Abstract
Vesicular stomatitis virus (VSV) is a negative-sense single-stranded RNA virus that closely resembles its deadly cousin, rabies virus. In mice, VSV elicits a rapid and severe T cell–independent encephalitis, indicating that resident glial cells play an important role in the initiation of central nervous system (CNS) inflammation. Recently, retinoic acid–inducible gene I (RIG-I)-like helicases have been shown to function as intracellular pattern recognition receptors for replicative viral RNA motifs. In the present study, the authors demonstrate that the expression of two members of this RIG-I–like receptor family (RLR), RIG-I and melanoma differentiation-associated antigen 5 (MDA5), are elevated in mouse brain tissue following intranasal administration of VSV. Using isolated cultures of primary murine glial cells, the authors demonstrate that microglia and astrocytes constitutively express both RIG-I and MDA5 transcripts and protein. Importantly, the authors show that such expression is elevated following challenge with VSV or another negative-sense RNA virus, Sendai virus. The authors provide evidence that such induction is indirect and secondary to the production of soluble mediators by infected cells. Circumstantial evidence for the functional nature of RLR expression in glial cells comes from the observation that microglia express the RLR downstream effector molecule, interferon promoter stimulator-1, and demonstrate diminished levels of the negative RLR regulator, laboratory of genetics and physiology 2, following viral challenge. These findings raise the exciting possibility that RLR molecules play important roles in the detection of viral CNS pathogens and the initiation of protective immune responses or, alternatively, the progression of damaging inflammation within the brain.
Keywords: astrocytes, microglia, pattern recognition receptors, rhabdoviruses, RIG-I-like receptors
Introduction
The order Mononegavirales includes numerous viral etiological agents of fatal human diseases with central nervous system (CNS) involvement, most notably rabies virus. Like rabies virus, vesicular stomatitis virus (VSV) is a negative-sense single-stranded RNA virus belonging to the Rhabdoviridae family, and closely resembles its deadly cousin. VSV is neurotropic in mice and has been shown to elicit rapid and severe encephalitis, with a high degree of mortality, following intranasal inoculation (Huneycutt et al, 1993). Interestingly, VSV-induced encephalitis appears to be T cell independent, having been observed in athymic mice after viral administration (Frei et al, 1989). As such, it is likely that the innate immune functions of resident CNS cells play an important role in the rapid inflammatory response following VSV infection.
There is a growing appreciation that resident CNS cells such as microglia and astrocytes can initiate and augment inflammation following trauma or infection. These important glial cells are ideally situated to detect traumatic injury or the presence of pathogens and microglia can respond by migrating to the site of injury where they proliferate. They become activated at the site of challenge and assume many of the immune effector functions typically associated with macrophages and dendritic cells, including the production of key proinflammatory molecules such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 (Streit et al, 1998; Kiefer et al, 1993). The ability of microglia and astrocytes to respond to VSV has previously been demonstrated (Bi et al; 1995, Christian et al, 1996), but the mechanisms underlying glial cell activation following infection with this viral pathogen have not been defined.
Microglia and astrocytes possess cell surface Toll-like receptors (TLRs), including TLR3, TLR7, TLR8, and TLR9, that can perceive viral motifs present in the extracellular milieu or that have been internalized (as reviewed in Konat et al, 2006). Recently, a newly described group of molecules have been shown to function as intracellular sensors for replicative viral RNA (Takeuchi and Akira, 2007). The retinoic acid–inducible gene I (RIG-I)–like helicases, RIG-I and melanoma differentiation-associated antigen 5 (MDA5), are soluble proteins found in the cytosol of many cell types and have been shown to mediate innate immune responses to viral RNA (Meylan and Tschopp, 2006). Interestingly, accumulating evidence suggests that these molecules can discriminate between different virus types despite utilizing similar signaling pathways. Genomic RNA from several Mononegaviruses, including VSV and the paramyxovirus, Sendai virus (SeV), has been demonstrated to specifically activate immune responses via RIG-I (Hiscott, 2007; Yoneyama and Fujita, 2007). In contrast, picornaviruses, which are positive-sense RNA viruses, and synthetic poly(I:C) molecules appear to initiate MDA5-mediated effects (Gitlin et al, 2006; Kato et al, 2006; Loo et al, 2008). To date, the expression of these novel pattern recognition receptors has not been investigated in murine microglia and astrocytes.
In the present study, we demonstrate that primary murine glial cells constitutively express RIG-I and MDA5 as well as their essential downstream effector molecules. Furthermore, we show that the expression of these molecules is significantly up-regulated following exposure to VSV or SeV. As such, the presence of these intracellular sensors for replicative viral motifs may reflect an important role for such molecules in the immune response of microglia and astrocytes following CNS infection.
Results
RIG-I and MDA5 are expressed in the CNS following VSV infection
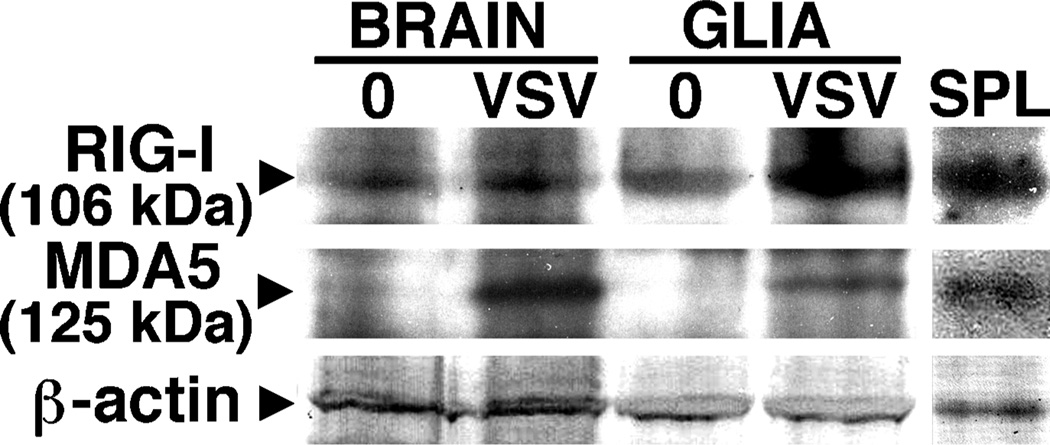
To begin to determine whether members of the RIG-I–like receptor (RLR) family of viral sensors are expressed by resident cells of the CNS, mice were infected intranasally with VSV (1 × 107 plaque-forming units [PFU]), and RIG-I and MDA5 protein expression were determined by immunoblot analyses of protein isolates prepared from whole-brain tissue homogenates or acutely isolated mixed glial cells. Reagents for murine RIG-I proteins are not currently available, but we employed polyclonal antibodies directed against human RIG-I molecules to detect the presence of the murine protein due to the 88% homology between the peptide used to generate this antibody and the corresponding sequence in the mouse protein. As shown in Figure 1, low level constitutive expression of RIG-I was detectable in both whole-brain tissue and glial cells. In whole-brain homogenates, RIG-I expression showed a modest in- crease (49 ± 7 to 68 ± 13 arbitrary densitometric units) following in vivo VSV infection, whereas levels of this protein showed a 3.3-fold induction in glial cells isolated from infected animals versus that seen in cells from uninfected mice (Figure 1). Brain tissue showed very low constitutive levels of MDA5 protein expression and very low levels were seen in isolated glial cells (Figure 1). Unexpectedly, expression of this RLR was significantly up-regulated in brain homogenates following VSV infection (18 ± 7 to 80 ± 14 arbitrary densitometric units) and, in contrast to cells from untreated mice, MDA5 was readily detectable in mixed glial cells from infected animals (Figure 1).
