoa-Mota M, Cabello N, Maseras F, Echavarren AM, Perez-Ramirez J, Lopez N. Chem Phys Chem. 2008;9:1624–1629. doi: 10.1002/cphc.200800246. [DOI] [PubMed] [Google Scholar]
- 3.Flügge S, Anoop A, Goddard R, Thiel W, Fürstner A. Chem. Eur. J. 2009;15:8558–8565. doi: 10.1002/chem.200901062. [DOI] [PubMed] [Google Scholar]
- 4.a Nechaev MS, Rayon VM, Frenking G. J. Phys. Chem. A. 2004;108:3134–3142. [Google Scholar]; b Hertwig RH, Koch W, Schröder D, Schwarz H, Hrusak J, Schwerdtfeger P. J. Phys. Chem. 1996;100:12253–12260. [Google Scholar]; c Ziegler T, Rauk A. Inorg. Chem. 1979;18:1558–1565. [Google Scholar]; d Salvi N, Belpassi L, Tarantelli F. Chem. Eur. J. 2010;16:7231–7240. doi: 10.1002/chem.201000608. [DOI] [PubMed] [Google Scholar]
- 5.Akana JA, Bhattacharyya KX, Müller P, Sadighi JP. J. Am. Chem. Soc. 2007;129:7736–7737. doi: 10.1021/ja0723784. [DOI] [PubMed] [Google Scholar]
- 6.For reviews on gold N-heterocyclic carbene complexes see: Marion N, Nolan SP. Chem. Soc. Rev. 2008;37:1776–1782. doi: 10.1039/b711132k. Díez-González S, Marion N, Nolan SP. Chem. Rev. 2009;109:3612–3676. doi: 10.1021/cr900074m.
- 7.a Lavallo V, Frey GD, Kousar S, Donnadieu B, Bertrand G. Proc. Natl. Acad. Sci. USA. 2007;104:13569–13573. doi: 10.1073/pnas.0705809104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lavallo V, Frey GD, Donnadieu B, Soleilhavoup M, Bertrand G. Angew. Chem. Int. Ed. 2008;47:5224–5228. doi: 10.1002/anie.200801136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Hooper TN, Green M, Russell CA. Chem. Commun. 2010:2313–2315. doi: 10.1039/b923900f. [DOI] [PubMed] [Google Scholar]; b Zuccaccia D, Belpassi L, Rocchigiani L, Tarantelli F, Macchioni A. Inorg. Chem. 2010;49:3080–3082. doi: 10.1021/ic100093n. [DOI] [PubMed] [Google Scholar]; c Shapiro ND, Toste FD. Proc. Natl. Acad. Sci. USA. 2008;105:2779–2782. [Google Scholar]
- 9.For examples of neutral two-coordinate and three-coordinate gold(I) π-palkyne complexes see: Wu J, Kroll P, Dias HVR. Inorg. Chem. 2009;48:423–425. doi: 10.1021/ic8020854. Dias HVR, Flores JA, Wu J, Kroll P. P.J. Am, Chem. Soc. 2009;131:11249–11255. doi: 10.1021/ja904232v. Schulte P, Behrens U. Chem. Commun. 1998:1633–1634. Köhler K, Silverio SJ, Hyla-Kryspin I, Gleiter R, Zsolnai L, Driess A, Huttner G, Lang H. Organometallics. 1997;16:4970–4979. Lang H, Köhler K, Zsolnai L. Chem. Commun. 1996:2043–2044.
- 10.Brown TJ, Dickens MG, Widenhoefer RA. J. Am. Chem. Soc. 2009;131:6350–6351. doi: 10.1021/ja9015827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown TJ, Dickens MG, Widenhoefer RA. Chem. Commun. 2009:6451–6453. doi: 10.1039/b914632f. [DOI] [PubMed] [Google Scholar]
- 12.For additional examples of cationic, two-coordinate gold(I) π-alkene complexes see: Schmidbaur H, Schier HA. Organometallics. 2010;29:2–23. de Frémont P, Marion N, Nolan SP. J. Organomet. Chem. 2009;694:551–560. Hooper TN, Green M, Mcgrady JE, Patel JR, Russell CA. Chem. Commun. 2009:3877–3879. doi: 10.1039/b908109g. Zuccaccia D, Belpassi L, Tarantelli F, Macchioni A. J. Am. Chem. Soc. 2009;131:3170–3171. doi: 10.1021/ja809998y.
- 13.a Scott NM, Clavier H, Mahjoor P, Stevens ED, Nolan SP. Organometallics. 2008;27:3181–3186. [Google Scholar]; b Scott NM, Nolan SP. Eur. J. Inorg. Chem. 2005:1815–1828. [Google Scholar]; c Jafarpour L, Stevens ED, Nolan SP. J. Organomet. Chem. 2000;606:49–54. [Google Scholar]
- 14.Nieto-Oberhuber C, Lopez S, Echavarren AM. J. Am. Chem. Soc. 2005;127:6178–6179. doi: 10.1021/ja042257t. [DOI] [PubMed] [Google Scholar]