Abstract
In Trypanosoma brucei, RNA interference (RNAi) and recombinant protein expression are established as powerful approaches for functional genomics, particularly when combined with inducible expression. The favoured methods involve exploiting homologous recombination to target expression cassettes to a chromosome sub-set to establish stable cell-lines. Unfortunately, bloodstream-form cells, those that cause disease in mammals, exhibit low efficiency stable transfection. Current expression systems can also exhibit other undesirable features, including variable position effects and leaky, inducible expression. We have developed systems in bloodstream-form cells that alleviate these problems. Using constructs for RNAi and expression of (GFP) tagged proteins, we target a (hyg) tagged ribosomal RNA (RRNA) locus which circumvents position effects and allows increased targeting efficiency. We also report a compatible double-inducible system for tight regulation of highly toxic products. This system exploits a new inducible RRNA promoter to drive T7 RNA polymerase (T7RNAP) transcription which then drives expression from inducible T7 promoters. The developments described should facilitate functional analysis and increased throughput.
Keywords: bloodstream-form, functional genomics, GFP, leaky, position effect, RNAi, transfection, T7 RNA polymerase
1. Introduction
Genetic manipulation and inducible expression, using tetracycline (Tet) or an analogue, are widely used in the African trypanosome, Trypanosoma brucei. Inducible expression is particularly useful since it allows the effect of altering the expression of a particular gene product to be assessed in a single cell-line. RNA interference (RNAi), described in organisms ranging from trypanosomes to humans, is a conserved biological response to double-stranded RNA (dsRNA) that involves ‘knock-down’ of homologous mRNA. Thus, strains can be established that express toxic proteins or, for RNAi, dsRNA against an essential gene [1]. Inducible expression of dsRNA is a particularly convenient method for examining gene function and the absence of RNAi in the South American trypanosome, Trypanosoma cruzi [2] and Leishmania major [3] has focussed emphasis on T. brucei as a model for trypanosomatid functional analysis.
Most of the T. brucei genome appears to be arranged in large polycistronic gene clusters. In most cases, it is desirable that integrated genetic elements should be isolated from native genes and regulatory elements and non-transcribed RRNA loci [4] and mini-chromosomes [5] have consequently been popular for targeting. The mechanisms limiting transcription at these loci are not fully understood however. In excess of 1000-fold induction has been reported using either locus but much lower levels of regulation have also been reported at RRNA loci [5]. Because multiple copies of the non-transcribed RRNA loci are present in the genome [6] the site of integration is unpredictable and can influence reporter gene expression [7]. In addition, the generation of recombinant bloodstream-form T. brucei cell-lines is laborious, largely due to the low efficiency of DNA-mediated transformation. Current protocols require ~10μg of construct DNA and ~2.5×107 cells to generate a single transformant with DNA integrated at an RRNA locus. This low efficiency represents a bottleneck for routine studies and a significant bottleneck for high throughput studies.
Widely used inducible promoters for T. brucei are the T. brucei EP/GPEET and phage T7 promoters [4]. These promoters have Tet operators (TetO) immediately downstream of the transcription start site and can constitute a simple ‘Tet-on’ system when imported into cells expressing the Tet-responsive repressor (TetR) [8]. RNA Polymerase (RNAP) I can transcribe mRNA in T. brucei due to trans-splicing of an RNAP II derived leader sequence onto every mRNA. Indeed the EP/GPEET promoter recruits RNAP I [9]. To use the phage T7 promoter, T7RNAP must be expressed [10]. T7RNAP does not require co-factors for activity and is used in many heterologous gene expression systems. The protein expressed in T. brucei has an SV40 large T antigen nuclear localisation signal (PKKKRKV) engineered at the N-terminus [10].
Although a range of inducible expression tools are available for functional analyses in T. brucei, current systems exhibit a range of problems in generation and maintenance of recombinant cell lines, some of which prove prohibitive when attempting high-throughput studies. With the completion of the core genome sequences for several trypanosomatids (http://www.genedb.org/), there is an urgent need for optimised genetic tools to improve efficiency, provide time and cost savings and improve data quality and consistency. Here, we report efforts that go some way towards alleviating the problems outlined above. All constructs have been tested in bloodstream-form cells but should work equally well in insect-stage cells.
