Abstract

Aiming at developing inhibitors of mannosyltransferases, the enzymes that participate in the biosynthesis of the cell envelope of Mycobacterium tuberculosis, the synthesis of a range of designed triazole-linked 1,6-oligomannosides up to a hexadecamer has been accomplished by a modular approach centred on the Cu(I)-catalyzed azide-alkyne cycloaddition as key process. The efficiency and fidelity of the cycloaddition are substantiated by high yields (76-96%) and exclusive formation of the expected 1,4-disubstituted triazole ring in all oligomer assembling reactions. Key features of oligomers thus prepared are the anomeric carbon-carbon bond of all mannoside residues and the 6-deoxymannoside capping residue. Suitable bioassays with dimer, tetramer, hexamer, octamer, decamer, and hexadecamer showed variable inhibitor activity against mycobacterial α-(1,6)-mannosyltransferases, the highest activity (IC50 = 0.14-0.22 mM) being registered with the hexamannoside and octamannoside.
Introduction
Among old infection diseases that were thought to be defeated in the last century, tuberculosis (TB) has reappeared overbearingly1 and is presently a leading cause of mortality in many parts of the world including Western countries. Tuberculosis is especially diffused in sub-Saharan Africa where is epidemic because of an increased susceptibility conferred by HIV infection.2 The convergence of TB and HIV poses difficult problems because antiviral and antitubercular drugs given together may present high levels of incompatibility. Various drugs are currently available with different modes of action to treat TB.3 Nevertheless, drug resistance continues to emerge and therefore there is an increasing demand for rapid development of new antitubercular drugs4 targeting essential functions of its etiological agent, Mycobacterium tuberculosis (Mtb). As this bacterium invades and colonizes macrophage cells, its ability to survive within this inhospitable environment has been attributed to its robust cell wall.5 Therefore an ideal TB drug target is the biosynthesis of the mycobacterial cell envelope. Indeed, two of the available antibiotics used to treat TB, ethambutol and isoniazid, act by preventing the formation of a complete cell wall.6 This is composed of various glycophospholipids such as mycolyl-arabinogalactan (mAG)7 and phosphatidylinositol mannosides (PIMs).8 The latter, in addition of being important in their own right, may also be hyperglycosylated to form other wall components such lipomannans (LMs) and lipoarabinomannans (LAMs).9 These glycolipids all contain a common α-1,6-linked mannoside core as shown, for example, in LMs and LAMs (Figure 1). Synthetic approaches to oligomannan fragments of Mtb cell wall were described in various instances.10 Moreover, in a series of papers Lowary and co-workers have described the preparation and biochemical evaluation of fluoro, methoxy, amino, deoxy analogues11 of octyl 1,6-α-D-dimannoside12 (α-D-Manp-(1,6)-α-D-Manp-O(CH2)7CH3) through selective substitution of the C6 hydroxyl group. Compounds have been also prepared in which two of the hydroxyl groups on the nonreducing residue of the above disaccharide were replaced.13 These compounds were all considered potential substrates and inhibitors for α-1,6-mannosyltransferase (α-(1,6)-ManT) activity required for the biosynthesis of LM and LAM mannan cores. On the same vein Watt and Williams prepared a series of hydrophobic octyl 6-deoxy α-1,6-linked oligomannosides up to a tetramer via iterative glycosylation.14 The rationale behind these synthetic efforts was that deoxygenation of the 6-position of the oligomannosyl chain should prevent these compounds acting as substrates for the α-(1,6)-ManTs. Oligomannosides prepared by Watt and Williams featured the natural O-glycosidic anomeric linkage and therefore were prone to undergo a facile cleavage by hydrolases. Moreover, the chain lengths of these compounds might be too short for an efficient mimicry of the oligomannoside chains of LMs and LAMs, which are in fact constituted of 10-20 mannose units. Hence we have developed a new approach to more stable and higher oligomers 1, all preserved from hydrolytic degradation by virtue of anomeric carbon-carbon bonds and triazole rings as interglycosidic linkers (Figure 1). In fact we envisaged the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC), first chronicled by Sharpless and Meldal groups,15 as a suitable tool for the assembly of a number of mannose residues via linear and/or convergent strategies. The usefulness of this valuable reaction for oligomannoside assembly was demonstrated in recent work from our laboratory.16 Moreover, we thought that in the oligomers thus obtained the triazole ring could act not only as a robust linker impervious to chemical and enzymatic degradation but could also participate in hydrogen-bonding and dipole interactions, thereby favoring molecular recognition processes.17
Figure 1.
Structures of lipomannans LMs and lipoarabinomannans (LAMs) of Mtb cell walls (R stands for various fatty acid acyl groups) and designed triazole-tethered oligomannosides 1 featuring a capping 6-deoxymannose fragment.
Results and Discussion
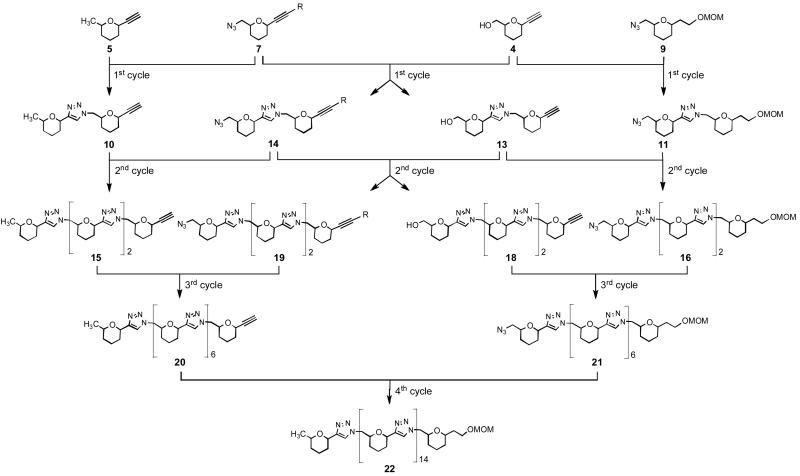
We set out to prepare triazole-linked oligomannosides (1, TOM) by a modular approach involving CuAAC reactions as shown in Scheme 1. While this scheme exemplifies only the synthesis of a high oligomer featuring sixteen mannose residues, it can be realized that the lower oligomers can be equally prepared from suitable precursors showed therein. Details on each reaction sequence constituted of the CuAAC and functional group transformation as well as the synthesis of a range of oligomers are presented below.
Scheme 1.
Convergent synthesis of triazole-linked hexadecamannoside. R stands for a protective group.
Preparation of starting monosaccharidic building blocks.
Although previously reported in a succinct manner,16 the synthetic routes to these building blocks are presented briefly in Scheme 2. Compounds 5 and 9 were finalized to act as a capping residue and a platform, respectively, in the prepared oligosaccharides while 4 and 7 were the first pair of elongating units. The methyl group in 5 constituted a key structural motif to prevent the enzyme-promoted oligomerization while the protected hydroxyethyl group at the anomeric carbon of 9 formed a stable terminal C-glycoside unit. Upon removal of the methoxymethyl (MOM) group by acid hydrolysis, the free hydroxyethyl group could serve as a point of the attachment of fatty acids via esterification. The 3-hydroxy-3-methylbutynyl (HMB) group in 7 acted as a masked ethynyl group. In fact HMB is a formal adduct of acetone to the ethynyl group from which the former can be easily removed (deacetonation) simply by basic treatment. This protection/deprotection strategy avoided homopolymer formation via intermolecular Cu(I)-catalyzed cycloaddition.18 The use of HMB represented two optimized conditions, i. e. the stability under the CuAAC conditions and the ease of deacetonation. On the other hand the ethynyl protection as cobalt hexacarbonyl complex or trimethylsilyl derivative 16b appeared unsatisfactory as these groups were partially removed under the CuAAC conditions.
Scheme 2.
