Abstract
Background
Complete Response (CR) at the primary tumor site as assessed by clinical examination following induction chemotherapy with cisPlatin and 5-Fluorouracil (5-FU)[PF] is a favorable predictive factor for overall survival and disease-control in patients with locally advanced squamous cell carcinoma of the head and neck. In most series, the rate of CR at the primary site after induction PF was 20–30%. We evaluated the efficacy and feasibility of induction nAb-paclitaxel and Cetuximab given with PF (ACPF) followed by definitive chemoradiation (CRT) in a phase II trial.
Methods
Patients with HNSCC were treated with ACPF (nab-paclitaxel 100 mg/m2/week; cetuximab 250 mg/m2/week; cisplatin 75 mg/m2 Day 1; 5-fluorouracil 750 mg/m2/day Days 1–3) every 21 days for 3 cycles followed by CRT (cisplatin 100 mg/m2 on days 1,22 and 43 of RT). CR at the primary tumor site after 2 cycles of ACPF was the primary endpoint.
Results
Thirty patients were enrolled, of which 22 (73%) had large (T3/T4) primary tumors. The CR rate at the primary tumor site after 2 cycles of ACPF was 53% and the overall response rate was 100%. Twenty-nine (96%) patients completed 3 cycles of ACPF, 26 (90%) completed definitive radiation therapy (RT) per protocol and 22 of the 27 evaluable patients (81%) received > 2 of the 3 planned doses of cisplatin with RT. The estimated 2-year overall and progression-free survivals were 84% and 65%, respectively.
Conclusion
Induction ACPF resulted in a high CR rate (53%) at the primary tumor site even in large tumors and did not adversely affect delivery of definitive CRT. Further investigation of ACPF is warranted.
Keywords: head and neck cancer, phase 2, nab-paclitaxel, cetuximab, cisplatin, 5-fluorouracil
INTRODUCTION
Squamous cell carcinoma of the head and neck (HNSCC) afflicts more than 500,000 patients annually worldwide.1 Most patients present with locally advanced disease and are often treated with definitive radiation therapy (RT). Chemotherapy given concurrently with RT (CRT) improved local-regional disease control and overall survival (OS) compared with RT alone but had minimal impact on the rate of distant metastases.2 Randomized trials of induction chemotherapy demonstrated a reduction in distant failure rates, but only two trials showed an improvement in OS.3,4 Recently, superior OS was observed with the addition of either docetaxel or cremaphor-based paclitaxel to induction cisplatin and 5-fluorouracil (5-FU)[PF] in patients subsequently treated with definitive RT5 or CRT.6,7 However, recurrent disease remains the primary cause for treatment failure following induction chemotherapy and definitive CRT.
Complete response (CR) at the primary site following induction PF correlated with improved OS and disease control after definitive RT.8,9 In most series, the rate of CR at the primary site after induction PF was 20–30%.4,9 Two strategies to improve CR rates at the primary site following induction chemotherapy include the use of novel taxanes and the addition of epidermal growth factor receptor (EGFR) inhibitors. Increased intratumoral paclitaxel accumulation and anti-tumor activity occurred with nanoparticle albumin-bound paclitaxel (nab-paclitaxel)(Celgene Corporation, Summit, NJ) compared to cremaphor-based paclitaxel in nude mice bearing several human tumor xenografts.10 In breast cancer, nab-paclitaxel resulted in higher tumor response rates in comparison to cremophor–based paclitaxel,11 which may be due to the high tumor expression of Secreted Protein Acidic and Rich in Cysteine (SPARC). SPARC plays a role in albumin receptor-mediated endothelial transport.12 SPARC expression is common in tumor and stromal cells of HNSCC but not in adjacent normal oral mucosa13 and correlated with tumor response to nab-paclitaxel in patients with HNSCC.14 More specifically, the concept is that high tumor expression of SPARC reflects tumor cells that have high rates of albumin-receptor-mediated endocytosis and will respond better to albumin-bound chemotherapeutic agents because they accumulate more albumin in the tumor cells. Inhibition of EGFR by cetuximab reduces proliferation and induces apoptosis of HNSCC cell lines, and enhances the activity of cisplatin in xenograft models.15 The addition of cetuximab to PF increased tumor response rates and OS in patients with metastatic HNSCC, and had an acceptable safety profile.16
We hypothesized that a novel induction regimen of weekly nab-paclitaxel and cetuximab given with every three week PF (ACPF) would result in a high favorable tumor response rate in patients with locally advanced HNSCC subsequently treated with definitive CRT. The primary efficacy endpoint was CR rate at the primary tumor site after two cycles of induction chemotherapy as assessed by clinical examinations as this endpoint represents a surrogate marker of improved disease control after definitive RT.8,9 We also sought to determine whether this novel regimen would be associated with an acceptable toxicity profile and whether it would adversely impact delivery of definitive CRT.