Figure 1.
Members of the RLR family of viral sensors are expressed in the CNS following in vivo infection with VSV. Mice were infected intranasally with VSV (1 × 107 PFU) and at 5 days post infection, protein isolates were prepared from whole brain tissue homogenates (Brain) or isolated mixed glial cells (Glia) and RIG-I and MDA5 protein expression were determined by immunoblot analyses. Immunoblots shown are representative of three separate experiments. For comparison purposes RLR protein expression in spleen tissue protein isolates is shown (SPL).
Primary microglia express RIG-I and MDA5
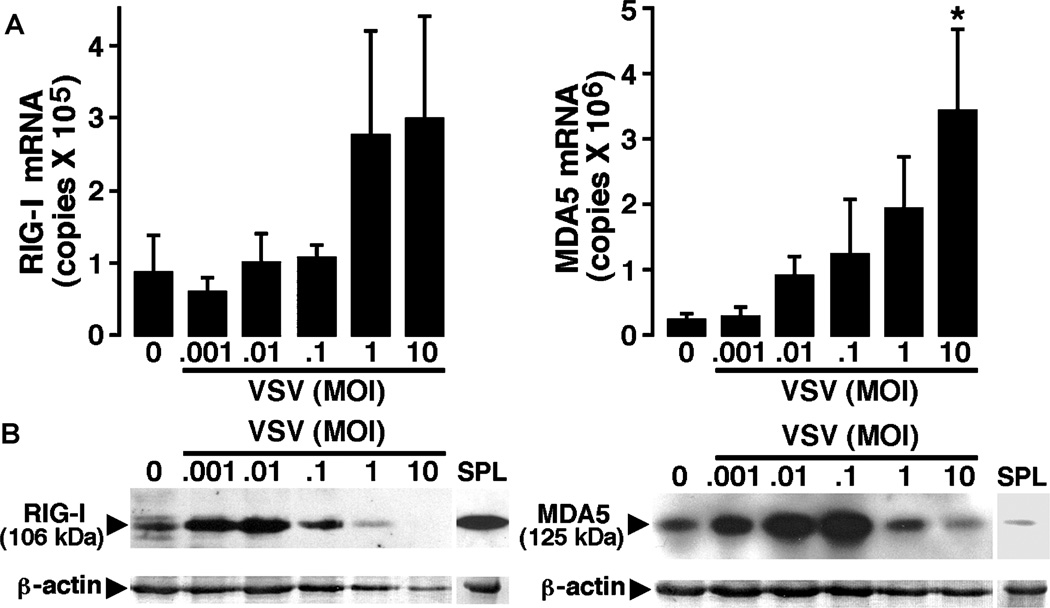
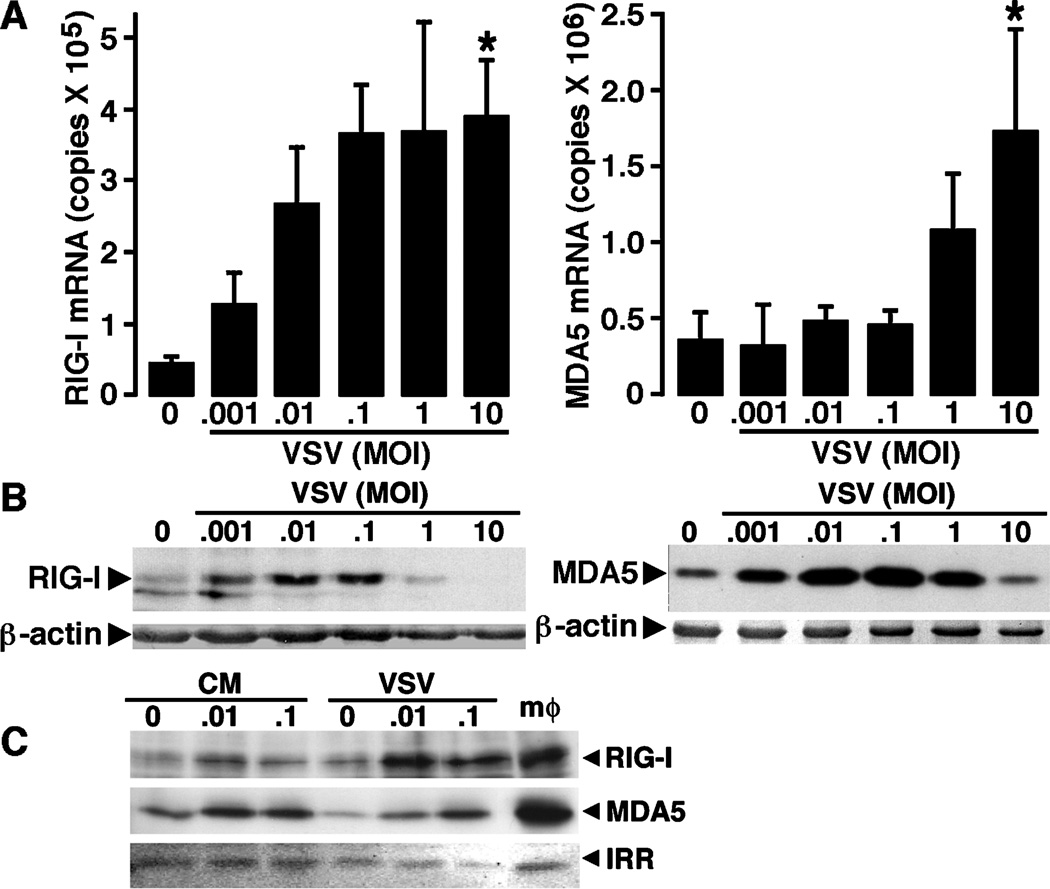
To determine whether microglia express these newly described pattern recognition receptors, we have assessed the level of mRNA expression encoding RIG-I and MDA5 in isolated cultures of murine microglia either at rest, or following challenge with VSV. Murine microglia were untreated or exposed to VSV at varying multiplicities of infection (MOIs) prior to RNA isolation at 4 and 8 h post infection. Real-time polymerase chain reaction (PCR) was performed for the presence of mRNA encoding RIG-I and MDA5. As shown in Figure 2A, microglia express high copy numbers of mRNA encoding RIG-I constitutively and there was a tendency for such expression to increase at 4 h following VSV infection, although this effect was not significant. Similarly, these resident sentinel CNS cells constitutively expressed high copy numbers of mRNA encoding MDA5 (Figure 2A). Interestingly, this pathogen elicited a significant and dose-dependent increase in the level of expression of MDA5mRNA at 4 (Figure 2A) and 8 (data not shown) h following viral challenge.
