Abstract
The differentiated state of somatic cells is highly stable but it can be experimentally reversed and the resulting cells can then be redirected into many different pathways. Nuclear reprogramming has been achieved by nuclear transfer to eggs, cell fusion, and by overexpression of transcription factors. The mechanisms of nuclear reprogramming are not understood, but some insight into them is provided by comparing the efficiencies of different reprogramming strategies. Here we compare these efficiencies by describing the frequency and rapidity with which reprogramming is induced and by the proportion of cells and level of expression in which reprogramming is achieved. We comment on the mechanisms that lead to successful somatic cell reprogramming, and those that resist to help to maintain the differentiated state of somatic cells.
Keywords: nuclear reprogramming, reprogramming efficiency, cell differentiation, egg, oocytes, nuclear transfer, cell fusion, iPS, epigenetic reprogramming, epigenetic memory
Introduction
We define nuclear reprogramming as a switch in gene expression from one cell type to that of an unrelated type. Although the differentiated state of somatic cells is extremely stable, certain experimental procedures can nevertheless reprogram somatic cells or their nuclei to acquire wide developmental plasticity. These procedures include nuclear transfer (to eggs or oocytes), cell fusion, and the overexpression of transcription factors. There are, however, enormous differences between procedures and cell types in the efficiency with which reprogramming takes place. With very low efficiency, there is the concern that an unidentified and minority cell type may be the only cell which is reprogrammed. In some procedures, multiple rounds of cell division take place before reprogramming is evident, and this may allow selection, making it difficult to determine the efficiency with which a cell is reprogrammed. Here, we review the efficiency of nuclear reprogramming in different systems and highlight striking differences in the reprogramming efficiency and speed of reprogramming according to the criteria used to assess reprogramming. There also appears to be great variation in the percentage of the starting population of cells that is reprogrammed, as well as in the level of expression of reprogrammed genes.
We consider that there are three reasons for interest in the efficiency of nuclear reprogramming. One is to help to identify the mechanisms by which reprogramming can be experimentally induced. Another reason is to understand the basis of the increasing resistance that cells show towards reprogramming as they become progressively more differentiated. The third interest is in reprogramming patient-specific somatic cells to provide genetically matched cells for cell replacement therapies.
The stability of the differentiated state
The fertilized egg is the most completely plastic cell in the sense that its mitotic products can form every cell in the body. This high degree of plasticity is also shared by certain embryonic cells. These include cells of the inner cell mass of the mouse embryo and the derived embryonic stem cells (ES cells); when implanted into a blastocyst, a contribution to all tissues and cell types of the body can occur. Likewise, the animal cap cells of the early amphibian embryo, when supplied with appropriate signals from other underlying cells or when cultured in activin- or retinoic acid-containing media, can form almost all cell types in the body (Asashima and Grunz 1983). However, soon after these early embryo stages, cells start to become restricted in their developmental capacity.
It is useful to distinguish commitment from determination (Slack 1983). Commitment is used to describe cells that will adopt their normal fate when explanted, that is cultured in a neutral medium. This means that these cells are sufficiently committed to their normal fate that they will follow that fate even in the absence of signals with which they would normally be provided when in a whole embryo. A more advanced stage of differentiation is described as determination. The test for this is to transplant cells into different parts of an embryo where they receive signals entirely different from those to which they would be exposed in normal development. A determined cell is unable to diverge from its normal fate, even in the presence of these unusual signals. The term transdetermination applies to those cells which can switch their differentiation pathway under certain conditions, even when they have reached, by normal criteria, a determined state. A number of examples of transdetermination have been reviewed by Okada (1991). The most striking example is that of lens regeneration in newts. In this case, pigmented cells of the iris epithelium can be observed to gradually change, in the course of many cell divisions and over many weeks, to produce lens or other cell types of the eye. Another well-known example is that of Drosophila imaginal disc cells. These cells retain their normal fate over many hundreds of cell divisions when serially transplanted to the adult abdomen; their fate is revealed by transplanting the cells to a larval abdomen and passing them through metamorphosis with exposure to ecdysone. Occasionally cells will switch from one fate to another, forming, for example, antenna as opposed to leg. The frequency of this example of transdetermination is very low, and has been estimated at a rate of 10−3 to 10−4 for most examples of a switch in cell fate (Hadorn 1963; Shearn et al. 1978).
The major conclusion from this section is that cells that have arrived at the determined state only switch to another fate with an extremely low frequency, in the course of numerous cell divisions and under special conditions. The determined state is therefore said to be extraordinarily stable, a very desirable situation so that we do not have inappropriate cells in most of our organs in the body.
Criteria for the efficiency of nuclear reprogramming
Many different measures have been used to estimate the efficiency of nuclear reprogramming. 1. One is to determine the frequency of new gene expression or transcription. The genes scored need to be those of an embryo cell or those of cell fates entirely unrelated to that of the starting cell. 2. More demanding is evidence of a functional cell-type, again unrelated to the cell undergoing reprogramming. 3. The ability to derive ES cells from a somatic cell nucleus opens a route towards several cell-types unrelated to that of the donor nucleus. 4. In some cases, attention is paid to the extinction of genes characteristically expressed in the cells that are subsequently reprogrammed. 5. Another important measure of reprogramming efficiency, usually not discussed, is the magnitude of the induced new gene expression. Complete reprogramming would ask for expression of the induced genes to be at the same level as that of equivalent cells in normal embryos or adult organs. 6. Finally, it is also important to determine the time and number of cell divisions that are required for the reprogrammed state to become evident. In some cases no cell division or DNA replication is required; in others, extended time and numerous cell divisions, and hence the possibility of cell selection, are required for the reprogrammed condition to become evident. We have assembled the results of different reprogramming procedures into tables, in order to compare the efficiencies of reprogramming by different routes (Table 1 – 2).
Table 1.
Efficiencies: Maximum number of reprogrammed cells and number of cell divisions.
| System | Starting Cell | Resulting Cells | Starting Nuclei Reprogrammed (%) |
Number of Cell Divisions |
Reference |
|---|---|---|---|---|---|
|
SCNT to amphibian egg
(MII oocyte) |
Blastulae cell nuclei | Blastula stage embryo |
32 | ~7 | (Gurdon 1960) |
|
SCNT to amphibian egg
(MII oocyte) |
Blastulae cell nuclei | Swimming tadpole | 22 | ~17 | (Gurdon 1960) |
|
SCNT to amphibian egg
(MII oocyte) |
Skin outgrowth | Swimming tadpole | 1,3 | ~17 | (Gurdon et al. 1975) |
|
SCNT to amphibian egg
(MII oocyte) |
Skin outgrowth | Swimming tadpole | 43 † | ~17 | (Gurdon et al. 1975; Byrne et al. 2002) |
|
SCNT to mouse MII oocyte
(egg) |
ES | Blastocyst | 29 | 5 | (Wakayama et al. 1999) |
|
SCNT to mouse MII oocyte
(egg) |
Cumulus | Blastocyst | 55 | 5 | (Wakayama et al. 1998) |
|
SCNT to mouse MII oocyte
(egg) |
Tail tip fibroblasts | Blastocyst | 50 | 5 | (Wakayama and Yanagimachi 1999) |
|
SCNT to mouse MII oocyte
(egg) |
ES | Live born mice | 2.4 | >25 | (Wakayama et al. 1999) |
|
SCNT to mouse MII oocyte
(egg) |
Cumulus | Live born mice | 1-3 | >25 | (Wakayama et al. 1998; Wakayama et al. 2005) |
|
SCNT to mouse MII oocyte
(egg) |
Tail tip fibroblasts | Live born mice | 1 | >25 | (Wakayama and Yanagimachi 1999; Wakayama et al. 2005) |
|
SCNT to mouse MII oocyte
(egg) |
ES | NT ES cells | 13 | >15 | (Blelloch et al. 2004) |
|
SCNT to mouse MII oocyte
(egg) |
Cumulus | NT ES cells | 5-10 | >15 | (Wakayama et al. 2005) |
|
SCNT to mouse MII oocyte
(egg) |
Tail tip fibroblasts | NT ES cells | 0.5-3 | >15 | (Wakayama et al. 2005) |
| NT Blastocyst | ES | NT ES cells | 10-50 | >10 | (Blelloch et al. 2004) |
| NT Blastocyst | Cumulus | NT ES cells | 12-19 | >10 | (Wakayama et al. 2005) |
| NT Blastocyst | Tail tip fibroblasts | NT ES cells | 13-33 | >10 | (Wakayama et al. 2005) |
|
Multiple mammalian NT to
Xenopus prophase I oocyte |
3 days RA mouse ES | Nuclear transfer oocyte |
>90* | 0 | (Jullien et al. 2010) |
| Cell fusion | Human fibroblasts × mouse ES |
Heterokaryons | 0.6-1* | 0 | (Bhutani et al. 2010) |
| Cell fusion | ES × NSC | Tetraploid hybrids | 0.00014* | ~10 | (Silva et al. 2006) |
| Cell fusion | ES × NSC + Nanog | Tetraploid hybrids | ~3* | ~10 | (Silva et al. 2006) |
| MyoD overexpression | Fibroblasts | Muscle myoblasts | 25-50 | 0 to 1 | (Davis et al. 1987) |
| C/EBPα overexpression | Pre-T cell | Macrophages | 60 | 0 to 2 | (Laiosa et al. 2006) |
|
Pdx1, Ngn3, Mafa:
adenovirus mediated expression |
in vivo adult pancreatic acinar cells |
β cells | 20 | 0 to 2 | (Zhou et al. 2008) |
|
OSKM: retrovirus
mediated overexpression |
MEFs | iPS colonies | <0.02 | >15 ? | (Takahashi and Yamanaka 2006) |
|
OSKM: dox inducible
lentivirus |
Tail tip fibroblasts | Oct4-GFP iPS colonies |
0.06-0.5 | >15 ? | (Stadtfeld et al. 2008; Sommer et al. 2009) |
|
OSKM: dox inducible
lentivirus + VPA |
MEFs | Oct4-GFP iPS colonies |
12 | >15 ? | (Huangfu et al. 2008) |
|
OSKM: retrovirus
mediated overexpression |
p53−/− MEFs | Nanog-GFP iPS colonies |
16* | >15 ? | (Hong et al. 2009) |
|
OSKM: dox inducible
lentivirus |
MEFs | Nanog+ Cdh1+ iPS colonies |
3.7 | >15 ? | (Smith et al. 2010) |
|
OSKM: dox inductiondox
inducible lentivirus |
MEFs | Nanog+ Cdh1+ iPS colonies |
1.15 Δ | >15 ? | (Smith et al. 2010) |
|
OSKM: dox inducible
lentivirus + p53 shRNA |
MEFs | Nanog+ Cdh1+ iPS colonies |
<0.2 Δ | >15 ? | (Smith et al. 2010) |
Experiment involves serial nuclear transfer.