MATERIALS AND METHODS
Patient Selection
Eligible patients were 18 years of age or older with untreated HNSCC stages III and IVa/b (T1 excluded) originating in the oropharynx, larynx and oral cavity.17 Other criteria included adequate performance status (ECOG 0–2) and vital organ function. Exclusion criteria included ≥ Grade 2 peripheral sensory neuropathy (PSN). The Washington University Human Research Protection Office approved the protocol and all study participants signed informed consent. This clinical trial was registered at ClinicalTrials.gov #NCT00736944.
Treatment Plan
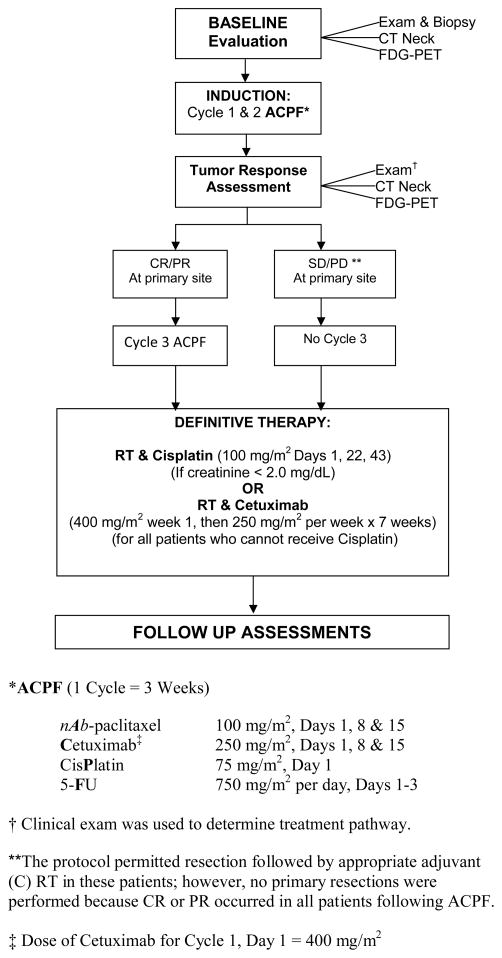
Induction therapy consisted of every three week cycles of intravenous (IV) nab-paclitaxel 100 mg/m2 weekly on days 1, 8, and 15, cetuximab 400 mg/m2 day 1 and 250 mg/m2 weekly subsequently, cisplatin 75 mg/m2 on day 1 and 5-FU 750 mg/m2 continuous infusion daily on days 1–3 (ACPF) (Figure 1). After two cycles of ACPF, patients underwent an assessment of tumor response at the primary site by clinical examination (laryngoscopy in office or operating room) by experienced oncologic surgeons using categorical outcomes commonly employed by others with slight modifications:8,18 CR-complete response, defined as complete resolution of lesion or near CR-minimal residual mucosal abnormality; partial response (PR)–50 to 94% decrease; stable disease (SD)–0 to 49% decrease; and progressive disease (PD)–any increase. Patients with favorable (CR, near CR, PR) tumor response at the primary site by clinical examination then received a third cycle of ACPF before definitive CRT whereas patients with an unfavorable (SD/PD) tumor response at the primary site by clinical examination proceeded directly to definitive CRT.
Figure 1.
Treatment and Study Design
Definitive CRT began within 22–56 days after the initiation of cycle 3 of ACPF. Intensity modulated radiation therapy (IMRT) was administered once daily, five days weekly. The total dose of RT to gross disease was 7000 cGy in 35 fractions of 200 cGy each over 7 weeks and areas at risk for microscopic disease in the ipsilateral and contralateral neck received 5600 cGy. A dose of 6300 cGy in 35 fractions was allowed to areas considered intermediate risk at physician discretion. Patients with serum creatinine < 2.0 mg/dL were scheduled to receive cisplatin 100 mg/m2 on days 1, 22 and 43 of RT. Patients who failed to meet this criteria and had no serious hypersensitivity reaction (HSR) with prior cetuximab, received cetuximab (400 mg/m2 IV loading dose one week before RT then 250 mg/m2 weekly × 7 doses) as a single agent concurrent with RT. Patients who failed to meet criteria for either cisplatin or cetuximab received RT alone.
