Abstract
Transcranial Electrical Stimulation (tES) encompasses all methods of non-invasive current application to the brain used in research and clinical practice. We present the first comprehensive and technical review, explaining the evolution of tES in both terminology and dosage over the past 100 years of research to present day. Current transcranial Pulsed Current Stimulation (tPCS) approaches such as Cranial Electrotherapy Stimulation (CES) descended from Electrosleep (ES) through Cranial Electro-stimulation Therapy (CET), Transcerebral Electrotherapy (TCET), and NeuroElectric Therapy (NET) while others like Transcutaneous Cranial Electrical Stimulation (TCES) descended from Electroanesthesia (EA) through Limoge, and Interferential Stimulation. Prior to a contemporary resurgence in interest, variations of trans-cranial Direct Current Stimulation were explored intermittently, including Polarizing current, Galvanic Vestibular Stimulation (GVS), and Transcranial Micropolarization. The development of these approaches alongside Electroconvulsive Therapy (ECT) and pharmacological developments are considered. Both the roots and unique features of contemporary approaches such as transcranial Alternating Current Stimulation (tACS) and transcranial Random Noise Stimulation (tRNS) are discussed. Trends and incremental developments in electrode montage and waveform spanning decades are presented leading to the present day. Commercial devices, seminal conferences, and regulatory decisions are noted. We conclude with six rules on how increasing medical and technological sophistication may now be leveraged for broader success and adoption of tES.
Keywords: Electrosleep, Electroanesthesia, Electronarcosis, TCES, CET, CES, TCET, EA, ES, EN, tDCS, HD-tDCS, tES, ECT, FEAST, NET, tPCS, Electrostimulation, tRNS, Limoge, Interferential, Stimulation, tACS, Historical, Dosage, Contemporary, Electrotherapy, Cranial, Transcerebral, Transcutaneous, History, Direct, Alternating, Pulsed
1. Scope and approach
Transcranial Electrical Stimulation (tES) encompasses all forms of research and clinical application of electrical currents to the brain non-invasively using (at least one) electrodes on the head. The dose of tES is defined by the electrode montage and the stimulation waveform applied to the electrode (Peterchev et al., 2012). There has been a resurgence of interest since 2000, but “modern” tES developed incrementally over a century. This review provides the first comprehensive organization of approaches and dose used in modern tES since 1900. Though ‘dose’ is used historically in different context, throughout this review we follow the strict convention of Peterchev 2012 (Peterchev et al., 2012), where tES dosage is defined by electrode parameters (including number, position, shape, and composition) and all details of stimulation waveform (including intensity and general waveform and when relevant pulse shape, amplitude, width, polarity, repetition frequency; duration of and interval between bursts or trains of pulses, interval between stimulation sessions and total number of sessions).
This process involves defining the litany of terminology that has developed and evolved around tES. We explain the terminology as used contemporarily by researchers. Particular attention is paid to historically linked categories of tES, “streams”, of which we identify four that span decades plus “contemporary” approaches (Fig. 1): (1) Cranial Electrical Stimulation (CES) descended from Electrosleep (ES) through Cranial Electro-stimulation Therapy (CET), Transcerebral Electrotherapy (TCET), and NeuroElectric Therapy (NET); (2) Electroanestheisia went through several periods of waning interest and resurgence when new waveform variations were proposed including Transcutaneous Cranial Electrical Stimulation (TCES), Limoge, and Interferential Stimulation; (3) Polarizing or Direct Current Stimulation includes recent transcranial Direct Current Stimulation, Transcranial Micropolarization, High-Definition transcranial Direct Current Stimulation (HD-tDCS) and Galvanic Vestibular Stimulation (GVS); (4) Electroconvulsive Therapy (ECT), initially called Electroshock Therapy, evolved in technique and dose, such as Focal Electrically Administered Seizure Therapy (FEAST); (5) Finally, we categorize “contemporary” approaches that have been explored intensely over the last decade, such as transcranial Alternating Current Stimulation (tACS), transcranial Sinusoidal Direct Current Stimulation (tSDCS), and transcranial Random Noise Stimulation (tRNS). Though analogs to these contemporary approaches can be identified in earlier literature, contemporary methods contain dose features that motivate us to consider them novel as a category. Contemporary approaches to some extent reflect a “reboot” of tES approach, typically employing basic, well documented, and well-defined waveforms (e.g. one sinusoid; Paulus, 2011) in contrast to the increasingly complex waveforms developed (though not always justified) over decades in some streams.