2. Materials & Methods
2.1 Plasmid constructs
pHD1313 [4] was used for TetR expression at the TUB locus. p2T7TAblue [4] (see http://homepages.lshtm.ac.uk/~ipmbdhor/dhhome.htm - Resources) was used as the ‘back-bone’ for the production of all other constructs reported here. pRPcMyc is similar to pRPGFP but with a cMyc-tag instead of a GFP-tag. All constructs were mapped using restriction enzymes and the ligated junctions were checked by DNA sequencing. In silico assembled sequences are available upon request.
2.2 T. brucei growth and manipulation
Bloodstream-form Lister 427 cells, clone 221a, were grown in HMI-11 [11]. For all transfections, 2.5×107 cells were electroporated with 10μg ml−1 linearised construct DNA as described [12]. Drugs were added <6 hours post-transfection at the following concentrations: blasticidin (Invitrogen), 10 μg ml−1; hygromycin (Sigma), 2.5μg ml−1; phleomycin (CayLa), 2 μg ml−1; puromycin (Calbiochem) 2 μg ml−1. Cell counts were carried out using a haemocytometer. Tet was from Sigma.
2.3 Western blotting and microscopy
Western blots were developed using primary mouse α-T7RNAP (Molecular Probes), mouse α-cMyc (Upstate) or rabbit α-GFP (Novagen) according to the manufacturers’ instructions, and signals were detected using an ECL+ kit (Amersham).
For microscopy, cells were washed in PBS and fixed overnight in 2% (v/v) formaldehyde in PBS at 4°C. Prior to analysis, cells were washed in PBS and 1% (w/v) BSA in water. After drying onto glass slides, cells were mounted in Vectashield (Vector Laboratories) containing the DNA counterstain, 4′,6-diamidino-2-phenylindole (DAPI). Slides were analysed on a Nikon Eclipse E600 epifluorescence microscope. Phase contrast and fluorescence images were captured using a Coolsnap FX (Photometrics) CCD camera and processed in Metamorph (Universal Imaging).
3. Results
3.1. A Tet-inducible ribosomal RNA promoter
Although fully active in insect-stage cells, the EP/GPEET promoter is down-regulated in the mammal-infective bloodstream stage [7]. In order to generate an inducible promoter that was not dependent on heterologous polymerase and that would be expected to function efficiently in both major life cycle stages we cloned a single TetO1 operator [13] adjacent to the transcription start site of a ribosomal RNA (RRNA) promoter ([14], see Fig. 1A). A similar inducible RRNA promoter has been used in Leishmania donovani [15]. Using pRPGFP (see Fig. 2A) which contains this promoter, we targeted a number of N-terminal GFP-tagged genes to RRNA loci in cells expressing TetR from the tubulin array [4]. The RRNA promoter system demonstrated robust inducible expression and can therefore be used as part of a T7RNAP-independent inducible expression system (Fig. 2) or for inducible expression of T7RNAP itself (Fig. 1).
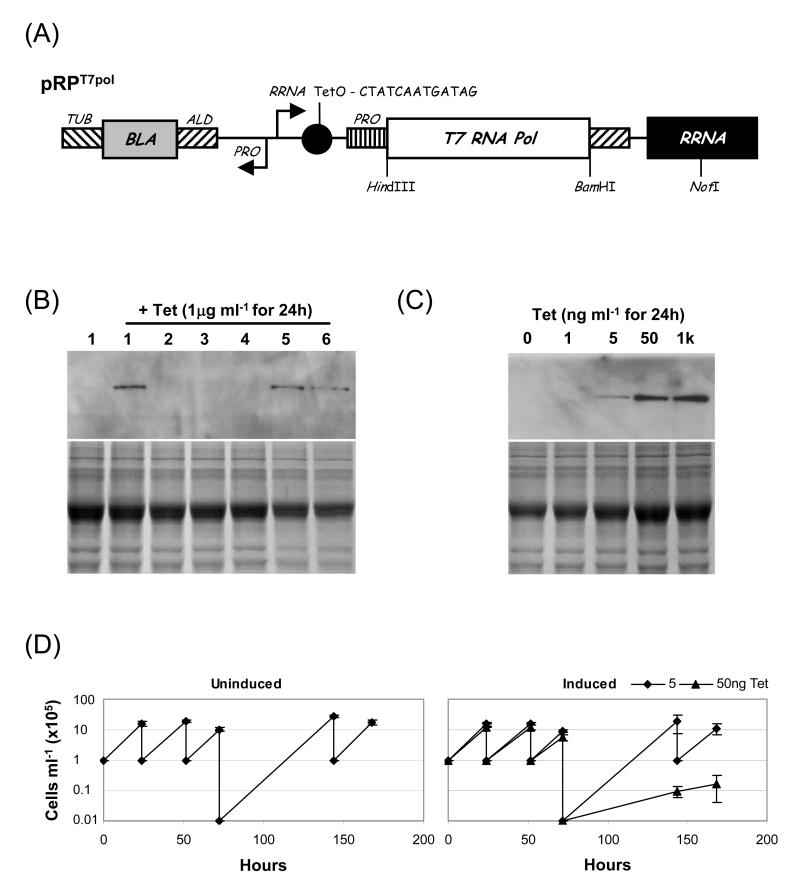
Fig 1.