Figure 2.
Primary murine microglia constitutively express members of the RLR family of cytosolic pattern recognition receptors and such expression is elevated following viral challenge. Cells (2 × 106) were untreated (0) or exposed to VSV (ratios of viral particles to cells: 0.001:1, 0.01:1, 0.1:1, 1:1, and 10:1). (A) At 4 h following infection, mRNA was isolated and real-time PCR performed to determine the level of expression of mRNA encoding RIG-I or MDA5. Data are presented as the mean ± SEM of four experiments normalized to the expression of G3PDH. Asterisk indicates a statistically significant difference from uninfected microglia. (B) At 24 h post infection, whole-cell protein isolates were prepared and RIG-I and MDA5 protein expression were determined by immunoblot analyses. Immunoblots shown are representative of four separate experiments. For comparison purposes RLR protein expression in spleen tissue protein isolates is shown (SPL).
To determine whether expression of mRNA encoding RLRs in microglia translates into expression of these proteins, immunoblot analyses were performed. As shown in Figure 2B and consistent with the RIG-I mRNA expression, murine microglia constitutively express robust levels of RIG-I protein. Importantly, exposure of these cells to low MOI of VSV for 12 (data not shown) and 24 (Figure 2B) h resulted in a significant increase in RIG-I protein expression, with a maximal 2.14 ± 0.18-fold induction at a MOI of 0.01 PFU/cell, as determined by densitometric analysis, whereas high viral titers diminished such expression perhaps due to cell death or impairment of protein translation as evidenced by concomitant reductions in β-actin levels (Figure 2B). Similarly, resting microglia expressed detectable levels of MDA5 (Figure 2B). Consistent with the ability of VSV to elevate MDA5 mRNA expression, this virus significantly increased MDA5 protein expression at 12 (data not shown) and 24 (Figure 2B) h post infection, with a maximal 2.50 ± 0.35-fold induction at a MOI of 0.1 PFU/cell, as determined by densitometric analysis. Indeed, such expression was markedly higher than that observed in spleen tissue used as a positive control for this RLR (Figure 2B).
SeV also up-regulates the expression of RIG-I and MDA5
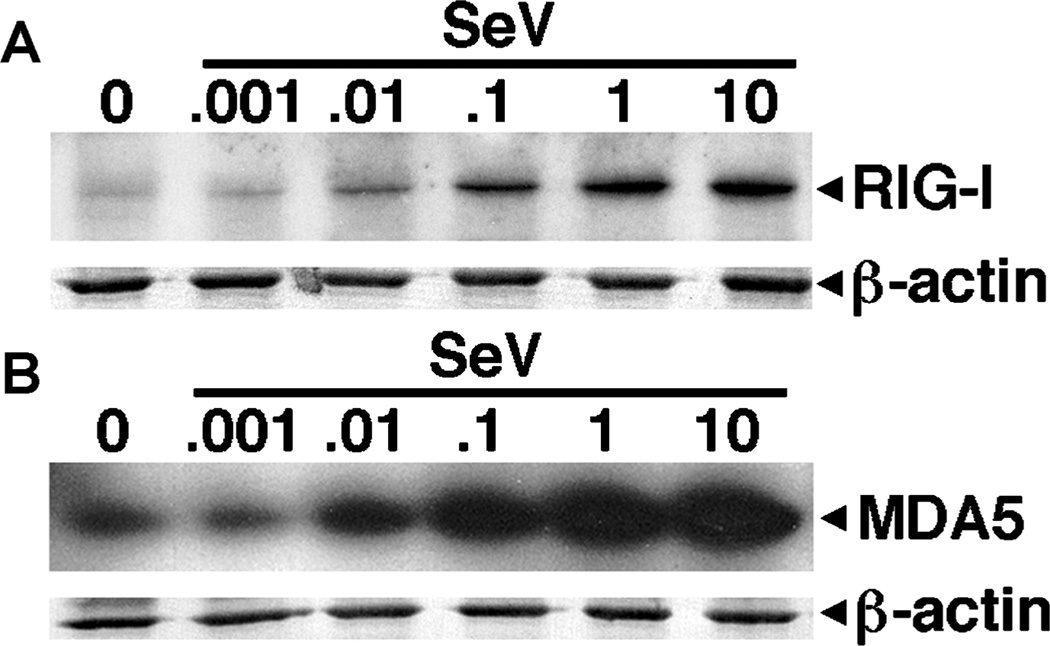
We have investigated the effect of another RIG-I–specific viral pathogen, the paramyxovirus SeV, on RLR expression in murine microglia. At 12 (data not shown) and 24 (Figure 3A) h following viral challenge, SeV elicited dose-dependent increases in RIG-I protein expression (6.2-fold induction at a MOI of 10 PFU/cell). Similar to VSV, SeV also elicited marked elevations in MDA5 expression (Figure 3B), with a maximal 3.3-fold induction at a MOI of 10 PFU/cell. It is noteworthy that high titers of this virus do not reduce RIG-I or MDA5 expression. This could be attributed to a lack of effect of this virus on microglial viability/protein production, as evidenced by the constant β-actin expression. Taken together, these data suggest that a distantly related virus, previously shown to be recognized by RIG-I, can elevate the expression of this viral sensor in microglia. However, it is apparent that VSV and SeV can also induce the expression of MDA5, a receptor that is not thought to be important in mediating cell responses to these viruses.
Figure 3.
Microglial RLR protein expression is less sensitive to the paramyxovirus Sendai virus than to VSV. Cells (2 × 106) were untreated (0) or exposed to Sendai virus (SeV; ratios of viral particles to cells: 0.001:1, 0.01:1, 0.1:1, 1:1, and 10:1). At 24 h post infection, whole-cell protein isolates were prepared and RIG-I (A) and MDA5 (B) protein expression were determined by immunoblot analyses. Immunoblots shown are representative of three separate experiments.
