Abstract
Phosphatase and tensin homolog (PTEN) is a tumor suppressor in the AKT/mTOR pathway. Animal model studies have shown that loss of PTEN function is involved in the progression of pancreatic cancer. However, the prognostic significance of loss of PTEN expression in pancreatic cancer is unclear. PTEN expression was evaluated by immunohistochemistry on tissue microarrays consisting of multiple cores of 133 resected stage II pancreatic ductal adenocarcinomas. A PTEN expression score was calculated as the product of the percentage of positive tumor cells and the intensity of PTEN staining. We categorized PTEN expression for each tumor as retained (PTEN score>5) or loss (PTEN score ≤5). Thirty-four (25.6%) patients had tumors with loss of PTEN expression, and 99 (74.4%) had tumors with retained PTEN expression. Recurrence/metastasis was observed in 88.2% (30/34) of patients whose tumors showed loss of PTEN compared to 68.7% (68/99) of patients whose tumors showed retained PTEN (p=0.03). Patients whose tumors showed loss of PTEN had a shorter overall survival (median: 19.9±3.6 months) than patients whose tumors had retained PTEN (32.7±5.0 months, p = 0.03). In a multivariate analysis, loss of PTEN expression was an independent prognostic factor for poor overall survival in patients with stage II PDA. No significant correlations between loss of PTEN expression and other clinicopathologic parameters were observed (p>0.05). Assessment of PTEN expression may be used as a prognostic marker for patients with resected pancreatic ductal adenocarcinoma.
Keywords: PTEN, Pancreatic cancer, Survival, Prognosis
INTRODUCTION
Pancreatic ductal adenocarcinoma is the fourth leading cause of cancer death in the United States (1, 2). Approximately 15–20% of patients present with localized disease, and the 5-year overall survival rate is less than 5% (3). Small tumor size, absence of lymph node metastases, and negative resection margins (R0) are independent prognostic factors predicting long term survival in patients with pancreatic ductal adenocarcinoma who have undergone pancreatectomy (4–6).
The development of pancreatic ductal adenocarcinoma represents the accumulation of successive mutations in proto-oncogenes and loss of tumor suppressor genes, typically beginning with activating mutations in KRAS followed by inactivation of tumor suppressor genes, including p16/INK4A, p53, and SMAD4/DPC4 (7–11). KRAS mutations are found in greater than ninety-percent of pancreatic ductal adenocarcinoma and occur early in tumorigenesis (9–11). Once activated, KRAS functions through multiple signaling pathways, including the phosphoinositide 3-kinase/AKT (PI3K/AKT) pathway. Coinciding with KRAS activation, Asano and colleagues have demonstrated that activation of the PI3K/AKT pathway and its downstream effectors, NF-κB and MYC, is essential for growth and survival of pancreatic ductal adenocarcinoma (12).
Phosphatase and tensin homolog (PTEN) is an important tumor suppressor encoded on chromosome 10q23.3 (13, 14). Loss of PTEN function has been implicated in the tumorigenesis of many human malignancies, including gliomas, endometrial cancers, thyroid cancers and pancreatic cancers. Loss of PTEN function can be due to multiple mechanisms, including biallelic or monoallelic deletions, mutations, gene silencing by promoter methylation or dysregulation of mRNA by microRNAs. In mouse model studies, pancreatic-specific knockout of Pten leads to ductal metaplasia from the expansion of centroacinar cells and the development of pancreatic ductal carcinoma (15). Single copy deletion of Pten in KrasG12D mice (Pdx1-Cre LSL-KrasG12D PtenL/+ mice) results in more extensive acinar-ductal metaplasia, pancreatic intra-epithelial neoplasia, increased epithelial proliferation, and higher frequencies of invasion and metastasis compared to Pdx1-Cre LSL-KrasG12D mice (16). Similar results were observed in the KrasG12D mice with conditional Pten knockout (Ptenlox/lox) (17). Pten also functions synergistically with the tumor suppressor, Smad4, during the development and progression of pancreatic cancer in mouse models (18). These data support the notion that PTEN is one of the major tumor suppressors in pancreatic tumorigenesis. Although deletion or loss of function mutations in PTEN have not been detected with significant frequency in pancreatic cancer (19, 20), low or no PTEN expression by immunohistochemistry has been reported in up to 70% of human pancreatic ductal adenocarcinoma samples (16). Recently, Feng et al. reported that PTEN expression in pancreatic ductal adenocarcinoma patients with liver metastasis is lower than those patients who had no liver metastasis (21). They also showed that high PTEN expression is associated with a better 5-year survival in pancreatic ductal adenocarcinoma patients with liver metastasis (21). However, the prognostic value of PTEN expression and its clinical impact in patients who underwent surgical resection for pancreatic ductal adenocarcinoma is not clear. In this study, we sought to evaluate by immunohistochemistry the frequency of loss of PTEN expression in 133 patients with stage II pancreatic ductal adenocarcinoma. The results of PTEN expression were correlated with clinicopathologic parameters and patient survival.