As shown in Scheme 2, the preparation of all starting building blocks relied on the use of tetra-O-benzyl-mannopyranosyl acetate 2 as the common precursor. Succinctly, the 3-hydroxy-3-methylbutynyl group was introduced by glycosylation of 2 with 1-tributylstannyl-3-methyl-3-trimethylsilyloxy-1-butyne followed by sequential desilylation-acetylation of the hydroxy group. Compound 6 thus obtained was selectively debenzylated, then deacetylated, and finally transformed into the target azide 7 by selective activation of the primary hydroxy group as tosylate and reaction with sodium azide.16b Coupling of 2 with 1-tributylstannyl-2-trimethylsilyl-ethyne, followed by desilylation, yielded the ethynyl glycoside 3 that was selectively debenzylated to afford 4.16a This compound was transformed into the target 6-deoxy derivative 5 by free hydroxy group iodination and hydride reduction.16b The preparation of the azide 9 involved first the protection of the free hydroxy group of 4 as silyl derivative, then hydroboration-oxidation of the alkyne function to give the hydroxyethyl C-mannoside 8. The latter was converted into the methoxymethyl (MOM) derivative that in turn was transformed into the target azide 9 by desilylation and treatment with diphenyl phosphoryl azide (DPPA) in the presence of 1,8-diazabicyclo[5.4.0.]undec-7-ene (DBU).16a
Synthesis of triazole-tethered 1,6-α-D-oligomannoside building blocks and assembly of the hexadecamer
To demonstrate the feasibility of the synthetic strategy outlined in Scheme 1, the synthesis of the hexadecasaccharide 1f was first performed. This involved execution of four consecutive cycles each one being constituted of CuAAC and azidation or/and deacetonation reactions. All cycloadditions were performed under the conditions optimized in our recent work,16b that is by microwave heating at 80 °C (external vessel wall temperature) for 15 min. of equimolar azide and alkyne mixture in DMF in the presence of catalytic CuI (0.2 equiv.) and N,N-diisopropylethylamine (2 equiv.) as a ligand. The deacetonation of the 3-hydroxy-3-methylbutynyl group to give the free ethynyl moiety was efficiently carried out under mild basic conditions (K2CO3, crown ether) in refluxing toluene. Finally, the transformation of the primary hydroxy group into azide was carried out by either direct azidation with DPPA or by one-pot tosylation and substitution with sodium azide.
1st Cycle
This involved: a) coupling of alkyne 5 with azide 7 followed by deacetonation to give the dimannoside 10 (Scheme 3, eq. a); b) coupling of alkyne 4 with azide 9 followed by azidation to give 11 (Scheme 3, eq. b); and c) coupling of alkyne 4 with azide 7 to give 12 and transformation of the latter into 13 and 14 by deacetonation and azidation, respectively (Scheme 4).
Scheme 3.
Scheme 4.
2nd Cycle
This involved: a) coupling of alkyne 10 with azide 14 followed by deacetonation to give the tetramannoside 15 (Scheme 5, eq. a); b) coupling of alkyne 13 with azide 11 followed by azidation to give 16 (Scheme 5, eq. b); c) coupling of alkyne 13 with azide 14 to give 17 and transformation of the latter into 19 and 18 by azidation and deacetonation, respectively (Scheme 6).
Scheme 5.
Scheme 6.
3rd Cycle
This involved: a) coupling of alkyne 15 with azide 19 followed by deacetonation to give the octamannoside 20 (Scheme 7, eq. a); b) coupling of alkyne 18 with azide 16 followed by azidation to give 21 (Scheme 7, eq. b).
Scheme 7.
4th Cycle
This involved only the final Cu(I)-catalyzed coupling of octamannosides 20 and 21 to give the protected hexadecamannoside 22 (Scheme 8). MALDI-TOF mass spectrometry of 22 revealed m/z = 7761.40 for the corresponding hydrogen adduct, as compared to the calculated m/z = 7761.15. This compound was subjected to the protecting group removal (demethoxymethylation by CF3CO2H and debenzylation by BCl3) and acetylation by Ac2O to give the peracetylated derivative 23, which was purified and characterized by NMR and MS analyses. Finally the removal of all acetyl protecting groups from 23 under mild basic conditions using NH3 in methanol and water at room temperature afforded the target TOM 1f (calcd m/z = 3413.10 [M+Na]+, found m/z = 3412.99) in 9.4% overall yield from the monosaccharidic building blocks.
Scheme 8.
Before closing this section a few aspects regarding Schemes 3-8 are worth to be considered. First of all it can be noticed that all intermolecular CuAAC reactions took place with great efficiency regardless of the complexity of the substrates to give exclusively a single cycloadduct. The structure of the 1,4 disubstituted 1,2,3-triazole moieties of the oligosaccharides was confirmed by 13C NMR spectroscopy.19 It can be also noticed that the two functional group transformations, i.e. deacetonation and azidation, were simple and efficient processes that were performed without any damage of the substrates.
Synthesis of a set of TOMs (1)
From an inspection of Scheme 1 it can be easily realized that the Cu(I)-catalyzed cycloadditions of the alkynes on the left side with the azides on the right side can give rise to the formation of all oligomers of type 1 starting from the dimannoside up to the hexadecamannoside. Even higher oligomers should be accessible by the extension of Scheme 1. Nevertheless, considering that the aim of the present work consisted of the discovery of Mtb cell envelope synthetase inhibitors, we decided to prepare only a set of compounds that could give some inside on the effect of the chain length on their biological activity. Suitable reaction partners required for the CuAAC-based synthesis of the target oligomers were synthesized as shown in Schemes 2, 3, 5. Thus, the CuAAC of alkyne 5 with azide 9 afforded the fully protected dimannoside 24 (Scheme 9, eq. a). The removal of MOM and benzyl protective groups and acetylation transformed this compound into the peracetylated derivative 25, which was purified and characterized by NMR and MS. Finally, the removal of all acetyl groups from 25 by NH3 in methanol afforded the target TOM 1a (71.5% yield from 5), suitable for the planned biological tests. The same procedure was followed for the preparation of the tetramannoside 1b starting from the cycloaddition of alkyne 10 with azide 11 (Scheme 9, eq. b) and octamannoside 1d starting from the cycloaddition of alkyne 15 with azide 16 (Scheme 9, eq. c). The oligomannosides 1b and 1d were obtained in 43.5% and 32.8% overall yield, respectively, from the monosaccharidic building blocks.
Scheme 9.
In vitro α-(1,6)-mannosyltransferases inhibition by TOMs
The inhibition of the mycobacterial α-(1,6)-mannosyltransferases by triazole-linked oligomannosides TOM-2 (1a), TOM-4 (1b), TOM-8 (1d), and TOM-16 (1f), as well as hexamannoside TOM-6 (1c) and decamannoside TOM-10 (1e), these being synthesized as reported,16b was evaluated in vitro using a well established assay11,12,13 based on membrane extracts from Mycobacterium smegmatis. These membrane extracts contain polyprenolphosphomannose (PPM)-dependent α-(1,6)-mannosyltransferases that are involved in the synthesis of the α-1,6-linked mannoside core present in the mycobacterial cell wall LAMs.20 It has been demonstrated that an octyl dimannoside [α-Manp(1,6)-α-Manp-O(CH2)7CH3] is the minimum acceptor substrate12 for the above α-(1,6)-mannosyltransferases, whereas their donor substrate, i.e. phosphodecaprenyl β-D-mannopyranoside, is formed from Guanosine Disphosphate (GDP)-Mannose.21
Each triazole-linked oligomannoside 1a – 1f (at 1.0 mM) was incubated at 37 °C for 30 min with 14C1-labeled phosphodecaprenyl β-D-mannopyranoside (generated in situ from GDP-[14C]Mannose), M. smegmatis membrane fractions, and α-Manp(1,6)-α-Manp-O(CH2)7CH3 disaccharidic acceptor (at 0.2 mM). After these experiments we were pleased to observe that all the oligomannosides led to a significant inhibition of [14C]Manp incorporation from decaprenyl P-[14C]Mannose onto the disaccharidic acceptor (101 cpm, no enzyme control; 13,627 cpm, no inhibitor; 8,174 cpm, TOM-2 (1a); 7,498 cpm, TOM-4 (1b); 401 cpm, TOM-6 (1c); 656 cpm, TOM-8 (1d); 6,680 cpm, TOM-10 (1e); 4,739 cpm, TOM-16 (1f). It has to be noted, however, that the hexamannoside TOM-6 (1c) and the octamannoside TOM-8 (1d), consistently over several independent experiments (Figure 2), showed the highest activity (ca. 95% inhibition). Subsequent experiments where TOM concentrations were varied provided inhibitory IC50 values for TOM-2 (1.24 mM), TOM-4 (1.15 mM), TOM-6 (0.14 mM), TOM-8 (0.22 mM), TOM-10 (0.95 mM), and TOM-16 (0.84 mM). These values demonstrated that TOM-6 and TOM-8 were stronger α-(1,6)-ManTs inhibitors than the octyl 6′-amino-O-disaccharides prepared by Tam and Lowary (IC50 = 0.8-1.2 mM).11c Very recent attempts to prepare other mycobacterial mannosyltransferase inhibitors were met with failure.22 In particular, a series of 15 monosaccharidic alkyl and cycloalkyl α-D-mannopyranoside derivatives, including 6-deoxygenated analogues, were prepared and evaluated as inhibitors. However, all these compounds were totally inactive toward the α-(1,6)-ManTs.22
Figure 2.