RLR expression is indirectly up-regulated by VSV infection
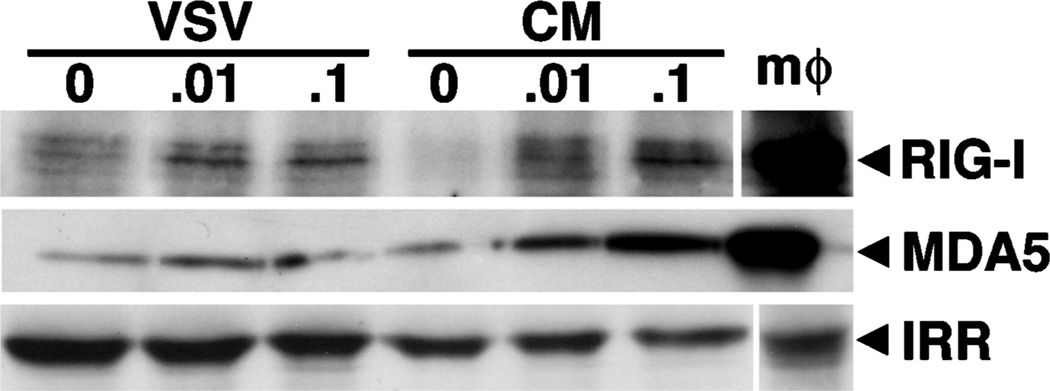
The ability of VSV to induce the expression of a second RLR member that is not thought to recognize this virus suggests that such regulation may be indirect. To confirm this hypothesis, cells were exposed to filtered conditioned medium devoid of infectious viral particles prepared from microglia that were exposed to low titers of VSV for 1 h prior to washing and 12 h of culture. As shown in Figure 4, conditioned medium was able to induce the protein expression of both RIG-I and MDA5 after 12 h in a similar manner to that seen in infected cells, with maximal 2.83 ± 1.29-fold and 1.90 ± 0.21-fold inductions, respectively, at a MOI of 0.1 PFU/cell, as determined by densitometric analysis. These data indicate that VSV-induced increases in RLR expression can occur via a soluble mediator acting in a paracrine or autocrine manner.
Figure 4.
Conditioned media from VSV-infected microglia can elevate RLR expression in uninfected cells. Cells (2 × 106) were untreated (0) or exposed to VSV (ratios of viral particles to cells: 0.01:1 and 0.1:1) for 1 h prior to washing. At 12 h following infection, whole-cell protein isolates were prepared and the culture medium was taken. This conditioned medium was filtered and placed on uninfected microglia cultures for a further 12 h prior to preparation of whole-cell protein isolates from these cells. RIG-I and MDA5 expression was then assessed by immunoblot analysis in isolates from VSV-infected cells (VSV) and those exposed to condition medium (CM). Immunoblots shown are representative of three separate experiments and the expression of an irrelevant protein is shown (IRR). For comparison purposes, RLR protein expression in a similar number of stimulated peritoneal macrophages is shown (mφ).
Microglia express downstream effector molecules for RLR signaling
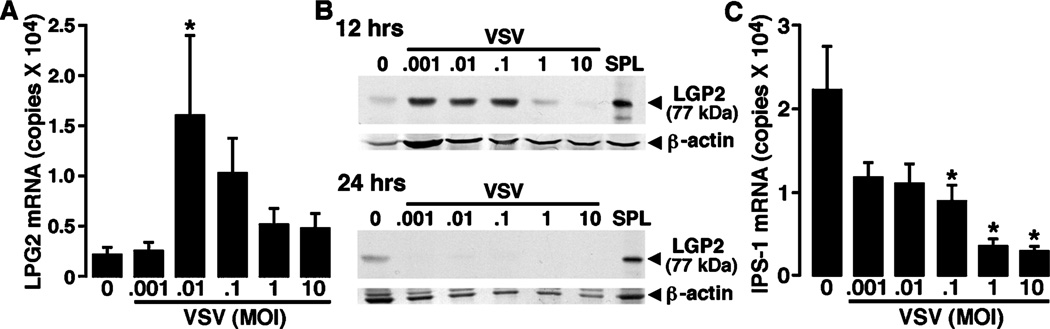
To begin to determine if RLR proteins are functional in microglia, we have investigated whether these cells express laboratory of genetics and physiology 2 (LGP2), a regulator of RLR-mediated cell signaling, and interferon promoter stimulator-1 (IPS-1), a critical downstream effector molecule in both RIG-I– and MDA5-mediated cellular activation (Kumar et al, 2006). As shown in Figure 5A, microglia constitutively express low levels of mRNA encoding LGP2. However, such expression is rapidly (4 h), and markedly, up-regulated following exposure to low titers of VSV (Figure 5A). We have confirmed that resting microglia express the LGP2 protein by immunoblot analysis and show that the rapid upregulation in LGP2 mRNA is mirrored by a maximal 5.6-fold induction in the expression of the protein at 12 h following VSV infection at a MOI of 0.1 PFU/cell (Figure 5B). Interestingly, this induction was short lived, as LGP2 protein levels were significantly reduced to almost undetectable levels at 24 h post infection (Figure 5B).
Figure 5.
Primary murine microglia constitutively express downstream regulators of RLR function and such expression is modulated following viral challenge. Cells (2 × 106) were untreated (0) or exposed to VSV (ratios of viral particles to cells: 0.001:1, 0.01:1, 0.1:1, 1:1, and 10:1). (A) At 4 h following infection, mRNA was isolated and real-time PCR performed to determine the level of expression of mRNA encoding LGP2. Data are presented as the mean ± SEM of six experiments normalized to the expression of G3PDH. Asterisk indicates a statistically significant difference from uninfected microglia. (B) At 12 and 24 h post infection, whole-cell protein isolates were prepared and LGP2 protein expression was determined by immunoblot analyses. Immunoblots shown are representative of four separate experiments. For comparison purposes, RLR protein expression in spleen tissue protein isolates is shown (SPL). (C) At 4 h following infection, mRNA was isolated and real-time PCR performed to determine the level of expression of mRNA encoding IPS-1. Data are presented as the mean ± SEM of three experiments normalized to the expression of G3PDH. Asterisk indicates a statistically significant difference from uninfected microglia.
Next, we have investigated whether murine microglia express mRNA encoding IPS-1. As shown in Figure 5C, microglia constitutively express IPS-1 mRNA. Surprisingly, mRNA levels of this downstream adaptor molecule are significantly reduced at 8 h following VSV challenge. It is presently unclear whether this decrease reflects diminished microglial viability/transcription or an increase in translation efficiency in these cells.
Primary astrocytes express RIG-I and MDA5
We have extended our studies to an additional resident CNS cell type, astrocytes. First, we investigated the expression of mRNA encoding RLRs in these cells. Murine astrocytes were untreated or exposed to VSV at varying MOIs prior to RNA isolation at 4 and 8 h post infection. Real-time PCR was performed for the presence of mRNA encoding RIG-I and MDA5. As shown in Figure 6A, astrocytes constitutively express mRNA encoding RIG-I, albeit at lower levels than that seen in microglia (Figure 2A). However, unlike microglia, RIG-I mRNA levels were significantly elevated in a dose-dependent manner at 8 h following VSV infection (Figure 6A). Similarly, these resident CNS cells showed constitutive expression of mRNA encoding MDA5 that was also significantly elevated at 8 h (Figure 6A) following viral challenge.
Figure 6.