MATERIALS AND METHODS
Patient population
Our study population consisted of 133 patients (78 male and 55 female) with stage II pancreatic ductal adenocarcinoma who underwent pancreatectomy as initial treatment for pancreatic ductal adenocarcinoma at our institution from 1990 to 2010. Patient age ranged from 24.9 to 84.8 years (median age: 64.6 years). Five patients with other disease stages (one patient each with stage IA, IB, or III disease and two patients with stage IV disease) were excluded because there were insufficient case numbers to be representative. Patients who received any form of neoadjuvant chemotherapy and/or radiation therapy were excluded. One hundred, thirteen patients (85.0%) underwent pancreaticoduodenectomy, 18 (13.5%) underwent distal pancreatectomy, and 2 (1.5%) underwent total pancreatectomy. One hundred eight patients (81.2%) had an R0 resection (all resection margins negative by histology) and 25 (18.8%) patients had an R1 resection (microscopic disease involving one or more margins). No patients had an R2 resection. Twenty-five (18.8%) patients received adjuvant chemotherapy alone, 73 (54.9%) received combined adjuvant chemoradiation therapy and 35 (26.3%) did not receive adjuvant therapy. The study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center.
Tissue microarray (TMA) construction
Hematoxylin and eosin (H & E) stained slides and their matched formalin-fixed paraffin embedded tissue blocks were retrieved and reviewed for representative areas of tumor and non-neoplastic pancreatic parenchyma. Two 1.0 mm cores from different areas of each tumor and one 1.0 mm core of the matched non-neoplastic pancreatic tissue were used for the TMA construction. The TMA was constructed as previously described using a tissue microarrayer (Beecher Instruments, Sun Prairie, WI)(22).
Immunohistochemistry for PTEN expression
PTEN immunohistochemistry was performed on 4 μm unstained sections from TMA blocks using a mouse monoclonal antibody against PTEN (DAKO clone 6H2.1, Carpeninteria, CA, 1:100 dilution). The immunohistochemical stained slides were evaluated independently by two pathologists (W.C.F. and H.W.) for percentage of tumor cells with PTEN immunoreactivity and the intensity of PTEN immunoreactivity (0-negative, 1-weak, 2-moderate, and 3-strong). The PTEN expression score was calculated as the product of the percentage of positive tumor cells and the intensity of PTEN staining. We categorized each tumor as either retained PTEN expression (defined as PTEN score >5) or loss of PTEN expression (defined as PTEN score ≤5). For tumors that retained PTEN expression, we further categorized those tumors as either PTEN-low (PTEN score >5, but ≤50) or PTEN-high (PTEN score>50). If there was a discrepancy in PTEN expression between two duplicate cores from the same tumor, the tumor was classified based on the result of the core with positive PTEN staining. All cases with a discrepancy in PTEN expression between pathologists were re-reviewed together, and the consensus results for PTEN expression were used.
Statistical analyses
The immunohistochemical staining results were correlated with clinicopathologic features and survival. Chi-square and Fisher’s exact tests were used to compare categorical data. Overall survival and disease-free survival curves were constructed using a Kaplan-Meier method, and log-rank tests were used to evaluate the statistical significance of differences. The prognostic significance of clinicopathologic parameters and PTEN expression was determined using a Cox-regression multivariate analysis via a backward stepwise procedure. Statistical calculations were performed using Statistical Package for Social Sciences software (version 12.0, SPSS Inc., Chicago, IL). A p-value of <0.05 were considered statistically significant.
