Abstract
Monoclonal antibodies (mAbs) against tumor-associated antigens are useful anticancer agents. Antibody-dependent cellular cytotoxicity (ADCC) is one of the major mechanisms responsible for initiating natural killer cell (NK)-mediated killing of tumors. However, the regulation of ADCC via NK cells is poorly understood. We have investigated the cytolytic activity of NK cells against pancreatic cancer cells that were coated with an antibody directed against the human tumor antigen, Mucin-1 designated HMFG-2, either alone or conjugated to CpG oligodeoxynucleotide (CpG ODN). Conjugated antibodies were tested for their ability to elicit ADCC in vitro and in vivo against pancreatic cancer cells. NK cells cultured in the presence of immobilized CpG ODN, HMFG-2 Ab, or CpG ODN-conjugated HMFG-2 Ab were able to up-regulate perforin similarly. Interestingly, a significant higher ADCC was observed when CpG ODN-conjugated HMFG-2-coated tumor cells were co-cultured with NK cells compared to unconjugated HMFG-2 Ab or CpG ODN alone. Moreover, MyD88-deficient NK cells can perform ADCC in vitro. Furthermore, intratumoral injections of CpG ODN-conjugated HMFG-2 induced a significant reduction in tumor burden in vivo in an established model of pancreatic tumor in nude mice compared to CpG ODN or the HMFG-2 alone. Depletion of macrophages or NK cells before treatment confirmed that both cells were required for the anti-tumor response in vivo. Results also suggest that CpG ODN and HMFG-2 Ab could be sensed by NK cells on the mAb-coated tumor cells triggering enhanced ADCC in vitro and in vivo.
Keywords: Immunoconjugates, Cancer vaccines, Immune response to cancer, Immunotherapy, Pancreatic cancer
Introduction
Monoclonal antibodies (mAbs) have been proven to be effective treatments for many malignant diseases due to their ability to initiate tumor antigen-specific immune responses, by promoting mAb-targeted cross-presentation of tumor antigens, triggering the idiotypic network, or antibody-dependent cellular cytotoxicity (ADCC) [1].
Antibody-dependent cellular cytotoxicity is a relevant tumoricidal mechanism that can occur when Fc receptors on effector cells bind Fc domains of Abs which simultaneously engage antigens on target cells through their Fab domains [2]. The strongest clinical evidence for an Fc receptor-mediated antitumor mechanism comes from the therapeutic use of humanized anti-CD20 mAb (rituximab) in patients with follicular lymphoma [3, 4]. Moreover, studies have shown that ADCC can be regulated by different types of Fc receptors on different cell populations, modulated by various cytokines and/or by engineered glycoforms of IgG1 [5–12].
Stimulation of immune-effector cells that mediate ADCC has been another strategy to improve the efficacy of therapeutic mAbs. Natural killer (NK) cells are key effector cells of the innate immune response against viruses and tumors [13]. ADCC is mediated through cross linking of the Fc gamma receptor IIIa (CD16) that is constitutively expressed on NK cells and appears to play a central role in NK cell activation with production of IFN-γ [14]. In addition, other cytokines such as interleukin-15 (IL-15) have been shown to stimulate NK cell proliferation, ADCC, and NK cell-macrophage interaction [15, 16].
Natural killer (NK) cells can also be activated by synthetic molecules such as the oligodeoxynucleotides (ODN) that contain unmethylated CpG dinucleotide motifs, similar to bacterial DNA. It does so by binding toll-like receptor (TLR) 9 intracellularly [17]. Three classes of CpGs have been identified: type A (CpG-A) is the most potent in inducing NK cell activity and the secretion of type I IFN; type B (CpG-B), a potent stimulator of B cells, is also shown to induce NK cell activity in vivo [18]; and type C (CpG-C) combines the characteristics of CpG-A and CpG-B [19]. More importantly, it has been shown by Roda et al. [20] that CpG ODN, acting through TLR9, could enhance cytokine production by NK cells in response to antibody-coated tumor cells. Thus, NK cells may contribute to the immunomodulatory effects of TLR9 agonist, which have been safely administered in healthy volunteers and patients with lymphoma [21, 22].
Despite accumulating evidence in support of several TLRs expressed on NK cells and an indirect effect of TLR agonists and ligands (including CpG) on NK cell stimulation [23], there is limited evidence of a direct effect of CpG on NK cell activation. In this study, we show evidence that CpG ODN conjugated to a specific IgG1 antibody against a tumor-associated antigen, Mucin-1 (MUC1), can trigger an enhanced ADCC response against antibody-coated tumor cells in vitro in a TLR9-independent fashion. We also show that antibody-conjugated CpG was effective in controlling tumor growth in vivo in nude mice. The anti-tumor effect was not only dependent on NK cells but also on the presence of macrophages. These findings suggest a pivotal role for NK cells during the initiation of an antitumor immune response mediated by therapeutic antibodies, and a potential approach to enhance ADCC by non-systemic delivery of CpG ODN as an adjuvant.
Materials and methods
Mice
Female age-matched nude (nu/nu Foxn1 nu) and C57BL/6 mice were purchased from Charles River Breeding Laboratory (Andover, MA, USA). All mice were maintained and treated in accordance with institutional guidelines for animal experimentation and American Association of Laboratory Animal Care regulations (AALAC). MyD88−/− mice were received from University of Alabama Birmingham [24].
Cell line
KCM pancreatic cell line was derived from a C57BL/6 mouse that developed spontaneous pancreatic ductal adenocarcinoma (PDA) that was crossed to human MUC1.Tg mice [25–28]. KCM cells constitutively express high levels of human MUC1. KCM cells were tested for mycoplasma by Charles River and maintained in DMEM medium supplemented with 10 % heat-inactivated FBS (Hyclone Lab.), 1 % glutamine, and 1 % penicillin–streptomycin.
Reagents: anti-NK1.1-PE, anti-CD3-FITC, and anti-mouse IgG-PE antibodies were purchased from BD Pharmingen (San Diego, CA, USA). IL-2 cytokine was ordered from Peprotech (Rocky Hill, NJ, USA). CpG ODN type B 1826 and FITC–CpG ODN were obtained from Trilink (San Diego, CA, USA). Anti-perforin-PE was purchased from eBiosciences (San Diego, CA, USA). Mouse anti-human MUC1 (HMFG-2, a murine mAb) was gifted by Dr. Taylor-Papadimitriou [29]. Chloroquine, pp2 (inhibitors SFK), and pp3 (pp2 control) were obtained from Calbiochem (San Diego, CA, USA). DMSO was purchased from Sigma (St. Louis, MO, USA).