Primary murine astrocytes constitutively express members of the RLR family of cytosolic pattern recognition receptors and such expression is elevated following viral challenge. Cells (2 × 106) were untreated (0) or exposed to VSV (ratios of viral particles to cells: 0.001:1, 0.01:1, 0.1:1, 1:1, and 10:1). (A) At 8 h following infection, mRNA was isolated and real-time PCR performed to determine the level of expression of mRNA encoding RIG-I or MDA5. Data are presented as the mean ± SEM of six experiments normalized to the expression of G3PDH. Asterisks indicate a statistically significant difference from uninfected astrocytes. (B) At 12 and 24 h post-infection, whole-cell protein isolates were prepared and MDA5 and RIG-I protein expression were determined by immunoblot analyses, respectively. Immunoblots shown are representative of five separate experiments. (C) Cells (2 × 106) were untreated (0) or exposed to VSV (ratios of viral particles to cells: 0.01:1 and 0.1:1). At 12 h following infection, whole-cell protein isolates were prepared and the culture media was taken. This conditioned medium was filtered and placed on uninfected astrocytes for a further 12 h prior to preparation of whole-cell protein isolates from these cells. RIG-I and MDA5 expression was then assessed by immunoblot analysis in isolates from VSV-infected cells (VSV) and those exposed to condition medium (CM). Immunoblots shown are representative of three separate experiments and the expression of an irrelevant protein is shown (IRR). For comparison purposes, RLR protein expression in a similar number of stimulated peritoneal macrophages is shown (mφ).
Next, we determined whether expression of mRNA encoding RLRs in astrocytes translated into expression of these proteins. As shown in Figure 6B, and consistent with our real-time PCR data, murine astrocytes constitutively express low levels of RIG-I protein. Importantly, exposure of these cells to low MOI of VSV for 12 (data not shown) and 24 (Figure 6B) h resulted in a significant increase in RIG-I protein expression, with maximal 2.36 ± 1.51-fold induction at a MOI of 0.1 PFU/cell, whereas high viral titers diminished such expression despite little change in β-actin expression (Figure 5B). Similarly, resting astrocytes expressed detectable levels of MDA5 and low VSV titers significantly elevated MDA5 protein expression at 12 (Figure 6B) and 24 h post infection (maximal 2.37 ± 0.58-fold induction at a MOI of 0.1 PFU/cell).
To determine whether RLR expression in astrocytes can be induced in an indirect manner similar to that seen in microglia (Figure 4), cells were exposed to filtered (0.1 µm) conditioned medium from astrocytes that were exposed to low titers of VSV for 12 h. As shown in Figure 6C, conditioned medium was able to induce the protein expression of both RIG-I and MDA5 after 12 h, with maximal 2.14 ± 0.63-fold and 1.42 ± 0.02-fold inductions, respectively, at a MOI of 0.1 PFU/cell, as determined by densitometric analysis. This indicates that RLR expression can be induced by soluble mediator(s) produced by VSV-infected astrocytes.
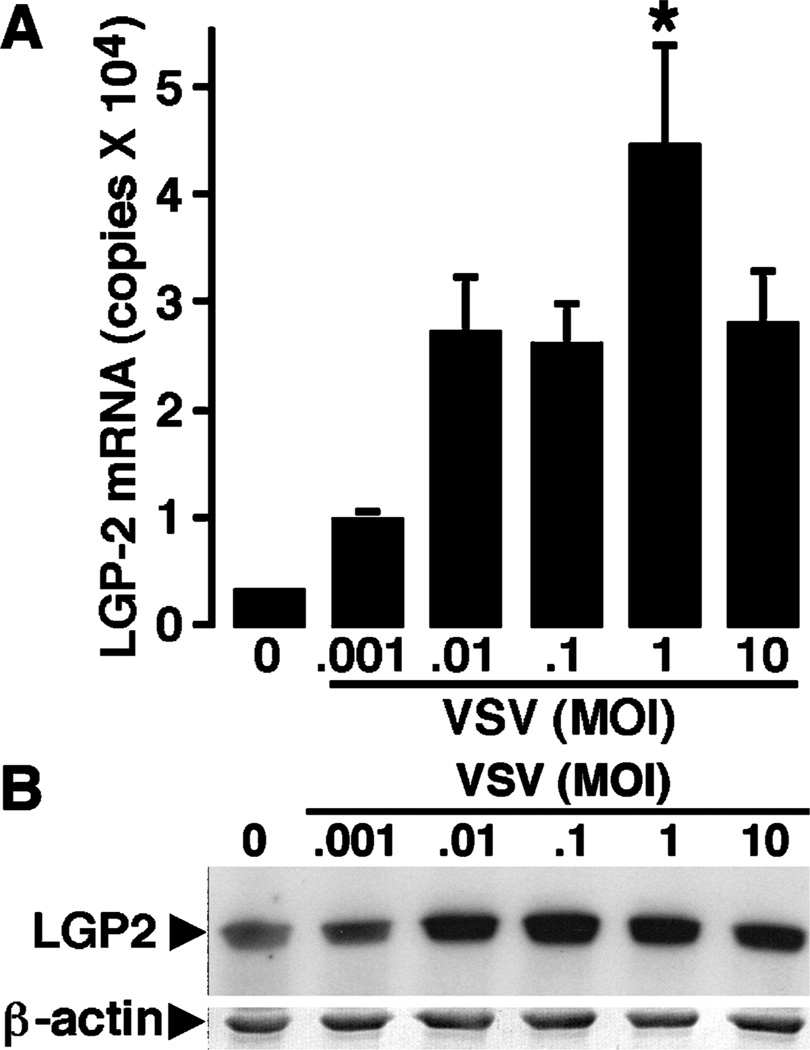
Finally, we have assessed the expression of the IPS-1 and LGP2 in astrocytes. We report that resting cultures of astrocytes express high mRNA copy numbers for the downstream adaptor molecule, IPS-1 (2.12 ± 0.26 × 105 copies). In addition, we have found that astrocytes constitutively express mRNA encoding LGP2 and such expression is up-regulated at 8 h following exposure to VSV (Figure 7A). Furthermore, we have found that resting astrocytes express robust levels of the LGP2 protein, as measured by immunoblot analysis, and show that this expression is also up-regulated at 12 h following VSV infection (data not shown); but, unlike microglia, this elevation is sustained at 24 h following viral challenge (Figure 7B).
Figure 7.
Astrocytes express a downstream regulator of RLR function. Cells (2 × 106) were untreated (0) or exposed to VSV (ratios of viral particles to cells: 0.001:1, 0.01:1, 0.1:1, 1:1, and 10:1). At 8 and 24 h following infection, mRNA and whole-cell protein isolates were prepared, respectively. Real-time PCR was performed to determine the level of expression of mRNA encoding LGP2 and LGP2 protein expression was determined by immunoblot analyses. Real-time PCR data are presented as the mean ± SEM of five experiments normalized to the expression of G3PDH. An asterisk indicates a statistically significant difference from uninfected astrocytes. Immunoblots shown are representative of four separate experiments.
Discussion
VSV is a rhabdovirus that is closely related to rabies virus and exhibits neurotropism in mice, eliciting severe and rapid encephalitis, with a high degree of morbidity and mortality, following intranasal inoculation (Huneycutt et al, 1993). Inoculation leads to rapid access of VSV to the CNS via the olfactory receptor neurons and then proceeds in a rostal-to-caudal direction throughout the brain where the virus replicates to high titers. Reiss and coworkers have demonstrated that resident glial cells respond to this virus both in vitro and in vivo (Bi et al, 1995; Christian et al, 1996). In vivo CNS infection is associated with astrocyte activation in the olfactory bulb as early as 24 h following infection and activated microglia are observed within 3 days of infection (Bi et al, 1995). However, T cells do not appear within the CNS until 4 days following challenge and the acute encephalitis caused by VSV appears to be T cell independent, having been observed in athymic mice following intrathecal viral administration (Frei et al, 1989). As such, it appears that the innate immune responses of resident glial cells are instrumental in the initiation and progression of inflammatory CNS damage associated with VSV infection.