In vitro inhibition of mycobacterial α-(1,6)-mannosyltransferase enzyme activity by TOM-2 – TOM-16 (1a-1f). The assay utilizes M. smegmatis membranes, α-Manp(1,6)-α-Manp-O(CH2)7CH3 disaccharidic acceptor (0.2 mM), 0.25 μCi of [14C]GDP-Man and 1a-1f (1.0 mM). The results are expressed as percentage inhibition of the incorporation of [14C]Mannose into the acceptor and are representative of three independent experiments with each assay performed in triplicate.
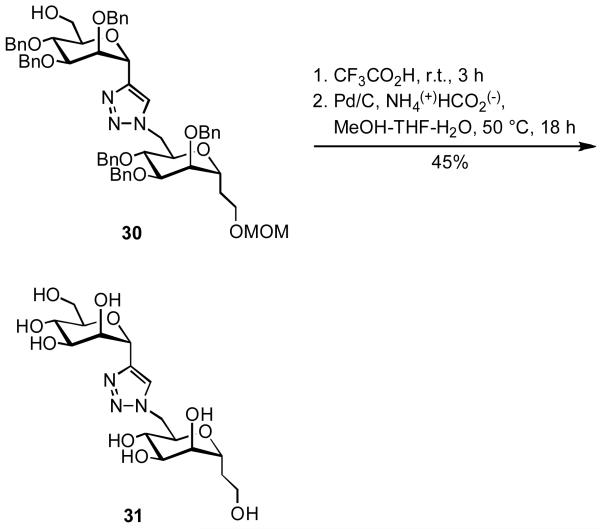
Following these results, it was important to establish whether the presence of a deoxygenated capping residue was a key structural feature for the mycobacterial α-(1,6)-mannosyltransferase inhibition. To this end the triazole-linked 1,6-dimannoside 31 was prepared from the known 16a protected derivative 30 (Scheme 10) and tested by the standard assay described for TOM-2. Quite surprisingly, this compound turned out to be not only a poor mannosyl acceptor up to 4 mM but also a weak inhibitor of the α-(1,6)-ManTs (IC50 = 0.96 mM). Thus, while the deoxygenated 6-position at the reducing end ensures the total inertness of TOMs toward mannosyltransferases, this structural feature appears to be not crucial for inhibition.
Scheme 10.
Conclusion
In summary, a modular strategy has been established for the preparation of a set of C-oligomannosides featuring the triazole ring as the interglycosidic linker. In fact, the key reaction for the assembly of these oligomers consists of the very efficient click azide-alkyne cycloaddition (CuAAC). The efficiency and fidelity of this reaction were maintained regardless of the structural complexity of building blocks to be assembled. The biological experiments indicated that TOM-6 (1c) and TOM-8 (1d) are endowed with the optimal chain length for the interaction with PPM-dependent α-(1,6)-mannosyltransferases. Although the core-α-(1,6)-LM is around 20 Man units in length, a TOM with a minor number of Man units binds efficiently to the α-(1,6)-mannosyltransferase, suggesting that the binding groove of the TOM-6 oligosaccharide fits optimally into the protein. Very likely the 1,4-disubstituted triazole ring spacers contribute substantially to the overall length of these non-natural oligomannosides. In addition, it is worth noting that the presence of a non-natural C-glycosidic linkage and triazole linker in oligomannosides 1a-1f does not perturb their molecular recognition properties toward these mycobacterial α-(1,6)-mannosyltransferases.
Experimental Section
General Experimental Section
All moisture-sensitive reactions were performed under a nitrogen atmosphere using oven-dried glassware. Anhydrous solvents were dried over standard drying agents 23 and freshly distilled prior to use. Reactions were monitored by TLC on silica gel 60 F254 with detection by charring with sulfuric acid. Flash column chromatography 24 was performed on silica gel 60 (40-63 μm). Optical rotations were measured at 20 ± 2 °C in the stated solvent; [α]d values are given in deg.mL.g−1.dm−1.1H NMR (300 and 400 MHz) and 13C NMR spectra (75 MHz) were recorded from CDCl3 solutions at room temperature unless otherwise specified. Peak assignments were aided by 1H-1H COSY and gradient-HMQC experiments. In the 1H NMR spectra reported below, the n and m values quoted in geminal or vicinal proton-proton coupling constants Jn,m refer to the number of the corresponding sugar protons. The closed vessel MW experiments were performed using a single-mode cavity Biotage Initiator microwave reactor; the temperature was measured externally on the outside vessel wall by an IR sensor; the reaction time was counted when the reaction mixture reached the preset temperature. Monosaccharides 4,16a 5,16b 6,16b 7,16b 8,16a 916a and disaccharides 10,16b 11,16a 30,16a were prepared as described in our previous papers.
MS Analysis
For accurate mass measurements the compounds were analyzed in positive ion mode by electrospray hybrid quadrupole orthogonal acceleration time-of-flight mass spectrometer (Q-TOF) fitted with a Z-spray electrospray ion source. The capillary source voltage and the cone voltage were set at 3500 V and 35 V, respectively; the source temperature was kept at 80 °C; nitrogen was used as a drying gas at a flow rate of ca. 50 L/h. The time-of-flight analyzer was externally calibrated with NaI from m/z 300 to 2000 to yield an accuracy near to 5 ppm. When necessary an internal lock mass was used to further increase the mass accuracy. Accurate mass data were collected by directly infusing samples (10 pmol/μL in 1:1 CH3CN-H2O containing 10 mM ammonium formate) into the system at a flow rate of 5 μL/min. Compounds 1b, 1d, 1f, 20-23, 28, and 29 were analysed by MALDI TOF mass spectrometry using a pulsed nitrogen laser (λ = 337 nm). Each compound was dissolved in H2O (1b, 1d, 1f) or 1:1 CHCl3-acetonitrile (20-23, 28, 29) and, prior to the acquisition of spectra, 1 μL of this solution was diluted with 1 μL of saturated α-cyano-4-hydroxycinnamic acid matrix solution (10 mg/mL in 1:1 EtOH-H2O, containing 0.1% of CF3CO2H). Ca. 1 μL of the resulting mixture was placed onto the mass spectrometer’s sample target and dried at room temperature. Once the liquid was completely evaporated, the sample was loaded into the mass spectrometer and analyzed. The instrument was operated in positive ion reflectron mode with the source voltage set to 12 kV. The pulse voltage was optimized at 1999 V, the detector and reflectron voltages were set to 5200 V and 2350 V, respectively. Measurements were performed in the mass range m/z 800-5000 with a suppression mass gate set to m/z 500 to prevent detector saturation from matrix cluster peaks and an extraction delay of 600 ns. The instrument was externally calibrated using a polyethylene glycol mix as standard. A mass accuracy near to the nominal (50 ppm) was achieved for each standard. The protonated monoisotopic mass of ACTH peptide (m/z 2465.199) was used as internal lock mass to further improve the peptide mass accuracy near to 10-20 ppm. The monoisotopic masses were calculated according to the reported 25 atomic weights of the elements.
3,7-Anhydro-4,5,6-tri-O-benzyl-1,2,8-trideoxy-1-C-[2-(2-hydroxy)propyl]-8-[4-(2′,3′,4′-tri-O-benzyl-α-d-mannopyranosyl)-1H-1,2,3-triazol-1-yl]-d-glycero-d-talo-oct-1-ynitol (12)
A mixture of alkyne 4 (420 mg, 0.92 mmol), azide 7 (497 mg, 0.92 mmol), N,N-diisopropylethylamine (320 μL, 1.84 mmol), CuI (34 mg, 0.18 mmol), and DMF (4 mL) in a vial sealed with a Teflon septum and aluminium crimp was subjected to microwave irradiation for 15 min at 80 °C, then cooled to room temperature, diluted with AcOEt (150 mL), washed with an aqueous solution of EDTA disodium salt (0.05 M, 3 × 30 mL), dried (Na2SO4), and concentrated. The residue was eluted from a column of silica gel with 3:2 cyclohexane-AcOEt to give 12 (818 mg, 89%) as an amorphous solid; [α]d = −13.6 (c 0.9, CHCl3). 1H NMR (400 MHz): δ 7.72 (s, 1H, H-5 Tr.), 7.46-7.26 (m, 30H, Ar), 5.23 (d, 1H, J1′,2′ = 1.8 Hz, H-1′), 4.96 and 4.71 (2 d, 2H, J = 11.0 Hz, PhCH2), 4.89 and 4.59 (2 d, 2H, J = 11.0 Hz, PhCH2), 4.82 and 4.77 (2 d, 2H, J = 12.5 Hz, PhCH2), 4.77 (dd, 1H, J2′,3′ = 3.0 Hz, H-2′), 4.74 (d, 1H, J3,4 = 2.0 Hz, H-3), 4.72 and 4.61 (2 d, 2H, J = 11.5 Hz, PhCH2), 4.68 (dd, 1H, J7,8a = 2.2, J8a,8b = 14.3 Hz, H-8a), 4.66 (s, 2H, PhCH2), 4.62 and 4.59 (2 d, 2H, J = 12.0 Hz, PhCH2), 4.52 (dd, 1H, J7,8b = 7.0 Hz, H-8b), 4.00-3.90 (m, 4H, H-3′, H-5, H-6, H-7), 3.80 (dd, 1H, J5′,6′a = 3.0, J6′a,6′b = 12.0 Hz, H-6′a), 3.72 (dd, 1H, J4,5= 3.0 Hz, H-4), 3.68 (dd, 1H, J5′,6′b = 6.2, H-6′b), 3.62 (dd, 1H, J3′,4′ = J4′,5 ′= 9.0 Hz, H-4′), 3.43 (ddd, 1H, H-5′), 2.85 (s, 1H, OH), 2.20 (bs, 1H, OH), 1.31 (s, 6H, 2 CH3). 13C NMR: δ 144.5 (C-4 Tr.), 138.4 (C), 138.0 (C), 137.9 (C), 137.70 (C), 137.67 (C), 128.5 (CH), 128.4 (CH), 128.3 (CH), 127.9 (CH), 127.8 (CH), 127.7 (CH), 127.6 (CH), 127.4 (CH), 123.7 (C-5 Tr.), 95.1 (C), 80.4 (CH), 79.4 (CH), 76.4 (CH), 75.3 (CH2), 75.1 (CH2), 75.0 (CH2), 74.8 (CH), 74.7 (CH), 74.3 (CH), 73.2 (CH), 72.8 (CH2), 72.1 (CH2), 71.9 (CH2), 71.5 (CH), 66.4 (CH), 64.4 (C), 62.9 (CH2), 50.8 (CH2), 31.2 (CH3), 30.9 (CH3). HRMS (ESI/Q-TOF) m/z calcd for C61 H66N3O10 (M+H)+ 1000.4748, found 1000.4714.