In vivo tumor experiments
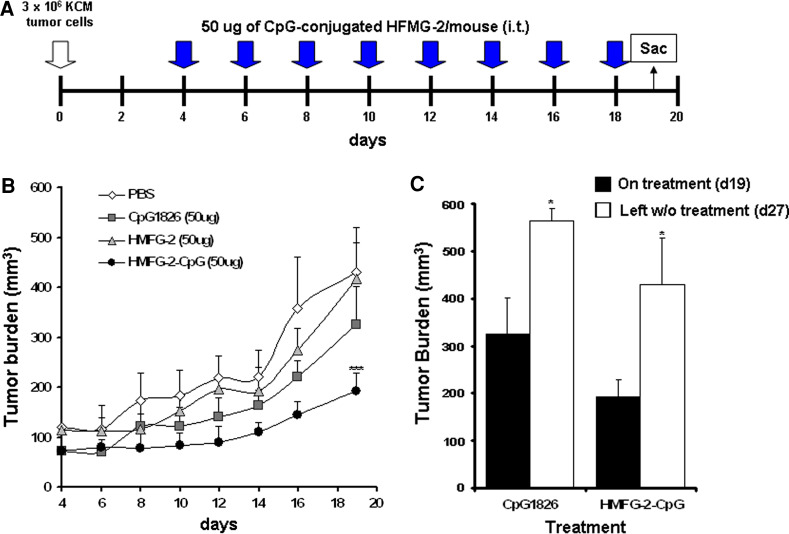
Nude mice were inoculated s.c. with 3 × 106 KCM tumor cells in the hind flank of mice. HMFG-2 mAb (50 µg/dose), CpG-HMFG-2 mAb (50 µg/dose), CpG ODN 1826 (50 µg/dose) treatments were commenced when tumors developed to a size of 100–150 mm3. Tumor sizes were measured with digital caliper every other day, and volumes were calculated using L × W2 × 0.5 formula. Working preparations were diluted in PBS, and mice received intratumoral (i.t.) injections on days indicated in each experiment (Figs. 5, 6). Control mice received an equal volume of PBS. The difference in tumor size between control and treatment groups was statistically analyzed using Student’s t test.
Fig. 5.
CpG ODN-conjugated HMFG-2 mAb treatment in vivo induced a significant tumor growth delay in immunodeficient nu/nu mice. a Schematic representation of the treatment regimen. Nude mice (nu/nu) were implanted s.c. with 3 × 106 KCM cells on day 0. On day 4, groups of mice (n = 4 mice/group) were treated i.t. with CpG ODN (50 µg), HMFG-2 mAb (50 µg), CpG ODN-conjugated HMFG-2 mAb, and PBS (50 µl). Each group of mice was treated every 48 h. Mice were randomized at day 4. b Tumor volume was measured with digital caliper every 2 days for 20 days and tumor growth is shown. Data shown are the mean ± SE of two experiments (***p < 0.001 compared to CpG ODN HMFG-2 mAb-treated mice vs. all other groups). c Tumor burden measured after treatment was suspended (n = 4 mice/group). Tumor burden increased in both the CpG ODN and CpG ODN-HMFG-2 mAb groups (*p = 0.038 and p = 0.027, respectively)
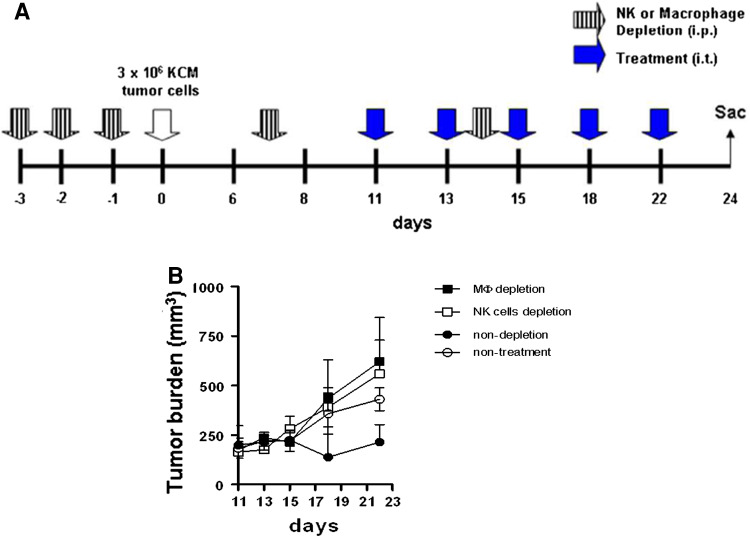
Fig. 6.
Enhanced ADCC triggered in vivo by CpG ODN-conjugated HMFG-2 mAb is mutually dependent on NK cells and macrophages. a Schematic representation of the treatment regimen (n = 4). Depletion of NK cells (anti-asialo GM1) or macrophages (carrageenan) was initiated 3 days prior to KCM tumor challenge. Depletion was carried out for 3 consecutive days prior to tumor challenge and then every 7 days to maintain depletion. Ctrl mice received i.p. injection of IgG isotype Ctrl antibody. At day 9 post tumor challenge, mice were treated with CpG ODN conjugated with HMFG-2 mAbs every 48 h. All mice were killed at day 24. b Tumor volume was measured with digital caliper every 2 days and resulting tumor burden is shown. Data shown are the mean ± SE of two experiments. Significantly lower tumor burden (***p < 0.001) in the non-depleted macrophages/NK cells group compared to macrophages and/or NK cells depleted groups
In vivo depletion
For NK cell depletion, 200 µl of rabbit anti-asialo GM1 polyclonal Ab (Wako Chemicals, Richmond, VA, USA) was injected i.p. 3 times/week before injection of tumor cells and every 7 days afterward. The control group was injected with 200 µl normal rabbit serum (Sigma-Aldrich, St. Louis, MO, USA). NK cell depletion was confirmed by FACS analysis (>95 %). Macrophage inactivation was induced via i.p. injection of 200 µl (2 mg/ml) carrageenan iota type (Sigma–Aldrich, St. Louis, MO, USA) for three consecutives days before tumor cell injection and every 7 days afterward [30].