Expression of T7RNAP from a Tet-inducible RRNA promoter. (A) NotI-digestion targets pRPT7pol to an RRNA locus. BLA, blasticidin resistance. Arrows represent promoters. RNA processing regions and promoters: ALD, aldolase; PRO, procyclin; TUB, tubulin. (B and C) Western blot analysis of T7RNAP expression. The blots were probed with primary mouse α-T7RNAP (1:5000). The lower panels show coomassie-stained gels as loading controls. (B) Six independent cell-lines expressing TetR and transfected with pRPT7pol express different levels of T7 polymerase upon induction with 1μg ml−1 Tet. Lane 1: un-induced cell line 1. (C) Induction of cell line 1 with 1ng to 1μg ml−1 Tet showed that T7 polymerase expression was dose dependent, peaking in the presence of 50ng ml−1 Tet. (D) Growth analysis was carried out in triplicate and populations were diluted as shown. Error bars: standard deviation.
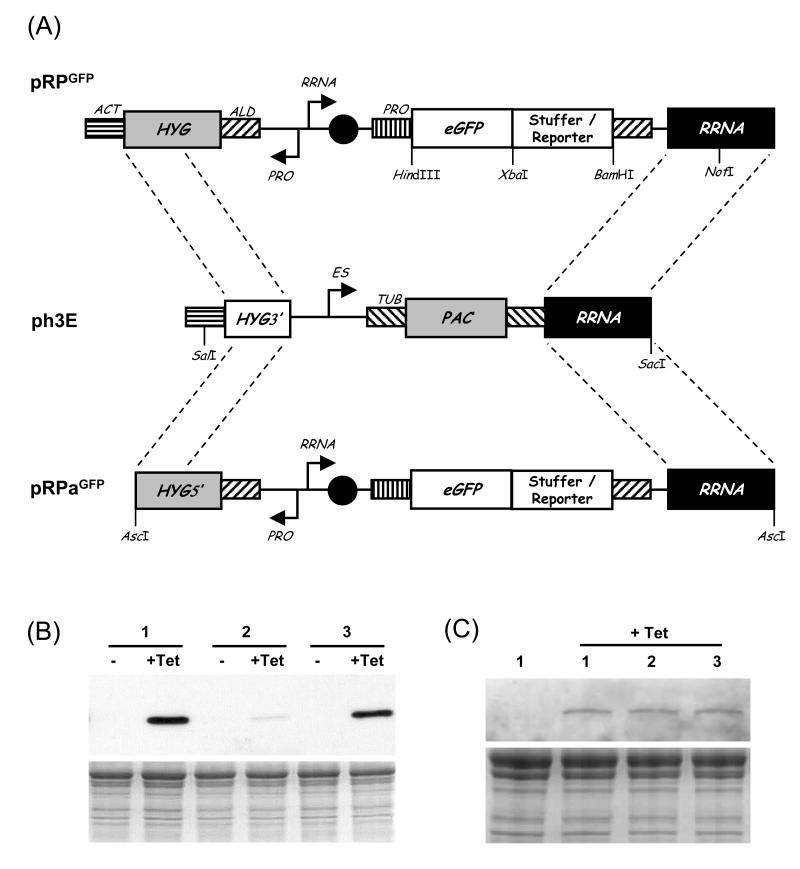
Fig. 2.