3,7-Anhydro-4,5,6-tri-O-benzyl-1,2,8-trideoxy-8-[4-(2′,3′,4′-tri-O-benzyl-α-d-mannopyranosyl)-1H-1,2,3-triazol-1-yl]-d-glycero-d-talo-oct-1-ynitol (13)
A mixture of 12 (355 mg, 0.36 mmol), anhydrous K2CO3 (20 mg, 0.15 mmol), 18-crown-6 ether (19 mg, 0.07 mmol), and toluene (3 mL) was stirred at 110 °C for 4 h, then concentrated. The residue was eluted from a column of silica gel with 2:1 cyclohexane-AcOEt to give 13 (308 mg, 92%) as an amorphous solid; [α]d = +28.9 (c 1.1, CHCl3). 1H NMR (400 MHz) selected data: δ 7.68 (s, 1H, H-5 Tr.), 7.43-7.21 (m, 30H, Ar), 5.25 (d, 1H, J1′,2′ = 2.0 Hz, H-1′), 4.54 (dd, 1H, J7,8a = 2.4, J8a,8b = 14.3 Hz, H-8a), 4.07 (ddd, 1H, J6,7 = 9.7, J7,8b = 5.2 Hz, H-7), 4.04 (dd, 1H, J4,5 = 3.0, J5,6 = 9.3 Hz, H-5), 4.00 (dd, 1H, J3′,4′ = 9.4, J4′,5′ = 9.0 Hz, H-4′), 3.91 (dd, 1H, J2′,3′ = 2.9 Hz, H-3′), 3.51 (dd, 1H, H-5), 3.48 (ddd, 1H, J5′,6′a = 3.2, J5′,6′b = 5.2 Hz, H-4′), 2.49 (d, 1H, J1,3 = 2.3, H-1), 2.07 (bs, 1H, OH). 13C NMR: δ 144.6 (C-4 Tr.), 138.4 (C), 138.0 (C), 137.8 (C), 137.5 (C), 128.4 (CH), 128.3 (CH), 127.9 (CH), 127.8 (CH), 127.7 (CH), 127.6 (CH), 127.5 (CH), 123.9 (C-5 Tr.), 80.2 (CH), 80.0 (CH), 78.2 (CH), 77.6 (C), 76.1 (CH), 75.3 (CH2), 75.2 (CH), 75.0 (CH2), 74.8 (CH), 74.2 (CH), 74.1 (CH), 73.1 (CH2), 72.7 (CH2), 72.1 (CH2), 72.0 (CH2), 71.8 (CH2), 71.4 (CH), 66.1 (CH), 62.7 (CH2), 50.6 (CH2). HRMS (ESI/Q-TOF) m/z calcd for C58H60N3O9 (M+H)+ 942.4330, found 942.4366.
3,7-Anhydro-8-[4-(6′-azido-2′,3′,4′-tri-O-benzyl-6′-deoxy-α-d-mannopyranosyl)-1H-1,2,3-triazol-1-yl]-4,5,6-tri-O-benzyl-1,2,8-trideoxy-1-C-[2-(2-hydroxy)propyl]-d-glycero-d-talo-oct-1-ynitol (14)
A mixture of alcohol 12 (440 mg, 0.44 mmol), freshly distilled Et3N (0.19 mL, 1.32 mmol), and p-toluensulfonyl chloride (126 mg, 0.66 mmol) in anhydrous CH2Cl2 (4 mL) was stirred at room temperature for 3 h, then diluted with CH2Cl2 (40 mL), washed with 1M phosphate buffer at pH 7 (2 × 15 mL), dried (Na2SO4), and concentrated. The residue was filtered through a short column of silica gel with 2:1 cyclohexane-AcOEt to give the 8′-O-tosyl derivative as a white solid (545 mg). A mixture of this compound, sodium azide (143 mg, 2.20 mmol), and anhydrous DMF (3 mL) was stirred at 55 °C for 19 h, then cooled to room temperature, diluted with AcOEt (40 mL), washed with H2O (2 × 15 mL), dried (Na2SO4), and concentrated. The residue was eluted from a column of silica gel with 3:1 cyclohexane-AcOEt to give 14 (352 mg, 78%) as a syrup; [α]d = +32.1 (c 0.8, CHCl3). 1H NMR (400 MHz) selected data: δ 7.81 (s, 1H, H-5 Tr.), 7.44-7.20 (m, 30H, Ar), 5.24 (d, 1H, J1′,2′ = 1.7 Hz, H-1′), 4.96 and 4.72 (2 d, 2H, J = 10.7 Hz, PhCH2), 4.89 and 4.57 (2 d, 2H, J = 11.0 Hz, PhCH2), 4.81 (dd, 1H, J2′,3′ = 3.0 Hz, H-2′), 4.77 (d, 1H, J3,4 = 2.0 Hz, H-3), 3.72 (dd, 1H, J4,5 = 3.5 Hz, H-4), 3.53 (ddd, 1H, J4′,5′ = 9.5, J5′,6a′ = 7.5, J5′,6b′ = 2.3 Hz, H-5′), 3.38 (dd, 1H, J6a′,6b′ = 13.0 Hz, H-6′a), 3.32 (dd, 1H, H-6′b), 2.40 (s, 1H, OH), 1.32 and 1.31 (2 s, 6H, 2 CH3). 13C NMR: δ 144.3 (C-4 Tr.), 138.2 (C), 137.9 (C), 137.7 (C), 137.6 (C), 137.5 (C), 128.3 (CH), 128.2 (CH), 127.9 (CH), 127.7 (CH), 127.6 (CH), 127.5 (CH), 127.4 (CH), 124.3 (C-5 Tr.), 94.9 (C), 80.3 (CH), 79.3 (CH), 76.4 (CH), 75.2 (CH), 75.2 (CH2), 74.9 (CH2), 74.39 (CH), 74.35 (CH), 73.9 (CH), 73.1 (CH), 72.5 (CH2), 71.9 (CH2), 71.69 (CH2), 71.65 (CH2), 71.1 (CH), 66.2 (CH), 64.4 (C), 51.5 (CH2), 50.6 (CH2), 31.1 (CH3), 30.8 (CH3). HRMS (ESI/Q-TOF) m/z calcd for C61H65N6O9 (M+H)+ 1025.4813, found 1025.4814.