Murine NK cells and LAK isolation
Splenocytes from C57BL/6 mice were grown in complete DMEM with 20 ng/ml human IL-2 (Peprotech, Rocky Hill, NJ, USA) for 3–4 days. Non-adherent cells were discarded, and adherent cells were cultured in 20 ng/ml human IL-2 for another 3–4 days. NK cells were harvested by negative selection, as described by manufacturer’s instructions (Miltenyi Biotec Kit). Isolated NK cells were routinely >95 % CD3− NK1.1+ by FACS analysis.
ADCC assay
Purified NK cells from wild type and MyD88−/− were plated in 96-well U-bottom plates in complete DMEM as the effector cells. The target cells were labeled with Na51CrO4 (100 µCi/106 cells; Perkin Elmer) for 1–2 h at 37 °C. Target cells were opsonized with different antibodies for 30 min at 37 °C. Subsequently, effector cells were plated at various E:T ratios and incubated for 8 h at 37 °C. Supernatants were harvested for quantification of chromium release. In TLR9/Src family kinases (SFK) inhibition experiments, NK cells were pre-treated with chloroquine (10 µM), pp2 (10 µM), pp3 (10 µM), and DMSO (0.002 %) for 15 min at 37 °C and washed 3 times before being plated. Chromium release was determined using TopCounter NT (Perkin Elmer). Percent lysis was calculated as 100 × [(release by NK − spontaneous release)/(maximal release − spontaneous release)]. Maximal release was determined by the addition of 1 % triton X-100.
Complement-dependent cytotoxicity (CDC) assay
Target cells (3 × 104 cells/well) were incubated in triplicate in 96-well plates in 100 μl DMEM with or without antibodies (10 μg/ml). After 20 min of incubation at RT, 100 µl DMEM with 30 % murine serum was added to a final volume of 200 μl. Positive control was obtained by TNP derivatization of KCM cells [31] and anti-DNP antibody from Abcam (Cambridge, MA, USA). After 1–2 h of incubation at 37 °C, the numbers of viable (PI−) cells were analyzed by flow cytometry. Viability was calculated as the percentage of PI− viable cells.
Proliferation and apoptosis assay
Target cells (8 × 103 cells/well) were incubated overnight in triplicate in 96-well plates in 100 μl DMEM with or without antibodies (15 μg/ml). Cell proliferation was measured by their ability to reduce tetrazolium products using the Celltiter-96® (Promega, Madison WI, USA) according the manufacturer’s instruction. Target cells (0.5 × 106 cells/well) were incubated in triplicate in 6-well plates overnight with or without antibodies (15 μg/ml). Apoptosis was determined via Annexin-V/7AAD cell staining following BD Biosciences kit instructions (San Jose, CA, USA).
Immobilized ODN and/or antibodies
Ten µg/ml ODN and/or antibodies were immobilized in 96-well flat microplates by overnight incubation in immobilization buffer (10 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA) followed by five washes with saline to remove unbound ODN, as described [32]. Effector cells were cultured overnight in triplicate for each condition. Brefeldin A (BD Golgiplug™) was added 6 h prior to the endpoint to enhance detection by FACS. Perforin levels were determined by intracellular staining (BD Biosciences, San Jose, CA, USA) following manufacturer’s instructions. FITC–CpG ODN internalization experiments were captured with Olympus IX70 inverted fluorescent microscope and analyzed with FACSCalibur flow cytometer.
Construction of CpG-conjugated anti-MUC1 mAb
5′-Amino-modified CpG ODN was subsequently modified to incorporate a 4FB-moiety by treatment with S-4FB. Anti-MUC1 antibody was modified to incorporate HyNic moieties on lysine residues using S-HyNic (Solulink Biosciences, San Diego, CA, USA). Synthesis of the conjugate was accomplished by mixing the 4FB-modified CpG oligo with the HyNic-modified anti-MUC1 antibody followed by purification by size exclusion chromatography × (Superdex 200, GE HealthCare). Purity of the conjugated antibody was confirmed by running the sample in an SDS-PAGE gel.
Statistical analysis
Statistical significance was determined by Student’s t test.
Results
CpG ODN-conjugated anti-MUC1 mAb retains its affinity for MUC1
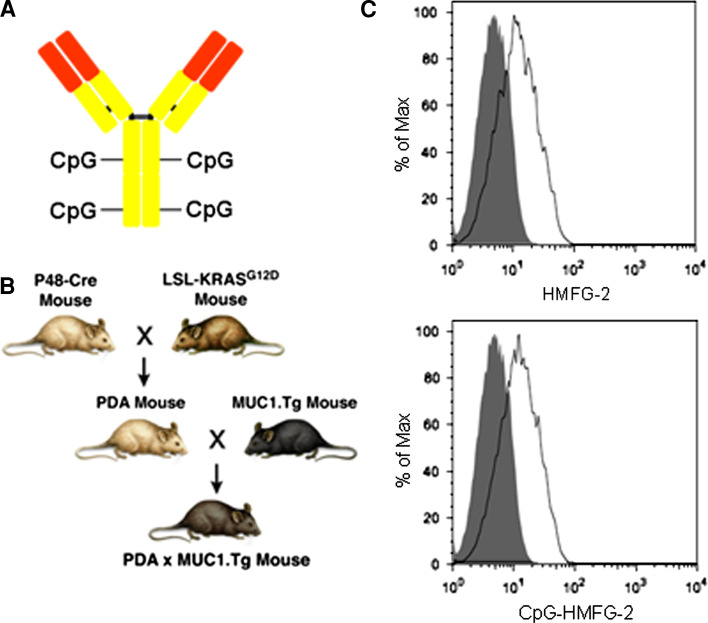
It has been shown in various immunotherapy protocols that CpG ODN acts as an adjuvant to enhance anti-tumor immune responses and that intra-tumoral injections of CpG ODN can be effective against established tumors [33]. We have conjugated 4 molecules of CpG ODN 1826 to an antibody directed against the tumor-associated form of human MUC1 (HMFG-2 mAb) (Fig. 1a). The presence of 4 molecules of CpG/antibody was confirmed by measuring the Ab concentration by BCA assay and the chromophore concentration by nanodrop.
Fig. 1.