Standard Assessments
Baseline assessments included history and physical examination, laryngoscopy, computed tomography (CT) of the neck, and body F-18 fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT). Assessments of patient symptoms and adverse events (AEs) were performed weekly during ACPF, every 3 weeks during and 4–6 weeks after RT using National Cancer Institute–Common Toxicity Criteria (NCI-CTC) version 3.0. After two cycles of ACPF and 10–12 weeks after RT, patients underwent assessment of tumor response by clinical examination, CT of the neck, and FDG-PET/CT. RECIST version 1.0 was used to determine anatomic tumor response by CT.19 Subsequently, patients were assessed for tumor response with physical examination and laryngoscopy every 3 months and CT of the neck and chest performed at 6, 12, 20, 28 and 36 months following RT. Patient’s co-morbidities were quantified using the Adult Co-morbidity Evaluation (ACE)-27 index.20
Immunohistochemistry
SPARC
SPARC expression was assessed by immunohistochemistry (IHC) performed on pretreatment formalin-fixed, paraffin-embedded primary tumor specimens using 4 μm sections. IHC was performed by hand using a commercially available monoclonal antibody for SPARC (osteonectin; clone 15G12; Vector Laboratories; dilution 1:50; antigen retrieval consisting of proteinase K digestion; 30 minute room temperature incubation with primary antibody followed by 30 minute room temperature incubation with secondary). A known SPARC expressing HNSCC was used as a positive control. Samples were analyzed semi-quantitatively by the study pathologist (JSL) reviewing 10 high power fields (HPF’s) with scoring of tumor cells for extent of staining in quartiles as: 0=no staining; 1+=0–24% staining; 2+=25–49% staining; 3+=50–74% staining; 4+=75–100% staining. Samples were also analyzed semi-qualitatively for staining intensity of tumor cells as: negative=none; 1+=weak; 2+=moderate; 3+=strong. Similar analyses were performed on the peri-tumoral stromal fibroblasts.
p16
For all patients with oropharyngeal primaries, IHC was performed for p16 as previously described.21 Slides were reviewed by the study pathologist and scored binarily as positive (staining in >50% of tumor cells) versus negative (no staining or staining in <50% of tumor cells).
Statistical Methods
Tumor Response and Survival
The primary objective was to determine the CR rate (defined as a composite of CR and near CR) at the primary tumor site using clinical examinations following two cycles of ACPF. We hypothesized that the CR rate at the primary tumor site would be at least similar to our historical experience with docetaxel, PF and cetuximab (TPF+Cet): 50% in T2, 20% in T3 and 0% in T4 disease.22 Given a sample size of 30 patients (10 in each T-classification stratum), the overall proportion with CR at the primary tumor site was estimated within 19%, as the 95% confidence interval for 7/30 patients (9.9%, 42.3%).
OS (time from diagnosis to death or to last follow-up alive) and progression-free survival (PFS, time from diagnosis to death due to disease progression, to disease progression or to last follow-up alive) were estimated by the Kaplan-Meier (KM) method.23 RT delivery (number of fractions, elapsed days and total dose) and chemotherapy administration (number of planned doses administered, total dose/m2) were determined and summarized for the group by descriptive statistics.
Safety Analysis and Planned Stopping Rule
The type and grade of each AE were documented in a frequency distribution for the whole group. An interim safety analysis was performed after the first 10 patients to confirm that the rate of grade 3–4 AEs during ACPF did not exceed the pre-determined maximum allowable frequency of 35%.
RESULTS
Patient Characteristics and SPARC Expression
Thirty patients were enrolled (Table 1). Most patients were older than 50 years and smokers. Significant co-morbidities ranging from moderate to severe (ACE-27 indices scores of 2–3) were present in 63% of patients. Most patients had large (T3,T4) primary tumors (74%) and bulky (>N2b) nodal disease in the neck (80%). Twenty-two patients (73%) had oropharyngeal cancers of which 17 of 21 (81%) tested were positive for p16, a surrogate marker for the human papilloma virus (HPV).
Table 1.
Patient and tumor characteristics
| Characteristic | No. | % |
|---|---|---|
| Age (years) Mean | 54.5 | |
| Range | 39–70 | |
| Sex | ||
| Male | 28 | 93 |
| Female | 2 | 7 |
| ECOG Performance Status | ||
| 0 | 25 | 83 |
| 1 | 5 | 17 |
| Smoking History | ||
| Yes | 27 | 90 |
| No | 3 | 10 |
| ACE Co-morbidity Index | ||
| 0 (none) | 9 | 30 |
| 1 (mild) | 2 | 7 |
| 2 (moderate) | 13 | 43 |
| 3 (severe) | 6 | 20 |
| Primary Site | ||
| Oropharynx | 22 | 73 |
| Oral Cavity | 1 | 3 |
| Larynx | 7 | 23 |
| T Classification | ||
| T2 | 8 | 27 |
| T3 | 11 | 37 |
| T4 | 11 | 37 |
| N Classification | ||
| N0 & N1 | 6 | 20 |
| N2b | 7 | 23 |
| N2c | 13 | 43 |
| N3 | 4 | 13 |
| p16 Positive | ||
| Oropharynx | 17/21 | 81 |
SPARC expression was assessed by IHC staining on 28 patients (Table 2). The proportion of cells staining for SPARC and the staining intensity of SPARC expression were greater in the stromal cells compared to the tumor cells. Stromal fibroblast staining for SPARC was present in all 28 evaluable cases (100%), while tumor cell staining was present in 10 of 28 evaluable cases (35.7%).
