Abstract
Positron emission tomography (PET) is a powerful noninvasive tool for acquisition of the physiological parameters in human and animals with the help of PET tracers. Among all the PET tracers, radiolabeled peptides have been widely explored for cancer-related receptor imaging due to their high affinity and specificity to receptors. But radiochemistry procedures for production of peptide-based PET tracers are usually complex, which makes large-scale clinical studies relatively challenging. New radiolabeling technologies which could simplify synthesis and purification procedures, are extremely needed. Over the last decade, microfluidics and lab-on-a-chip (LOC) technology have boomed as powerful tools in the field of organic chemistry, which potentially provide significant help to the PET chemistry. In this minireview, microfluidic radiolabeling technology is described and its application for synthesis of peptide-based PET tracers is summarized and discussed.
1. Introduction
Positron emission tomography (PET) is increasingly being used for in vivo biochemical, physiological, and pharmacological process visualization and is also routinely used for screening, diagnosing, and staging of cancer. With the help of PET tracers and PET scanners, physiological parameters (like blood flow, metabolism, receptor properties, drug distribution, and gene expression) in the living human and animal bodies could be studied noninvasively. These PET tracers, including small molecules, peptides, antibodies, are radiolabeled with short-lived radioisotopes. Among these tracers, radiolabeled peptides have been widely explored for cancer imaging due to their high affinity and specificity to many kinds of cancer-related receptors. Peptide-based PET tracers have the following advantages: (1) peptides usually have very high affinity and specificity to the target (receptor); (2) compared to biomacromolecules like antibodies, peptides are easy to synthesize and characterize; (3) rapid clearance from the blood and nontarget tissues. A lot of peptide-based PET tracers are undergoing clinical trials, like the 18F-labeled RGD and 68Ga-labeled Octreotide [1, 2]. However, large-scale clinical studies and applications are relatively challenging due to the complex radiochemistry procedures for peptide-based PET tracers, especially for 18F-labeled tracers which usually require a laborious and time-consuming multistep process. Radiochemists are continually working on the development of new methods and technologies for the preparation of PET tracers.
During the last several decades, microfluidics and lab-on-a-chip (LOC) technologies have boomed as powerful tools in the field of organic chemistry, showing characteristics like enhanced heat and mass transfer, reduction of reagent consuming and hazardous waste, which potentially provide significant help to the PET chemistry. Applications of microfluidic chemistry in radiopharmaceutical synthesis have also drawn increasing attention [5–9]. Microfluidic system could increase the overall efficiency of radiolabeling reaction remarkably. 18F-FDG production by the microfluidic system has been successfully demonstrated [10], and a lot of other PET tracers have been produced successfully. Compared with small molecules, synthesis of peptide-based PET tracers requires milder reaction conditions and strict chromatographic purification. Microfluidic system allows lower precursor consumption which simplified the purification, and the enhanced heat and mass transfer in microfluidic reactors can provide higher labeling yields under milder reaction conditions, so microfluidic system has good potential to play an important role in production of peptide-based PET tracers.
In this minireview, microfluidic radiolabeling technology is described and its application for synthesis of peptide-based PET tracers is summarized and discussed.
2. Peptide-Based PET Tracers
Biological active peptides are involved in many biochemical processes, like immune response and information transmission, and they play important roles in cellular communication and cell proliferation. On the other hand, a variety of receptors are found to be expressed on the membrane and possess very high affinity to specific peptides. Particular receptors are often massively overexpressed in cancer tissues, so peptide ligands could be utilized as targeting tools for PET imaging. Representative targets for peptide-based PET tracers are listed in Table 1.