Includes incompletely reprogrammed cells.
normalized efficiency. NT, Nuclear transfer. SCNT, Single Cell Nuclear Transfer. MII, Metaphase II. O, Oct4. S, Sox2. K, KLF4. M, c-Myc. MEFs, Mouse embryonic fibroblasts. NSC, Neural stem cell. RA, Retinoic Acid. VPA, Valproic Acid.
Table 2.
Efficiencies: Frequency, speed and magnitude of response to reprogramming cue.
| System | Starting cell | Frequency of gene reactivation (%) |
Magnitude of gene reactivation (%) |
Starting cell gene extinction (%) |
Time | Reference |
|---|---|---|---|---|---|---|
|
SCNT to amphibian
egg (MII oocyte) |
Stage 21 somite |
Xbra, >90 | 100 | 50 | < 10 hours |
(Ng and Gurdon 2005) |
|
SCNT to mouse MII
oocyte (egg) |
Cumulus | Oct4, 88 | 0-400? | ~Yes | 3.5 days | (Boiani et al. 2002; Bortvin et al. 2003) |
|
Multiple mammalian
NT to Xenopus prophase I oocyte |
3 day RA mouse ES |
Sox2, >90 | 100 of ES level | No | 24 h | (Jullien et al. 2010) |
|
Multiple mammalian
NT to Xenopus prophase I oocyte |
C2C12 | Sox2, >90 | 0.4 of ES level | No | 1-3 days | (Halley-Stott et al. 2010) |
| Cell fusion | Human fibroblasts × mouse ES |
Oct4, >70 | <0.01 | Sometimes | 24-48h | (Pereira et al. 2008; Lee et al. 2009; Bhutani et al. 2010) |
|
C/EBPα
overexpression |
pre-B cell | Mac1, 60 | 100 | Yes | 4 days | (Xie et al. 2004) |
|
Pdx1, Ngn3, Mafa
overexpression |
in vivo adult pancreatic acinar cells |
Insulin, 25 | low (3 days) high (1 month) |
Yes | 3 days to 1 month |
(Zhou et al. 2008) |
|
OSKM: retrovirus
mediated overexpression |
MEFs | Oct4 | 100 | Yes | 2 weeks | (Takahashi and Yamanaka 2006) |
|
OSKM: retrovirus
mediated overexpression |
MEFs | Oct4-GFP, 0.03 |
100 | Yes | 1 week | (Huangfu et al. 2008) |
|
OSKM: retrovirus
mediated overexpression + VPA |
MEFs | Oct4-GFP, 11.8 |
100 | Yes | 1 week | (Huangfu et al. 2008) |
|
OSK: retrovirus
mediated overexpression |
MEFs | Nanog-GFP, 0.22 |
1 to 100 | Yes | 1 month | (Hong et al. 2009) |
|
OSK: retrovirus
mediated overexpression |
p53 −/− MEFs | Nanog-GFP, 10 | 1 to 100 | Yes, but not always |
1 month | (Hong et al. 2009) |
NT, Nuclear transfer. SCNT, Single Cell Nuclear Transfer. MII, Metaphase II. O, Oct4. S, Sox2. K, KLF4. M, c-Myc. MEFs, Mouse embryonic fibroblasts. NSC, Neural stem cell. RA, Retinoic Acid. VPA, Valproic Acid.
Nuclear transfer to enucleated eggs (metaphase II oocytes)
The original design of nuclear transfer experiments, first established in amphibia, involves the injection of a nucleus (ruptured cell) into an enucleated, unfertilized egg (Fig. 1A) (Briggs and King 1952). Most of the resulting injected eggs begin development as shown by activation (cortical rotation and early cleavage) of the egg. Some of these then cleave normally and develop through normal embryogenesis, eventually reaching adulthood (Gurdon et al. 1958). When a nucleus from an embryonic cell, such as a blastula cell, is used, a high proportion of the embryos reach normal blastula stages (32%) and of these a variable number, but up to 30% in good experiments, will reach adulthood (Gurdon 1960). However, when the nucleus of a specialized cell is used, the success rate is markedly less. For example, the nucleus of an endoderm or skin cell promotes the formation of complete blastulae in 12% and 5% of the total nuclear transfers, respectively (Fig. 2A) (Gurdon et al. 1975). Most of these first nuclear transfer embryos do not develop into tadpoles and therefore do not reach adulthood. Therefore, the efficiency of nuclear reprogramming by nuclear transfer decreases when the nuclei of cells of increasingly differentiated state are used as donor. These and other results are summarized in Table 1.
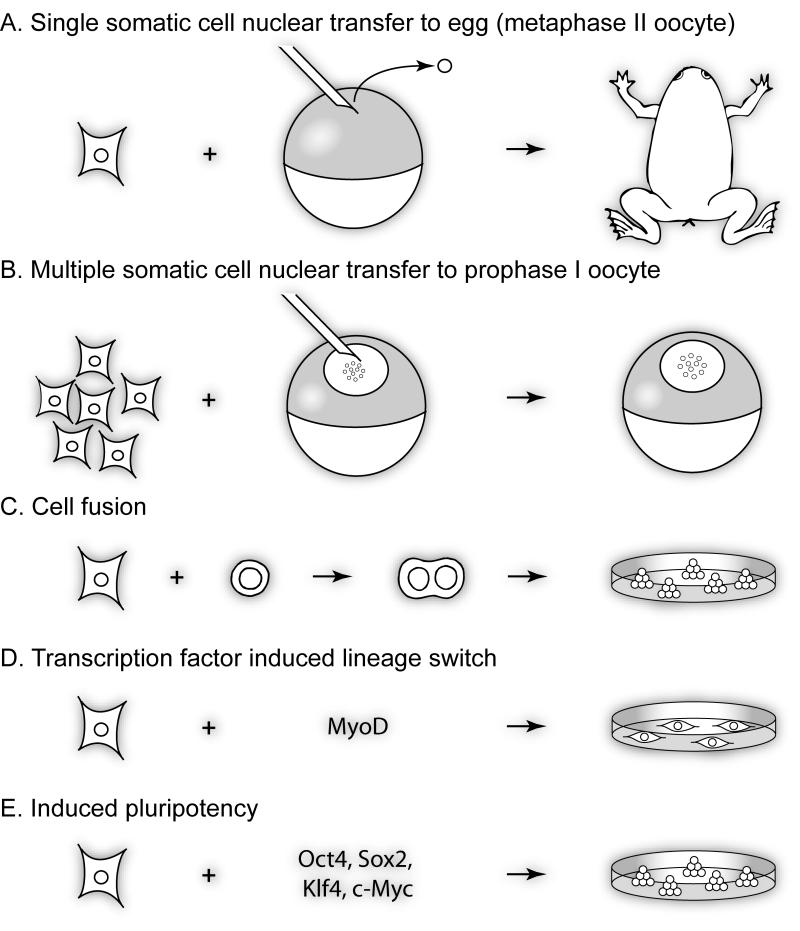
Figure 1. Nuclear reprogramming strategies.