CpG ODN-conjugated HMFG-2 mAb retains specificity to endogenous human MUC1 expressed on KCM cells. a Schematic representation of CpG ODN-conjugated HMFG-2 mAb. b Schematic representation of PDA × MUC1.Tg mice from which KCM cells were derived. c Staining of MUC1+ KCM tumor cells with HMFG-2 antibody before and after conjugation with CpG ODN. Antibody-binding capability was revealed using a PE-conjugated anti-IgG mouse antibody. Flow cytometry analysis of unconjugated mAb (top) and CpG ODN-conjugated mAb (bottom) is shown (15 µg/mL). Similar results were obtained with 1, 5, 7, and 11 µg/mL. Isotype Ctrl is shown as a closed histogram
To evaluate whether HMFG-2 mAb conjugated to CpG ODN retains its affinity/avidity for human MUC1, we utilized a primary pancreatic cancer cell line (KCM cells) derived from a PDA × MUC1.Tg mouse (Fig. 1b) that spontaneously develops pancreatic adenocarcinoma and constitutively expresses the human MUC1 molecule [27, 28]. KCM cells were stained with unconjugated and CpG ODN-conjugated HMFG-2 mAb and analyzed for MUC1 expression by flow cytometry. Results showed that CpG ODN-conjugated HMFG-2 mAb retains its ability to bind with similar affinity/avidity to MUC1 as the unconjugated HMGF-2 mAb at all concentrations tested (Fig. 1c). It has already been shown that CpG ODN conjugated to mAbs retains their immune stimulatory capacity to activate dendritic cells and induce IL-12 secretion [34].
In vitro ADCC against tumor cells is enhanced when HMFG-2 is conjugated to CpG ODN
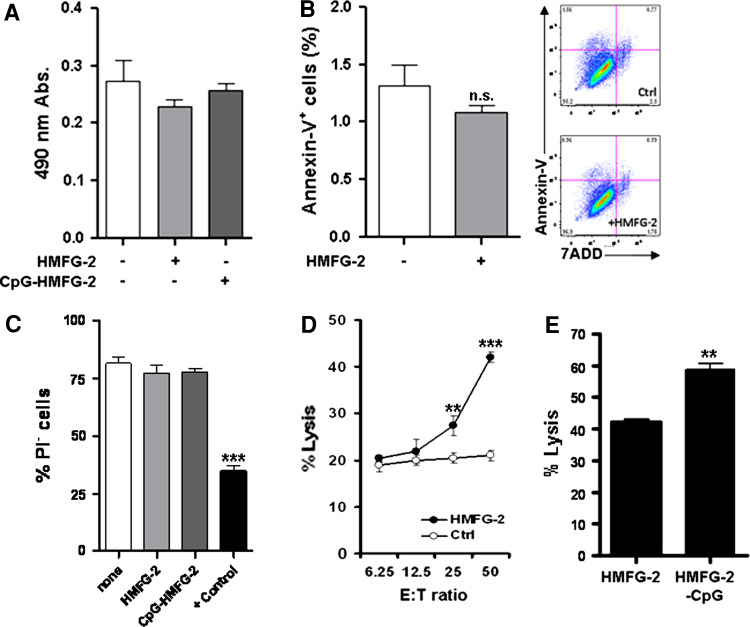
Many mechanisms have been proposed to explain the antitumor effect of mAbs in immunotherapy. Immune-modulation of tumor antigen-specific immune responses by specific mAbs may be conducted via (1) alteration of signaling pathways, (2) induction of apoptosis, (3) complement-dependent cytotoxicity (CDC), or (4) ADCC [35]. Therefore, we tested which of the above mechanisms may preponderate when unconjugated or CpG ODN-conjugated HMFG-2 mAb is utilized in vitro. First, we evaluated whether the presence of conjugated or unconjugated HMFG-2 mAb could alter the cellular proliferation rate of tumor cells. KCM tumor cells were cultured with CpG ODN conjugated or unconjugated HMFG-2 mAb for 24 h, and cellular proliferation was determined by their ability to reduce tetrazolium products. Our results indicate that both HMFG-2 mAb were unable to impede cellular growth (Fig. 2a). To determine whether HMFG-2 mAb can trigger apoptosis of tumor cells, levels of phosphatidylserine exposed on the surface of the cells were measured by flow cytometry. KCM tumor cells were co-cultured with HFMG-2 mAb overnight. Annexin-V assay revealed that HMFG-2 mAb could not significantly increase cell death in tumor cells (Fig. 2b). Additionally, a CDC assay was performed to analyze the potential role of complement. Target cells were co-cultured with CpG-conjugated and non-conjugated HMFG-2 mAb in the presence of 30 % cold-murine serum. Positive control was obtained by TNP derivatization of KCM cells [31]. Cytotoxicity was measured independently by a standard 51Cr release assay, and by a propidium iodide exclusion viability assay. Both results indicated no significant difference between control and antibody-treated tumor cell viability suggesting that complement was not activated through conjugated or non-conjugated HMFG-2 mAb (Fig. 2c). Data from PI-positive cell viability experiments are shown in Fig. 2c. Finally, to determine whether HMFG-2 mAb mediated ADCC through FcR+ effector cells, a standard 51Cr release assay was performed. Purified, freshly isolated, NK/LAK cells derived from healthy mouse spleen were used as effector cells with KCM cells as the target. Assays were conducted at E:T ratios of 6.25:1, 12.5:1, 25:1, and 50:1 using 15 µg/ml of HMFG-2 mAb. As was expected, NK/LAK cells in the presence of HMFG-2 mAb could kill MUC1+ tumor cells more efficiently than effector cells with an IgG control (Ctrl) antibody (Fig. 2d). Interestingly, ADCC was significantly more effective when a CpG ODN-conjugated HMFG-2 mAb was used to coat the target cells (Fig. 2e). These results may suggest that CpG ODNs conjugated with a non-cleavable linker to a specific antibody could directly enhance NK/LAK cells’ cytotoxic activity. CpG ODN alone had no effect in these experiments (data not shown).
Fig. 2.