Tagging an RRNA locus. (A) NotI-digested pRPGFP and SalI/SacI-digested ph3E were used to identify an RRNA locus with no evidence of position effect repression and to add the hyg-tag to that locus respectively. The resultant tag can then be replaced using AscI-digested pRPaGFP. HYG, hygromycin resistance; PAC, puromycin resistance; ACT, actin; ES, variant surface glycoprotein Expression-Site. (B) Western blot analysis of inducible expression in three independent clones generated using non-tagged cells and pRPcMyc with a reporter gene (GeneDB: Tb927.4.2520). The blot was probed with primary mouse α-cMyc (1:2000). (C) Western blot analysis of induced expression in three independent and representative clones generated using tagged RRNA locus cells and pRPaGFP with a reporter gene (GeneDB: Tb11.01.3380). Lane 1: un-induced cell line 1. The blot was probed with primary rabbit α-GFP (1:4000). Other details as in Fig. 1.
A potential solution to problems associated with constitutive T7RNAP expression was ‘double-inducible’ expression whereby an inducible but truncated EP/GPEET promoter drives T7RNAP expression which then drives expression from inducible T7-promoters. This approach was successful in insect-stage cells but was unsuccessful in bloodstream-form cells, apparently due to down-regulation of the EP/GPEET promoter [4]. We therefore decided to test the inducible RRNA promoter, considered to be a ‘strong’ promoter, for expression of T7RNAP in bloodstream-form cells.
We replaced the GFP gene downstream of the Tet-inducible RRNA promoter in pRPGFP with the T7RNAP gene and targeted the resulting pRPT7pol construct (Fig. 1A) to an RRNA locus in cells expressing TetR. Robust inducible T7RNAP expression was possible but independent clones expressed different maximal levels of T7RNAP (Fig. 1B). We selected clone 1 for further analysis and the data show that induction was Tet dose-dependent (Fig. 1C) as expected. In the absence of Tet, cell line 1 exhibits the same growth dynamics as wild-type cells (not shown), increasing ~10-fold every 24h (Fig. 1D, left-hand plot). Induction with 5ng ml−1 does not affect growth rate while induction with 50ng ml−1 Tet leads to a significant decrease in growth rate (right-hand plot). Thus, T7RNAP is expressed at a sub-toxic level when induced with 5ng ml−1 Tet but cell growth is severely retarded following induction with 50ng ml−1 Tet. Tet is generally used between 0.1 and 1μg ml−1 in T. brucei cultures and no Tet-toxicity has been reported under these conditions so we conclude that T7RNAP expression is responsible for toxicity.
3.2. Circumventing position effect using cells with a tagged locus
We observed highly variable maximal expression levels in individual clones when targeting the non-transcribed RRNA loci using a target sequence spanning the region from 1595-1031 nt upstream of the transcription start site (Fig. 1B, 2B and our unpublished observations). Several clones could be generated and tested using each construct but clearly this approach is time-consuming and incompatible with high-throughput analysis. Since DNA transformation is predominantly via homologous recombination in T. brucei [16], a locus tagged with a unique sequence should allow constructs to be reproducibly inserted into the same genomic location, thus circumventing position effects. This would be useful when analysing putative regulatory elements and should also improve reproducibility for a range of other experiments, including those involving inducible expression. We first used pRPGFP to integrate an inducible reporter into RRNA loci in cells expressing TetR (Fig. 2A, top). We selected a cell line with no evidence of leaky expression and robust inducible expression (i.e. no evidence of position effect repression) and using ph3E replaced the reporter cassette at the chosen target with a ‘hyg-tag’, a portion of the HYG selectable marker (Fig. 2A, middle). These cells (TetRBLE:TagPAC) can then be used for specific targeting to a locus validated for robust inducible expression by including the remainder of the HYG gene in the construct of choice (Fig. 2A, bottom). Tag-compatible constructs contain a truncated HYG gene that lacks the last four codons and shares 604 bp with the tag. We added AscI sites to pRPGFP to generate pRPaGFP which is now compatible with hyg-tagged cells. Figure 2B again shows that inducible reporter expression varies between clones when several different RRNA loci are targeted. In contrast, reporter expression is reproducible among independent clones when the hyg-tagged RRNA locus is targeted (Fig. 2C and our unpublished observations with a number of different GFP-tagged proteins).