Table 1.
| Receptors | Peptide | Cancer | Labeling |
|---|---|---|---|
| Somatostatin receptor | Somatostatin | Neuroendocrine tumors | 68Ga-DOTA, 68Ga-NOTA, 64Cu-TETA, 64Cu-DOTA, 64Cu-NOTA, 64Cu-CB-TE2A, 18F-NFP, 18F-SFB, and so forth |
| Gastrin-releasing peptide receptor (GRPR) | Bombesin | Prostate cancer, breast cancer, Gastrointestinal stromal tumor | |
| α v β 3-integrin | RGD | Brain cancer, lung cancer, breast cancer, and so forth | |
|
| |||
| Melanocortin 1 receptor (MC1R) | α-MSH | Melanomas | 18F-SFB, 68Ga-DOTA, 64Cu-TETA, 64Cu-DOTA, 64Cu-CB-TE2A |
|
| |||
| Cholecystokinin B/gastrin receptor (CCK2/CCK-B) | CCK/gastrin | Medullary thyroid cancer | 18F-SFB, 68Ga-DOTA, 64Cu-DOTA |
|
| |||
| Glucagon-like peptide-1 receptor (GLP-1) | Exendin | Insulinoma cancer | 18F-FBEM, 68Ga-DOTA |
|
| |||
| Neurotensin receptor (NTR1) | Neurotensin | Small cell lung cancer, colon cancer, and so forth |
68Ga-DOTA 18F-FB |
|
| |||
| Neuropeptide Y receptor (Y1) | NPY | Breast cancer, prostate cancer | 64Cu-DOTA |
|
| |||
| Luteinizing hormone-releasing hormone receptor (LHRH-R) | LHRH | Prostate cancer, breast cancer, and so forth | 68Ga-DOTA |
|
| |||
| Neurokinin 1 receptor (NK-1) | Substance P | Glioblastoma | |
|
| |||
| Vasoactive intestinal peptide receptor (VIP-R) | Vasoactive intestinal peptide (VIP) | Prostate cancer |
64Cu-DOTA 18F-FB |
|
| |||
| Pituitary adenylate cyclase-activating peptide (PACAP) receptor | Pituitary adenylate cyclase-activating peptide (PACAP) | Breast cancer | 64Cu-DOTA |
|
| |||
| Chemokine receptor 4 (CXCR4) | CXCR4 | Lymphatic system, lung cancer, and so forth | 18F-SFB, 68Ga-DOTA, 64Cu-DOTA |
Common radioisotopes used for peptide radiolabeling are listed in Table 2; half-lives of these radioisotopes (18F: t 1/2 = 109.8 min, 68Ga: t 1/2 = 67.6 min, 64Cu: t 1/2 = 12.8 h) are suitable for the pharmacokinetics of most peptides. 18F is one of the most widely used radionuclides for diagnostic PET imaging because of its unique nuclear and chemical properties [15]. 18F-labeling of peptides can be achieved via prosthetic groups, such as N-succinimidyl-4-18F-fluorobenzoate (18F-SFB) [16] and 4-nitrophenyl 2-18F-fluoropropionate (18F-NFP) [17]. In recent years, a facile, 1-step Al18F method has been developed and demonstrated as a very promising method for radiofluorination of peptides, which does not require on-site cyclotron by use of the commonly available sodium 18F-fluoride solutions [18–20]. 68Ga is available from an in-house generator rendering 68Ga radiopharmacy independent of an on-site cyclotron, which is a big advantage for clinical use. 68Ga-labeled peptides have been developed for the targeting of somatostatin receptors, the melanocortin 1 receptor, the bombesin receptor, HER2 receptor and so forth [21, 22]. 64Cu is another widely used metallic positron emitter, which can be produced on a large scale with a medical cyclotron, and the half-life (12.7 h) and decay properties make it an ideal radioisotope for PET imaging and radiotherapy [23, 24]. Besides, coordination chemistry of copper is well established and a wide variety of chelator systems are available. 64Cu-labeled peptides have also been developed for the targeting of a variety of receptors.
Table 2.
Commonly used radioisotopes for peptide-based PET tracer.
| Radionuclide | Half-life | E max (β +)/abundance | Production | Chemistry |
|---|---|---|---|---|
| 18F | 109.8 min | 634 Kev/97% | Cyclotron | Organic chemistry Chelation chemistry |
| 64Cu | 12.8 h | 656 Kev/19% | Cyclotron | Chelation chemistry |
| 68Ga | 67.6 min | 1899 Kev/89% | Generator | Chelation chemistry |
Conventional methods for radiolabeling of peptides can be divided into two catalogs: labeling with radiometals via chelation chemistry [25] (Figure 1) and labeling through prosthetic groups [26] (Figure 2). For example, the BBN peptide and its analogues have been radiolabeled with various radionuclides including 64Cu, 68Ga, and 18F for GRPR-related cancer imaging [27–30]. In addition to the above two methods, direct radiolabelling of peptide analogues with a leaving group with 18F-fluoride was also reported [31]. Generally speaking, labeling through prosthetic groups is more time consuming, and labeling with radiometals via chelation chemistry is straightforward and usually has higher labeling yields. But some chelation reactions also require high temperature and long reaction time in order to get high yields. New technologies are always desired to improve the radiolabeling efficacy and efficiency.