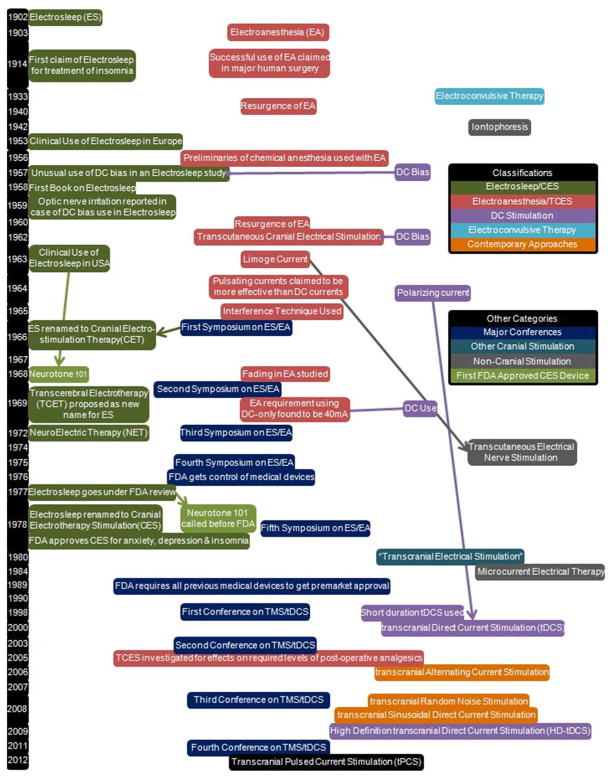
Fig. 1.
A general timeline of ES/EA noting key points in the history from 1902 until 2011 as well as their relation to DC stimulation. A brief history of DC stimulation is also presented in this table. Other cranial therapies are mentioned for a complete cranial stimulation history and non-cranial therapies are mentioned for their connection to ES/EA. Arrows are used to connect historically related points while the horizontal purple lines are used to point out DC use in historically pulsed applications.
As our technical focus is on dose clarification and classification, we minimize comments on the clinical efficacy or safety of any approaches except in special cases where findings resulted in historically notable and sudden changes in dose or terminology. We note specific conferences and regulatory agencies that helped identify and shape the field of transcranial Electrical Stimulation including establishing terminology. Commercial (brand) names of devices are noted ad hoc for context and linked to dose terms where appropriate. We do not comment directly on mechanisms but emphasize that dose determines electric field in the brain (Peterchev et al., 2012) which, in turn, gives rise to neurophysiological responses (Bikson et al., 2004) and sustained brain function changes (which may depend on N-methyl-D-aspartate receptor; Li and Tsien, 2009); thus understanding the dose is a prerequisite to understanding mechanisms.
We do not address magnetic stimulation approaches or electrical stimulation approaches not targeting the brain, or non-electrical therapies, except in specific cases to indicate the terminology used in these other approaches for the purpose of overall clarity of nomenclature. We did not attempt to perform an exhaustive cataloging of tES publications.
Though we do not comment on efficacy, the nominal indications for tES use (intended clinical outcomes) are noted when contextually relevant, especially for many historical streams (defined above). There are instances in which researchers used terminology to describe a dose in a manner potentially inconsistent with typical historical norms of dose associated with that terminology -when these papers provide sufficient dose details these deviations are noted. Our summary aims to reflect the most typical doses used across the majority of studies (Table 1). In addition, to promote a more comprehensive and systematic dose classification, we propose new categories for those waveforms using pulsed stimulation in Table 2 (transcranial Pulsed Current Stimulation, tPCS).
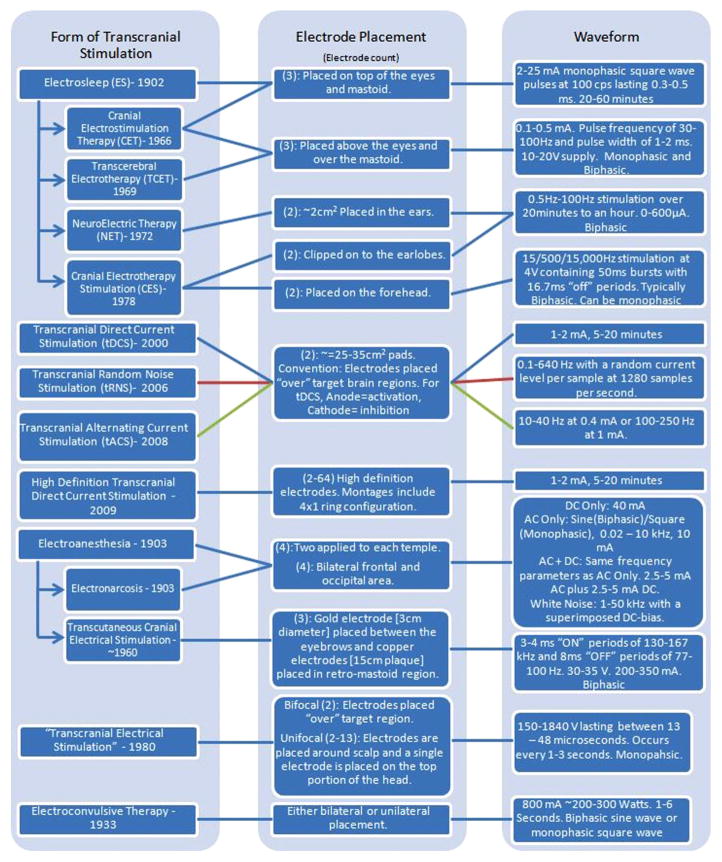
Table 1.