3.3. Improved targeting efficiency in cells with a tagged locus
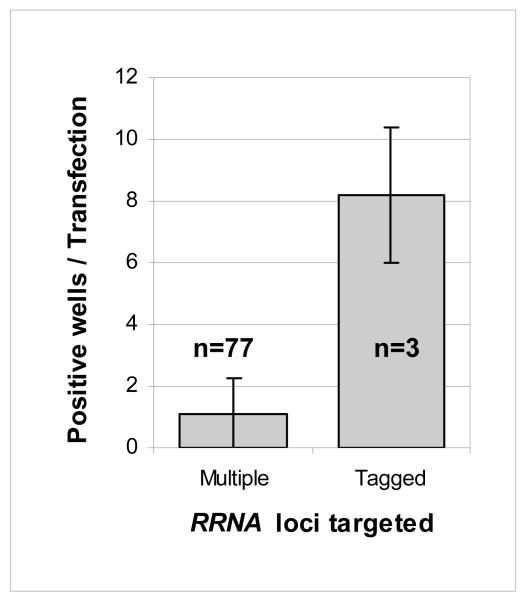
There are ~10 RRNA loci in the T. brucei genome but target copy number is not thought to be proportional to targeting efficiency [6] so we were not in a position to predict transformation efficiency when targeting a single hyg-tagged locus. Remarkably, comparison of transformation efficiency indicated that the single tagged locus was targeted at significantly increased efficiency relative to ~10 un-tagged loci (Fig. 3). This increase in efficiency facilitates scaling in that T. brucei recombinants should now be reliably generated using only ~5μg of construct DNA, less than the typical yield from a plasmid Miniprep (Qiagen).
Fig. 3.
Increased transfection efficiency in cells with a hyg-tagged RRNA locus. HYG selection was administered <6h following electroporation to minimise cell division prior to distribution of cells in 96-well plates. Data for multiple targets was derived over several time points. Error bars: standard deviation.
In cells with a tagged RRNA locus, only correct targeting was expected to generate a complete HYG gene while integration elsewhere in the genome was not expected to generate hygromycin-resistant cells. Indeed, we were unable to transform cells lacking a tagged locus using pT7aGFP (data not shown). Correct targeting in tagged cells is expected to remove the PAC gene (see Fig. 2A) and this was confirmed by sensitivity to puromycin in >60% of the hygromycin resistant clones (HygR:PacS). Using these clones, the PAC selectable marker is available for further manipulation. Loss of the PAC gene from the genome confirms correct integration circumventing the need to map the integration site and also avoiding problems associated with unexpected targeting (see [17] for example).
Interestingly, HygR:PacR clones showed no evidence of position effects (data not shown) suggesting recombination with the tag and a second, possibly tandem, RRNA target and/or duplication of the tagged locus in some cells prior to assembly of an intact HYG gene. If the former explanation is correct, it should be possible to increase the proportion of HygR:PacS clones by using constructs with unique targeting sequences at both ends. Regardless of the explanation for the generation of HygR:PacR clones, the system described significantly improves transfection efficiency and circumvents position effects.
3.4. Double-inducible expression in cells with a tagged locus
To generate cells for double-inducible expression that are not prone to position effects, we introduced pRPT7pol (see section 3.1) into cells with a hyg-tagged RRNA locus (see section 3.2). The combination should allow inducible T7RNAP to drive inducible T7 promoters. We selected a clone with undetectable T7RNAP when un-induced and expressing the highest level of T7RNAP when induced. We used 5ng ml−1 Tet for these experiments since we knew that T7RNAP would be toxic if Tet were applied at significantly higher concentration (see Fig. 1D). These (TetRBLE:TagPAC:T7RNAPBLA or T7i:Tag) cells showed no growth retardation or other discernable phenotype following induction for one week in 5ng ml−1 Tet (data not shown).