NK cell-mediated ADCC against KCM cells is enhanced when HMFG-2 mAb is conjugated to CpG ODN. a Cell proliferation assays were performed with KCM cells co-cultured with CpG ODN-conjugated or unconjugated HMFG-2 mAb (15 µg/ml). Proliferation was determined by bioreduction of a tetrazolium salt to a formazan at 490 nm. b Antibody-induced apoptosis analysis was determined in the presence of 15 µg/ml HMFG-2 mAb by flow cytometry. Annexin-V/7ADD staining was used to detect apoptotic cells. c Tumor cells (target cells) were mixed with CpG ODN-conjugated or unconjugated HMFG-2 (15 µg/ml) and 30 % cold-murine serum and cultured for 2 h at 37 °C. CDC was quantified by propidium iodide exclusion assay (PI−) on target cells. Positive Ctrl (TNP derivatization of KCM cells plus anti-DNP antibodies) showed significantly lower cell viability (***p = 0.0002 as compared to all other groups). d ADCC activity in KCM tumor cells coated with unconjugated HMFG-2 mAb as targets. Freshly isolated NK/LAK cells were used as effectors cells at E:T ratios of 50:1, 25:1, 12.5:1, 6.25:1. Significantly higher lysis (**p = 0.019 and ***p = 0.0001) in the HMFG-2 mAb-treated cells compared to IgG Ctrl-treated cells. E) ADCC activity using CpG ODN-conjugated HMFG-2 mAb (15 µg/ml) coated KCM tumor target cells versus unconjugated HMFG-2-treated cells. Isolated NK/LAK cells were used as effectors cells at E:T ratios of 50:1. Significantly higher lysis (***p = 0.0005) observed in CpG ODN-conjugated HMFG-2 treatment versus unconjugated HMFG-2 treatment. Each assay was repeated at least three times. Data shown are the mean ± SE of triplicate determinations
IL-2-activated NK cells stimulated with immobilized CpG ODN and/or HMFG-2 mAb upregulate perforin
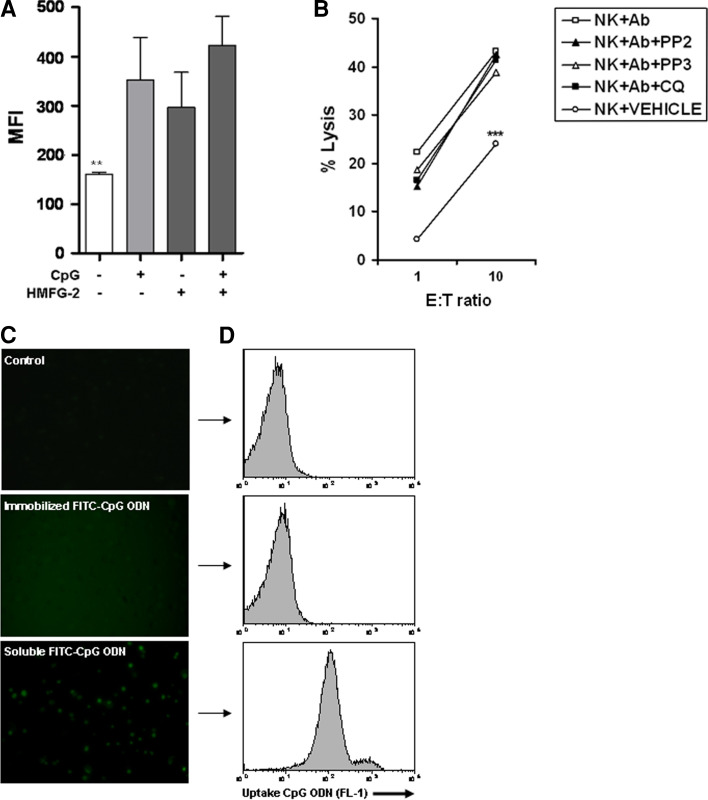
Can activated NK cells sense CpG ODNs directly on the surface of CpG ODN-conjugated HMFG-2-coated tumor cells? To determine whether activated NK cells are able to detect extracellular CpG ODN, we immobilized CpG ODN and/or HMFG-2 mAb on flat-bottom 96-wells plates. Activated NK1.1+ NK cells were cultured with immobilized CpG ODN (10 µg/ml), immobilized HMFG-2 mAb (10 µg/ml), or CpG ODN-conjugated HMFG-2 mAb (10 µg/ml) overnight. Golgi plug™ was added 6 h prior to assaying for intracellular levels of perforin in NK cells for each condition by flow cytometry. Perforin levels were significantly increased in all three conditions (Fig. 3a), suggesting that CpG ODNs can be sensed by activated NK cells without internalization. To confirm that CpG ODNs can be sensed in a TLR9-independent fashion, activated NK cells were pretreated with chloroquine, which was shown to block CpG and TLR9 interaction [36], before ADCC assay was performed. In addition, a potent inhibitor of SFK, pp2, was tested to investigate whether the SFK (Hck and Lyn) are involved, through tyrosine phosphorylation, in enhanced ADCC [36]. As Ctrls, activated NK cells were treated with DMSO (vehicle) or pp3 (Ctrl for the inhibitor, pp2). As shown in Fig. 3b, activated NK cells pretreated with chloroquine were still able to induce enhanced ADCC activity when co-cultured with CpG ODN-conjugated HMFG-2 mAb-coated tumor cells (Ab). Our results suggest that activated NK cells could sense CpG ODN without internalization, and in a TLR9-independent pathway. Interestingly, inhibition of Hck and Lyn kinases in NK cells did not diminish their ability to kill CpG ODN-conjugated antibody-coated tumor cells when compared to Ctrl treatment (Fig. 3b). We next verified that immobilized ODN is not released and internalized by NK cells. We immobilized FITC–CpG ODN (10 µg/ml) and cultured with activated NK cells overnight. As shown in Fig. 3c, FITC–CpG ODN is not internalized by NK cells when it was previously immobilized. On the contrary, soluble FITC–CpG ODN was taken by NK cells and internalized, as shown in Fig. 3c, d by fluorescent microscopy and flow cytometry, respectively. Together, these results suggest that the enhanced ADCC observed with CpG ODN-conjugated HMFG-2 is independent of TLR9-CpG ODN interaction, internalization, and SFK activation in NK cells.
Fig. 3.