Table 2.
SPARC Expression assessed by IHC*
| SPARC IHC | Stroma (#pts) | Tumor (#pts) |
|---|---|---|
|
| ||
| Proportion of Cells Positive in 10 HPF’s | ||
| - | 0 | 20 |
| 1+ | 2 | 4 |
| 2+ | 4 | 2 |
| 3+ | 7 | 2 |
| 4+ | 15 | 0 |
|
| ||
| Intensity of Cell Staining | ||
| - | 0 | 20 |
| 1+ | 1 | 2 |
| 2+ | 11 | 5 |
| 3+ | 16 | 1 |
|
| ||
| Overall Positive Staining | 28/28 (100%) | 10/28 (35.7%) |
Two patients’ slides were not available.
IHC=immunohistochemistry
HPF=high power fields
pts=patients
Primary Tumor Site Response to ACPF
After two cycles of ACPF, the composite CR rate at the primary tumor site as assessed by clinical examination was 53.3% (CR-40% [12 patients]; Near CR-13.3% [4 patients]); whereas, the rate of PR was 46.7% (14 patients). On univariate analysis, variables associated with attaining a CR at the primary tumor site following two cycles of ACPF included fewer packs per day smoked and absence of SPARC staining in the tumor (Table 3).
Table 3.
Variables associated with primary tumor site response to two cycles of ACPF
| Variable | Primary Tumor Site Response
|
||
|---|---|---|---|
| CR | PR | p-value | |
|
| |||
| Ever Smoker | 0.23** | ||
| Yes | 13 | 14 | |
| No | 3 | 0 | |
|
| |||
| Packs per Day Smoked | 0.0091*** | ||
| Median | 1.00 | 1.25 | |
|
| |||
| Years Smoked | 0.56*** | ||
| Median | 34.5 | 37.0 | |
|
| |||
| T Classification | 0.86* | ||
| T2 | 4 | 4 | |
| T3 | 6 | 5 | |
| T4 | 6 | 5 | |
|
| |||
| Tumor Site | 0.10** | ||
| Oropharynx | 14 | 8 | |
| Larynx/Oral Cavity | 2 | 6 | |
|
| |||
| Proportion of Tumor Cells SPARC Positive in 10 HPFs | 0.0092* | ||
| - | 14 | 6 | |
| 1+ | 0 | 4 | |
| 2+ | 1 | 1 | |
| 3+ | 0 | 2 | |
| 4+ | 0 | 0 | |
|
| |||
| Intensity of SPARC staining in Tumor | 0.0083* | ||
| - | 14 | 6 | |
| 1+ | 0 | 2 | |
| 2+ | 1 | 4 | |
| 3+ | 0 | 1 | |
|
| |||
| Proportion of Stromal Cells SPARC Positive in 10 HPFs | 0.43* | ||
| - | 0 | 0 | |
| 1+ | 1 | 1 | |
| 2+ | 3 | 1 | |
| 3+ | 4 | 3 | |
| 4+ | 7 | 8 | |
|
| |||
| Intensity of SPARC staining in Tumor | 0.87* | ||
| - | 0 | 0 | |
| 1+ | 1 | 0 | |
| 2+ | 5 | 6 | |
| 3+ | 9 | 7 | |
|
| |||
| p16 Status | 0.14** | ||
| Positive | 11 | 5 | |
| Negative | 4 | 8 | |
Jonckheere-Terpstra Test
Fisher’s Exact Test
Kruskal-Wallis Test
Neck Nodal and Overall Tumor Response to ACPF
The tumor response rates at neck nodal sites after two cycles of ACPF based on clinical examinations were 61% CR (11 patients) and 39% PR (7 patients). Twelve patients were not evaluable because of initial absence of nodal disease on clinical examination. On univariate analysis, the only variable associated with attaining a CR at the neck nodal sites following two cycles of ACPF was absence of smoking history (CR in 3/3 nonsmokers versus 8/15 smokers, p=0.049). Table 4 summarizes and correlates the tumor response rates at the primary site and at the neck nodes after two cycles of ACPF based on clinical examination, CT, and FDG-PET/CT scans.