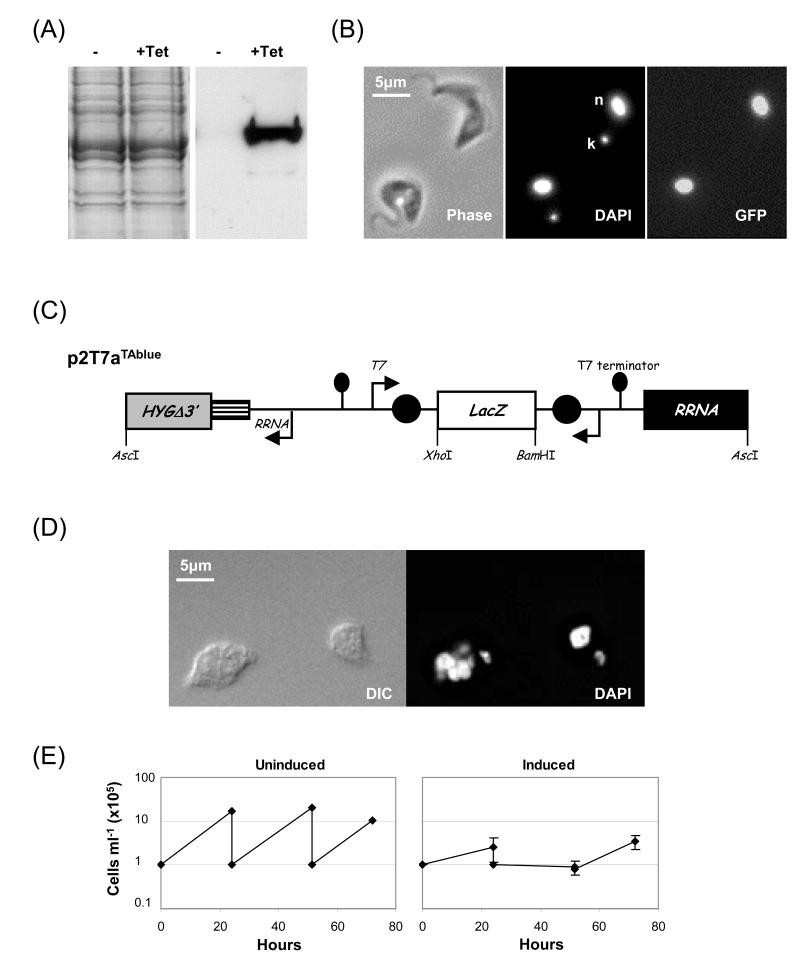
We first tested pT7aGFP, a construct similar to pRPaGFP (Fig. 2A) but with a T7 promoter instead of an RRNA promoter. A GFP-tagged gene in this construct, demonstrated tight regulation, robust induction (Fig. 4A) and, as expected, nuclear localisation of the product (Fig. 4B). We then modified the p2T7TAblue inducible RNAi construct [4] to generate p2T7aTAblue (Fig. 4C), an RNAi construct compatible with the tagged locus. We easily generated clones with this construct containing a large α/β-tubulin insert which previously failed to transform ‘single inducible’ cells [4]. In the double-inducible system, this construct exhibited the expected [18] FAT cell phenotype (Fig. 4D) in the majority of cells and generated a severe growth defect when induced (Fig. 4E). RNAi revertants begin to grow out on the third day following induction (Fig. 4E) consistent with the idea that ~10−3 cells escape the RNAi phenotype. Using a similar system, this has been shown to occur predominantly through deletion of the RNAi target sequence [19].
Fig. 4.
Double-inducible expression in cells with a hyg-tagged RRNA locus. (A and B) A reporter gene (GeneDB: Tb10.6k15.3240) was cloned in pT7aGFP (similar to pRPaGFP but with a T7 promoter in place of an RRNA promoter) and the nuclear GFP-tagged protein was expressed in T7i:Tag cells. (A) Induction was with 5ng ml−1 Tet for 24h. The western blot was probed with primary rabbit α-GFP (1:4000). The left hand panel shows a coomassie-stained gel as a loading control. (B) Direct detection of the GFP-tagged protein. DNA counterstained with (DAPI); k, kinetoplast; n, nucleus. (C) The p2T7aTAblue RNAi construct. Details as in Fig. 1A. (D and E) dsRNA against α/β-tubulin was expressed in T7i:Tag cells using p2T7aTUB, a derivative of p2T7aTAblue. Details as above. (D) Microscopy. Induction was with 5ng ml−1 Tet for 20h. (E) Growth analysis. Details as in Fig. 1D.