RESULTS
PTEN expression in pancreatic ductal adenocarcinoma
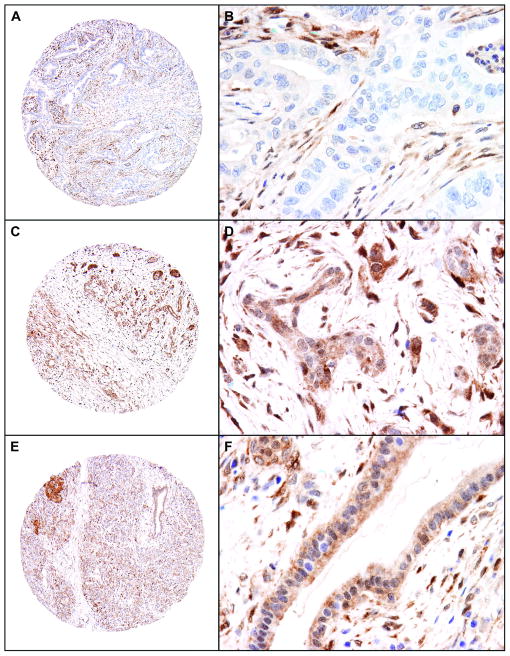
Of the 133 samples, 34 tumors (25.6%) showed loss of PTEN expression (Figure 1A & 1B) and 99 tumors (74.4%) showed retained PTEN expression. In the cases that had retained expression for PTEN, the tumor showed both cytoplasmic and/or nuclear staining (Figure 1C & 1D). Forty-three (32.3%) cases were PTEN-low and 56 (42.1%) cases were PTEN-high. Heterogenous PTEN expression between the two tissue cores of the tumor was observed in 22 (16.5%) cases; in these cases, the tumor was classified based on the core with positive staining. All 22 tumors were classified as retained PTEN expression. The matched non-neoplastic pancreatic tissue cores retained PTEN expression in all cases examined and served as the internal positive controls for our immunohistochemical staining (Figure 1E and F).
Figure 1.
Representative micrographs show PTEN expression in pancreatic ductal adenocarcinoma samples and benign pancreatic tissue. A and B, a moderately differentiated pancreatic ductal adenocarcinoma with negative staining for PTEN; C and D, strong cytoplasmic and nuclear staining for PTEN in a moderately differentiated pancreatic ductal adenocarcinoma; E and F, Representative benign pancreatic tissue that is strongly positive for PTEN (Original magnification, 40× for A, C, and E; 400× for B, D, and F).
Association of PTEN expression and clinicopathologic features
The correlations between PTEN expression and clinicopathologic features are summarized in Table 1. Of 34 patients whose tumor lost PTEN expression, 30 (88.2%) had local recurrence or distant metastasis compared to 68.7% (68/99) of patients whose tumor retained PTEN expression (p=0.03). Lymph node metastasis was present in 88.2% (30/34) in cases that lost PTEN expression, compared to 71.7% (71/99) in the patients whose tumor retained PTEN expression (p=0.05). We did not observe significant correlation between the loss of PTEN expression and patient gender, age, tumor differentiation, tumor size, resection margin status or post operative chemo/radiation therapies (p>0.05, Table 1). Similarly, we did not observe significant difference in any of the clinicopathologic factors between the group with PTEN-low tumors and the group with PTEN-high tumors (data not shown).
Table 1.
Clinicopathologic features correlated with PTEN expression in stage II pancreatic ductal adenocarcinomas
| Characteristics | n | Retained PTEN (n = 99) | Loss of PTEN (n = 34) | p value |
|---|---|---|---|---|
| Age (y) | 0.90 | |||
| <60 | 39 | 28 | 11 | |
| 60–70 | 57 | 43 | 14 | |
| >70 | 37 | 28 | 9 | |
| Gender | 0.67 | |||
| Male | 78 | 57 | 21 | |
| Female | 55 | 42 | 13 | |
| Tumor size (cm) | 0.74 | |||
| <= 2.0 | 22 | 17 | 5 | |