A. Nuclear reprogramming by nuclear transfer involves the transplantation of a somatic cell nucleus into an enucleated egg (metaphase II oocyte), which can lead, in some cases, to the production of fertile adults, genetically identical to the donor nucleus. B. Direct transplantation of multiple mammalian somatic cell nuclei into the nucleus of a Xenopus prophase oocyte I does not lead to new cells, but induces fast and direct transcriptional reprogramming of previously silenced genes. C. Reprogramming of somatic cells by cell fusion produces heterokaryons and cell hybrids, possessing many molecular characteristics of pluripotency when pluripotent cells are fused to somatic ones. D. Overexpression of transcription factors can induce a lineage switch. E. Ground state pluripotency can be induced in somatic cells by overexpression of a few factors such as Oct4, Sox2, KLF4 and c-Myc.
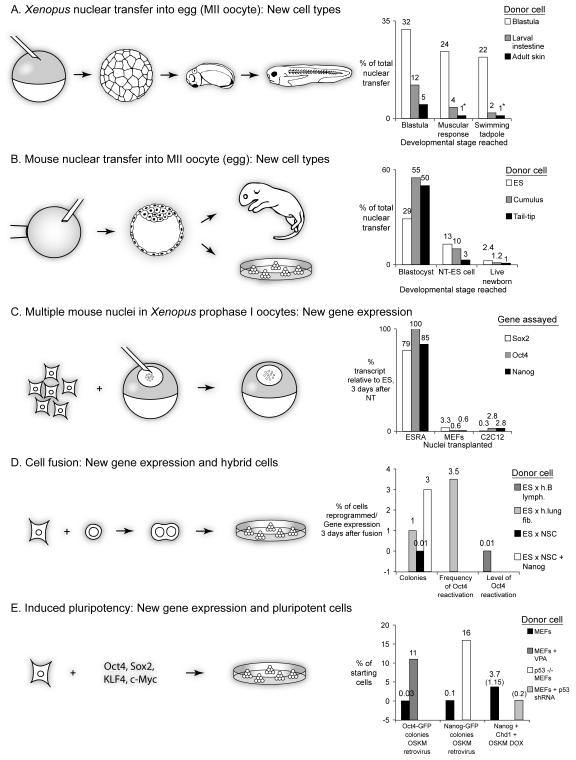
Figure 2. Nuclear reprogramming efficiencies judged by different criteria.
The left part of the figure shows the reprogramming steps of each system. The graphs on the right indicate the efficiency (%) of reprogramming as judged by different criteria. The origin of donor nuclei is indicated. A. Efficiency judged by the proportion of nuclear transfer Xenopus embryos obtained from somatic cell nuclear transfers to egg (metaphase II oocytes) that reach a particular stage of development. * Includes results from serial nuclear transfer experiments. B. Efficiency judged by the proportion of mouse embryos obtained by somatic cell nuclear transfers to mouse eggs (MII oocyte) that reach a particular stage of development. C. Efficiency judged by the level of transcriptional reactivation of pluripotency genes after nuclear transfer to Xenopus oocytes. D. Reprogramming efficiency after cell fusion, as judged by the number of colonies obtained or the extent of gene reactivation. E. Induced pluripotency: efficiency judged by the number of colonies obtained from starting cells by using various factor delivery approaches. Numbers in parentheses indicate normalized efficiencies (see text).
A stringent test of efficiency asks what proportion of nuclear transplant embryos reach a stage of development in which functional cell-types entirely unrelated to that of the donor are expressed (criterion 2). For example, tadpoles which respond to a stimulus by movement must have functional muscle and nerve cells. If nuclei are transplanted from skin cells, about 30% of such nuclear transplant embryos will develop into partial or complete blastulae, but only a very small proportion will develop into swimming tadpoles (1.3%, Fig 2A). However, when the partial blastulae obtained from such skin cell first nuclear transfers are used for a second round of nuclear transfers, up to 43% of these now reach swimming tadpole stages (Gurdon et al. 1975). This means that serial nuclear transfer can induce a switch in cell lineage with a high frequency. This frequency is up to 30%, when considering a switch between a functional endoderm cell nuclei into functional muscle and nerve cells, by a combination of serial nuclear transfer and grafts (Byrne et al. 2002). This process of reprogramming by single nuclear transfer to the egg involves several cell divisions. For example the muscular response stage of Xenopus embryos consists of about 150,000 cells, resulting from an average of 17 cell divisions.
Transplantation of the nucleus of a mammalian somatic cell into an enucleated egg (MII oocyte) also leads to the development of nuclear transfer embryos (Wilmut et al. 1997; Wakayama et al. 1998). The efficiency in mammalian nuclear transfer can be measured as the proportion of nuclear transfer embryos that reaches a particular stage. For example, 50% of mouse tail tip fibroblasts nuclear transfer embryos develop to the blastocyst stage, but only a very few survive to birth (Fig. 2B) (Wakayama and Yanagimachi 1999; Wakayama et al. 2005). For comparison, the efficiency of morula/blastocyst formation by ES cell nuclear transfer is 44%, with up to 3.3% giving rise to live newborn (Wakayama et al. 1999; Blelloch et al. 2004). Another way of assessing nuclear reprogramming efficiency is to measure the number of ES cell lines that can be derived from nuclear transfer blastocysts (criterion 3). When considering derivation efficiencies of NT ES cells from ES and fibroblast nuclear transfer blastocysts, as much as half or a third of these can give rise to ES cell lines, respectively (Blelloch et al. 2004; Wakayama et al. 2005). While adult nuclear transfer mice can be derived with high efficiencies by using the nuclei of pluripotent ES cells (8%) (Wakayama et al. 1999), those from highly specialized and differentiated cells lead to much lower rates. The efficiencies of mammalian nuclear transfer have been reviewed in detail (Meissner and Jaenisch 2006).
Nuclear reprogramming by nuclear transfer to MII oocytes may involve a very high number of cell divisions. For example, obtaining germ cells by nuclear transfer of mouse somatic cell nuclei involves an average of 25 cell divisions. Yet Oct4 gene reactivation occurs within 1 or 2 cell divisions after nuclear transfer (Boiani et al. 2005; Egli et al. 2008). Similarly, developmentally regulated induction of pluripotency genes in the ICM and reactivation of the silent X chromosome occur within very few cell divisions (Eggan 2000; Bao et al. 2005; Silva et al. 2009).
There are two important conclusions from nuclear transfer experiments. The first is that the nuclei of even the most differentiated cells can be fully reprogrammed efficiently to functional new cell types by nuclear transfer, by the natural components of the egg (MII oocyte) and developmental programmes of the embryo. The second is that the process of cellular differentiation entails a major resistance of somatic cell nuclei to nuclear reprogramming by eggs and oocytes, such that reprogramming is always less efficient for nuclei of cells of increased differentiation states.
Nuclear transfer to oocytes in first meiotic prophase
An altogether different design of nuclear transplant experiment involves transplanting multiple cell nuclei into the germinal vesicle of an amphibian first meiotic prophase oocyte (Fig. 1B) (Byrne et al. 2003). The oocyte germinal vesicle (GV) contains a high concentration of components that are eventually distributed to the egg, after meiotic maturation, and that are necessary for embryonic development (Gao et al. 2002). Importantly, this reprogramming system does not lead new cell types. Somatic nuclei transplanted to an oocyte germinal vesicle do not undergo DNA synthesis or cell division, but are intensely active in RNA synthesis as is the host oocyte itself. This system makes it possible to transplant multiple nuclei from mammalian cells into the amphibian oocyte and to see the activation of many genes expressed in normal early development, including pluripotency marker genes (criterion 1). An advantage of this system is that it is possible to see the direct activation of previously silenced genes in adult somatic nuclei in the total absence of DNA replication. There is therefore a direct switch in gene transcription from a somatic to an oocyte-like type without the intervention of or need for DNA replication. The exchange of molecules involved therefore reflects directly the process of transcriptional reprogramming. In contrast, nuclear transfer experiments to unfertilized eggs (above) are complicated by a period of intense DNA replication and cell division and absence of transcription immediately after nuclear transfer (criterion 6). The time when transcriptional reprogramming takes place is very hard to determine or analyze in egg nuclear transfer experiments.