The recent description of RLRs, cytosolic DExD/H box RNA helicases that can recognize early viral replicative intermediates (Takeuchi and Akira, 2007), raises the possibility that such molecules might play an important role in the detection of RNA viruses in glial cells in an analogous manner to the role played by NOD2 in the perception of bacterial pathogens (Sterka et al, 2006; Sterka and Marriott, 2006). RIG-I and MDA5 are members of the RLR family found in the cytosol of fibroblasts and dendritic cells and have been shown to mediate immune responses to viral RNA through a complex pathway involving their caspase recruitment domains, or CARDs (as reviewed in Takeuchi and Akira, 2007). RIG-I and MDA5 utilize these CARDs to initiate downstream signaling events that ultimately result in antiviral cytokine production. Both MDA5 and RIG-I CARD domains interact with the adaptor molecule, IPS-1 (Kumar et al, 2006), to induce type I interferon gene expression via TRAF3 and IRF3/IRF-7 pathways, and activate nuclear factor (NF)-κB in a caspase 8 and 10–dependent manner to induce inflammatory cytokine production in macrophages and fibroblasts (Hiscott, 2007). In the present study, we describe the expression of these novel intracellular viral sensors in primary cultures of microglia and astrocytes. We show that these cells constitutively express robust levels of mRNA encoding RIG-I and MDA5 and show detectable levels of these proteins in whole-cell protein isolates prepared from resting cells. These data are in agreement with a recent study demonstrating the expression of RIG-I by cultured human astrocytes and astrocytoma cells as determined by immunocytochemical techniques (Yoshida et al, 2007). Furthermore, in the present study we demonstrate that glia constitutively express mRNA encoding IPS-1, a critical downstream adaptor molecule for RLR signaling.
Interestingly, we have found that RIG-I protein expression is rapidly and markedly elevated following exposure to VSV in both microglia and astrocytes. Although mRNA levels were not significantly altered, it is important to note that mRNA levels do not always directly correlate with protein expression levels, and the increase in protein levels may be due to changes in translation efficiency rather than alterations in mRNA transcription. In addition, a second Mononegavirus, SeV, can also induce the protein expression of this intracellular viral sensor, albeit with lower efficacy. Because RIG-I has been shown to mediate cellular responses to VSV and SeV (Takeuchi and Akira, 2007; Loo et al, 2008), it is tempting to suggest that perception of these viruses by this sensor promotes further RIG-I expression in a feed-forward manner. However, we have found that VSV also induces elevated MDA5 expression by murine glia following in vivo and in vitro infection, despite the documented specificity of RIG-I for this virus (Takeuchi and Akira, 2007; Loo et al, 2008). Similarly, SeV also up-regulates this seemingly inappropriate RLR. An explanation for these findings may come from the observations that RIG-I and MDA5 protein expression can be induced in uninfected cells by filtered conditioned medium from cells exposed to this virus. As such, these data suggest that increases in RLR expression occur, at least in part, indirectly by the autocrine or paracrine action of soluble mediators produced following viral challenge. Such a hypothesis is supported by several recent studies that inflammatory cytokines, including TNF-α (Matikainen et al, 2006; Kitamura et al, 2007) and IL-1β (Sakaki et al, 2005), can elevate RIG-I expression in a number of cell types.
Although both microglia and astrocytes demonstrate similar patterns of RIG-I andMDA5 expression constitutively and following viral challenge, there are differences in the level of expression of RLRs and their effector molecules between these two glial cell types, as summarized in Table 1. For example, levels of mRNA encoding RIG-I were approximately twofold higher in microglia than astrocytes and this difference was also apparent in the resting protein levels of this receptor. Furthermore, maximum increases in MDA5 mRNA and protein levels following VSV infection were also greater in this resident myeloid cell type than in astrocytes. This higher expression in microglia was seen despite an apparent ability of VSV to decrease total protein content as assessed by β-actin levels, indicative of either greater cell death in this cell type or inhibition of protein synthesis. Finally, we have found that both astrocytes and microglia express a third member of the RLR family, LGP2, that lacks the CARD domain and has been reported to function as a negative regulator of RIG-I and MDA5 signaling (Hiscott, 2007), with constitutively higher LGP2 protein levels in astrocytes than microglia. Interestingly, following a transient increase in expression, LGP2 expression in microglia was virtually abolished 24 h following VSV infection in contrast to the high expression maintained in infected astrocytes. Such a difference may reflect the central role thought to be played by microglia in the generation of inflammatory host responses within the CNS.
Table 1.
Summary of relative expression of RLR and their associated molecules in microglia and astrocytes constitutively (Resting) or following VSV challenge (+VSV)
| Microglia | Astrocytes | |||
|---|---|---|---|---|
| Molecule | Resting | +VSV | Resting | +VSV |
| RIG-I | ||||
| mRNA | ++ | ++ | + | +++ |
| Protein | ++ | +++ | + | ++ |
| MDA5 | ||||
| mRNA | ++ | +++ | ++ | +++ |
| Protein | ++ | ++++ | ++ | +++ |
| LGP2 | ||||
| mRNA | + | ++ | + | ++ |
| Protein | ||||
| 12 hours | + | ++ | ++ | +++ |
| 24 hours | + | − | ++ | +++ |
| IPS-1 | ||||
| mRNA | +++ | + | ++++ | ++ |
Taken together, the present studies show that two resident glial cells, known to contribute to inflammatory immune responses following microbial challenge, express members of the RLR family of proteins that can function as intracellular pattern recognition receptors for RNA motifs associated with replicating viruses. Furthermore, such expression is up-regulated in both astrocytes and microglia following viral challenge. Although RLR-mediated signaling induces type I interferon (IFN) gene expression in other cell types and such production is thought to play a critical role in the control of VSV infection in peripheral organs of mice, it is important to note that IFN-α and IFN-β play little or no role in the CNS. This is not due to a lack of sensitivity of resident CNS cells to type I interferons rather, this difference appears to be due to the absence of these molecules in the CNS during acute infection (Trottier et al, 2005). However, both RIG-I and MDA5 signaling can activate NF-κB to induce inflammatory cytokine production (Hiscott et al, 2007). Accordingly, the expression of RLRs by microglia and astrocytes may represent an important mechanism underlying the lethal inflammation associated with neurotropic RNA virus infections.