Tetramannoside alkyne (15)
A mixture of alkyne 10 (110 mg, 0.12 mmol), azide 14 (122 mg, 0.12 mmol), N,N-diisopropylethylamine (42 μL, 0.24 mmol), CuI (5 mg, 24 μmol), and DMF (0.6 mL) in a vial sealed with a Teflon septum and aluminium crimp was subjected to microwave irradiation for 15 min at 80 °C, then cooled to room temperature, diluted with AcOEt (50 mL), washed with 0.05 M aqueous solution of EDTA (3 × 10 mL), dried (Na2SO4), and concentrated. A mixture of this residue, anhydrous K2CO3 (7 mg, 50 μmol), 18-crown-6 ether (6 mg, 24 μmol), and toluene (1 mL) was stirred at 110 °C for 4 h, then concentrated. The residue was eluted from a column of silica gel with 2:1 cyclohexane-AcOEt to give 15 (176 mg, 78%), identical to the product described by us in a previous paper.16b
Tetramannoside azide (16)
A mixture of alkyne 13 (68 mg, 65 μmol), azide 11 (62 mg, 65 μmol), N,N-diisopropylethylamine (23 μL, 0.13 mmol), CuI (2.5 mg, 13 μmol), and DMF (1 mL) in a vial sealed with a Teflon septum and aluminium crimp was subjected to microwave irradiation for 15 min at 80 °C, then cooled to room temperature, diluted with AcOEt (50 mL), washed with 0.05 M aqueous solution of EDTA (2 × 15 mL), dried (Na2SO4), and concentrated. A mixture of this residue, diphenyl phosphoryl azide (43 μL, 0.20 mmol), 1,8-diazabicyclo[5.4.0.]undec-7-ene (19 μL, 0.13 mmol), sodium azide (22 mg, 0.33) and anhydrous DMF (1 mL) in a vial sealed with a Teflon septum and aluminium crimp was subjected to microwave irradiation for 2 h at 130 °C, then cooled to room temperature, diluted with AcOEt (50 mL), washed with saturated aqueous Na2CO3 (20 mL), dried (Na2SO4), and concentrated. The residue was eluted from a column of silica gel with 3:1 cyclohexane-AcOEt to give 16 (81 mg, 61%), identical to the product described by us in a previous paper.16a
Protected tetramannoside alkyne (17)
The coupling of azide 14 (178 mg, 0.17 mmol) with alkyne 13 (165 mg, 0.14 mmol) was performed as described for the synthesis of 12 to give, after column chromatography on silica gel (from 2:1 to 1:1 cyclohexane-AcOEt), 17 (259 mg, 76%) as an amorphous solid; [α]d = +6.8 (c 0.8, CHCl3). 1H NMR (400 MHz) selected data: δ 7.79 (s, 1H, H-5 Tr.), 7.39-7.12 (m, 62H, Ar, 2 H-5 Tr.), 5.36, 5.25, and 5.23 (3 d, 3H, J1′,2′ = 1.5 Hz, J1′′,2′′ = 1.5 Hz, J1′′′,2′′′ = 1.5 Hz, H-1′, H-1′′, H-1′′′), 4.96 (d, 1H, J3,4 = 1.5 Hz, H-3), 3.03 (s, 1H, OH), 2.40 (dd, 1H, J6′′′a,OH = 5.0, J6′′′b,OH = 7.2 Hz, OH), 1.24 and 1.22 (2 s, 6H, 2 CH3). 13C NMR: δ 144.5 (C-4 tr.), 143.4 (C-4 Tr.), 138.5 (C), 138.3 (C), 138.1 (C), 138.0 (C), 137.9 (C), 137.6 (C), 128.7-127.5 (CH), 124.3 (C-5 Tr.), 124.0 (C-5 Tr.), 95.0 (C), 80.0 (CH), 79.5 (CH), 76.6 (CH), 75.3 (CH), 75.1 (CH2), 74.9 (CH2), 74.6 (CH), 73.1 (CH), 72.6 (CH2), 72.4 (CH2), 72.3 (CH), 71.8 (CH2), 71.6 (CH2), 71.4 (CH2), 71.1 (CH), 66.1 (CH), 64.2 (C), 62.7 (CH2), 51.1 (CH2), 50.5 (CH2), 31.1 (CH3), 30.9 (CH3). HRMS (ESI/Q-TOF) m/z calcd for (C119H125N9O18)/2 (M+2H)2+ 983.9571, found 983.9586.
Tetramannoside alkyne (18)
The deprotection of tetramannoside 17 (75 mg, 0.04 mmol) was performed as described for the synthesis of 13, to give, after column chromatography on silica gel (2:1 cyclohexane-AcOEt), 18 (65 mg, 89%) as an amorphous solid; [α]d = +2.8 (c 1.1, CHCl 1 3). 1H NMR (400 MHz) selected data: δ 7.79 (s, 1H, H-5 Tr.), 7.47 (s, 1H, H-5 Tr.), 7.42-7.17 (m, 61H, Ar, 1 H-5 Tr.), 5.35, 5.26, and 5.23 (3 d, 3H, J1′,2′ = 1.7 Hz, J1′′,2′′ = 1.7 Hz, J1′′′,2′′′ = 1.7 Hz, H-1′, H-1′′, H-1′′′), 2.33 (bs, 1H, OH), 2.32 (d, 1H, J1,3 = 2.2 Hz, H-1). 13 C NMR selected data: δ 144.6 (C-4 Tr.), 143.8 (C-4 Tr.), 143.6 (C-4 Tr.), 138.5-137.5 (C), 128.9-127.6 (CH), 124.4 (C-5 Tr.), 124.2 (C-5 Tr.), 124.0 (C-5 Tr.), 67.8 (CH), 62.8 (CH2), 51.1 (CH2), 50.9 (CH2), 50.5 (CH2). HRMS (ESI/Q-TOF) m/z calcd for (C116H119N9O17)/2 (M+2H)2+ 954.9362, found 954.9412.
Tetramannoside azide (19)
The alcohol 17 (150 mg, 0.08 mmol) was azidated as described for the preparation of 14 to give, after column chromatography on silica gel (2:1 cyclohexane-AcOEt), 19 (118 mg, 79%) as a syrup; [α]d = +4.3 (c 1.1, CHCl3). 1H NMR (400 MHz) selected data: δ 7.91 (1s, 1H, H-5 Tr.), 741-7.06 (m, 62H, Ar, 2 H-5 Tr.), 5.40, 5.27, and 5.26 (3 d, 3H, J1′,2′ = 1.5 Hz, J1′′,2′′ = 1.5 Hz, J1′′′,2′′′ = 1.5 Hz, H-1′, H-1′′, H-1′′′), 4.99 (d, 1H, J3,4 = 2.0 Hz, H-3), 2.80 (s, 1H, OH), 1.23 and 1.19 (2 s, 6H, 2 CH3). 13 C NMR: δ 144.5 (C-4 tr.), 143.5 (C-4 Tr.), 138.5 (C), 138.4 (C), 138.3 (C), 138.1 (C), 138.0 (C), 137.6 (C), 128.7-127.5 (CH), 124.7 (C-5 Tr.), 124.4 (C-5 Tr.), 124.1 (C-5 Tr.), 95.0 (C), 80.3 (CH), 80.2 (CH), 80.0 (CH), 79.6 (CH), 77.2 (CH), 76.7 (CH), 75.5 (CH), 75.2 (CH), 75.0 (CH2), 74.7 (CH), 74.5 (CH), 73.9 (CH), 73.2 (CH), 72.6 (CH), 72.5 (CH2), 72.3 (CH), 71.8 (CH2), 71.6 (CH2), 71.4 (CH2), 71.1 (CH), 70.6 (CH2), 70.3 (CH2), 70.0 (CH2), 66.1 (CH), 64.2 (C), 61.8 (CH2), 51.8 (CH2), 51.3 (CH2), 51.0 (CH2), 50.5 (CH2), 31.2 (CH3), 30.9 (CH3). HRMS (ESI/Q-TOF) m/z calcd for (C119H124N12O)/2 (M+2H)2+ 996.4604, found 996.4620.
Octamannoside alkyne (20)
The alkyne 15 (97 mg, 0.05 mmol) was allowed to react with azide 19 (102 mg, 0.05 mmol) as described for the preparation of 15 to give, after trituration with CH3OH, 20 (101 mg, 52%) as an amorphous solid; [α]d = -172.3 (c 0.7, CHCl3). 1H NMR (400 MHz) selected data: δ 7.84 (s, 1H, H-5 Tr.), 6.02 (bs, 2H, 2 anom. H), 5.96, 5.88, 5.80, 5.47, and 5.41 (5 bs, 5H, 5 anom. H), 1.40 (d, 3H, J = 6.0 Hz, CH3). 13C NMR selected data: δ 145.0 (C-4 Tr.), 143.5 (C-4 Tr.), 143.3 (C-4 Tr.), 143.0 (C-4 Tr.), 139.6-137.5 (C), 128.5-126.7 (CH), 124.9-123.9 (C-5 Tr.), 18.5 (CH3). MALDI-TOF MS: m/z calcd for C232H234N21O32 (M+H)+ 3828.53, found 3828.41.