Using the multiple nuclei to oocyte GV assay system, one important conclusion is that the frequency and speed of gene reactivation is very high. With HeLa cell nuclei, reactivation of Oct4 can be seen in as little as 8 hours at 18°C (Koziol et al. 2007). When transcriptional reactivation of Sox2 is assayed, reprogramming is seen in all experiments within 48 hours (Halley-Stott et al. 2010). Therefore, Xenopus oocytes must contain factors that are able to efficiently induce transcriptional reprogramming. The oocyte type linker histone B4 was recently identified as a factor necessary for efficient induction of gene reactivation (Jullien et al. 2010). After nuclear transfer, B4 is incorporated into transplanted nuclei, an event associated with loss of somatic linker histone H1. This occurs in over 90% of nuclei in most experiments. Genome-wide resetting of histone post-translational modifications of transplanted nuclei has also been shown (Murata et al. 2010). Thus, after nuclear transfer, there is efficient loss of somatic nuclear proteins and incorporation of oocyte factors.
A more stringent measure of reprogramming efficiency includes the magnitude of gene reactivation (criterion 5). With the Xenopus oocyte system, the maximum level of gene reactivation is related to the differentiated state of donor nuclei. ES cells that have been induced to differentiate for 3 days by retinoic acid (RA) reactivate transcription of the silenced pluripotency genes Oct4, Sox2 and Nanog to 100% of the level in transplanted ES cells within 4 days (Table 2) (Halley-Stott et al. 2010). However, when the nuclei from specialized cells such as thymus, C2C12 or MEFs are used, the same genes reactivate to a maximum level ranging from 0.3% to 3.3% of the transplanted ES nuclei levels (Fig. 2C). Therefore, the process of differentiation entails a great resistance to transcriptional nuclear reprogramming by oocytes. An important conclusion is that Xenopus oocytes have the ability to efficiently induce gene reactivation, in the absence of cell division and within a short time period, using the natural components of the oocyte. Therefore, multiple mammalian nuclear transfer to Xenopus oocytes is a good system to investigate the mechanisms of transcriptional reprogramming. Moreover, their large size (1.2 mm) makes them an ideal source of material for the identification of new reprogramming factors.
Cell fusion
Cell fusion can be experimentally induced such that several nuclei are forced to reside within a common cytoplasm (Fig. 1C) (Davidson et al. 1966; Harris et al. 1966). This procedure provides short term multinucleated cells, termed heterokaryons, in which nuclear reprogramming is induced. One cell type usually shows dominance over the other, such that it imposes its state on another nucleus. The early experiments demonstrated that the nuclei of mature hen erythrocytes are induced to resume RNA synthesis when fused to rapidly dividing HeLa cells, an event associated with nuclear swelling (Harris 1967). Reprogramming of previously silenced genes can occur in the absence of cell division in heterokaryons, such that non-muscle amniotic human cells are induced to express muscle genes when they are fused to mouse muscle cells (Blau et al. 1983).
Initiation of reprogramming of gene expression in heterokaryons is direct, rapid (1 to 2 days), and independent of DNA synthesis (Han et al. 2008; Palermo et al. 2009). For example, ES cells that have been treated with the cell division inhibitor Mitomycin C are able to induce reactivation of pluripotency marker Oct4-GFP when fused to neural stem cells (NSCs) (Do and Scholer 2004). In cell fusion experiments, only a minor proportion of cells are induced to fuse (0.6-1%), and a strong selection of fused cells is usually required. However, when ES cells are used, a very high proportion of the fused cells is induced to reactivate previously silent genes (criterion 1). For example, the frequency of pluripotency gene reactivation from human fibroblasts after fusion with mouse ES cells is very high (>70%) (Bhutani et al. 2009). However, the level of gene reactivation (criterion 5) obtained in heterokaryons with lymphocytes is usually very low. One study estimates these levels as <0.01-1% of ES cell levels for Oct4, several days after fusion (Fig. 2D) (Pereira et al. 2008). Nevertheless, because gene reactivation is initiated frequently in heterokaryons, they constitute a good system to study the mechanisms that initiate gene reactivation.
Heterokaryons eventually give rise to proliferating cell hybrids, following fusion of several nuclei, after nuclear membrane breakdown at cell division. Reprogramming of somatic cells in cell hybrids has been achieved by fusion with ES, embryonic germ (EG) and embryo carcinoma (EC) cells (Miller and Ruddle 1976; Tada et al. 1997; Matveeva et al. 1998; Kimura et al. 2004). Pluripotent cell types are usually dominant over the somatic cell types in cell hybrids, and induce phenotypic and epigenetic changes so that somatic nuclei are induced to resemble pluripotent ones. Full level Oct4-GFP reactivation can be obtained within 2 days of neural stem cells (NSCs) fusion with ES cells (Do et al. 2007). Tissue specific gene extinction can also be seen in pluripotent hybrids (criterion 4). However, gene extinction does not to occur in some cases, and is dependant on the cell types and their ratio before fusion (Lee et al. 2009; Kruglova et al. 2010). Somatic gene extinction was shown not to be required for gene reactivation in heterokaryons of human B lymphocytes fused to mouse myotubes (Terranova et al. 2006). Somatic cells, such as thymus cells, are induced to adopt epigenetic modifications resembling those of pluripotent cells when fused to EG cells (Tada et al. 1997). These include reactivation of pluripotency genes and of the inactive X chromosome, self-renewal, and DNA demethylation; reviewed in (Do et al. 2006; Pralong et al. 2006). Cell hybrids obtained by fusion to pluripotent cells therefore possess all the molecular characteristics of pluripotent cells. Tetraploid hybrids contribute to all three germ lineages when introduced into blastocysts, as judged by transgene expression analaysis (Matveeva et al. 1998; Tada et al. 2001; Ying et al. 2002). Because evidence for germline contribution has not been reported, whether tetraploid cell hybrids are truly pluripotent remains to be confirmed.
One study established that the efficiency of cell hybrid formation from ES cell fusion to NSCs can be increased by 200-fold (from <0.014% to ~3%) following overexpression of the pluripotency conferring factor Nanog (Silva et al. 2006). When fibroblasts and thymoctyes were used, the effect was also seen, but to a lower extent. Cell fusion experiments have the remarkable property of inducing active DNA demethylation in the absence of cell division. For example, the promoters of the pluripotency genes Oct4 and Nanog become demethylated within a day in mouse ES cells fused to human B cells (Pereira et al. 2008). Cell fusion provides a powerful system for the analysis of nuclear reprogramming. The presence of multiple genomes in the resulting hybrids precludes their use for therapy, but efforts to remove the genome of the dominant cells have been reported (Matsumura et al. 2007).
Several important conclusions can be drawn from cell fusion studies. First, transcriptional reactivation of previously silent genes can be efficiently initiated in the absence of cell division. Second, molecules of differentiated cells can reprogram the nuclei of other somatic cells.
Transcription factor induced lineage switch
It has been known for a long time from developmental biology studies that overexpression of one or a few transcription factors can have profound effects on cell phenotypes and identity. Pioneering studies in this field include the discovery of MyoD, a transcription factor sufficient to convert fibroblasts into myoblasts when overexpressed by transfection (Fig. 1D) (Davis et al. 1987). In these experiments, the proportion of responding cells is very high. For example, about 25 to 50% of fibroblasts are reprogrammed to myoblasts and up to 60% of pre-T cells are reprogrammed to become marcophages by overexpression of MyoD and C/EBPα, respectively (Laiosa et al. 2006). This kind of reprogramming usually involves several cell divisions, and hence the possibility for selection. However, cultured pancreatic exocrine cells can be transdifferentiated into hepatocytes, in the absence of cell division, following the overexpression of C/EBPα (Shen et al. 2000). The magnitude of gene reactivation can be very high in the reprogrammed cells. For instance, the macrophage marker Mac1 is reactivated to 100% of its marcrophage level within 4 days when C/EBPα is overexpressed in pre-B cells (Xie et al. 2004). Moreover, genes that are expressed in the starting cells are also turned off, indicating that reprogramming is complete.