Table 4.
Tumor response at primary and neck nodal sites as assessed by clinical examination, CT, and FDG-PET/CT following two cycles of ACPF
| Treatment | Primary Site | Neck Nodes | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Eval (#) | CR (%) | PR (%) | SD/PD (%) | Eval (#) | CR (%) | PR (%) | SD/PD (%) | Eval (#) | CR (%) | PR (%) | SD/PD (%) | |
| Post 2 Cycles ACPF | ||||||||||||
| Clinical Exam | 30 | 53* | 47 | 0 | 18 | 61 | 39 | 0 | 30 | 43* | 57 | 0 |
| CT Scan | 28 | 33 | 41 | 26 | 24 | 30 | 48 | 22 | 29 | 14 | 50 | 36 |
| FDG-PET/CT | 28 | 32 | 61 | 7 | 25 | 36 | 56 | 8 | 29 | 24 | 66 | 10 |
includes near CR’s
Treatment Delivery
Thirty patients completed two cycles and 29 of the patients completed three cycles of ACPF, and 28 patients underwent definitive radiation-based therapy per protocol. One patient died after cycle 2 of ACPF due to treatment-related mortality (TRM) and one was removed from study following ACPF due to non-compliance but was treated with definitive CRT.
During ACPF, the proportion of planned doses of nab-paclitaxel, cisplatin, cetuximab and 5-FU administered were 92%, 97%, 87% and 99%, respectively. During definitive RT, 26 patients received cisplatin, 1 received cetuximab, 1 received RT alone and 1 received cisplatin off protocol. Twenty-six of the 29 evaluable patients (90%) completed the planned 35 fractions of RT, whereas two elected to stop RT early and one received RT off protocol. The median (range) elapsed days and dose (Gy) of RT administered were 50 (8–69) and 70 (14–72), respectively. The proportions of the 27 evaluable patients given 3, 2, 1 or 0 doses of cisplatin during RT were 48%(13), 33%(9), 15%(4) and 4%(1), respectively. Across the planned sequential therapy with ACPF and CRT, the median (range) cumulative dose of cisplatin delivered was 425 (150–525) mg/m2.
Adverse Events During ACPF
Table 5 summarizes the grade 3–4 AE’s related to ACPF. The proportion of the 30 patients who experienced maximum grade 3, 4, or 5 AEs were 33%(10), 3%(1) and 3%(1), respectively. Some patients experienced more than one > grade 3 AE. Most grade 3 AE’s that occurred, such as acneiform rash, asymptomatic neutropenia, fatigue and mucositis were manageable. One patient experienced a grade 4 HSR due to cetuximab. The single grade 5 AE was a neutropenia-related pneumonia. The safety analysis demonstrated that the incidence of grade 3–4 AE’s in the first ten patients was 30%, within the pre-determined acceptable range (<35%).
Table 5.
Grade 3–4 AE’s that occurred during ACPF
| Toxicity | During ACPF (N=30)
|
|||||
|---|---|---|---|---|---|---|
| 3 | 4 | 3–5 combined | ||||
|
| ||||||
| No. | % | No. | % | No. | % | |
| Allergy | ||||||
| Infusion Reaction – Cetuximab | 0 | 0 | 1 | 3 | 1 | 3 |
| Constitutional | ||||||
| Fatigue | 3 | 10 | 0 | 0 | 3 | 10 |
| Dermatologic | ||||||
| Acneiform Rash | 4 | 13 | 0 | 0 | 4 | 13 |
| Rash – Chemoradiation | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | ||||||
| Colitis | 1 | 3 | 0 | 0 | 1 | 3 |
| Dysphagia/Odynophagia | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucositis | 1 | 3 | 0 | 0 | 1 | 3 |
| Nausea/Vomiting | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematological | ||||||
| Anemia | 1 | 3 | 0 | 0 | 1 | 3 |
| Neutropenia | 5 | 17 | 1 | 3 | 6 | 20 |
| Thrombocytopenia | 0 | 0 | 1 | 3 | 1 | 3 |
| Infection | ||||||
| Febrile Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 0 | 0 | 1* | 3* |
| Metabolic/Laboratory | ||||||
| Creatinine/Creatinine Clearance | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperkalemia | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypokalemia | 2 | 7 | 1 | 3 | 3 | 10 |
| Hypomagnesemia | 0 | 0 | 0 | 0 | 0 | 0 |
| Neurological | ||||||
| Sensory Neuropathy – Peripheral | 0 | 0 | 0 | 0 | 0 | 0 |
| Vascular | ||||||
| Thrombosis | 5 | 17 | 0 | 0 | 5 | 17 |
One patient expired during ACPF due to pneumonia (Grade 5) in association with Grade 4 neutropenia
Peripheral Sensory Neuropathy (PSN) and Gastrostomy Tubes
Nineteen (63%) patients developed (grade 1–3) PSN (G1:12;G2:6;G3:1): 6 during ACPF, 2 during CRT, and 11 after CRT. At last follow-up, only 5 patients still complained of PSN. Twenty patients (67%) required placement of a gastrostomy tube. Three (14%) of the 21 patients who had reached the 1 year post treatment follow-up had a gastrostomy tube in place.