Octamannoside azide (21)
The alkyne 18 (55 mg, 0.03 mmol) was allowed to react with azide 16 (58 mg, 0.03 mmol) as described for the preparation of 16 to give, after trituration with CH3OH, 21 (71 mg, 63%) as an amorphous solid; [α]d = −138.6 (c 1.1, CHCl3). 1H NMR (400 MHz) selected data: δ 7.92 (s, 1H, H-5 Tr.), 5.98 (bs, 2H, 2 anom. H), 5.94, 5.86, 5.71, 5.50, and 5.44 (5 bs, 5H, 5 anom. H), 3.10 (s, 3H, CH3). 13C NMR selected data: δ 144.5-143.1 (C-4 Tr.), 139.5-137.6 (C), 128.3-126.7 (CH), 125.5-123.9 (C-5 Tr.). MALDI-TOF MS: m/z calcd for C234H241N24O34 (M+H)+ 3933.63, found 3933.40.
Hexadecamannoside (22)
The alkyne 20 (50.6 mg, 13 μmol) was allowed to react with azide 21 (52 mg, 13 μmol) as described for the preparation of 12 to give, after trituration with CH3CN, 22 (93 mg, 92%) as an amorphous solid; [α]d = −3.2 (c 0.5, CHCl3). 1H NMR (400 MHz) selected data: δ 7.88 (s, 1H, H-5 Tr.), 3.17 (s, 3H, CH3). 13C NMR selected data: δ 143.4 (C-4 Tr.), 139.8-137.5 (C), 128.2-126.7 (CH), 124.8 (C-5 Tr.). MALDI-TOF MS: m/z calcd for C466H474N45O66 (M+H)+ 7761.15, found 7761.40.
Hexadecamannoside (23)
To a solution of 22 (75 mg, 9.7 μmol) in anhydrous CH2Cl2 (5 mL) was added trifluoroacetic acid (200 μL). The solution was kept at room temperature for 3 h, then concentrated. To a stirred, cooled (−60 °C) solution of the residue in anhydrous CH2Cl2 (2 mL) was added a 1 M solution of BCl3 in CH2Cl2 (930 μL, 0.93 mmol). The solution was stirred at −60 °C for 1 h and, after an additional 1 h stirring at 0 °C, diluted with MeOH (0.5 mL), stirred at 0 °C for 30 min, diluted with Et3N (0.5 mL), concentrated, and dried under high vacuum to give a white solid. A suspension of the residue in pyridine (2 mL) and acetic anhydride (1 mL) was stirred at room temperature for 24 h, then diluted with MeOH (1 mL) and concentrated. The residue was eluted from a column of silica gel with AcOEt to give 23 (18 mg, 40%) as an amorphous solid; [α]d = +32.9 (c 0.4, CHCl3). 1H NMR (300 MHz) selected data: δ 7.99 (s, 1H, H-5 Tr.), 7.73-7.62 (14 s, 14H, 14 H-5 Tr.). MALDI-TOF MS: m/z calcd for C226H279N45NaO114 (M+Na)+ 5472.93, found 5472.88.
Hexadecamannoside (1f)
To a solution of 23 (18 mg, 3.8 μmol) in MeOH (2 mL) was added a 2 M solution of ammonia in MeOH (2 mL). The solution was kept at room temperature overnight, then concentrated to give 1f (10 mg, 91%) as an amorphous solid; [α]d = +4.7 (c 0.3, DMSO). 1H NMR (300 MHz, D2O) selected data: δ 7.80-7.36 (15 s, 15H, 15 H-5 Tr.). MALDI-TOF MS: m/z calcd for C128H181N45NaO65 (M+Na)+ 3413.10, found 3412.99.
3,7-Anhydro-4,5,6-tri-O-benzyl-2,8-dideoxy-1-methoxymethyl-8-[4-(2′,3′,4′-tri-O-benzyl-6′-deoxy-α-d-mannopyranosyl)-1H-1,2,3-triazol-1-yl]-d-glycero-d-talo-octitol (24)
The coupling of azide 9 (12 mg, 0.023 mmol) with alkyne 8 (10 mg, 0.023 mmol) was performed as described for the synthesis of 22 to give, after column chromatography on silica gel (3:1 cyclohexane-AcOEt), 24 (21 mg, 96%) as an amorphous solid; [α]d = +21.7 (c 1.1, CHCl3). 1H NMR (400 MHz) selected data: δ 7.65 (s, 1H, H-5 Tr.), 7.43-7.22 (m, 30H, Ar), 5.18 (d, 1H, J1′,2′ = 2.0 Hz, H-1′), 4.92 and 4.60 (2 d, 2H, J = 10.8 Hz, PhCH2), 4.74 (dd, 1H, J7,8a = 6.5, J8a,8b = 14.3 Hz, H-8a), 4.47 (dd, 1H, J7,8b = 3.0 Hz, H-8b), 4.18 (ddd, 2H, J = 4.3, 5.0, 9.0 Hz, 2 H-1), 3.80 (dd, 1H, J3,4 = 3.0, J4,5 = 7.5 Hz, H-4), 3.62 (dd, 1H, H-3), 3.54 (dd, 1H, J5,6 = 6.8 Hz, H-5), 3.28 (s, 3H, CH3), 1.80-1.70 (m, 2H, 2 H-2), 1.33 (d, 3H, J = 6.0 Hz, 3 H-6′). 13C NMR: δ 145.2 (C-4 Tr.), 138.7 (C), 138.6 (C), 138.0 (C), 137.8 (C), 128.5 (CH), 128.4 (CH), 128.2 (CH), 128.1 (CH), 127.9 (CH), 127.8 (CH), 127.7 (CH), 127.5 (CH), 127.4 (CH), 123.8 (C-5 Tr.), 96.4 (CH2), 80.5 (CH), 80.2 (CH), 77.2 (CH), 75.9 (CH), 75.1 (CH2), 74.9 (CH), 74.0 (CH2), 72.6 (CH), 72.5 (CH2), 72.4 (CH2), 71.8 (CH2), 71.0 (CH), 70.4 (CH), 70.2 (CH), 63.6 (CH2), 55.2 (CH), 50.5 (CH2), 29.8 (CH2), 26.9 (CH2), 18.2 (CH3). HRMS (ESI/Q-TOF) m/z calcd for C60H67N3NaO10 (M+Na)+ 1012.4724, found 1012.4786.
1,4,5,6-Tetra-O-acetyl-3,7-anhydro-2,8-dideoxy-8-[4-(2′,3′,4′-tri-O-acetyl-6′-deoxy-α-d-mannopyranosyl)-1H-1,2,3-triazol-1-yl]-d-glycero-d-talo-octitol (25)
The deprotection of 24 (18 mg, 18 μmol) was performed as described for the synthesis of 23 to give, after column chromatography on silica gel (AcOEt), 25 (10 mg, 81%) as an amorphous solid; [α]d = +118.7 (c 0.6, CHCl3). 1H NMR (300 MHz) selected data: δ 7.83 (s, 1H, H-5 Tr.). 13C NMR selected data: δ 170.1 (C=O), 141.8 (C-4 Tr), 124.3 (C-5 Tr), 50.5 (CH2), 29.8 (CH2), 27.9 (CH2), 17.6 (CH3). HRMS (ESI/Q-TOF) m/z calcd for C30H42N3O16 (M+H)+ 700.2565, found 700.2619.
3,7-Anhydro-2,8-dideoxy-8-[4-(6′-deoxy-α-d-mannopyranosyl)-1H-1,2,3-triazol-1-yl]-D-glycero-d-talo-octitol (1a)
The deprotection of 25 (8 mg, 11 μmol) was performed as described for the synthesis of 1f to give, after a column chromatography on Sephadex LH20 (1:1 CH2Cl2-MeOH), 1a as an amorphous solid (4.2 mg, 92%). 1H NMR (300 MHz, D2O) selected data: δ 7.96 (s, 1H, H-5 Tr.), 5.10 (bs, 1H, H-1′). HRMS (ESI/Q-TOF) m/z calcd for C16H28N3O9 (M+H)+ 406.1826, found 406.1815.
Tetramannoside (26)
The coupling of azide 11 (15 mg, 0.015 mmol) with alkyne 10 (13.8 mg, 0.015 mmol) was performed as described for the synthesis of 22, to give, after column chromatography on silica gel (2:1 cyclohexane-AcOEt), 26 (26 mg, 88%) as an amorphous solid; [α]d = −34.5 (c 0.7, CHCl3). 1H NMR (400 MHz) selected data: δ 7.80, 7.69 and 7.43 (3 s, 3H, 3 H-5 Tr.), 7.42-7.14 (m, 60H, Ar) 5.29, 5.23, and 5.20 (3 d, 3H, J1′,2′ = 1.8 Hz, J1′′,2′′ = 1.8 Hz, J1′′′,2′′′ = 1.8 Hz, H-1′, H-1′′, H-1′′′), 3.22 (s, 3H, CH3), 1.34 (d, 3H, J = 6.0 Hz, CH3). 13C NMR selected data: δ 145.2 (C-4 Tr.), 143.9 (C-4 Tr.), 143.8 (C-4 Tr.), 138.7-137.9 (C), 128.5-127.3 (CH), 124.4 (C-5 Tr.), 124.3 (C-5 Tr.), 123.9 (C-5 Tr.), 96.4 (CH2), 63.8 (CH2), 55.2 (CH), 50.8 (CH2), 50.7 (CH2), 29.7 (CH2), 18.4 (CH3).