| > 2.0 | 111 | 82 | 29 | |
| Tumor differentiation | 0.92 | |||
| Well | 10 | 8 | 2 | |
| Moderate | 88 | 65 | 23 | |
| Poor | 35 | 26 | 9 | |
| Margin status | 0.07 | |||
| Negative | 108 | 84 | 24 | |
| Positive | 25 | 15 | 10 | |
| Lymph node status (stage) | 0.05 | |||
| Negative (IIA) | 32 | 28 | 4 | |
| Positive (IIB) | 101 | 71 | 30 | |
| Adjuvant chemo/radiation | 0.26 | |||
| No | 35 | 29 | 6 | |
| Yes | 98 | 70 | 28 | |
| Recurrence or distant metastasis | 0.009 | |||
| No | 35 | 31 | 4 | |
| Local recurrence | 26 | 14 | 12 | |
| Distant metastasis | 72 | 54 | 18 |
Loss of PTEN expression correlated with poor overall survival
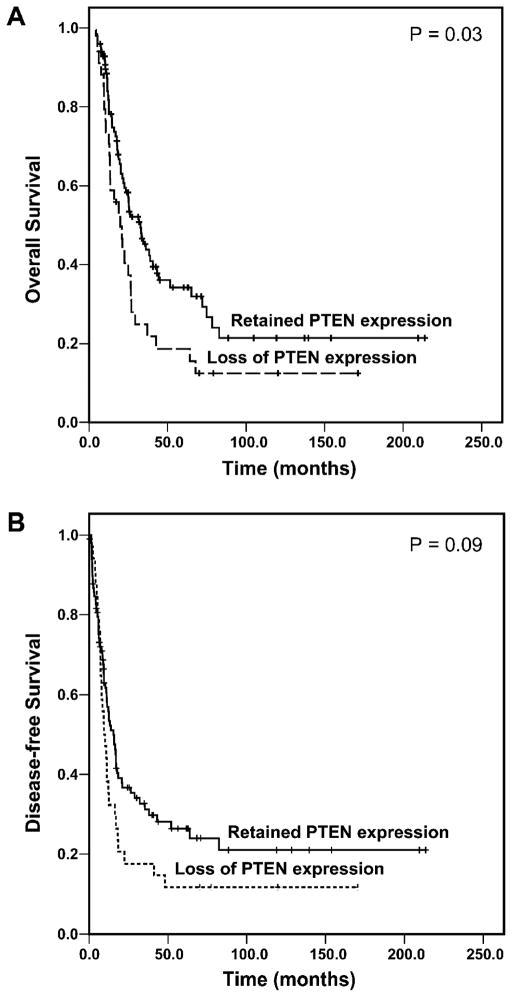
The median follow up time was 18.5 months (range: 4.2 to 82.9 months) for 88 patients who died of disease. For 45 patients who did not die from the disease, the follow-up time ranged from 7.1 months to 214.0 months with the median follow-up time of 40.7 months after pancreatectomy. No patients died in the immediate postoperative period, and none were lost to follow up. The median disease free survival and overall survival in this study population were 12.1 ± 1.9 months and 25.2 ± 3.0 months, respectively. Loss of PTEN expression correlated with poor overall survival. For the patients whose tumor retained PTEN expression, the median overall survival was 32.7 ± 5.0 months compared to the median overall survival of 19.9 ± 3.6 months for patients whose tumor lost PTEN expression (p=0.03, log-rank method, Figure 2A). Patients whose tumor retained PTEN expression also had longer disease free survival (median: 15.4 ± 2.0 months) than those whose tumor lost PTEN expression (median: 9.4 ± 1.7 months) but was not statistically significant (p=0.09, log-rank method, Figure 2B). The univariate analyses of overall survival in correlation with the clinicopathologic parameters and PTEN expression are shown in Table 2. In addition to the loss of PTEN protein expression, overall survival was also associated with tumor size, margin status, and lymph node metastasis (p<0.05) but not other clinicopathologic factors (p≥0.05). No significant difference in either disease free survival or overall survival between the group with PTEN-low tumors and the group with PTEN-high tumors was observed (data not shown). In multivariate analysis, loss of PTEN expression was a prognostic factor for overall survival in patients with stage II disease independent of tumor size, margin status, and lymph nodal metastases (Table 3).
Figure 2.
Kaplan-Meier curves for overall survival (A) and disease-free survival (B) by PTEN expression in patients with stage II pancreatic ductal adenocarcinoma. Patients whose tumors lost PTEN expression had shorter overall survival than patients whose tumors retained PTEN expression.
Table 2.