Figure 1.
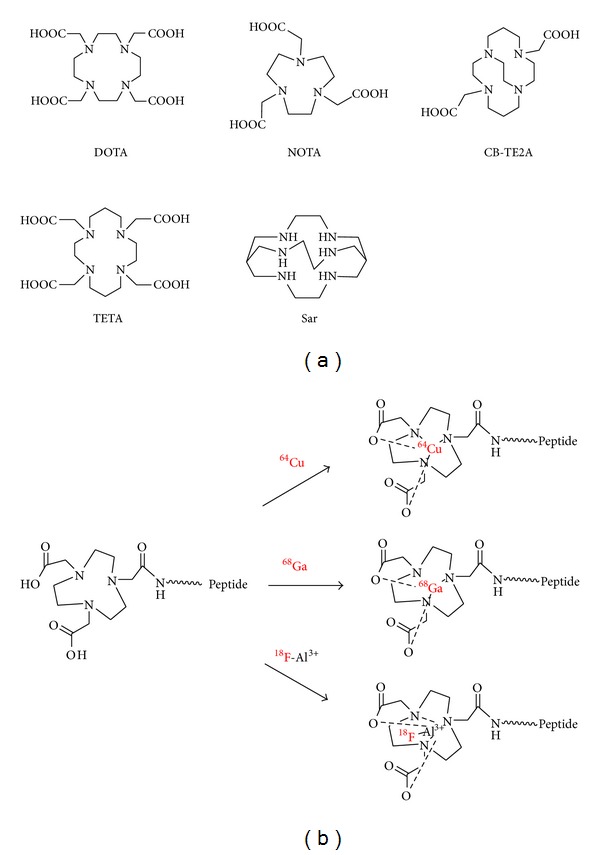
Basic bifunctional chelators (a) and schematic procedure for radiolabeling of peptides (b).
Figure 2.
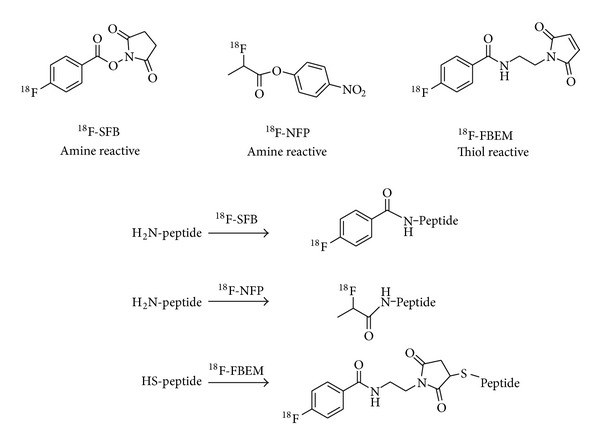
Representative prosthetic groups for radiolabeling of peptides.
3. Microfluidic Reactors
Microfluidic reactors, which generally consist of a network of micron-sized channels (typically 10–500 μm) embedded in a glass, metal or plastic solid substrate, have already found broad applications in the fields of organic synthesis [32] and biomolecular labeling [33]. The basic aspects of microfluidic reactors have been well summarized in other reviews [34–37]. In recent years, multistep synthesis has been performed on multistep continuous-flow synthesis systems [38–40], which could be utilized as promising tools for the multistep synthesis and purification of radiopharmaceuticals for PET.