a Immobilized CpG ODN-conjugated HMFG-2 mAb induced perforin up-regulation in NK cells. b Inhibition of Src family kinases or TLR9 signaling does not impair enhanced ADCC activity. a Immobilization of CpG ODN, HMFG-2 mAbs, or CpG ODN-conjugated HMFG-2 mAbs all induced upregulation of perforin on isolated NK cells when cultured for 18 h. Cells were incubated with PE-conjugated anti-NK1.1, and an intracellular staining with FITC-conjugated anti-perforin performed. Data shown are the mean ± SE of triplicate determinations (**p < 0.01 when compared to all treatment groups). b ADCC using isolated NK cells pre-incubated with pp2 (a Src inhibitor) (10 µM) or chloroquine (TLR9 inhibitor) (10 µM), and Ctrls (pp3, vehicle, or non-pretreatment) before the assay was performed. Data shown are the mean ± SE of triplicate determinations (***p < 0.001 for all groups compared to the vehicle Ctrl). c,d Fluorescent microscopy (×40) and flow cytometry analysis of immobilized FITC–CpG ODN incubated with activated NK cells. Immobilized FITC–CpG ODN is not internalized after overnight incubation with activated NK cells
CpG ODN-conjugated HMFG-2 mAb does not require MyD88 to trigger an enhanced ADCC
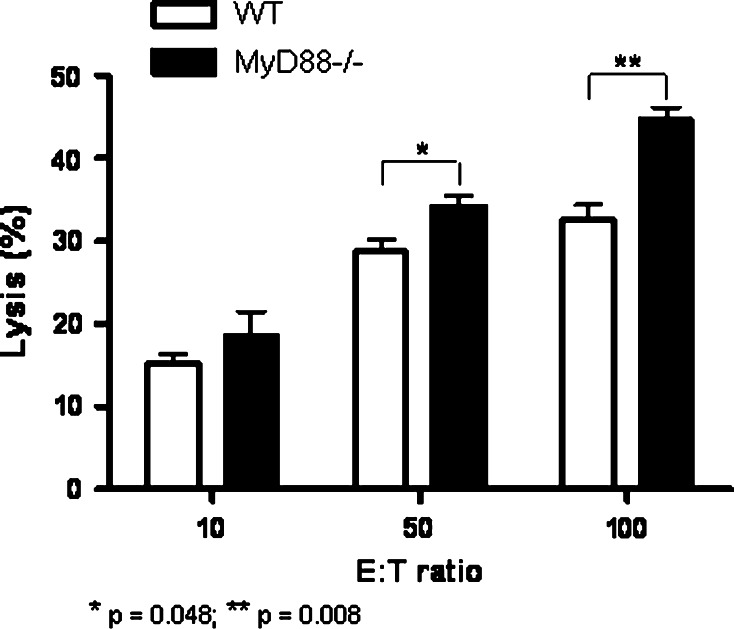
To further confirm TLR independency, we tested ADCC using NK cells from MyD88−/− mice that have impaired TLR9 signaling [37]. In contrast to other TLRs, TLR9 is known to signal in a strictly MyD88-dependent manner and is localized in the endosome. Opsonized KCM tumor cells coated with CpG ODN-conjugated mAb (15 µg/ml) were used as the target cells. As shown in Fig. 4, NK cells from MyD88−/− mice were able to trigger ADCC significantly better than wild-type NK cells in the presence of CpG-conjugated HMFG-2 mAb (p < 0.008). Data clearly suggest that TLR9 in the NK cells is not necessarily required for enhanced ADCC. However, further examination needs to be done to understand the significant increase of ADCC in the MyD88−/− NK cells.
Fig. 4.
CpG ODN-conjugated HMFG-2 mAb does not require MyD88 to trigger enhanced NK cell-mediated ADCC. ADCC activity was analyzed on opzonized KCM tumor cells using CpG ODN-conjugated mAb (15 µg/ml). Isolated NK cells from wild type, MyD88−/− mice were used as effectors cells at E:T ratios of 100:1, 50:1, 10:1. Compared to the MyD88−/− NK cells, NK cells from WT mice were significantly lower (*p = 0.048 and **p = 0.008). Each assay was repeated at least three times
Significant delay in tumor growth in nu/nu mice when treated with CpG ODN-conjugated HMFG-2 mAb
To determine the in vivo role of innate immune-effector cells, and their ability to initiate an immune response by ADCC, we injected 3 × 106 KCM cells subcutaneously (s.c.) in nu/nu mice in which T and B cells are non-functional. For treatment, KCM-bearing mice were injected intratumorally with CpG ODN-conjugated HMFG-2 mAb every 2 days for 2 weeks, as shown in Fig. 5a. As Ctrls, mice received PBS, CpG ODN, or HMFG-2 mAb. Mice were randomized prior to treatment, and tumor burden was measured every 2 days with digital caliper. A sample size of n = 4 gave us a power of 0.81 that is above the standards of adequacy. Results indicate that mice treated with CpG ODN-conjugated HMFG-2 mAb had a significantly reduced tumor burden compared to mice that received CpG ODN or HMFG-2 alone (p < 0.001; Fig. 5b). In addition, effective therapeutic intervention of CpG-conjugated HMFG-2 mAb was determined by tumor burden progression after suspension of the specific treatment at day 18. Tumor-bearing mice under CpG ODN or CpG-conjugated HMFG-2 mAb treatment were left without treatment until day 27. As shown in Fig. 5c, tumor burden increased significantly in both groups after treatment was suspended. These results indicate that the immune-effector cells in nude mice were able to Ctrl the tumor burden in the presence of CpG ODN-conjugated mAb in vivo. Thus, delivery of CpG ODN-conjugated mAbs at the tumor site has shown to be more efficient to trigger an innate-restricted antitumor response than its unconjugated version, and/or soluble CpG ODN.
In vivo antitumoral action of CpG ODN-conjugated HMFG-2 mAbs is mediated through NK cells and macrophages
Accumulating evidence suggests that macrophages upon activation can cooperate with NK cell activation through secretion of various cytokines [15]. Therefore, we determined whether ADCC enhanced by CpG ODN-conjugated mAbs on the surface of tumor cells is dependent on macrophages. To determine the in vivo role of macrophages, and their ability to cooperate with NK cell activation, we depleted macrophages and/or NK cells in vivo before tumor challenge. Depletion of macrophages and NK cells were induced by carrageenan iota type [30] and anti-asialo GM1 antibody, respectively (Fig. 6). Immunodeficient mice (nu/nu) were treated with anti-asialo GM1 antibody, or carrageenan (i.p.), as described in Fig. 6a for depletion of NK cells or macrophages, respectively. At day 0, all mice received 3 × 106 KCM cells. Treatment with CpG ODN-conjugated HMFG-2 mAb began when the tumor on the flank reached the size of (100–150 mm3). Anti-asialo GM1 antibody or carrageenan injections were repeated every 7 days post tumor challenge (Fig. 6a). Splenocytes were analyzed by flow cytometry using F4/80 and Nk1.1 antibodies showing actual depletion (data not shown). Our results show that depletion of macrophages accelerates the tumor burden in nude mice under CpG ODN-conjugated mAb treatment in comparison with the non-depleted mice (Fig. 6b). These data indicate that macrophages have an important role in the initiation of an antitumoral (innate-restricted) immune response. In vivo, cooperation of macrophages was necessary for enhancing NK cell-mediated ADCC. As was expected, NK cell depletion also accelerated tumor growth (Fig. 6b). Taken together, these results imply that localized CpG ODN delivery as a conjugate to HMFG-2 mAbs can initiate a T-cell-independent antitumor response via macrophage-dependent enhanced ADCC.