Based on a very elegant strategy designed by Shinya Yamanaka (see induced pluripotency section below), the direct conversion of fibroblasts into functional neurons by defined factors was recently reported (Vierbuchen et al. 2010). A set of 19 genes that are specifically expressed in neural tissues were found to induce neuronal cells when overexpressed in mouse embryonic fibroblasts (MEFs). These experiments suggest that transcription factor overexpression may allow the reprogramming of somatic cells into any desired cell type. However, the conversion of somatic cells into other cell types by transcription factor overexpression is, at present, limited to certain cell types, usually, but not always, of closely related lineages. For instance, MyoD does not lead to myoblasts when expressed in certain non-muscle cell types, such as liver (Davis et al. 1987; Schafer et al. 1990).
A recent study reported the in vivo conversion of mouse pancreatic acinar cells into insulin producing β cells, following adenoviral mediated delivery of Pdx1, Ngn3 anf Mafa, 3 key genes with established functions in pancreatic embryonic development (Zhou et al. 2008). Insulin was induced in a high proportion of the infected cells (25%), from a low level after 3 days, to a high level after one month. The newly producing insulin cells possessed all the phenotypic characteristics of pancreatic β cells. The conversion seemed to occur with very limited, if any, cell divisions. Altogether, transcription factors involved in the early stages of embryonic development of specific cell types and organs appear as an appealing route for the induction of nuclear reprogramming of one kind of cell into another.
Induced pluripotency
A remarkable advance in the reprogramming field came from the elegant work of Takahashi and Yamanaka (2006), which demonstrated that overexpression of a set of four factors, namely Oct3/4, Sox2, KLF4 and c-Myc into mouse adult fibroblasts can induce nuclear reprogramming to produce pluripotent cells that closely resemble ES cells (Fig. 1E). The new cells pass the most stringent pluripotency tests, and share many characteristics with mouse ES cells, including morphology, growth, transcriptome and epigenome. Hence they were termed induced pluripotent stem cells (iPS cells). iPS cells show reactivation of the inactive X chromosome (Stadtfeld 2008), restore telomerase activity, and show genome-wide histone methylation patterns characteristic of ES cells (Takahashi and Yamanaka 2006; Maherali et al. 2007). Thus, it is possible to directly reprogram somatic cells from many different origins to acquire pluripotency directly by overexpression of a few factors. Nuclear reprogramming by defined factors has been extensively reviewed by others (Yamanaka 2007; Jaenisch and Young 2008; Amabile and Meissner 2009; Hochedlinger and Plath 2009; Kiskinis and Eggan 2010)
Of special interest here is the efficiency with which somatic cells can be reprogrammed to produce iPS cells. This was originally calculated as the number of iPS cell colonies obtained 2 weeks after addition of the four factors to MEFs (Takahashi and Yamanaka 2006). By this route, 0.01% to 0.05% of the starting cells are induced to form iPS cell colonies over 2 weeks (Takahashi and Yamanaka 2006; Maherali et al. 2007; Meissner et al. 2007; Okita et al. 2007; Wernig et al. 2007). This raises the possibility that reprogrammed cells arise from a subpopulation of cells, more prone to reprogramming. Variable reprogramming efficiencies of iPS formation have been reported (Kiskinis and Eggan 2010). During the reprogramming process, the reactivation of pluripotency markers AP1, SSEA1 and Oct4 was shown to be gradual (Brambrink 2008), culminating in the reactivation of Nanog and the acquisition of ground state pluripotency (Silva et al. 2009). It is thought that induction of pluripotency during iPS cell formation occurs stochastically (Hanna et al. 2009). When successful, the full pluripotency transcriptional network is induced, such that pluripotency genes are reactivated to the same levels as in ES cells (criterion 5), and differentiation genes initially expressed in the starting cells are silenced (criterion 4). As for nuclear transfer, the efficiency of nuclear reprogramming by defined factors decreases with the increased differentiation state of cells. For example, the reprogramming efficiency is much higher from less differentiated cells compared with terminally differentiated cells (Eminli et al. 2009). When the four factors are expressed in donor cells by means of stably integrated inducible expressing cassettes, the overall efficiency is increased (from 0.05% to 0.5%) but still remains low (Stadtfeld et al. 2008). Striking improvements in efficiencies have been reported following the use of small molecules (Feng et al. 2009). The histone deacetylase inhibitor valproic acid (VPA) (Huangfu et al. 2008), DNA methyltransferase inhibitors (Huangfu et al. 2008; Mikkelsen et al. 2008), histone methyltransferase inhibitor BIX-01294 (Shi et al. 2008) and Vitamin C (Esteban et al. 2009) have been shown to substantially increase the efficiency of nuclear reprogramming, and even to substitute for some of the factors, such VPA for Klf4 and c-Myc. VPA was found to increase the frequency of Oct4-GFP reactivation in MEFs from 0.03% to 11% (Fig. 2E) (Huangfu et al. 2008).
Because nuclear reprogramming of somatic cells by defined factors is a long process, the origins of iPS cell colonies have been hard to investigate. A recent study addressed this issue by using time-lapse imaging to track the reprogramming process over several weeks from MEFs to pluripotent iPS cells (Smith et al. 2010). This allowed a calculation of the normalized efficiency of nuclear reprogramming, taking into account only those iPS cell colonies that can be tracked back to single MEFs. It is found that apart from those colonies that originate from reprogramming events of single MEFs, secondary satellite iPS colonies also appear during the culture process, and cannot be tracked back to single cells; these therefore do not represent new reprogramming events. Moreover, iPS colonies were stringently assessed for full acquisition of pluripotency as judged by expression of AP, Nanog and Chd1. The use of less rigorous criteria can lead to an overestimate of reprogramming efficiencies (Smith et al. 2010). For example, the normalized efficiency of MEFs nuclear reprogramming to Cdh1 + Nanog + iPS cell colonies is 1.15%, to be compared with 3.7% obtained without normalization. A series of earlier reports which established that the deletion of p53 in MEFs is sufficient to increase the efficiency of Nanog-GFP positive colony formation from 0.2% to 20% (Hong et al. 2009; Kawamura et al. 2009; Li et al. 2009). However, it is noteworthy that the efficiency of iPS formation from p53 depleted MEFs, as judged by the number of Cdh1 and Nanog positive colonies is markedly less, approaching 0.2% (Fig. 2E) (Smith et al. 2010). The main conclusions from iPS cell work is that overexpression of a set of few factors can induce, with low frequency, the full reactivation of genes leading to the complete erasure of somatic cell identity, providing pluripotent cells capable of differentiating into all other cell types. Because iPS cells can be generated from human patient cells, without the need for oocytes, this is the most hopeful route to regenerative medicine.
Mechanisms
Efficient reprogramming requires opening up of the genome
One of the mechanisms of nuclear reprogramming is an efficient opening up of the genome. This is required in order to give reprogramming factors access to regulatory regions, facilitate chromatin remodelling, and mediate changes in gene expression. Eggs and oocytes very efficiently induce nuclear swelling and decondensation after somatic cell nuclear transfer (Gurdon 1968; Gurdon 1976). Moreover, nuclear decondensation correlates with nuclear reprogramming in many reprogramming systems. These include nuclear transfer to eggs and oocytes, cell fusion (Harris 1967), and reprogramming in the mouse germline (Hajkova et al. 2008). Nuclear swelling may also help removal of chromosomal proteins present in the nuclei undergoing reprogramming, which could contribute to chromatin decondensation, through loss of proteins such as Ring1b, shown to mediate chromatin condensation independently of its catalytic domain (Eskeland et al. 2010). Reprogramming by eggs uses the natural components of the egg, which have been synthesised and stored in the oocyte during oogenesis. These factors are used at fertilization to reprogram sperm and induce its reprogramming with a 100% efficiency. By use of egg extracts, Nucleoplasmin, an abundant egg protein, was identified as a factor greatly contributing to decondensation of sperm and somatic nuclei (Philpott et al. 1991; Tamada et al. 2006). It is noteworthy that factors required for opening up of the genome, such as Chd1, are reactivated and required for the induction of pluripotency in iPS cells (Gaspar-Maia et al. 2009). The main concept here is that efficient reprogramming requires opening up the genome, in order to give transcriptional regulators access to regulatory regions. This is done very efficiently by eggs, which possess the natural ability to decondense and remodel sperm with high efficiencies.