Figure 3 showed representative microfluidic devices and the PET tracers synthesized [3]. Low precursor and reagent consumption, efficient heat transfer, and enhanced mixing are quite beneficial for the overall efficiency of radiolabelling reaction processes. Radiolabeling carried on microfluidic reactors usually results in purer products, higher yields, greater selectivities, and shorter reaction times than conventional methods. Furthermore, more benign and milder reaction conditions could be applied for certain reactions within microfluidic devices, which is very helpful for maintenance of the bioactivities of some peptides.
Figure 3.
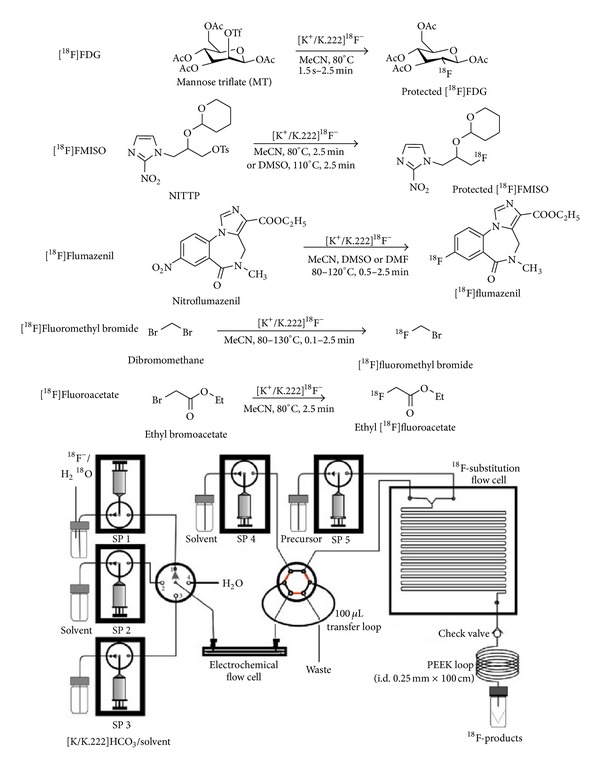
Microfluidic platform showing reaction setup using an electrochemical concentration chip and a reaction flow cell. 18F-fluorination yields for the four 18F-labeled compounds (protected 18F-FDG: 98%, protected 18F-FMISO: 80%, 18F-flumazenil: 20%, 18F-fluoromethyl bromide: 60%) were comparable to or higher than those obtained by conventional means (reproduced from [3] with permission from Elsevier).
4. Preparation of Peptide-Based PET Tracers with Microfluidic Reactors
Conventional methods for radiolabeling of peptides have several limitations. (1) Large excess of precursors are needed to promote rapid and efficient labeling, and then extensive purification is required in order to separate the product from precursors; otherwise these cold precursors would occupy the targets and result in lower imaging quality. (2) Strict reaction conditions, which are hard to bear for some peptides, are needed, especially in the case of F-18 labeling. It would be promising to apply microfluidics technology to prepare peptide-based PET tracers.
Recently, a PDMS microreactor was fabricated and tested for the labeling of bifunctional chelate conjugated biomolecules with PET radiometals 64Cu and 68Ga in aqueous solutions (Figure 4) [4, 41]. The results showed that the microfluidic approach overall outperforms conventional radiosynthetic methods. The PDMS microreactor had a serpentine microchannel for mixing, a series of reservoirs for the incubation of the radiometal-ligand mixture, and a thin-film heater for heating the mixture. The reservoir was composed of 5 hexagonal chambers connected in series, with a total volume of 50 μL. DOTA-RGD conjugate was labeled with 64Cu at mild reaction condition (23–47°C, 5–20 minutes). The incorporation yield is considerably better (~90%) than that obtained in classical vessel radiochemistry (~60%). The authors further investigated radiolabeling of both DOTA-peptides and NOTA-peptides conjugate with 68Ga, and similar conclusions were drawn. These results demonstrated that it was possible to achieve high radiolabeling yields without using excess of peptide precursors, and this would eliminate the need for chromatographic purification of the product to remove unlabeled peptides.
Figure 4.