Survival
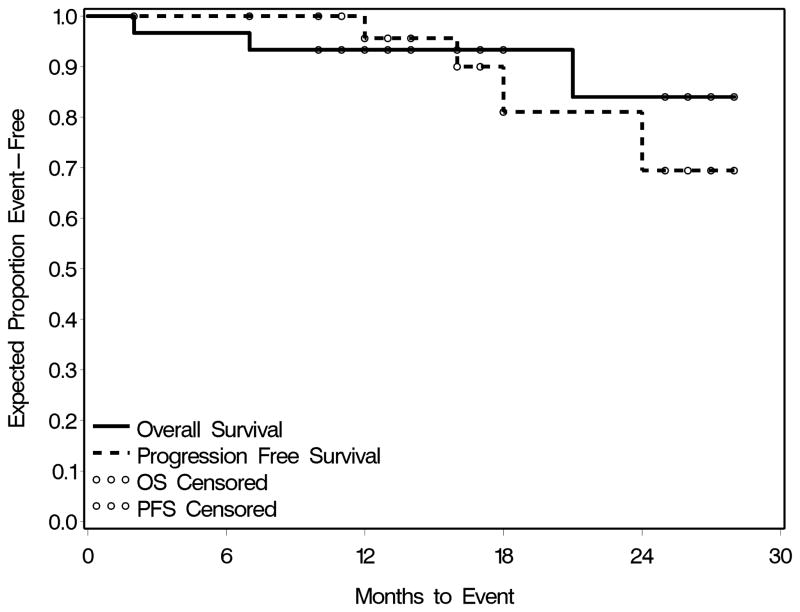
In this intent-to-treat analysis, 24 (80%) patients were alive and disease-free, 3 (10%) were alive with disease, one (3%) died due to disease, one (3%) died due to inter-current illness (acute on chronic hepatitis C), and one (3%) died due to TRM. Sites of failure included local regional (3) or distant (1). The median (range) follow-up of the patients was 25 (2–39) months. OS and PFS are depicted in Figure 2. The estimated 2-year OS for the whole group was 84%, and for the oropharynx and laryngeal cancer subsites were 80% and 86%, respectively. The estimated 2-year PFS for the whole group was 65%, and for the oropharynx and laryngeal cancer subsites were 62% and 64%, respectively.
Figure 2.
Overall and Progression-free Survival Curves
Surgical Treatment Following Definitive Therapy
One patient underwent a neck dissection for a residual neck mass and four underwent biopsies of the primary tumor site to evaluate indeterminate residual mucosal abnormalities. All showed no evidence of malignancy.
DISCUSSION
CR at the primary tumor site as assessed by clinical examination following induction chemotherapy with PF is a favorable predictive factor for OS and disease-control in patients with HNSCC subsequently treated with definitive RT.8,9 In this study, we observed a high CR rate (53%) at the primary tumor site following two cycles of ACPF. These data compare favorably to the likelihood of achieving a CR at the primary site as assessed by clinical examination with induction PF: 21% in laryngeal HNSCC,18 20% in oropharyngeal carcinoma,4 and 33% in oral cavity carcinoma.24 Large primary tumors are associated with a low rate of CR after induction PF.25 In our study, 37% of patients had T4 tumors compared to <16% of patients in these trials with induction PF.