Efficient reprogramming of gene expression requires epigenetic reversal
Epigenetic regulation of gene expression mediated by DNA methylation, histone modification and chromatin remodelling plays an important role in the efficiency of nuclear reprogramming. Epigenetic defects have been widely documented in cloned embryos (Bourc’his et al. 2001; Dean et al. 2001). These result from the inability to revert donor cell epigenetic states, causing some genes to be inconsistently expressed in reprogrammed cells or embryos. The failure to reverse an epigenetic state is seen in the continuing expression of MyoD in muscle nuclei transplanted to amphibian eggs, but in the wrong lineages, such that endoderm cells are found to express muscle specific genes (Ng and Gurdon 2008).
Another example of epigenetic defects in cloned embryos comes from studies on the inactive X chromosome of female mouse somatic cells. After nuclear transfer from female somatic cells, the inactive X chromosome is reactivated upon developmentally programmed induction of pluripotency in the ICM (Eggan et al. 2000; Silva et al. 2009). However, the chromatin state of the inactive X chromosome is not fully reversed in early nuclear transfer embryos (Bao et al. 2005), and aberrant expression of the X inactivation inducing non-coding RNA Xist is seen in the differentiating cells of the epiblast, with cells harbouring one, two, or no Xist clouds (Nolen et al. 2005). These defects indicate that the Xist locus is incompletely reversed in some cells of cloned embryos. This could be due to DNA methylation (see below).
Epigenetic defects can also arise during the reprogramming process itself. One such recent development in this area includes the inactivation of the imprinted Igf2 locus during iPS cell formation (Stadtfeld et al. 2010). This leads to iPS colonies with limited developmental potential. The resulting iPS colonies share all the epigenetic characteristics of ES cells except for this locus, which can nevertheless be reactivated by exposure to TSA, providing fully pluripotent iPS cells.
The low efficiencies of nuclear reprogramming have been suggested to result from incomplete epigenetic reprogramming. Epigenetic defects are best revealed by the inefficiency of gene reactivation. Inconsistent reactivation of pluripotency genes in nuclear transfer embryos may be correlated with the inability to demethylate gene promoters (Boiani et al. 2002; Yamazaki et al. 2006). The general conclusion here is that during cell differentiation, cells progressively acquire epigenetic modifications that make them resistant to nuclear reprogramming, and decrease its efficiency.
Efficient reprogramming requires DNA demethylation
Efficient nuclear reprogramming requires epigenetic reversal of donor cell nuclei. It is well known that the inefficiency of nuclear reprogramming by nuclear transfer to enucleated eggs correlates with DNA methylation. Nuclear transfer embryo development is often associated with defects in DNA methylation (Dean et al. 2001; Kang et al. 2001; Ohgane et al. 2001; Yamazaki et al. 2006). Surprisingly, treatment of donor nuclei with unmethylatable cytosine analogue 5-Aza-dC is usually not found to increase the efficiency of nuclear transfer embryo development (Enright et al. 2003; Enright et al. 2005). However, when using Dnmt1 mutant MEFs, the efficiency of NT ES cell derivation from nuclear transfer blastocysts is increased threefold (Blelloch et al. 2006). Efficient active DNA demethylation in heterokaryons was recently suggested to require AID (Activation-induced cytidine deaminase) (Bhutani et al. 2010). Another study demonstrated genetically that efficient DNA demethylation during germline reprogramming involves AID (Popp et al. 2010). There are several other factors that could initiate active DNA demethylation, and may include components of the DNA repair pathway (Niehrs 2009). The major conclusion here is that efficient reprogramming requires DNA demethylation of silenced genes, a process that can be achieved passively, when cell divisions occur, or actively, by mechanisms that remain to be further explored.
Histone modifications
Epigenetic memory of gene silencing could also involve mechanisms that are independent of DNA methylation. During cell differentiation, gene silencing is generally seen to precede DNA methylation. Post-translational modifications of histone tails is well known to play an important role in regulation of gene expression (Kouzarides 2007). In particular, histone acetylation loosens the highly compact nucleosomal structure, making chromatin more accessible to transcriptional regulators such as transcription factors. Part of the epigenetic restriction imposed by modifications such as DNA methylation and histone modifications result in the recruitment of histone deacetylases (HDAC). HDAC inhibition after nuclear transfer was shown to enhance chromatin remodelling, histone modification, DNA replication and transcriptional activity (Kishigami et al. 2006; Bui et al. 2010). The efficiency of gene reactivation is also dramatically increased in iPS by HDAC inhibitors (Huangfu et al. 2008).
Cell divisions are not required for initiation of nuclear reprogramming
Transitions through mitosis are opportunities to reverse somatic cell characteristics, and has been proposed to greatly facilitate nuclear reprogramming (Egli et al. 2008). For example, many factors such as HP1β, Bmi1 or Brg1 are excluded from mitotic chromatin. After cell division, the pool of transcriptional regulators present in the cell instructs the nucleus which products to make. However we see that while cell divisions facilitate reprogramming, these are not strictly required for the induction of transcriptional reprogramming. Gene reactivation has been demonstrated to occur in the complete absence of cell division after nuclear transfer to Xenopus oocytes and in heterokaryons (Blau et al. 1983; Byrne et al. 2003). Furthermore, previously methylated pluripotency genes can be reactivated in the absence of cell division, as demonstrated by cell fusion experiments (Pereira et al. 2008). In addition, cell type switches can occur without cell divisions (Shen et al. 2000; Zhou et al. 2008). Altogether, we conclude that the initiation of transcriptional reprogramming does not require cell division.
Conclusion
We have discussed six criteria that have been used to assess the efficiency of nuclear reprogramming by nuclear transfer, cell fusion, and transcription factor overexpression. A general concept that emerges from a comparison of these procedures is that the differentiated state of a cell is very dynamic; it seems to be continually maintained by a cyclic reprogramming of gene expression by cytoplasmic factors that are encoded by the nucleus. We suggest that the proteins synthesized in the cytoplasm under the direction of the nucleus include ones, like transcription factor, that move back into the nucleus, and continually reprogram genes characteristic of that cell. The differentiated state will then persist in that cell and in its mitotic progeny. When the cytoplasmic environment around a nucleus is changed by cell fusion, and even more massively by nuclear transfer to eggs, then these reprogramming proteins will be changed. In the case of iPS, the enforced overexpression of transcription factors will have a similar effect. The survey of efficiencies, described here, shows that the transcription of nuclei from even the most differentiated cells can be rapidly re-set. The efficiency of reprogramming is much greater by the natural components of eggs and oocytes than by the overexpression of a few transcription factors. Also the efficiency of reprogramming as judged by new gene expression (criterion 1) is much greater than by fully functional cell types or ES cells (criteria 2 and 3).
The second generalization based on our survey of efficiencies is that, as cells differentiate, they become increasingly resistant to the reprogramming components of eggs and cultured cells, and to transcription factor overexpression. This is seen by the decreasing percent of cells that become reprogrammed (criterion 4) as well as by the reduced magnitude of reprogramming in a cell (criterion 5). This resistance is not explained by the lower division rate of more specialized cells (criterion 6). It is thought to reflect a progressively tighter association of repressors with the regulatory regions of pluripotency genes (Gurdon and Melton 2008). To identify the molecular basis of this resistance is a major challenge for the future.
Acknowledgments
The J.B.G. laboratory is supported by grants from the Wellcome Trust. V.P. is supported by a Wellcome Trust PhD scholarship (081277). K.M. is funded by JSPS Postdoctoral Fellowships for Research Abroad.