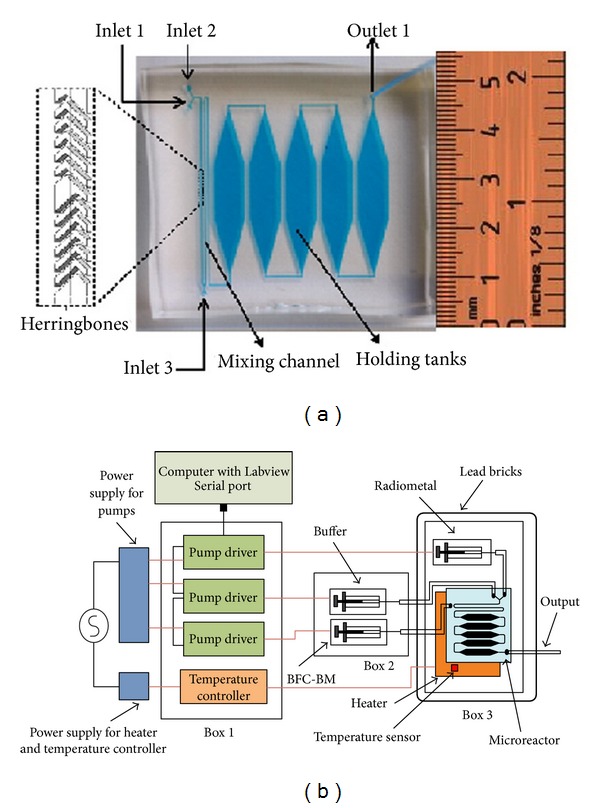
Optical micrograph of the microreactor (a) and schematic of the microreactor system for radiolabeling (b). (Reproduced from [4] with permission from Elsevier).
Synthesis and purification methods of the widely used prosthetic group 18F-SFB based on microreactor have been developed [42]. It was a good example for multistep synthesis and purification of radiopharmaceuticals for PET. Aqueous 18F-fluoride (100–500 MBq) was concentrated and further eluted to a microreactor for evaporation. 18F-fluorination of the precursor (1.5 mg) was carried out at high temperature (200°C for 4 minutes), followed by hydrolysis and subsequent activation of the 4-18F-fluorobenzoyl group. Purification was performed on a miniaturized solid phase extraction column. It took about 25 minutes, and 18F-SFB was obtained with 55 ± 6% yield (not decay-corrected) and >98% radiochemical purity.
Radiolabeling of peptides with 18F-SFB utilizing microfluidic technology was reported [43]. In their study, 18F-SFB was firstly synthesized by conventional method and concentrated to a final volume of 50~100 μL in CH3CN for further use. Then two different approaches were applied for the radiolabeling of the phosphopeptide-cell-penetrating peptide dimers: conventional labeling and microfluidic technology. The isolated radiochemical yields using microfluidic technology (~26%) were much higher than those using conventional labeling methods (2% to 4%). And it was also found that the N-terminal acylation of 18F-SFB was more selective in the microfluidic-based reaction compared to the conventional radiolabeling procedure. Liu et al. reported a labeling system for biomolecules via 18F-SFB utilizing a digital microfluidic droplet generation (DMDG) chip which allowed rapid scouting of reaction conditions in nanoliter volumes [44]. This system required only very small amounts of precursors (200- to 2000-fold reduction than conventional method). And it might be utilized for radio-labeling of a diverse spectrum of biomolecules including intact antibodies and their fragments, other proteins, and peptides.
Direct radiolabelling of peptides with 18F-fluoride was also successfully achieved using the Advion NanoTek continuous flow microreactor [45]. These results showed radiochemical yields were dependent on the leaving group, precursor concentration, reaction temperature, and flow rate. The optimal temperature in the microreactor was 70–80°C yielding labeling efficiency up to 90%. They had some very promising findings: (1) the reaction progressed even at 35°C with 30–40% labelling yields; (2) reactions could be performed even at a concentration of 0.5 mg mL−1 with reasonable yields. These results demonstrated that the microreactor may be used for labeling of thermally labile peptide molecules with 18F radioisotope under very mild conditions.