Reported CR rates at the primary site with other induction regimens that included a taxane and/or cetuximab have been variable, but generally lower than comparably staged patients in our ACPF trial. In our historical experience with TPF+Cet, the CR rate at the primary site by clinical examination was only 14% overall (T2:50%;T3:20%;T4:0%).22 Higher rates of CR were reported with a similar TPF+Cet regimen.26,27 Rates of CR at the primary site with TPF varied from 8–40%.28,29 Cremaphor-based paclitaxel added to PF increased the CR rate at the primary site from 33% to 49%.7 A high rate of CR (70%) at the primary site was reported with weekly paclitaxel, cetuximab and carboplatin, but most patients had T1 or T2 tumors.30
Our patient population was enriched in HPV-related oropharyngeal SCC (56%) which could have affected the primary endpoint since such tumors are more chemosensitive.31 However, p16 status did not significantly associate with achieving a CR at the primary tumor site following two cycles of ACPF. This may have occurred because most patients had additional adverse characteristics such as smoking history (90%) and large (T3,4) primary tumors (73%) that offset the favorable predictive effect of HPV status on primary tumor site response to induction chemotherapy.32
Most patients in this study had significant co-morbidities (63%), were smokers, and were older. In this context, the treatment delivery of induction ACPF and of definitive CRT was very good. Nearly all patients (96%) completed three cycles of ACPF with the proportion of planned doses of each drug administered being 87–99%. Of the 29 evaluable patients, 26 (90%) completed the planned 35 fractions of definitive RT per protocol and 81% received >2 of the 3 planned doses of cisplatin concurrently. In comparison, delivery of >2 of 3 planned doses of cisplatin concurrent with definitive RT was reported to be 74% following induction PF33 and 43–66% following induction TPF.34,35
The frequency and distribution of AE’s during ACPF were expected for induction chemotherapy administered to patients with locally advanced HNSCC. Overall, 12 (40%) patients experienced >Grade 3 AE’s, of which 1 (3%) resulted in a TRM. However, several of the Grade 3–4 AE’s (rash, neutropenia, and fatigue) were manageable and did not result in serious sequelae. For comparison, reported rates of ≥ Grade 3 AE’s during induction TPF were 27–85%35,36 during induction docetaxel, cisplatin and cetuximab was 77%26 and during weekly paclitaxel, carboplatin and cetuximab was 45%.30
Consistent with prior reports, we found SPARC expression to be common in tissue specimens obtained from patients with HNSCC.13 In contrast to a prior study, we observed an inverse relationship between SPARC expression in tumor cells and primary tumor site response to ACPF.14 The reason for these disparate findings is unclear. SPARC expression has been shown to correlate with worse prognosis so perhaps this is overcoming the relationship with increased accumulation of tumor cell nab-paclitaxel.
The 2-year OS and PFS for our patients treated with ACPF followed by definitive CRT were 84% and 65%, respectively. These outcomes compare favorably to other reports using induction regimens of taxane and platinum with or without cetuximab.6,7,26,30,35,36,37 Our data are limited by the median follow-up and the heterogeneity in tumor characteristics.
In conclusion, the novel induction regimen of ACPF was feasible and resulted in a high CR rate (53%) at the primary tumor site even in large (T3,4) primary tumors. ACPF did not adversely affect delivery of definitive CRT. Two-year survival outcomes with ACPF followed by definitive CRT were favorable and warrant further investigation.
Acknowledgments
The authors recognize the contributions by Jose Iglesias MD, Rick Hippert, Bruce Haughey MBBS, Jason Diaz MD, Randall Paniello MD, Eric Hallowell, and Nancy Gregory.
This research project was supported in part by the Celgene Corporation and by the Biostatistics Core and Imaging and Response Assessment Core of the Alvin J. Siteman Cancer Center.
Footnotes
Poster presentations were made at American Society of Clinical Oncology meetings of 2010 and 2011.