References
- Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Asashima M, Grunz H. Effects of inducers on inner and outer gastrula ectoderm layers of Xenopus laevis. Differentiation. 1983;23:206–12. doi: 10.1111/j.1432-0436.1982.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Bao S, Miyoshi N, Okamoto I, Jenuwein T, Heard E, Azim Surani M. Initiation of epigenetic reprogramming of the X chromosome in somatic nuclei transplanted to a mouse oocyte. EMBO Rep. 2005;6:748–54. doi: 10.1038/sj.embor.7400461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–7. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–80. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Blelloch R, Wang Z, Meissner A, Pollard S, Smith A, Jaenisch R. Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells. 2006;24:2007–13. doi: 10.1634/stemcells.2006-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blelloch RH, Hochedlinger K, Yamada Y, Brennan C, Kim M, Mintz B, Chin L, Jaenisch R. Nuclear cloning of embryonal carcinoma cells. Proc Natl Acad Sci USA. 2004;101:13985–90. doi: 10.1073/pnas.0405015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiani M, Eckardt S, Scholer HR, McLaughlin KJ. Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev. 2002;16:1209–19. doi: 10.1101/gad.966002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiani M, Gentile L, Gambles VV, Cavaleri F, Redi CA, Scholer HR. Variable reprogramming of the pluripotent stem cell marker Oct4 in mouse clones: distinct developmental potentials in different culture environments. Stem Cells. 2005;23:1089–104. doi: 10.1634/stemcells.2004-0352. [DOI] [PubMed] [Google Scholar]
- Bortvin A, Eggan K, Skaletsky H, Akutsu H, Berry DL, Yanagimachi R, Page DC, Jaenisch R. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development. 2003;130:1673–80. doi: 10.1242/dev.00366. [DOI] [PubMed] [Google Scholar]
- Bourc’his D, Le Bourhis D, Patin D, Niveleau A, Comizzoli P, Renard JP, Viegas-Péquignot E. Delayed and incomplete reprogramming of chromosome methylation patterns in bovine cloned embryos. Curr Biol. 2001;11:1542–6. doi: 10.1016/s0960-9822(01)00480-8. [DOI] [PubMed] [Google Scholar]
- Brambrink T. Sequential Expression of Pluripotency Markers during Direct Reprogramming of Mouse Somatic Cells. Cell stem cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R, King TJ. Transplantation of Living Nuclei From Blastula Cells into Enucleated Frogs’ Eggs. Proc Natl Acad Sci U S A. 1952;38:455–63. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui HT, Wakayama S, Kishigami S, Park KK, Kim JH, Van Thuan N, Wakayama T. Effect of Trichostatin A on Chromatin Remodeling, Histone Modifications, DNA Replication, and Transcriptional Activity in Cloned Mouse Embryos. Biol Reprod. 2010 doi: 10.1095/biolreprod.109.083337. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Simonsson S, Gurdon JB. From intestine to muscle: nuclear reprogramming through defective cloned embryos. Proc Natl Acad Sci USA. 2002;99:6059–63. doi: 10.1073/pnas.082112099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JA, Simonsson S, Western PS, Gurdon JB. Nuclei of adult mammalian somatic cells are directly reprogrammed to oct-4 stem cell gene expression by amphibian oocytes. Curr Biol. 2003;13:1206–13. doi: 10.1016/s0960-9822(03)00462-7. [DOI] [PubMed] [Google Scholar]
- Davidson RL, Ephrussi B, Yamamoto K. Regulation of pigment synthesis in mammalian cells, as studied by somatic hybridization. Proc Natl Acad Sci U S A. 1966;56:1437–40. doi: 10.1073/pnas.56.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dean W, Santos F, Stojkovic M, Zakhartchenko V, Walter J, Wolf E, Reik W. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci USA. 2001;98:13734–8. doi: 10.1073/pnas.241522698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do J, Han D, Gentile L, Sobek-Klocke I, Stehling M, Lee H, Scholer H. Erasure of Cellular Memory by Fusion with Pluripotent Cells. Stem Cells. 2007;25:1013–1020. doi: 10.1634/stemcells.2006-0691. [DOI] [PubMed] [Google Scholar]
- Do JT, Han DW, Schöler HR. Reprogramming somatic gene activity by fusion with pluripotent cells. Stem cell reviews. 2006;2:257–64. doi: 10.1007/BF02698052. [DOI] [PubMed] [Google Scholar]
- Do JT, Scholer HR. Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells. 2004;22:941–9. doi: 10.1634/stemcells.22-6-941. [DOI] [PubMed] [Google Scholar]
- Eggan K. X-Chromosome Inactivation in Cloned Mouse Embryos. Science. 2000;290:1578–1581. doi: 10.1126/science.290.5496.1578. [DOI] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Hochedlinger K, Rideout W, Yanagimachi R, Jaenisch R. X-Chromosome inactivation in cloned mouse embryos. Science. 2000;290:1578–81. doi: 10.1126/science.290.5496.1578. [DOI] [PubMed] [Google Scholar]
- Egli D, Birkhoff G, Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nat Rev Mol Cell Biol. 2008;9:505–16. doi: 10.1038/nrm2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, Hock H, Hochedlinger K. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–978. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright BP, Kubota C, Yang X, Tian XC. Epigenetic characteristics and development of embryos cloned from donor cells treated by trichostatin A or 5-aza-2′-deoxycytidine. Biol Reprod. 2003;69:896–901. doi: 10.1095/biolreprod.103.017954. [DOI] [PubMed] [Google Scholar]
- Enright BP, Sung LY, Chang CC, Yang X, Tian XC. Methylation and acetylation characteristics of cloned bovine embryos from donor cells treated with 5-aza-2′-deoxycytidine. Biol Reprod. 2005;72:944–8. doi: 10.1095/biolreprod.104.033225. [DOI] [PubMed] [Google Scholar]
- Eskeland R, Leeb M, Grimes GR, Kress C, Boyle S, Sproul D, Gilbert N, Fan Y, Skoultchi AI, Wutz A, Bickmore WA. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell. 2010;38:452–64. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, Chen K, Li Y, Liu X, Xu J, Zhang S, Li F, He W, Labuda K, Song Y, Peterbauer A, Wolbank S, Redl H, Zhong M, Cai D, Zeng L, Pei D. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2009;6:71–9. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4:301–12. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Gao S, Gasparrini B, McGarry M, Ferrier T, Fletcher J, Harkness L, De Sousa P, Wilmut I. Germinal vesicle material is essential for nucleus remodeling after nuclear transfer. Biol Reprod. 2002;67:928–34. doi: 10.1095/biolreprod.102.004606. [DOI] [PubMed] [Google Scholar]
- Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, Ramalho-Santos J, McManus MT, Plath K, Meshorer E, Ramalho-Santos M. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460:863–8. doi: 10.1038/nature08212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J, Melton D. Nuclear Reprogramming in Cells. Science. 2008;322:1811–1815. doi: 10.1126/science.1160810. [DOI] [PubMed] [Google Scholar]
- Gurdon JB. The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. Journal of embryology and experimental morphology. 1960;8:505–26. [PubMed] [Google Scholar]
- Gurdon JB. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. Journal of embryology and experimental morphology. 1968;20:401–14. [PubMed] [Google Scholar]
- Gurdon JB. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. Journal of embryology and experimental morphology. 1976;36:523–40. [PubMed] [Google Scholar]
- Gurdon JB, Elsdale TR, Fishberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182:64–5. doi: 10.1038/182064a0. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Laskey RA, Reeves OR. The developmental capacity of nuclei transplanted from keratinized skin cells of adult frogs. Journal of embryology and experimental morphology. 1975;34:93–112. [PubMed] [Google Scholar]
- Hadorn E. Differenzierungsleistungen wiederhelt fragmentierter Teilstiicke miinnlicher Genital scheiben von Drosophila melanogaster nach Kultur in vivo. Dev. Biol. 1963;7:617–629. [Google Scholar]
- Hajkova P, Ancelin K, Waldmann T, Lacoste N, Lange UC, Cesari F, Lee C, Almouzni G, Schneider R, Surani MA. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature. 2008;452:877–81. doi: 10.1038/nature06714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halley-Stott RP, Pasque V, Astrand C, Miyamoto K, Simeoni I, Jullien J, Gurdon JB. Mammalian Nuclear Transplantation to Germinal Vesicle stage Xenopus Oocytes - A method for Quantitative Transcriptional Reprogramming. Methods. 2010;51:56–65. doi: 10.1016/j.ymeth.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DW, Do JT, Gentile L, Stehling M, Lee HT, Schöler HR. Pluripotential reprogramming of the somatic genome in hybrid cells occurs with the first cell cycle. Stem Cells. 2008;26:445–54. doi: 10.1634/stemcells.2007-0553. [DOI] [PubMed] [Google Scholar]
- Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. The reactivation of the red cell nucleus. J Cell Sci. 1967;2:23–32. doi: 10.1242/jcs.2.1.23. [DOI] [PubMed] [Google Scholar]