5. Conclusion
Peptide-based PET tracers are valuable tools for peptide receptor imaging in clinical oncology and a large variety of radiolabeled peptides analogues have been developed for in vivo detection of tumors overexpressing relevant receptors. And many of them were under clinical and preclinical investigation. Large-scale clinical studies are still relatively challenging due to the complex radiochemistry procedures for peptide-based PET tracers, such as multistep synthesis and purification. Convenient radiolabeling technology, which could simplify the synthesis and purification procedures, is needed. In the last decade, remarkable progress has been achieved in the field of microfluidics-based PET radiochemistry. With the help of microfluidics system, rapid and efficient preparation of peptide-based PET tracers might be achieved. By now, some microfluidic systems have been explored for peptide radiolabeling, which may provide great versatility for the production of imaging agents in a doses-on-demand way for clinic use. In conclusion, microfluidics is a very promising technology to meet the increased demand for peptide-based PET tracers.
Conflict of Interests
No potential conflict of interests was disclosed.
Acknowledgments
This work is partly sponsored by Grants from the Zhejiang Provincial Natural Science Foundation of China (Z2110230), Health Bureau of Zhejiang Province (2010ZA075, 2011ZDA013), Science and Technology Bureau of Zhejiang Province (2012R10040), National Science Foundation of China (81327004, 81000630), China Postdoctoral Science Foundation (201104729), and Ministry of Science and Technology of China (2012BAI13B06).
References
- 1.Chin FT, Shen B, Liu S, et al. First experience with clinical-grade ([18F]FPP(RGD(2)): an automated multi-step radiosynthesis for clinical PET studies. Molecular Imaging and Biology. 2012;14:88–95. doi: 10.1007/s11307-011-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittra ES, Goris ML, Iagaru AH, et al. Pilot pharmacokinetic and dosimetric studies of18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology. 2011;260(1):182–191. doi: 10.1148/radiol.11101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong R, Iwata R, Saiki H, Furumoto S, Ishikawa Y, Ozeki E. Reactivity of electrochemically concentrated anhydrous [18F]fluoride for microfluidic radiosynthesis of18F-labeled compounds. Applied Radiation and Isotopes. 2012;70(1):193–199. doi: 10.1016/j.apradiso.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Zeng DX, Desai AV, Ranganathan D, Wheeler TD, Kenis PJA, Reichert DE. Microfluidic radiolabeling of biomolecules with PET radiometals. Nuclear Medicine and Biology. 2013;40:42–51. doi: 10.1016/j.nucmedbio.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elizarov AM. Microreactors for radiopharmaceutical synthesis. Lab on a Chip. 2009;9(10):1326–1333. doi: 10.1039/b820299k. [DOI] [PubMed] [Google Scholar]
- 6.Audrain H. Positron emission tomography (PET) and microfluidic devices: a breakthrough on the microscale? Angewandte Chemie. 2007;46(11):1772–1775. doi: 10.1002/anie.200603509. [DOI] [PubMed] [Google Scholar]
- 7.Miller PW, Audrain H, Bender D, et al. Rapid carbon-11 radiolabelling for PET using microfluidics. Chemistry. 2011;17(2):460–463. doi: 10.1002/chem.201002644. [DOI] [PubMed] [Google Scholar]
- 8.Pascali G, Watts P, Salvadori PA. Microfluidics in radiopharmaceutical chemistry. Nuclear Medicine and Biology. 2013;40(6):776–787. doi: 10.1016/j.nucmedbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Lin W, Liu K, Masterman-Smith M, Shen CK. Microfluidics for positron emission tomography probe development. Molecular Imaging. 2010;9(4):175–191. [PMC free article] [PubMed] [Google Scholar]
- 10.Arima V, Pascali G, Lade O, et al. Radiochemistry on chip: towards dose-on-demand synthesis of PET radiopharmaceuticals. Lab on a Chip. 2013;13:2328–2336. doi: 10.1039/c3lc00055a. [DOI] [PubMed] [Google Scholar]