Materials and methods
Preparation of viral stocks
Viral stocks were prepared by infecting monolayer cultures of baby hamster kidney (BHK-21) cells (ATCC, CCL-10) with VSV (Indiana strain) or by infecting African green monkey kidney cells (Vero; ATCC, CCL-81) with Sendai virus (SeV; Fushimi strain) with an enhanced green fluorescent protein (eGFP) gene just downstream of the P gene (described in Bitzer et al, 2003) at a low multiplicity of infection (MOI) of 0.05 plaque-forming units (PFU) per cell and incubated for 48 h at 34°C in SFM4Mega Vir protein-free medium (Hyclone). The cell-free medium containing released viruses was collected and viral titers were quantified using a standard plaque assay of serial dilutions of VSV or SeV on BHK-21 or Vero cells, respectively, at 34°C. Wild-type SeV-GFP virus was kindly provided to Dr. Grdzelishvili by Dr. Wolfgang J. Neubert (Department of Molecular Virology, Max-Planck-Institute of Biochemistry, Germany).
In vivo infection of mice
VSV was administered to wild-type C57BL/6 mice (Jackson Laboratories) via intranasal (i.n.) infection essentially as previously described by our laboratory (Gasper-Smith et al, 2006). Anesthetized animals were untreated or received i.n. administration of VSV (1 × 107 PFU) in phosphate-buffered saline PBS (final volume of 1 µl). At 5 days post infection, protein isolates were prepared from whole-brain tissue homogenates or mixed glial cells isolated via Percoll gradient as previously described by our laboratory (Chauhan et al, 2008). All studies were performed in accordance with relevant federal guidelines and institutional policies regarding the use of animals for research purposes.
Isolation of primary murine microglia
Mouse neonatal brain microglia were isolated as described previously by our laboratory (Rasley et al, 2004a, 2004b, 2006; Sterka and Marriott, 2006; Chauhan et al, 2008) and cultured in RPMI 1640 with 10% fetal bovine saline (FBS) and 20% conditioned medium from LADMAC cells (ATCC number CRL-2420) as a source of CSF-1. Cells isolated in thismanner are >95% authentic microglia as assessed by their characteristic amoeboid and unramified morphology characteristic of nonquiescent cells and expression of CD11b and F4/80.
Isolation and characterization of primary murine astrocytes
Mouse neonatal brain astrocytes were isolated as described previously by our laboratory (Bowman et al, 2003; Rasley et al, 2004a; Sterka et al, 2006; Chauhan et al, 2008) and cultured in RPMI 1640 containing 10% FBS. Cells isolated in this manner are >97% authentic astrocytes due to their characteristic morphology, expression of glial fibrillary acidic protein, and the absence of CD11b as determined by immunofluorescent microscopy.
In vitro infection of glial cells
Microglia and astrocytes were infected with VSV or SeV at MOIs of 0.001, 0.01, 0.1, 1, and 10 PFU per cell and the virus was allowed to adsorb for 1 h. Nonadherent viral particles were removed by washing followed by the addition of RPMI 1640 medium supplemented with 10% FBS and 20% LADMAC (microglia) or 10% FBS (astrocytes). Glial cells were found to be susceptible to viral infection and harbored replicative virus as determined by the presence of GFP-expressing viral particles and the expression of native viral gene products in microglia and astrocytes following exposure to VSV of SeV (unpublished observations). Cultures were maintained for 4 and 8 h or 12 and 24 h for isolation of mRNA and whole-cell protein isolates, respectively. These time points were selected to assess early versus delayed induction events.
Isolation of RNA and quantitative real-time PCR
Poly(A) + RNA was isolated from microglia and astrocytes and reverse transcribed as previously described (Bowman et al, 2003; Rasley et al, 2004a, 2004b, 2006; Sterka and Marriott, 2006; Sterka et al, 2006; Chauhan et al, 2008). Real-time PCR was performed to determine the expression of mRNA encoding RIG-1, MDA5, interferon promoter stimulator-1 (IPS-1), laboratory of genetics and physiology 2 (LGP2), and glyceraldehyde 3-phosphate dehydrogenase (G3PDH) using the SYBR Green approach described previously (Sterka et al, 2006; Chauhan et al, 2008) on a Lightcycler device (Roche Applied Science). PCR conditions included an initial denaturation step of 95°C for 15 min, followed by 40 cycles of 94°C (15 s), 60°C (20 s), and 72°C (25 s). A melt-curve protocol was employed immediately followed amplification (94°C for 1 min and 50°C for 30 s, followed by repeats of heating, starting at 50°C up to 99°C with 0.2°C/s increments) and melting curve analysis was performed to determine the melting temperature of each sample to indicate the purity of the reaction product and denote absence of primer dimers or other nonspecific PCR products. Acquisition points were set at appropriate temperatures to exclude primer dimers.
Positive- and negative-strand PCR primers used, respectively, were 5′-GCCAGAGTGTCAGAATCTCAGTCAG-3′ and 5′-GAGAACACAGTTGCCTGCTGCTCAT-3′ to amplify mRNA encoding murine RIG-1 (285-bp fragment); 5′-CGATGTCTTGGACACTTGCTTCGAG-3′ and 5′-GCAGGCAGAAGACACTCATTAGCTG-3′ to amplify mRNA encoding murine MDA5 (285-bp fragment); 5′-TAGACAGAGGCAAGGTGGTGGTACT-3′ and 5′-TGGTGTTGTAGACGGTGTCCTTGTG-3′ to amplify mRNA encoding murine LGP2 (300-bp fragment); and 5′-CCAGAGAGCATCAAGAGCAAGAACC-3 and 5′-AAGGCACTGAGCTGGTAGTCACAGA-3 to amplify mRNA encoding murine IPS-1 (300-bp fragment). All primers were designed by using OligoCalc (Kibbe, 2007; www.basic.northwestern.edu/biotools/oligocalc.html) based on their location in different exons of the genomic sequences and their lack of significant homology to sequences present in GenBank (MacVector Sequence analysis software; IBI, New Haven, CT). The identity of the PCR-amplified fragments was further verified by size comparison with DNA standards (Promega) and by direct DNA sequencing of representative fragments (Davis Sequencing, Davis, CA).
Levels of mRNA were quantified from a standard curve generated using known concentrations of amplicons developed by conventional PCR. Seven 100-µl PCR reactions were performed using positive-control samples exhibiting strong expression of the gene of interest. Products were isolated from an agarose electrophoresis gel using a Qiagen Gel extraction kit (Valencia, CA) and their concentrations were determined using GeneSpec III (MiraiBio, San Francisco, CA). The number of RNA copies/µl in the stock solution was then calculated and 1:10 serial dilutions performed to generate amplicons for use in real-time PCR. All RNA expression levels in the present study are reported normalized to the expression of the housekeeping gene G3DPH determined in parallel real-time PCR reactions.