Univariate analysis of overall survival in patients with stage II pancreatic ductal adenocarcinomas
| Variables | n | HR (95% CI) | p value |
|---|---|---|---|
| Age | |||
| <60 years | 39 | 1.00 | |
| 60 – 70 years | 57 | 1.07 (0.65 – 1.77) | |
| >70 years | 37 | 1.45 (0.83 – 2.55) | 0.39 |
| Gender | |||
| Female | 55 | 1.00 | 0.14 |
| Male | 78 | 0.73 (0.48 – 1.11) | |
| Tumor differentiation | |||
| Well | 10 | 1.00 | |
| Moderate | 88 | 2.70 (1.08 – 6.76) | |
| Poor | 35 | 2.62 (0.99 – 6.96) | 0.05 |
| Tumor size (cm) | |||
| <= 2.0 | 22 | 1.00 | |
| > 2.0 | 111 | 3.25 (1.56 – 6.77) | 0.002 |
| Margin status | |||
| Negative | 108 | 1.00 | |
| Positive | 25 | 1.75 (1.04 – 2.93) | 0.04 |
| Lymph node status (stage) | |||
| Negative (IIA) | 32 | 1.00 | |
| Positive (IIB) | 101 | 2.05 (1.18 – 3.55) | 0.01 |
| Adjuvant chemo/radiation | |||
| No | 35 | 1.00 | |
| Yes | 98 | 0.69 (0.42 – 1.14) | 0.15 |
| PTEN expression | |||
| Retained | 99 | 1.00 | |
| Loss | 34 | 1.65 (1.06 – 2.58) | 0.03 |
Abbreviations: HR, hazard ratio; CI, confidence interval
Table 3.
Multivariate analysis of overall survival in patients with stage II pancreatic ductal adenocarcinomas
| Variables | n | HRa (95% CIa) | p value |
|---|---|---|---|
| Tumor size (cm) | |||
| <= 2.0 | 22 | 1.00 | |
| > 2.0 | 111 | 3.47 (1.66 – 7.25) | 0.001 |
| Margin status | |||
| Negative | 108 | 1.00 | |
| Positive | 25 | 1.39 (0.82 – 2.36) | 0.228 |
| Lymph node status (stage) | |||
| Negative (IIA) | 32 | 1.00 | |
| Positive (IIB) | 101 | 1.52 (0.83 – 2.80) | 0.178 |
| PTEN expression | |||
| Retained | 99 | 1.00 | |
| Loss | 34 | 1.79 (1.14 – 2.80) | 0.011 |
Abbreviations: HR, hazard ratio; CI, confidence interval
DISCUSSION
In this study, we examined the PTEN expression by immunohistochemistry in 133 patients with stage II pancreatic ductal adenocarcinoma. We found that loss of PTEN protein expression correlated with tumor recurrence/distant metastasis after pancreatectomy and shorter overall survival. Loss of PTEN protein expression was an independent prognostic factor in our patient population. Our data supports the notion that PTEN is an important tumor suppressor in the tumorigenesis and progression of pancreatic ductal adenocarcinoma.
PTEN has been implicated in the tumorigenesis and progression of pancreatic ductal adenocarcinoma. It has been demonstrated that heterozygous Pten deletion in the pancreas accelerates the development of pancreatic intraepithelial neoplasia and pancreatic ductal adenocarcinoma in KrasG12D pancreatic cancer mouse model(17) and that Pten haploinsufficiency was sufficient to induce development of pancreatic ductal adenocarcinoma in murine models. Moreover, mouse survival was dictated in a Pten dose-dependent fashion(16). To test this hypothesis in human pancreatic ductal adenocarcinoma samples, we compared the clinicopathologic parameters and survival between a group of patients’ tumors with retained PTEN expression and another with loss of PTEN expression. In this study, low or no PTEN expression was observed in 57.9% of stage II pancreatic ductal adenocarcinoma samples. Our findings were consistent with the previous reports, which identified 38.8% and 70% of pancreatic ductal adenocarcinoma samples have low or no PTEN expression (16, 21). We found loss of PTEN expression in 25.6% of our cohort, which was similar to results reported by others using immunohistochemistry performed on both whole tissue and TMA sections (12, 23–25). Given that our study included tumor samples from 133 patients, our results in conjunction with earlier studies show that loss of PTEN staining is not an infrequent occurrence in pancreatic ductal adenocarcinoma.