Although Tet at 5ng ml−1 is sufficient for the expression cassettes described above, this is not sufficient to induce the expected phenotype for several other RNAi constructs (data not shown). Significantly lower constitutive T7RNAP expression does give the expected phenotypes so this limitation is probably due to insufficient activation of the inducible T7 promoters by 5ng ml−1 Tet. Since increased Tet will lead to toxic T7RNAP expression, the utility of the current double-inducible system is probably limited to expression of particularly potent products. We have clones that express less T7RNAP (see Fig. 1B) but a truncated RRNA promoter (see [20]) should facilitate the construction of a more versatile system that tolerates more Tet.
4. Discussion
Clearly, technology enables scientific discovery. Now that several trypanosomatid genomes have been sequenced, there is an urgent need for new and optimised genetic tools. We identified a number of problems and bottlenecks associated with genetic manipulation and inducible expression procedures in T. brucei and have systematically endeavoured to overcome each in turn.
We have established cells with a unique target sequence at a single RRNA locus that has been validated for robust inducible expression and we show that a single modified locus is targeted at higher efficiency relative to ~10 unmodified loci. The system circumvents position effects and the need to map the insertion site. The parameters that influence targeting efficiency in T. brucei are not completely understood but it could be increased transcription of the tagged target that is responsible for increased efficiency (see Fig. 2A). Whatever the explanation, the system should facilitate increased throughput genetic manipulation.
High-level T7RNAP expression is thought to be toxic in T. brucei [4] but this interpretation was indirect, based on the inability to establish clones with T7 transcription driven by a ‘strong’ promoter. We use a new inducible RRNA promoter to directly demonstrate T7RNAP toxicity. Fortunately, lower, sub-toxic level expression is sufficient to drive significant transcription and strains have been established that constitutively express T7RNAP using ‘weakened’ promoters [4]. These strains have been used for a wide range of analyses but the consequences of long term constitutive expression of T7RNAP have not been assessed. It remains possible that low-level T7RNAP toxicity confers a selective advantage on altered cells. Leaky inducible expression can also compromise the ability to maintain cells transfected with toxic ‘un-induced’ expression cassettes [4]. Inducible expression of T7RNAP, as part of a double-inducible system, could help alleviate problems associated with toxicity and leaky expression. Our demonstration T7RNAP toxicity however underscores the importance of achieving the correct RNAP expression level in a double-inducible system. Alternatively, the inducible RRNA promoter can be used to circumvent the need for heterologous polymerase altogether.
Although there are clearly a number of outstanding issues to be addressed if we are to develop an optimised toolkit for manipulation of gene expression in T. brucei, the new constructs reported here should help to alleviate some current problems. We demonstrate utility at several levels. Position effects can be circumvented, targeting efficiency can be significantly increased and cells expressing highly toxic products can be generated. These tools should facilitate the application of scaleable protocols for functional genomics in T. brucei.
Acknowledgements
We thank Christine Clayton (ZMBH, Heidelberg) for pHD1313, George Cross (Rockefeller University, New York) for pLew13 and Martin Taylor (LSHTM) for comments on the manuscript. This work was funded by The Wellcome Trust (069909 and 064563).