- Harris H, Watkins JF, Ford CE, Schoefl GI. Artificial heterokaryons of animal cells from different species. J Cell Sci. 1966;1:1–30. doi: 10.1242/jcs.1.1.1. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–23. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–5. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen A, Melton D. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–82. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien J, Astrand C, Halley-Stott RP, Garrett N, Gurdon JB. Characterization of somatic cell nuclear reprogramming by oocytes in which a linker histone is required for pluripotency gene reactivation. Proc Natl Acad Sci U S A. 2010;107:5483–8. doi: 10.1073/pnas.1000599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YK, Koo DB, Park JS, Choi YH, Chung AS, Lee KK, Han YM. Aberrant methylation of donor genome in cloned bovine embryos. Nat Genet. 2001;28:173–7. doi: 10.1038/88903. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009 doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Tada M, Nakatsuji N, Tada T. Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol Cell Biol. 2004;24:5710–20. doi: 10.1128/MCB.24.13.5710-5720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun. 2006;340:183–9. doi: 10.1016/j.bbrc.2005.11.164. [DOI] [PubMed] [Google Scholar]
- Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest. 2010;120:51–9. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Koziol MJ, Garrett N, Gurdon JB. Tpt1 activates transcription of oct4 and nanog in transplanted somatic nuclei. Curr Biol. 2007;17:801–7. doi: 10.1016/j.cub.2007.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglova AA, Matveeva NM, Gridina MM, Battulin NR, Karpov A, Kiseleva EV, Morozova KN, Serov OL. Dominance of parental genomes in embryonic stem cell/fibroblast hybrid cells depends on the ploidy of the somatic partner. Cell Tissue Res. 2010;340:437–450. doi: 10.1007/s00441-010-0987-3. [DOI] [PubMed] [Google Scholar]
- Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–44. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bugarija B, Millan EJ, Walton NM, Gaetz J, Fernandes CJ, Yu WH, Mekel-Bobrov N, Vallender TW, Snyder GE, Xiang AP, Lahn BT. Systematic identification of cis-silenced genes by trans complementation. Human Molecular Genetics. 2009;18:835–46. doi: 10.1093/hmg/ddn409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1141. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R. Directly Reprogrammed Fibroblasts Show Global Epigenetic Remodeling and Widespread Tissue Contribution. Cell stem cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Tada M, Otsuji T, Yasuchika K, Nakatsuji N, Surani A, Tada T. Targeted chromosome elimination from ES-somatic hybrid cells. Nat Methods. 2007;4:23–5. doi: 10.1038/nmeth973. [DOI] [PubMed] [Google Scholar]
- Matveeva NM, Shilov AG, Kaftanovskaya EM, Maximovsky LP, Zhelezova AI, Golubitsa AN, Bayborodin SI, Fokina MM, Serov OL. In vitro and in vivo study of pluripotency in intraspecific hybrid cells obtained by fusion of murine embryonic stem cells with splenocytes. Mol Reprod Dev. 1998;50:128–38. doi: 10.1002/(SICI)1098-2795(199806)50:2<128::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Meissner A, Jaenisch R. Mammalian nuclear transfer. Dev Dyn. 2006;235:2460–9. doi: 10.1002/dvdy.20915. [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–81. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:794. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Ruddle FH. Pluripotent teratocarcinoma-thymus somatic cell hybrids. Cell. 1976;9:45–55. doi: 10.1016/0092-8674(76)90051-9. [DOI] [PubMed] [Google Scholar]
- Murata K, Kouzarides T, Bannister AJ, Gurdon JB. Histone H3 lysine 4 methylation is associated with the transcriptional reprogramming efficiency of somatic nuclei by oocytes. Epigenetics Chromatin. 2010;3:4. doi: 10.1186/1756-8935-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng RK, Gurdon JB. Epigenetic memory of active gene transcription is inherited through somatic cell nuclear transfer. Proc Natl Acad Sci USA. 2005;102:1957–62. doi: 10.1073/pnas.0409813102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng RK, Gurdon JB. Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nat Cell Biol. 2008;10:102–9. doi: 10.1038/ncb1674. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Active DNA demethylation and DNA repair. Differentiation. 2009;77:1–11. doi: 10.1016/j.diff.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Nolen LD, Gao S, Han Z, Mann MR, Gie Chung Y, Otte AP, Bartolomei MS, Latham KE. X chromosome reactivation and regulation in cloned embryos. Dev Biol. 2005;279:525–40. doi: 10.1016/j.ydbio.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Ohgane J, Wakayama T, Kogo Y, Senda S, Hattori N, Tanaka S, Yanagimachi R, Shiota K. DNA methylation variation in cloned mice. Genesis. 2001;30:45–50. doi: 10.1002/gene.1031. [DOI] [PubMed] [Google Scholar]
- Okada TS. Transdifferentiation : flexibility in cell differentiation. Clarendon Press; Oxford: 1991. [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Palermo A, Doyonnas R, Bhutani N, Pomerantz J, Alkan O, Blau HM. Nuclear reprogramming in heterokaryons is rapid, extensive, and bidirectional. FASEB J. 2009;23:1431–40. doi: 10.1096/fj.08-122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira CF, Terranova R, Ryan NK, Santos J, Morris KJ, Cui W, Merkenschlager M, Fisher AG. Heterokaryon-based reprogramming of human B lymphocytes for pluripotency requires Oct4 but not Sox2. PLoS Genet. 2008;4:e1000170. doi: 10.1371/journal.pgen.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott A, Leno GH, Laskey RA. Sperm decondensation in Xenopus egg cytoplasm is mediated by nucleoplasmin. Cell. 1991;65:569–78. doi: 10.1016/0092-8674(91)90089-h. [DOI] [PubMed] [Google Scholar]
- Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–5. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong D, Trounson AO, Verma PJ. Cell fusion for reprogramming pluripotency: toward elimination of the pluripotent genome. Stem Cell Rev. 2006;2:331–40. doi: 10.1007/BF02698060. [DOI] [PubMed] [Google Scholar]
- Schafer BW, Blakely BT, Darlington GJ, Blau HM. Effect of cell history on response to helix-loop-helix family of myogenic regulators. Nature. 1990;344:454–8. doi: 10.1038/344454a0. [DOI] [PubMed] [Google Scholar]
- Shearn A, Davis KT, Hersperger E. Transdetermination of Drosophila imaginal discs cultured in vitro. Dev Biol. 1978;65:536–40. doi: 10.1016/0012-1606(78)90049-0. [DOI] [PubMed] [Google Scholar]
- Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol. 2000;2:879–87. doi: 10.1038/35046522. [DOI] [PubMed] [Google Scholar]
- Shi Y, Taedo J, Desponts C, Hahm H, Scholer H, Ding S. A Combined Chemical and Genetic Approach for the Generation of Induced Pluripotent Stem Cells. Cell stem cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Silva J, Chambers I, Pollard S, Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2006;441:997–1001. doi: 10.1038/nature04914. [DOI] [PubMed] [Google Scholar]
- Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–37. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JMW. From egg to embryo : determinative events in early development. Cambridge University Press, Cambridge Cambridgeshire; New York: 1983. [Google Scholar]
- Smith ZD, Nachman I, Regev A, Meissner A. Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nat Biotechnol. 2010;28:521–6. doi: 10.1038/nbt.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–9. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M. Defining Molecular Cornerstones during Fibroblast to iPS Cell Reprogramming in Mouse. Cell stem cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–81. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell stem cell. 2008;2:230–40. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Tada T, Lefebvre L, Barton SC, Surani MA. Embryonic germ cells induce epigenetic reprogramming of somatic nucleus in hybrid cells. Embo J. 1997;16:6510–20. doi: 10.1093/emboj/16.21.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–8. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tamada H, Van Thuan N, Reed P, Nelson D, Katoku-Kikyo N, Wudel J, Wakayama T, Kikyo N. Chromatin decondensation and nuclear reprogramming by nucleoplasmin. Mol Cell Biol. 2006;26:1259–71. doi: 10.1128/MCB.26.4.1259-1271.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova R, Pereira CF, Du Roure C, Merkenschlager M, Fisher AG. Acquisition and extinction of gene expression programs are separable events in heterokaryon reprogramming. J Cell Sci. 2006;119:2065–72. doi: 10.1242/jcs.02945. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama S, Ohta H, Kishigami S, Thuan NV, Hikichi T, Mizutani E, Miyake M, Wakayama T. Establishment of male and female nuclear transfer embryonic stem cell lines from different mouse strains and tissues. Biol Reprod. 2005;72:932–6. doi: 10.1095/biolreprod.104.035105. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–74. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Rodriguez I, Perry AC, Yanagimachi R, Mombaerts P. Mice cloned from embryonic stem cells. Proc Natl Acad Sci USA. 1999;96:14984–9. doi: 10.1073/pnas.96.26.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R. Cloning of male mice from adult tail-tip cells. Nat Genet. 1999;22:127–8. doi: 10.1038/9632. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–76. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Fujita T, Low E, Alarcón V, Yanagimachi R, Marikawa Y. Gradual DNA demethylation of theOct4 promoter in cloned mouse embryos. Mol Reprod Dev. 2006;73:180–188. doi: 10.1002/mrd.20411. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–8. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]