Disclosures: Honoraria–Bristol-Myers Squibb, Eli Lilly; Research funding-Celgene
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Pignon J, le Maitre A, Maillard E, Bourhis J, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Paccagnella A, Orlando A, Marchiori C, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86:265–272. doi: 10.1093/jnci/86.4.265. [DOI] [PubMed] [Google Scholar]
- 4.Domenge C, Hill C, Lefebvre JL, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d’Etude des Tumeurs de la Tete et du Cou (GETTEC) Br J Cancer. 2000;83:1594–1598. doi: 10.1054/bjoc.2000.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 6.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 7.Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23:8636–8645. doi: 10.1200/JCO.2004.00.1990. [DOI] [PubMed] [Google Scholar]
- 8.Ensley JF, Jacobs JR, Weaver A, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54(5):811–814. doi: 10.1002/1097-0142(19840901)54:5<811::aid-cncr2820540508>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Spaulding MB, Fischer SG, Wolf GT. Tumor response, toxicity, and survival after neoadjuvant organ-preserving chemotherapy for advanced laryngeal carcinoma. The Department of Veterans Affairs Cooperative Laryngeal Cancer Study Group. J Clin Oncol. 1994;12:1592–1599. doi: 10.1200/JCO.1994.12.8.1592. [DOI] [PubMed] [Google Scholar]
- 10.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 11.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 12.John TA, Vogel SM, Tiruppathi C, Malik AB, Minshall RD. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284:L187–196. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 13.Chin D, Boyle GM, Williams RM, et al. Novel markers for poor prognosis in head and neck cancer. Int J Cancer. 2005;113:789–797. doi: 10.1002/ijc.20608. [DOI] [PubMed] [Google Scholar]
- 14.Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC Expression Correlates with Tumor Response to Albumin-Bound Paclitaxel in Head and Neck Cancer Patients. Transl Oncol. 2009;2:59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- 16.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer Staging Manual. 7. New York: Springer; 2009. [Google Scholar]
- 18.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer-specific comorbidity index. Otolaryngol Head Neck Surg. 2002;128:1172–1179. doi: 10.1001/archotol.128.10.1172. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JS, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal Squamous cell carcinoma: An entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34(8):1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuperman DI, Nussenbaum B, Thorstad W, et al. Retrospective analysis of the addition of cetuximab to induction chemotherapy (IC) with docetaxel, cisplatin, and 5-fluorouracil (TPF-C) for locally advanced squamous cell carcinoma of the head and neck (LA-HNSCC) ASCO Meeting Abstracts. 2007;25:6072. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 24.Lefebre J, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx Preservation in pyriform sinus cancer: Preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. J Natl Cancer Inst. 1996;88(13):890–899. doi: 10.1093/jnci/88.13.890. [DOI] [PubMed] [Google Scholar]
- 25.Licitra L, Grandi C, Guzzo M, et al. Primary chemotherapy in resectable oral cavity squamous cell cancer: A randomized controlled trial. J Clin Oncol. 2003;21(2):327–333. doi: 10.1200/JCO.2003.06.146. [DOI] [PubMed] [Google Scholar]
- 26.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin and cetuximab followed by concurrent radiotherapy cisplatin and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28(36):5294–5300. doi: 10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddad RI, TIshler RB, Norris C, et al. Phase I study of C-TPF in patients with locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(27):4448–4453. doi: 10.1200/JCO.2009.22.1333. [DOI] [PubMed] [Google Scholar]
- 28.Schrijvers D, Van Herpen C, Kerger J, et al. Docetaxel, cisplatin and 5-fluorouracil in patients with locally advanced unresectable head and neck cancer: a phase I–II feasibility study. Annals of Oncol. 2004;15(4):638–645. doi: 10.1093/annonc/mdh145. [DOI] [PubMed] [Google Scholar]
- 29.Posner MR, Glisson B, Frenette G, et al. Multicenter phase I–II trial of docetaxel, cisplatin, and fluorouracil induction chemotherapy for patients with locally advanced squamous cell cancer of the head and neck. J Clin Oncol. 2001;19(4):1096–1104. doi: 10.1200/JCO.2001.19.4.1096. [DOI] [PubMed] [Google Scholar]
- 30.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: Results from a phase II prospective trial. J Clin Oncol. 2009;28(1):8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 32.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: Response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26(19):3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urba SC, Moon J, Shankar Giri PG, et al. Organ preservation for advanced resectable cancer of the base of tongue and hypopharynx: A southwest oncology group trial. J Clin Oncol. 2005;23(1):88–95. doi: 10.1200/JCO.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Lefebvre J, Pointreau Y, Rolland F, et al. Sequential chemoradiotherapy (SCRT) for larynx preservation (LP). Preliminary results of the randomized phase II TREMPLIN study. J Clin Oncol. 2009;27(15 supp):303s. [Google Scholar]
- 35.Prestwich RJ, Colpan Oksuz D, Dyker K, Coyle C, Sen M. Feasibility and efficacy of induction docetaxel, cisplatin and 5-fluorouracil chemotherapy combined with cisplatin concurrent chemoradiotherapy for nonmetastatic stage IV head-and-neck squamous cell carcinomas. Int J Rad Oncol Biol Phys. 2011;81(4):e237–e243. doi: 10.1016/j.ijrobp.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 36.Adelstein DJ, Moon J, Hanna E, et al. Docetaxel, cisplatin and fluorouracil induction chemotherapy followed by accelerated fractionation/concomitant boost radiation and concurrent cisplatin in patients with advanced squamous cell head and neck cancer: A Southwest Oncology Group phase II trial. Head & Neck. 2010;32:221–228. doi: 10.1002/hed.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paccagnella A, Ghi MG, Loreggian L, et al. Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5-flurouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol. 2010;21(7):1515–1522. doi: 10.1093/annonc/mdp573. [DOI] [PubMed] [Google Scholar]