- 11.Fani M, Maecke HR. Radiopharmaceutical development of radiolabelled peptides. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39(supplement 1):S11–S30. doi: 10.1007/s00259-011-2001-z. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosini V, Fani M, Fanti S, Forrer F, Maecke HR. Radiopeptide imaging and therapy in Europe. Journal of Nuclear Medicine. 2011;52(supplement 2):42S–55S. doi: 10.2967/jnumed.110.085753. [DOI] [PubMed] [Google Scholar]
- 13.Graham MM, Menda Y. Radiopeptide imaging and therapy in the United States. Journal of Nuclear Medicine. 2011;52:56S–63S. doi: 10.2967/jnumed.110.085746. [DOI] [PubMed] [Google Scholar]
- 14.Schottelius M, Wester H. Molecular imaging targeting peptide receptors. Methods. 2009;48(2):161–177. doi: 10.1016/j.ymeth.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Varagnolo L, Stokkel MPM, Mazzi U, Pauwels EKJ. 18F-labeled radiopharmaceuticals for PET in oncology, excluding FDG. Nuclear Medicine and Biology. 2000;27(2):103–112. doi: 10.1016/s0969-8051(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Z, Zhang L, Graves E, et al. Small-animal PET of melanocortin 1 receptor expression using a 18F-labeled α-melanocyte-stimulating hormone analog. Journal of Nuclear Medicine. 2007;48(6):987–994. doi: 10.2967/jnumed.107.039602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Moore SJ, Liu S, et al. A novel radiofluorinated agouti-related protein for tumor angiogenesis imaging. Amino Acids. 2013;44:673–681. doi: 10.1007/s00726-012-1391-y. [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg DM, Sharkey RM, McBride WJ, Boerman OC. Al18F: a new standard for radiofluorination. Journal of Nuclear Medicine. 2013;54:p. 1170. doi: 10.2967/jnumed.113.125245. [DOI] [PubMed] [Google Scholar]
- 19.McBride WJ, Sharkey RM, Goldenberg DM. Radiofluorination using aluminum-fluoride (Al18F) EJNMMI Research. 2013;3:p. 36. doi: 10.1186/2191-219X-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Liu H, Jiang H, Xu Y, Zhang H, Cheng Z. One-step radiosynthesis of18F-AlF-NOTA-RGD2 for tumor angiogenesis PET imaging. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38(9):1732–1741. doi: 10.1007/s00259-011-1847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maecke HR, Hofmann M, Haberkorn U. 68Ga-labeled peptides in tumor imaging. Journal of Nuclear Medicine. 2005;46:172S–178S. [PubMed] [Google Scholar]
- 22.Ren G, Zhang R, Liu Z, et al. A 2-helix small protein labeled with 68Ga for PET imaging of HER2 expression. Journal of Nuclear Medicine. 2009;50(9):1492–1499. doi: 10.2967/jnumed.109.064287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shokeen M, Anderson CJ. Molecular imaging of cancer with copper-64 radiopharmaceuticals and positron emission tomography (PET) Accounts of Chemical Research. 2009;42(7):832–841. doi: 10.1021/ar800255q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SV. Molecular imaging with copper-64. Journal of Inorganic Biochemistry. 2004;98(11):1874–1901. doi: 10.1016/j.jinorgbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Bartholomä MD. Recent developments in the design of bifunctional chelators for metal-based radiopharmaceuticals used in Positron Emission Tomography. Inorganica Chimica Acta. 2012;389:36–51. [Google Scholar]
- 26.Li Z, Conti PS. Radiopharmaceutical chemistry for positron emission tomography. Advanced Drug Delivery Reviews. 2010;62(11):1031–1051. doi: 10.1016/j.addr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Garrison JC, Rold TL, Sieckman GL, et al. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using Cu-64 bombesin analogs: side-by-side comparison of the CB-TE2A and DOTA chelation systems. Journal of Nuclear Medicine. 2007;48(8):1327–1337. doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Cai W, Cao F, et al. 18F-labeled bombesin analogs for targeting GRP receptor-expressing prostate cancer. Journal of Nuclear Medicine. 2006;47(3):492–501. [PubMed] [Google Scholar]