Discussion
It is well documented that upon activation with CpG ODN, B cells and/or dendritic cells secrete TNFα, IL-12, or type I IFNs that subsequently activate NK cells to produce IFNγ [38]. In parallel, CpG ODN can also activate NK cells directly in a TLR9-dependent manner without the help of antigen-presenting cells [20]. Regardless of alternative models, there is accumulating evidence indicating that various immune cells can sense CpG ODNs through a TLR9-independent mechanism [32, 36, 39–41]. In our study, we show that treatment with a CpG ODN-conjugated mAb could activate NK cells through a TLR9-independent mechanism. To the best of our knowledge, this is the first study to demonstrate that a non-cleavable CpG ODN-conjugated mAb can enhance ADCC by NK cells in vitro in a TLR9-independent pathway and delay in vivo tumor growth in immunodeficient nude mice that have a functional innate immune system.
To investigate the direct role of CpG ODNs on activation of NK cells during ADCC, we designed a molecule in which CpG ODN was conjugated in a non-cleavable manner to a mAb against MUC1. Thus, non-soluble CpG ODNs and the Fc domains could be simultaneously recognized by NK cells during an ADCC assay. Each mAb molecule was successfully conjugated to 4 CpG ODN molecules. Our results and previous studies indicate that antibody-conjugated CpG ODNs do not interfere with the affinity/avidity of the antibody (Fig. 1c) nor does the CpG ODN’s immune-modulatory capacity compromised [34].
CpG ODNs are well known as adjuvants for tumor vaccines; however, due to off target effects, its use has not been extensively explored in vivo. We show that targeting CpG ODN directly to MUC1-expressing tumors significantly impeded tumor growth in vivo (Fig. 5). The underlying mechanism of anti-tumor response was NK cell-mediated ADCC that required both NK cells and macrophages (Fig. 6). ADCC occurs when the Fc receptors on NK cells are engaged with the Fc domains of antibody-coated targets. As was expected, unconjugated HMFG-2 mAb was effective in triggering ADCC and activating NK cells (Fig. 2d). However, to our surprise, the CpG ODN-conjugated HMFG-2 mAb was able to significantly increase NK cell-mediated ADCC and its killing capacity (Fig. 2e). These data may suggest that NK cells are activated directly by CpG ODN. However, it is unlikely that CpG ODNs directly activate NK cells via TLR9 because CpG ODNs are conjugated with a non-cleavable linker. Although our data do not fit the current model of activation of effector cells by CpG ODN, it may suggest that CpG ODN enhances NK cells through a TLR9-independent pathway as implied in Fig. 3. Recently, TLR9-independent pathways of activation have been described in inflammatory cells. Although mechanisms vary, it has been suggested that CpG ODN may signal via scavenger receptor B1 and trigger a calcium entry on primary B cells [39]. Moreover, bacterial DNA has been shown to stimulate neutrophils without being internalized or interacting with TLR9 [32]. Our data suggest that CpG ODN may signal through an alternative pathway on NK cells, which do not require CpG internalization.
There are three classes of ODN established as immunomodulators: type A, B, and C ODN. Although type B ODN (ODN 1826) used in our study has a low NK cell activation profile, soluble ODN 1826 has been shown to induce NK cell activity in vivo like other ODN types and can be internalized to activate TLR9 in the endosomes [18]. Upon recognition of ODNs in the endosomes, TLR9 initiates a conserved TLR signaling cascade that begins with the recruitment of the adaptor MyD88. The adaptor protein MyD88 plays an important role in transducing signals from TLR family members. The TLR9 inhibitor chloroquine inhibits immune stimulation by bacterial DNA and CpG ODN. The inhibition of TLR9 occurs in endosomes. This is where chloroquine was shown to block CpG and TLR9 interaction [42]. On the other hand, two Src family kinases, Hck and Lyn, have been found to be phosphorylated upon CpG ODN stimulation. The SFK-initiated tyrosine phosphorylation cascade activates signaling proteins implicated in reorganization, adhesion, and motility. Moreover, it has been indicated that this pathway of phosphorylation is triggered upstream of the traditional TLR9 pathway upon CpG stimulation. Evidence suggests that the Src family pathway intersects the TLR9–MyD88 pathway by promoting tyrosine phosphorylation of TLR9 and the recruitment of Src [36]. Our present data strongly suggest that blocking Src family pathway or blocking TLR9 interaction with CpG in endosomes with Src inhibitor or chloroquine, respectively, does not block the activation of NK cells by CpG ODN and rather triggers an enhanced ADCC (Fig. 3b). To rule out the involvement of TLR9, isolated NK cells from MyD88−/− mice were used as effectors cells in an ADCC assay. These results have shown that NK cells with impaired TLR9 signaling can still trigger an enhanced ADCC as wild-type NK cells do (Fig. 4). Surprisingly, we observed higher ADCC with MyD88−/− NK cells as compared to wild-type NK cells, suggesting that MyD88−/− NK cells may have been involved in the down-regulation of ADCC. Taken together, data suggest that CpG ODN may signal in an alternative pathway, where internalization or Src/TLR9 signaling pathways are not necessary.
It has been previously reported that the TLR9 agonist are unable to induce the production of cytokines or increase NK cell cytotoxicity against tumors in the absence of IL-12 or -18 [43]. Although this concept was applied to the indirect effect of CpG ODN on NK cells, Roda et al. [20] have shown that those cytokines are not absolutely necessary for the induction of NK cell cytokine secretion in response to CpG ODN. However, in our macrophage/NK cell depletion experiments (Fig. 6a), we found that macrophages are essential for an anti-tumor response in nude mice under treatment with CpG ODN-conjugated HMFG-2 mAb. These results suggest that macrophage cooperation in vivo is required to initiate and sustain a NK cell-mediated anti-tumor activity (Fig. 6b). We cannot rule out an indirect effect from macrophages. However, isolated NK cells in vitro respond significantly better to CpG-conjugated antibody-coated tumor cells (no presence of macrophages). This also suggests a direct effect on NK cells, which might be enhanced even further by an indirect effect of macrophages. As previously established, NK cells, in synergistic cooperation with macrophages, play a central role for an antitumor response triggered by mAb-based therapy [44]. Our data present proof of concept that CpG ODN-conjugated mAbs are able to enhance NK cell-mediated ADCC in vitro and in vivo. In the absence of T cells and Treg cells, an enhanced ADCC activity of NK cells abetted by macrophages can delay tumor growth when a non-cleavable CpG ODN-conjugated HMFG-2 Ab is present [34]. In summary, we provide a rationale to deliver locally and safely CpG ODNs into the tumor as a new strategy for traditional immunotherapy.