PTEN functions as an important tumor suppressor in the tumorigenesis of many human malignancies. Loss of PTEN protein expression is reported to be associated with worse survival in advanced endometrial cancer, lung, breast, and colon cancers (26–28), (29, 30). The prognostic significance of loss of PTEN expression in patients with pancreatic ductal adenocarcinoma was unclear. Feng et al reported high levels of PTEN expression in 61.2% pancreatic ductal adenocarcinoma patients without liver metastasis compared to 29.9% in pancreatic ductal adenocarcinoma patients who had liver metastasis (21). Their data suggest that loss of PTEN plays a role in liver metastasis in patients with pancreatic ductal adenocarcinoma. Consistent with this notion, we found that loss of PTEN expression was associated with higher frequency of recurrence/distant metastasis in patients with stage II pancreatic ductal adenocarcinoma compared to those patients whose tumor retained PTEN expression. In addition, we also found that loss of PTEN expression was associated with poor overall survival and was an independent prognostic factor in our patient population with stage II pancreatic ductal adenocarcinoma by multivariate analysis. Loss of nuclear staining for PTEN has been reported to be associated with poor survival in colon cancer (29, 30). In this study, we did not observe a significant correlation in the loss of nuclear staining for PTEN with either disease free survival or overall survival in our study population. Our data did not support the previous report by Pham and colleagues, who found a higher percentage of stage I and II tumors had low levels of PTEN protein expression than stage III and stage IV tumors (25). The limitation of our study was the inclusion of only patients with stage II disease as there were only a small number of patients with other stages of disease. However, our findings are corroborated by a recent report demonstrating high levels of PTEN expression associated with a better 5-year survival rate compared to those with no or low PTEN expression in patients with pancreatic ductal adenocarcinoma metastatic to the liver (21).
The mechanisms of loss PTEN expression in pancreatic ductal adenocarcinoma are unclear. Recently, Ying et al demonstrated the deletion of one or two copies of the PTEN locus on 10q23 in 15% of the 61 pancreatic ductal adenocarcinoma samples examined using high resolution array comparative genomic hybridization (16). This finding suggested that loss of PTEN expression in pancreatic ductal adenocarcinoma may be due to the deletion of PTEN locus, which had been reported in other malignancies. The loss of PTEN expression is accompanied by the gain/amplification in AKT2 locus and activation of AKT. The aberrant activation of PI3K/AKT pathway by loss of PTEN and/or activation of AKT accelerates mutant KRAS driven malignant progression of pancreatic ductal adenocarcinoma (31). Our findings that loss of PTEN expression correlated with recurrence/distant metastasis and poor overall survival in patients with pancreatic ductal adenocarcinoma provides additional support for the functions of PTEN/PI3K pathway in pancreatic cancer progression. Therefore, targeting PI3K/mTOR pathway in combination with other treatment modality may provide more effective treatment for pancreatic ductal adenocarcinoma.
In summary, loss of PTEN is not an infrequent occurrence in pancreatic ductal adenocarcinoma. Loss of PTEN expression correlates with recurrence/distant metastasis and survival in patients with stage II pancreatic ductal adenocarcinoma. Thus, loss of PTEN expression supports the implication that PTEN function plays an important role in progression of pancreatic ductal adenocarcinoma. Evaluation of PTEN expression by immunohistochemistry has prognostic implications in patients with stage II pancreatic cancer and may have therapeutic implications as well. As such, the utility of targeting the PI3K/Akt pathway via PI3K inhibitors and mTOR inhibitors should be evaluated in pancreatic ductal adenocarcinoma.