Tetramannoside (27)
The deprotection of 26 (20 mg, 10 μmol) was performed as described for the synthesis of 23 to give, after column chromatography on silica gel (AcOEt), 27 (10 mg, 73%) as an amorphous solid; [α]d = +3.9 (c 0.7, CHCl3). 1H NMR (400 MHz) selected data: δ 8.15, 7.77, and 7.68 (3 s, 3H, 3 H-5 Tr.). 13C NMR selected data: δ 170.5-170.0 (C=O), 29.7 (CH2), 20.8-20.7 (CH3), 17.6 (CH3). HRMS (ESI/Q-TOF) m/z calcd for C58H76N9O30 (M+H)+ 1378.4698, found 1378.4749.
Tetramannoside (1b)
The deprotection of 27 (9 mg, 6.5 μmol) was performed as described for the synthesis of 1f to give 1b as an amorphous solid (5 mg, quantitative). HRMS (MALDI-TOF) m/z calcd for C32H49N9NaO17 (M+Na)+ 854.3144, found 854.3214.
Octamannoside (28)
The coupling of azide 16 (21 mg, 10.6 μmol) with alkyne 15 (20 mg, 10.6 μmol) was performed as described for the synthesis of 22 to give, after trituration with CH3OH, 28 (35 mg, 85%) as an amorphous solid; [α]d = −150.4 (c 1.0, CHCl3). 1H NMR (400 MHz) selected data: δ 7.88, 6.50, 6.38, 6.36, and 6.24 (5 s, 5H, 5 H-5 Tr.), 6.00 (bs, 2H, 2 anom. H), 5.94, 5.87, 5.71, 5.46, and 5.41 (5 bs, 5H, 5 anom. H), 3.09 (s, 3H, CH3), 1.39 (d, 3H, J = 6.0 Hz, CH3). 13C NMR selected data: δ 145.0-143.1 (C-4 Tr.), 139.5-137.5 (C), 128.4-126.7 (CH), 125.0-124.0 (C-5 Tr.), 96.0 (CH2), 64.2 (CH2), 54.8 (CH), 18.5 (CH3). MALDI-TOF MS: m/z calcd for C234H242N21O34 (M+H)+ 3892.62, found 3892.57.
Octamannoside (29)
The deprotection of 28 (34 mg, 8.7 μmol) was performed as described for the synthesis of 23 to give, after column chromatography on silica gel (AcOEt), 29 (19 mg, 78%) as an amorphous solid; [α]d = −52.1 (c 0.5, CHCl3). 1H NMR (300 MHz) selected data: δ 7.98 (s, 1H, H-5 Tr.), 7.72 (s, 2H, 2 H-5 Tr.), 7.70 (s, 2H, 2 H-5 Tr.), 7.68 and 7.63 (2 s, 2H, 2 H-5 Tr.), 1.20 (d, 3H, J = 6.0 Hz, CH3). MALDI-TOF MS: m/z calcd for C114H143N21NaO58 (M+Na)+ 2758.49, found 2758.48.
Octamannoside (1d)
The deprotection of 29 (9 mg, 3.4 μmol) was performed as described for the synthesis of 1f to give 1d (6 mg, quantitative) as an amorphous solid. HRMS (MALDI-TOF) m/z calcd for C64H94N21O33 (M+H)+ 1684.6323, found 1684.5648.
3,7-Anhydro-2,8-dideoxy-8-[4-(α-d-mannopyranosyl)-1H-1,2,3-triazol-1-yl]-d-glycero-d-talo-octitol (31)
To a solution of 30 (22 mg, 0.02 mmol) in anhydrous CH2Cl2 (5 mL) was added trifluoroacetic acid (400 μL). The solution was kept at room temperature for 3 h, then concentrated. To a solution of the residue in 4:2:1 CH3OH-THF-H2O (4 mL) were added ammonium formate (110 mg, 1.75 mmol) and 10% palladium on carbon (212 mg, 0.20 mmol). The mixture was stirred at 50 °C for 18 h in a sealed vial, then cooled to room temperature, filtered through a pad of Celite, and concentrated. The residue was eluted from a column of Sephadex LH20 (1 × 20 cm, d × h) with 3:1 H2O-CH3OH to give 31 (4 mg, 45%), as an amorphous solid; [α]d = −6.1 (c 0.4, H2O). 1H NMR (400 MHz, D2O) selected data: δ 7.97 (s, 1H, H-5 Tr.), 5.10 (d, 1H, J1′,2′ = 2.2 Hz, H-1′), 4.46 (dd, 1H, J7,8b = 9.0, J8a,8b = 14.2 Hz, H-8a), 4.41 (dd, 1H, J2′,3′ = 3.2 Hz, H-2′). 13C NMR (D2O): δ 146.5 (C-4 Tr.), 128.3 (C-5 Tr.), 78.2 (CH), 77.7 (CH), 75.7 (CH), 75.2 (CH), 74.1 (CH), 73.7 (CH), 73.4 (CH), 72.7 (CH), 71.4 (CH), 69.9 (CH), 63.7 (CH2), 60.3 (CH2), 54.2 (CH2), 32.3 (CH2). HRMS (ESI/Q-TOF) m/z calcd for C16H28N3O10 (M+H)+ 422.1775, found 422.1767.
Evaluation of inhibitor activity of 1a-1f in the PPM1:α(1,6)-mannosyltransferase assay
The α-Manp(1,6)-α-Manp-O(CH2)7CH3 disaccharide acceptor 12 (0.8 μL) stored as a 20 mM ethanol stock) was dried under a stream of argon in a microcentrifuge tube (1.5 mL), placed in a vacuum desiccator for 15 min to remove any residual solvent and then resuspended in 8 μL of a 1% aqueous solution of Igepal. Assays were performed by incubating each TOM compound (1a-1f) at a final concentration of 1.0 mM with 2.4 μM GDP-[U-14C]mannose (321 mCi/mmol, 0.25 μCi; Dupont-New England Nuclear), 62.5 μM ATP, 10 μM MgCl2, 62.5 μM DTT, 12.5 μM NaF, 0.25 mM decaprenolphosphate (in 1% CHAPS) and M. smegmatis membrane fractions corresponding to 500 μg. The final volume of the assays was adjusted to 80 μL with 50 mM MOPS (pH 7.9), with a 0.2 mM final concentration of α-Manp(1,6)-α-Manp-O(CH2)7CH3 acceptor. The reaction mixtures were then incubated at 37 °C for 30 min. A 1:1 CHCl3/CH3OH (533 μL) solution was added to the incubation tubes and the entire contents centrifuged at 18,000 × g. The supernatant was recovered and dried under a stream of argon and re-suspended in 1:1 EtOH/H2O (1 mL) and loaded onto a pre-equilibrated (1:1 EtOH/H2O) 1 mL Whatmann strong anion exchange (SAX) cartridge, after which was washed with 3 mL of EtOH. The eluate was dried and the resulting products partitioned between the two phases arising from a mixture of n-butanol (3 mL) and H2O (3 mL). The resulting organic phase was recovered following centrifugation at 3500 × g and the aqueous phase was again extracted twice with 3 mL of water saturated n-butanol, the pooled extracts were back-washed twice with water saturated with n-butanol (3 mL). The water saturated n-butanol fraction was dried and re-suspended in 200 μL of n-butanol. The total counts per minute of radiolabeled material extractable into the n-butanol phase was measured by scintillation counting using 10% of the labeled material and 10 mL of EcoScintA (National Diagnostics, Atlanta, GA, USA). The incorporation of [14C]Man was determined by subtracting counts present in control assays (incubation of the reaction components in the absence of α-Manp(1,6)-α-Manp-O(CH2)7CH3).
Supplementary Material
Copies of the NMR spectra of the new products. This material is also available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
GSB acknowledges support in the form of a Personal Research Chair from Mr. James Bardrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow, the Medical Research Council and The Wellcome Trust (081569/Z/06/Z).