Acknowledgments
NIH 1R01CA118944, P50 CA102701, and PanCan Pilot Project (P. Mukherjee). We thank Dr. S. M. Michalek (UAB) for generous supply of MyD88−/− mice; all members in the animal facility at Mayo Clinic Scottsdale, AZ and UNCC, NC. Special thanks to Dr. Joyce Taylor-Papadimitriou and Dr. Joy Burchell for providing the HMFG-2 antibody.
Conflict of interest
No potential conflicts of interest were disclosed.
Footnotes
Amritha Kidiyoor and Dahlia M. Besmer contributed equally to the manuscript.
References
- 1.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373(9668):1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23(9):1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 3.Cartron G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 4.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 6.Clynes RA, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 7.Sulica A, et al. Ig-binding receptors on human NK cells as effector and regulatory surface molecules. Int Rev Immunol. 2001;20(3–4):371–414. doi: 10.3109/08830180109054414. [DOI] [PubMed] [Google Scholar]
- 8.Peipp M, et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112(6):2390–2399. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- 9.Leidi M, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182(7):4415–4422. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 10.Karagiannis SN, et al. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179(5):2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 11.Khan KD, et al. A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12(23):7046–7053. doi: 10.1158/1078-0432.CCR-06-1571. [DOI] [PubMed] [Google Scholar]
- 12.Umana P, et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17(2):176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 13.Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118(1):1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Bowles JA, Weiner GJ. CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J Immunol Methods. 2005;304(1–2):88–99. doi: 10.1016/j.jim.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.V97.1.14. [DOI] [PubMed] [Google Scholar]
- 16.Moga E, et al. NK cells stimulated with IL-15 or CpG ODN enhance rituximab-dependent cellular cytotoxicity against B-cell lymphoma. Exp Hematol. 2008;36(1):69–77. doi: 10.1016/j.exphem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 18.Ballas ZK. Modulation of NK cell activity by CpG oligodeoxynucleotides. Immunol Res. 2007;39(1–3):15–21. doi: 10.1007/s12026-007-0066-3. [DOI] [PubMed] [Google Scholar]
- 19.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 20.Roda JM, Parihar R, Carson WE., III CpG-containing oligodeoxynucleotides act through TLR9 to enhance the NK cell cytokine response to antibody-coated tumor cells. J Immunol. 2005;175(3):1619–1627. doi: 10.4049/jimmunol.175.3.1619. [DOI] [PubMed] [Google Scholar]
- 21.Krieg AM, et al. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27(6):460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Link BK, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29(5):558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 23.Andoniou CE, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6(10):1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 24.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1 and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/S1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 25.Tinder TL, et al. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol. 2008;181(5):3116–3125. doi: 10.4049/jimmunol.181.5.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee P, et al. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J Immunol. 2009;182(1):216–224. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besmer DM, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 2011;71(13):4432–4442. doi: 10.1158/0008-5472.CAN-10-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy LD, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30(12):1449–1459. doi: 10.1038/onc.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor-Papadimitriou J, et al. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- 30.Grosso JF, et al. MUC1/sec-expressing tumors are rejected in vivo by a T cell-dependent mechanism and secrete high levels of CCL2. J Immunol. 2004;173(3):1721–1730. doi: 10.4049/jimmunol.173.3.1721. [DOI] [PubMed] [Google Scholar]
- 31.Vitetta ES, Hart DA, Forman J. Relationship between trinitrophenol and H-2 antigens on trinitrophenyl-modified spleen cells. III. Quantitative aspects of trinitrophenol binding on cells treated with trinitrobenzene sulfonic acid. J Immunol. 1978;121(3):997–1001. [PubMed] [Google Scholar]
- 32.Trevani AS, et al. Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol. 2003;33(11):3164–3174. doi: 10.1002/eji.200324334. [DOI] [PubMed] [Google Scholar]
- 33.Sfondrini L, et al. Prevention of spontaneous mammary adenocarcinoma in HER-2/neu transgenic mice by foreign DNA. FASEB J. 2002;16(13):1749–1754. doi: 10.1096/fj.02-0383com. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S, et al. Systemic targeting of CpG–ODN to the tumor microenvironment with anti-neu-CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB-neuT tolerant mice. Cancer Res. 2008;68(18):7530–7540. doi: 10.1158/0008-5472.CAN-08-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zafir-Lavie I, Michaeli Y, Reiter Y. Novel antibodies as anticancer agents. Oncogene. 2007;26(25):3714–3733. doi: 10.1038/sj.onc.1210372. [DOI] [PubMed] [Google Scholar]
- 36.Sanjuan MA, et al. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol. 2006;172(7):1057–1068. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liese J, Schleicher U, Bogdan C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur J Immunol. 2007;37(12):3424–3434. doi: 10.1002/eji.200737182. [DOI] [PubMed] [Google Scholar]
- 38.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9(7):831–835. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 39.Zhu P, et al. Mechanism and regulatory function of CpG signaling via scavenger receptor B1 in primary B cells. J Biol Chem. 2009;284(34):22878–22887. doi: 10.1074/jbc.M109.018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trieu A, et al. TLR9-independent effects of inhibitory oligonucleotides on macrophage responses to S. typhimurium . Immunol Cell Biol. 2009;87(3):218–225. doi: 10.1038/icb.2008.95. [DOI] [PubMed] [Google Scholar]
- 41.Hokeness-Antonelli KL, et al. IFN-alphabeta-mediated inflammatory responses and antiviral defense in liver is TLR9-independent but MyD88-dependent during murine cytomegalovirus infection. J Immunol. 2007;179(9):6176–6183. doi: 10.4049/jimmunol.179.9.6176. [DOI] [PubMed] [Google Scholar]
- 42.Rutz M, et al. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur J Immunol. 2004;34(9):2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- 43.Sivori S, et al. Comparison of different CpG oligodeoxynucleotide classes for their capability to stimulate human NK cells. Eur J Immunol. 2006;36(4):961–967. doi: 10.1002/eji.200535781. [DOI] [PubMed] [Google Scholar]
- 44.Lauzon NM, Mian F, Ashkar AA. Toll-like receptors, natural killer cells and innate immunity. Adv Exp Med Biol. 2007;598:1–11. doi: 10.1007/978-0-387-71767-8_1. [DOI] [PubMed] [Google Scholar]