Western blot analyses for RIG-1, MDA5, and LGP2
Western blot analyses for the presence of RIG-1, MDA5, and LGP2 in microglia and astrocytes were performed as described previously by our laboratory (Bowman et al, 2003; Rasley et al, 2006; Sterka and Marriott, 2006; Sterka et al, 2006). The primary antibodies used were a purified rabbit polyclonal antibody directed against human RIG-1 (Abgent, San Diego, CA), a recombinant rabbit polyclonal antibody directed against mouse MDA5 (Alexis Biochemicals, San Diego, CA), and an affinity-purified rabbit polyclonal antibody directed against human LGP2 (ProteinTech Group, Chicago, IL). To assess total protein loading in each well, immunoblots were reprobed for the with a goat anti-mouse β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Treatment with conditioned medium
Microglia or astrocytes were untreated or infected with VSV at MOIs of 0.01 and 0.1 PFU per cell and the virus was allowed to adsorb for 1 h. Nonadherent viral particles were removed by repeated washing and cultures were maintained for 12 h. Medium was then collected and filtered with 0.1-µm syringe filters (Sterlitech, Kent, WA) to remove residual viral particles (0.1 to 0.4 µm in length) prior to addition to uninfected glial cells. Cells were cultured with conditioned medium for 12 h and whole-cell protein isolates prepared for immunoblot analyses. The absence of infectious viral particles in the filtered conditioned medium was verified using GFP-expressing VSV. Glial cells were infected for 12 h with GFP-expressing viral particles (MOIs of 0.01 and 0.1 PFU per cell) and medium collected. These media were unfiltered or filtered, and placed on uninfected cell cultures. At 18 h following exposure, GFP-associated fluorescence was visualized and was only found to be present in cells treated with unfiltered conditioned media (data not shown).
Densitometric analyses
Densitometric analyses of immunoblots were performed using NIH Image (obtained from the NIH Web site http://rsb.info.nih.gov/nih-image). Results are presented as mean values of arbitrary densitometric units corrected for background intensity normalized to the expression of β-actin, or as fold increases over levels in unstimulated cells.
Statistical analysis
Results of the present studies were tested statistically by one-way analysis of variance (ANOVA) and Tukey’s post hoc test using commercially available software (SAS Institute, Cary, NC). Results were determined to be statistically significant when a P value of less than .05 was obtained.
Acknowledgments
This work was supported by grants NS050325 and NS057434 to I.M. from the National Institutes of Health.
References
- Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer M, Ungerechts G, Bossow S, Graepler F, Sedlmeier R, Armeanu S, Bernloehr C, Spiegel M, Gross CD, Gregor M, Neubert WJ, Lauer UM. Negative-strand RNA viral vectors: intravenous application of Sendai virus vectors for the systemic delivery of therapeutic genes. Mol Ther. 2003;7:210–217. doi: 10.1016/s1525-0016(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express Toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Chauhan VS, Sterka DG, Jr, Gray DL, Bost KL, Marriott I. Neurogenic exacerbation of microglial and astrocyte responses to Neisseria meningitidis and Borrelia burgdorferi. J Immunol. 2008;180:8241–8249. doi: 10.4049/jimmunol.180.12.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian AY, Barna M, Bi Z, Reiss CS. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol. 1996;9:195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Gasper-Smith N, Marriott I, Bost KL. Murine gamma-herpesvirus 68 limits naturally occurring CD4+CD25+ T regulatory cell activity following infection. J Immunol. 2006;177:4670–4678. doi: 10.4049/jimmunol.177.7.4670. [DOI] [PubMed] [Google Scholar]
- Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18:483–490. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Huneycutt BS, Bi Z, Aoki CJ, Reiss CS. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kiefer R, Lindholm D, Kreutzberg GW. Interleukin-6 and transforming growth factor-beta 1 mRNAs are induced in rat facial nucleus following motoneuron axotomy. Eur J Neurosci. 1993;5:775–781. doi: 10.1111/j.1460-9568.1993.tb00929.x. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Matsuzaki Y, Kimura K, Nakano H, Imaizumi T, Satoh K, Hanada K. Cytokine modulation of retinoic acid-inducible gene-I (RIG-I) expression in human epidermal keratinocytes. J Dermatol Sci. 2007;45:127–134. doi: 10.1016/j.jdermsci.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Konat GW, Kielian T, Marriott I. The role of Toll-like receptors in CNS responses to microbial challenge. J Neurochem. 2006;99:1–12. doi: 10.1111/j.1471-4159.2006.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, Yamamoto M, Uematsu S, Ishii KJ, Takeuchi O, Akira S. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, García-Sastre A, Katze MG, Gale M., Jr Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen S, Sirén J, Tissari J, Veckman V, Pirhonen J, Severa M, Sun Q, Lin R, Meri S, Uzé G, Hiscott J, Julkunen I. Tumor necrosis factor alpha enhances influenza A virus-induced expression of antiviral cytokines by activating RIG-I gene expression. J Virol. 2006;80:3515–3522. doi: 10.1128/JVI.80.7.3515-3522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Rasley A, Bost KL, Marriott I. Murine gammaherpesvirus-68 elicits robust IL-12p40 expression, but not IL-12p70 production, by murine microglia and astrocytes. J NeuroVirol. 2004a;10:171–180. doi: 10.1080/13550280490444119. [DOI] [PubMed] [Google Scholar]
- Rasley A, Marriott I, Halberstadt CR, Bost KL, Anguita J. Substance P augments B. burgdorferi-induced PGE2 production by murine microglia. J Immunol. 2004b;172:5707–5713. doi: 10.4049/jimmunol.172.9.5707. [DOI] [PubMed] [Google Scholar]
- Rasley A, Tranguch SL, Rati DM, Marriott I. Murine microglia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia. 2006;53:583–592. doi: 10.1002/glia.20314. [DOI] [PubMed] [Google Scholar]
- Sakaki H, Imaizumi T, Matsumiya T, Kusumi A, Nakagawa H, Kubota K, Nishi N, Nakamura T, Hirashima M, Satoh K, Kimura H. Retinoic acid-inducible gene-I is induced by interleukin-1beta in cultured human gingival fibroblasts. Oral Microbiol Immunol. 2005;20:47–50. doi: 10.1111/j.1399-302X.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Sterka D, Jr, Rati DM, Marriott I. Functional expression of NOD2, a novel pattern recognition receptor for bacterial motifs, in primary murine astrocytes. Glia. 2006;53:322–330. doi: 10.1002/glia.20286. [DOI] [PubMed] [Google Scholar]
- Sterka D, Jr, Marriott I. Characterization of nucleotide-binding oligomerization domain (NOD) protein expression in primary murine microglia. J Neuroimmunol. 2006;179:65–75. doi: 10.1016/j.jneuroim.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Trottier MD, Jr, Palian BM, Reiss CS. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology. 2005;333:215–225. doi: 10.1016/j.virol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. RIG-I family RNA helicases: cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007;18:545–551. doi: 10.1016/j.cytogfr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Imaizumi T, Lee SJ, Tanji K, Sakaki H, Matsumiya T, Ishikawa A, Taima K, Yuzawa E, Mori F, Wakabayashi K, Kimura H, Satoh K. Retinoic acid-inducible gene-I mediates RANTES/CCL5 expression in U373MG human astrocytoma cells stimulated with double-stranded RNA. Neurosci Res. 2007;58:199–206. doi: 10.1016/j.neures.2007.02.017. [DOI] [PubMed] [Google Scholar]