References
- 1.(a) Gaudy M, Zumla A, editors. The Return of the White Plague: Global Poverty and the New Tuberculosis. Verso; London: 2003. [Google Scholar]; (b) Ryan F. The Forgotten Plague: How the Battle Against Tuberculosis Was Won - and Lost. Back Bay Books; New York: 1994. [Google Scholar]
- 2.(a) Corbett EL, Marston B, Churchyard GJ, De Cock KM. Lancet. 2006;367:926–937. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]; (b) Maartens G, Wilkinson RJ. Lancet. 2007;370:2030–2043. doi: 10.1016/S0140-6736(07)61262-8. [DOI] [PubMed] [Google Scholar]
- 3.Lenaerts AJ, Degroote MA, Orme IM. Trends Microbiol. 2008;16:48–54. doi: 10.1016/j.tim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 4.(a) Zhao Y, Bacher A, Illarionov B, Fischer M, Georg G, Ye Q-Z, Fanwick PE, Franzblau SG, Wan B, Cushman M. J. Org. Chem. 2009;74:5297–5303. doi: 10.1021/jo900768c. [DOI] [PubMed] [Google Scholar]; b) Hung AW, Silvestre HL, Wen S, Ciulli A, Blundell TL, Abell C. Angew. Chem. Int. Ed. 2009;48:8452–8456. doi: 10.1002/anie.200903821. [DOI] [PubMed] [Google Scholar]; (c) Tan LP, Wu H, Yang P-Y, Kalesh KA, Zhang X, Hu M, Srinivasan R, Yao SQ. Org. Lett. 2009;11:5102–5105. doi: 10.1021/ol9023419. [DOI] [PubMed] [Google Scholar]
- 5.(a) Brennan PJ, Crick DC. Curr. Top. Med. Chem. 2007;7:475–488. doi: 10.2174/156802607780059763. [DOI] [PubMed] [Google Scholar]; (b) Berg S, Kaur D, Jackson M, Brennan PJ. Glycobiology. 2007;17:35R–56R. doi: 10.1093/glycob/cwm010. [DOI] [PubMed] [Google Scholar]; (c) Tam P-H, Lowary TL. Curr. Opin. Chem. Biol. 2009;13:618–625. doi: 10.1016/j.cbpa.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Janin YL. Bioorg. Med. Chem. 2007;15:2479–2513. doi: 10.1016/j.bmc.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Crick DC, Mahapatra S, Brennan PJ. Glycobiology. 2001;11:107R–118R. doi: 10.1093/glycob/11.9.107r. [DOI] [PubMed] [Google Scholar]
- 8.Morita YS, Patterson JH, Billman-Jacobe H, McConville MJ. Biochem. J. 2004;378:589–597. doi: 10.1042/BJ20031372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Chatterjee D, Hunter SW, McNeil M, Brennan PJ. J. Biol. Chem. 1992;267:6228–6233. [PubMed] [Google Scholar]; (b) Khoo KH, Dell A, Morris HR, Brennan PJ, Chatterjee D. Glycobiology. 1995;5:117–127. doi: 10.1093/glycob/5.1.117. [DOI] [PubMed] [Google Scholar]; (c) Alderwick LJ, Birch HL, Mishra AK, Eggeling L, Besra GS. Biochem. Soc. Trans. 2007;35:1325–1328. doi: 10.1042/BST0351325. [DOI] [PubMed] [Google Scholar]
- 10.(a) Jayaprakash KN, Lu J, Fraser-Reid B. Angew. Chem. Int. Ed. 2005;44:5894–5898. doi: 10.1002/anie.200500505. [DOI] [PubMed] [Google Scholar]; (b) Fraser-Reid B, Lu J, Jayaprakash KN, Cristóbal López J. Tetrahedron: Asymmetry. 2006;17:2449–2463. [Google Scholar]; (c) Liu X, Stocker BL, Seeberger PH. J. Am. Chem. Soc. 2006;128:3638–3648. doi: 10.1021/ja0565368. [DOI] [PubMed] [Google Scholar]; (d) Hoelemann A, Stocker BL, Seeberger PH. J. Org. Chem. 2006;71:8071–8088. doi: 10.1021/jo061233x. [DOI] [PubMed] [Google Scholar]; (e) Jayaprakash KN, Chaudhuri SR, Murty CVSR, Fraser-Reid B. J. Org. Chem. 2007;72:5534–5545. doi: 10.1021/jo070018t. [DOI] [PubMed] [Google Scholar]; (f) Fraser-Reid B, Chaudhuri SR, Jayaprakash KN, Lu J, Ramamurty CVS. J. Org. Chem. 2008;73:9732–9743. doi: 10.1021/jo802000p. [DOI] [PubMed] [Google Scholar]; (g) Boonyarattanakalin S, Liu X, Michieletti M, Lepenies B, Seeberger PH. J. Am. Chem. Soc. 2008;130:16791–16799. doi: 10.1021/ja806283e. [DOI] [PubMed] [Google Scholar]; (h) Patil PS, Hung S-C. Chem. Eur. J. 2009;15:1091–1094. doi: 10.1002/chem.200802189. [DOI] [PubMed] [Google Scholar]
- 11.(a) Subramaniam V, Gurcha SS, Besra GS, Lowary TL. Bioorg. Med. Chem. 2005;13:1083–1094. doi: 10.1016/j.bmc.2004.11.027. [DOI] [PubMed] [Google Scholar]; (b) Tam P-H, Lowary TL. Carbohydr. Res. 2007;342:1741–1772. doi: 10.1016/j.carres.2007.05.001. [DOI] [PubMed] [Google Scholar]; (c) Tam P-H, Lowary TL. Org. Biomol. Chem. 2010;8:181–192. doi: 10.1039/b916580k. [DOI] [PubMed] [Google Scholar]
- 12.This disaccharide is a known substrate for α-(1,6)-ManT, the enzyme promoting the biosynthesis of the mannan core in LM and LAM of Mtb. See: Brown JR, Field RA, Barker A, Guy M, Grewal R, Khoo K-H, Brennan PJ, Besra GS, Chatterjee D. Bioorg. Med. Chem. 2001;9:815–824. doi: 10.1016/s0968-0896(00)00300-x.
- 13.Subramaniam V, Gurcha SS, Besra GS, Lowary TL. Tetrahedron: Asymmetry. 2005;16:553–567. [Google Scholar]
- 14.Watt JA, Williams SJ. Org. Biomol. Chem. 2005;3:1982–1992. doi: 10.1039/b503919c. [DOI] [PubMed] [Google Scholar]
- 15.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057–3062. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 16.(a) Cheshev P, Marra A, Dondoni A. Org. Biomol. Chem. 2006;4:3225–3227. doi: 10.1039/b609734k. [DOI] [PubMed] [Google Scholar]; (b) Lo Conte M, Chambery A, Marra A, Dondoni A. Synlett. 2009:2679–2681. [Google Scholar]
- 17.Horne WS, Yadav MK, Stout CD, Ghadiri MR. J. Am Chem. Soc. 2004;126:15366–15367. doi: 10.1021/ja0450408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.It is well known that the CuAAC takes place with terminal alkynes. Exceptionally, iodoalkynes do react as well. See: Hein JE, Tripp JC, Krasnova L, Sharpless BKB, Fokin VV. Angew. Chem. Int. Ed. 2009;48:8018–8021. doi: 10.1002/anie.200903558.
- 19.The 1,4-disubstitution pattern of the triazole ring in all prepared oligomannosides was supported by the large and positive Δ(δC4 - δC5) value (17-21 ppm) in their 13C NMR spectra as observed in other compounds obtained by click CuAAC in our laboratory. See: ref.14 and Dondoni A, Marra A. J. Org. Chem. 2006;71:7546–7557. doi: 10.1021/jo0607156. Dondoni A, Giovannini PP, Massi A. Org. Lett. 2004;6:2929–2932. doi: 10.1021/ol048963g. Marra A, Vecchi A, Chiappe C, Melai B, Dondoni A. J. Org. Chem. 2008;73:2458–2461. doi: 10.1021/jo7026454.
- 20.Besra GS, Morehouse CB, Rittner CM, Waechter CJ, Brennan PJ. J. Biol. Chem. 1997;272:18460–18466. doi: 10.1074/jbc.272.29.18460. [DOI] [PubMed] [Google Scholar]
- 21.Gurcha SS, Baulard AR, Kremer L, Locht C, Moody DB, Muhlecker W, Costello CE, Crick DC, Brennan PJ, Besra GS. Biochem. J. 2002;365:441–450. doi: 10.1042/BJ20020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poláková M, Belánová M, Petrus L, Mikusová K. Carbohydr. Res. 2010;345:1339–1347. doi: 10.1016/j.carres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Armarego WLF, Chai CLL. Purification of Laboratory Chemicals. 5th ed. Butterworth- Heinemann; Amsterdam: 2003. [Google Scholar]
- 24.Still WC, Kahn M, Mitra A. J. Org. Chem. 1978;43:2923–2925. [Google Scholar]
- 25.Pure Appl. Chem. 1991;63:975–990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Copies of the NMR spectra of the new products. This material is also available free of charge via the Internet at http://pubs.acs.org.