Acknowledgments
Supported by the National Institutes of Health grant (1R21CA149544-01A1) and G. S. Hogan Gastrointestinal Cancer Research Fund at The University of Texas M.D. Anderson Cancer Center
Footnotes
DISCLOSURE/CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 6.Tempero MA, Arnoletti JP, Behrman S, Ben-Josef E, Benson AB, 3rd, Berlin JD, Cameron JL, Casper ES, Cohen SJ, Duff M, Ellenhorn JD, Hawkins WG, Hoffman JP, Kuvshinoff BW, 2nd, Malafa MP, Muscarella P, 2nd, Nakakura EK, Sasson AR, Thayer SP, Tyler DS, Warren RS, Whiting S, Willett C, Wolff RA. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8:972–1017. doi: 10.6004/jnccn.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 8.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 9.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Kloppel G, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 10.Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140–2143. [PubMed] [Google Scholar]
- 11.Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988;16:7773–7782. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 14.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23. 3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 15.Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng KH, McLaughlin M, Brown D, Depinho RA, Wu H, Melton DA, Dor Y. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H, Fletcher-Sananikone E, Zhang H, Liu Y, Wang W, Ren X, Zheng H, Kimmelman AC, Paik JH, Lim C, Perry SR, Jiang S, Malinn B, Protopopov A, Colla S, Xiao Y, Hezel AF, Bardeesy N, Turley SJ, Wang YA, Chin L, Thayer SP, Depinho RA. Pten is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov. 2011;1:158–169. doi: 10.1158/2159-8290.CD-11-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, Dry S, Wu H. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010;70:7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Ehdaie B, Ohara N, Yoshino T, Deng CX. Synergistic action of Smad4 and Pten in suppressing pancreatic ductal adenocarcinoma formation in mice. Oncogene. 2010;29:674–686. doi: 10.1038/onc.2009.375. [DOI] [PubMed] [Google Scholar]
- 19.Okami K, Wu L, Riggins G, Cairns P, Goggins M, Evron E, Halachmi N, Ahrendt SA, Reed AL, Hilgers W, Kern SE, Koch WM, Sidransky D, Jen J. Analysis of PTEN/MMAC1 alterations in aerodigestive tract tumors. Cancer Res. 1998;58:509–511. [PubMed] [Google Scholar]
- 20.Sakurada A, Suzuki A, Sato M, Yamakawa H, Orikasa K, Uyeno S, Ono T, Ohuchi N, Fujimura S, Horii A. Infrequent genetic alterations of the PTEN/MMAC1 gene in Japanese patients with primary cancers of the breast, lung, pancreas, kidney, and ovary. Jpn J Cancer Res. 1997;88:1025–1028. doi: 10.1111/j.1349-7006.1997.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng C, Yao R, Huang F, Liu X, Nie W. High Level of PTEN Expression Is Associated with Low-grade Liver Metastasis and Satisfactory Patient Survival in Pancreatic Cancer. Archives of medical research. 2011;42:584–588. doi: 10.1016/j.arcmed.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain pathology. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellizzi AM, Bloomston M, Zhou XP, Iwenofu OH, Frankel WL. The mTOR pathway is frequently activated in pancreatic ductal adenocarcinoma and chronic pancreatitis. Appl Immunohistochem Mol Morphol. 2010;18:442–447. doi: 10.1097/PAI.0b013e3181de115b. [DOI] [PubMed] [Google Scholar]
- 24.Ebert MP, Fei G, Schandl L, Mawrin C, Dietzmann K, Herrera P, Friess H, Gress TM, Malfertheiner P. Reduced PTEN expression in the pancreas overexpressing transforming growth factor-beta 1. Br J Cancer. 2002;86:257–262. doi: 10.1038/sj.bjc.6600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham NA, Schwock J, Iakovlev V, Pond G, Hedley DW, Tsao MS. Immunohistochemical analysis of changes in signaling pathway activation downstream of growth factor receptors in pancreatic duct cell carcinogenesis. BMC Cancer. 2008;8:43. doi: 10.1186/1471-2407-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanamori Y, Kigawa J, Itamochi H, Sultana H, Suzuki M, Ohwada M, Kamura T, Sugiyama T, Kikuchi Y, Kita T, Fujiwara K, Terakawa N. PTEN expression is associated with prognosis for patients with advanced endometrial carcinoma undergoing postoperative chemotherapy. Int J Cancer. 2002;100:686–689. doi: 10.1002/ijc.10542. [DOI] [PubMed] [Google Scholar]
- 27.Sawai H, Yasuda A, Ochi N, Ma J, Matsuo Y, Wakasugi T, Takahashi H, Funahashi H, Sato M, Takeyama H. Loss of PTEN expression is associated with colorectal cancer liver metastasis and poor patient survival. BMC Gastroenterol. 2008;8:56. doi: 10.1186/1471-230X-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Hsu CP, Kao TY, Chang WL, Nieh S, Wang HL, Chung YC. Clinical significance of tumor suppressor PTEN in colorectal carcinoma. Eur J Surg Oncol. 2011;37:140–147. doi: 10.1016/j.ejso.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Jang KS, Song YS, Jang SH, Min KW, Na W, Jang SM, Jun YJ, Lee KH, Choi D, Paik SS. Clinicopathological significance of nuclear PTEN expression in colorectal adenocarcinoma. Histopathology. 2010;56:229–239. doi: 10.1111/j.1365-2559.2009.03468.x. [DOI] [PubMed] [Google Scholar]
- 31.Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Skele KL, Hoffman JP, Testa JR. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem. 2002;87:470–476. doi: 10.1002/jcb.10287. [DOI] [PubMed] [Google Scholar]