References
- [1].Djikeng A, Shen S, Tschudi C, Ullu E. Analysis of gene function in Trypanosoma brucei using RNA interference. Methods Mol Biol. 2004;270:287–98. doi: 10.1385/1-59259-793-9:287. [DOI] [PubMed] [Google Scholar]
- [2].DaRocha WD, Otsu K, Teixeira SM, Donelson JE. Tests of cytoplasmic RNA interference (RNAi) and construction of a tetracycline-inducible T7 promoter system in Trypanosoma cruzi. Mol Biochem Parasitol. 2004;133:175–86. doi: 10.1016/j.molbiopara.2003.10.005. [DOI] [PubMed] [Google Scholar]
- [3].Robinson KA, Beverley SM. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2003;128:217–28. doi: 10.1016/s0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- [4].Alibu VP, Storm L, Haile S, Clayton C, Horn D. A doubly inducible system for RNA interference and rapid RNAi plasmid construction in Trypanosoma brucei. Mol Biochem Parasitol. 2005;139:75–82. doi: 10.1016/j.molbiopara.2004.10.002. [DOI] [PubMed] [Google Scholar]
- [5].Wickstead B, Ersfeld K, Gull K. Targeting of a tetracycline-inducible expression system to the transcriptionally silent minichromosomes of Trypanosoma brucei. Mol Biochem Parasitol. 2002;125:211–6. doi: 10.1016/s0166-6851(02)00238-4. [DOI] [PubMed] [Google Scholar]
- [6].Wickstead B, Ersfeld K, Gull K. The frequency of gene targeting in Trypanosoma brucei is independent of target site copy number. Nucleic Acids Res. 2003;31:3993–4000. doi: 10.1093/nar/gkg445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Biebinger S, Rettenmaier S, Flaspohler J, Hartmann C, Pena-Diaz J, Wirtz LE, Hotz HR, Barry JD, Clayton C. The PARP promoter of Trypanosoma brucei is developmentally regulated in a chromosomal context. Nucleic Acids Res. 1996;24:1202–11. doi: 10.1093/nar/24.7.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wirtz E, Clayton C. Inducible gene expression in trypanosomes mediated by a prokaryotic repressor. Science. 1995;268:1179–83. doi: 10.1126/science.7761835. [DOI] [PubMed] [Google Scholar]
- [9].Gunzl A, Bruderer T, Laufer G, Schimanski B, Tu LC, Chung HM, Lee PT, Lee MG. RNA polymerase I transcribes procyclin genes and variant surface glycoprotein gene expression sites in Trypanosoma brucei. Eukaryot Cell. 2003;2:542–51. doi: 10.1128/EC.2.3.542-551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- [11].Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75:985–9. [PubMed] [Google Scholar]
- [12].Ingram AK, Cross GA, Horn D. Genetic manipulation indicates that ARD1 is an essential N-acetyltransferase in Trypanosoma brucei. Mol Biochem Parasitol. 2000;111:309–17. doi: 10.1016/s0166-6851(00)00322-4. [DOI] [PubMed] [Google Scholar]
- [13].Orth P, Schnappinger D, Hillen W, Saenger W, Hinrichs W. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat Struct Biol. 2000;7:215–9. doi: 10.1038/73324. [DOI] [PubMed] [Google Scholar]
- [14].White TC, Rudenko G, Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986;14:9471–89. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yan S, Myler PJ, Stuart K. Tetracycline regulated gene expression in Leishmania donovani. Mol Biochem Parasitol. 2001;112:61–9. doi: 10.1016/s0166-6851(00)00345-5. [DOI] [PubMed] [Google Scholar]
- 16].Eid J, Sollner-Webb B. Stable integrative transformation of Trypanosoma brucei that occurs exclusively by homologous recombination. Proc Natl Acad Sci USA. 1991;88:2118–21. doi: 10.1073/pnas.88.6.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Motyka SA, Zhao Z, Gull K, Englund PT. Integration of pZJM library plasmids into unexpected locations in the Trypanosoma brucei genome. Mol Biochem Parasitol. 2004;134:163–7. doi: 10.1016/j.molbiopara.2003.11.013. [DOI] [PubMed] [Google Scholar]
- [18].Ngo H, Tschudi C, Gull K, Ullu E. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc Natl Acad Sci USA. 1998;95:14687–92. doi: 10.1073/pnas.95.25.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen Y, Hung CH, Burderer T, Lee GS. Development of RNA interference revertants in Trypanosoma brucei cell lines generated with a double stranded RNA expression construct driven by two opposing promoters. Mol Biochem Parasitol. 2003;126:275–9. doi: 10.1016/s0166-6851(02)00276-1. [DOI] [PubMed] [Google Scholar]
- [20].Vanhamme L, Pays A, Tebabi P, Alexandre S, Pays E. Specific binding of proteins to the noncoding strand of a crucial element of the variant surface glycoprotein, procyclin, and ribosomal promoters of Trypanosoma brucei. Mol Cell Biol. 1995;15:5598–606. doi: 10.1128/mcb.15.10.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]