- 29.Schuhmacher J, Zhang H, Doll J, et al. GRP receptor-targeted PET of a rat pancreas carcinoma xenograft in nude mice with a 68Ga-labeled bombesin(6–14) analog. Journal of Nuclear Medicine. 2005;46(4):691–699. [PubMed] [Google Scholar]
- 30.Chen X, Park R, Hou Y, et al. MicroPET and autoradiographic imaging of GRP receptor expression with Cu-64-DOTA-Lys(3) bombesin in human prostate adenocarcinoma xenografts. Journal of Nuclear Medicine. 2004;45(8):1390–1397. [PubMed] [Google Scholar]
- 31.Honer M, Mu L, Stellfeld T, et al. 18F-labeled bombesin analog for specific and effective targeting of prostate tumors expressing gastrin-releasing peptide receptors. Journal of Nuclear Medicine. 2011;52(2):270–278. doi: 10.2967/jnumed.110.081620. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher PDI, Haswell SJ, Pombo-Villar E, et al. Micro reactors: principles and applications in organic synthesis. Tetrahedron. 2002;58(24):4735–4757. [Google Scholar]
- 33.Wang C, Ouyang J, Ye DK, Xu JJ, Chen HY, Xia XH. Rapid protein concentration, efficient fluorescence labeling and purification on a micro/nanofluidics chip. Lab on a Chip. 2012;12:2664–2671. doi: 10.1039/c2lc20977b. [DOI] [PubMed] [Google Scholar]
- 34.deMello AJ. Control and detection of chemical reactions in microfluidic systems. Nature. 2006;442(7101):394–402. doi: 10.1038/nature05062. [DOI] [PubMed] [Google Scholar]
- 35.Geyer K, Codée JDC, Seeberger PH. Microreactors as tools for synthetic Chemists—the chemists’ round-bottomed flask of the 21st century? Chemistry. 2006;12(33):8434–8442. doi: 10.1002/chem.200600596. [DOI] [PubMed] [Google Scholar]
- 36.Hartman RL, Jensen KF. Microchemical systems for continuous-flow synthesis. Lab on a Chip. 2009;9(17):2495–2507. doi: 10.1039/b906343a. [DOI] [PubMed] [Google Scholar]
- 37.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 38.Sahoo HR, Kralj JG, Jensen KF. Multistep continuous-flow microchemical synthesis involving multiple reactions and separations. Angewandte Chemie. 2007;46(30):5704–5708. doi: 10.1002/anie.200701434. [DOI] [PubMed] [Google Scholar]
- 39.Pagano N, Heil ML, Cosford NDP. Automated multistep continuous flow synthesis of 2-(1H-Indol-3-yl)thiazole derivatives. Synthesis-Stuttgart. 2012;44:2537–2546. doi: 10.1055/s-0031-1290953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu XY, Jensen KF. Multistep synthesis of amides from alcohols and amines in continuous flow microreactor systems using oxygen and urea hydrogen peroxide as oxidants. Green Chemistry. 2013;15:1538–1541. [Google Scholar]
- 41.Wheeler TD, Zeng D, Desai AV, Önal B, Reichert DE, Kenis PJA. Microfluidic labeling of biomolecules with radiometals for use in nuclear medicine. Lab on a Chip. 2010;10(24):3387–3396. doi: 10.1039/c0lc00162g. [DOI] [PubMed] [Google Scholar]
- 42.Bejot R, Elizarov AM, Ball E, et al. Batch-mode microfluidic radiosynthesis of N-succinimidyl-4-[18F]fluorobenzoate for protein labelling. Journal of Labelled Compounds and Radiopharmaceuticals. 2011;54(3):117–122. [Google Scholar]
- 43.Richter S, Bouvet V, Wuest M, et al. F-labeled phosphopeptide-cell-penetrating peptide dimers with enhanced cell uptake properties in human cancer cells. Nuclear Medicine and Biology. 2012;39(18):1202–1212. doi: 10.1016/j.nucmedbio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Liu K, Lepin EJ, Wang M, et al. Microfluidic-based18F-labeling of biomolecules for immuno-positron emission tomography. Molecular Imaging. 2011;10(3):168–176, 161–167. [PMC free article] [PubMed] [Google Scholar]
- 45.Selivanova SV, Mu L, Ungersboeck J, et al. Single-step radiofluorination of peptides using continuous flow microreactor. Organic and Biomolecular Chemistry. 2012;10(19):3871–3874. doi: 10.1039/c2ob00015f. [DOI] [PubMed] [Google Scholar]