Dosages of the various cranial stimulation methods are shown. The year at which the form of stimulation came about is written with the stimulation method. Each method is connected to an electrode placement as well as a waveform used.
|
Table 2.
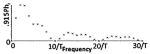
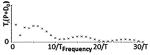
Different classes of tPCS are summarized including temporal waveform (function), the associated magnitude spectrum (frequency content), and clinical references including dose using “CES”. The Fourier series were generated using the same parameters for T, τ, and A across all classes and the same parameters for h, D0, Ton, and Toff where applicable. Note n is a discrete function of 1/T (or Toff in the case of Class III). In Class III, the CES case would have D0 set to zero which would lower the peak at zero. In Class II, hr = (h + 1)/h, in Class III, Tr = Ton /Toff and in all classes, P = A(τ/T).
| Waveform | Magnitude Spectrum | Notes | |
|---|---|---|---|
| Class I(A) - Monophasic Pulse |
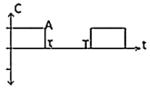
|
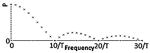
|
-ES2 -CET6 -EA2 |
| Class I(B) - Monophasic Pulse with DC offset |
|

|
-ES2,6 -EA2 -CET2,6 -TCET6,2 |
| Class II(A) – Biphasic pulse |
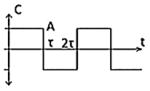
|
|
-CES7 |
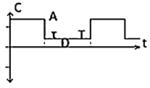
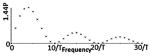
| Class II(B) - Biphasic Pulse with delay |
|
|
-TCET -CET -NET4 -CES5 |
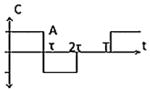
| Class II(C) - Asymmetric Biphasic Pulse |
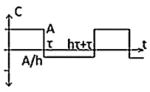
|
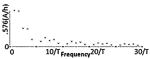
|
|
| Class II(D) – Asymmetric Biphasic Pulse with delay |
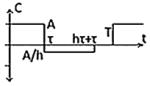
|
|
-CES3 -NET |
| Pulse Trains |

|
|
-LC1 -TCES1 -CES8 |
| Class I Train (50/55) (TON/TOFF) |
The references indicated are:
Liss Stimulator Manual, Model No. SBL-502-B;
Liss Stimulator Manual, Model No. SBL-501-M.
Adapted from Datta et al. (2013).
It is important to emphasize that the specifics of tES dose (electrode montage, waveform, intensity and duration) determine brain modulation – evidently the given therapy name is incidental and often reflects a historical bias and varying intended use. In this sense, a strict approach would involve ignoring all historical nomenclature and consideration of specific dose. However, this ideal approach is problematic due to the following reasons: firstly, in most cases the complete dose details are not provided (e.g. electrode size, waveform details); secondly, investigators often adjusted (tweaked) dose on a case-by-case basis, thus resulting in hundreds of potential categories.
Though decisions by regulatory agencies, notably the Food and Drug Administration which regulates the marketing of medical devices in the US, plays heavily in the commercial viability of various tES technologies we note that: (1) FDA approval is a negotiation around a specific product and settings initiated between the company and the FDA; (2) A majority of devices and waveforms have simply not been reviewed by the FDA, thereby a lack of FDA approval is not a verdict on safety or efficacy; (3) FDA approval for an indication and inclusion criterion is by definition limited to this “label”; (4) FDA “cleared” devices have ‘grand-fathered’ regulatory clearance with no verdict on efficacy or safety and moreover devices with a “510k” FDA clearance based on these devices often provide varied doses. In Europe, a CE-mark can typically be obtained by compliance with electrical and mechanical production standards with no further endorsement of safety or efficacy in medical use. Much of the historical development of tES predates any regulatory environment and a majority of ongoing research is not designed to satisfy the FDA. The regulatory status of tES remains in infancy.
Ultimately, this review should serve as a road map for further investigation of classical techniques and appreciation of the origin of recent techniques. Even experienced researchers may remain unclear about basic features in classical literature, for instance, did Electrosleep or CES use DC? At the same time, the broad view taken in this review should be a useful introduction to new investigators and clinicians. More generally, we are interested in the narrative of tES development with respect to current tES clinical studies. Research into tES mechanisms in clinical outcomes has been active for over a century. Some specific dose approaches (with indications) generated increased interest only later to be largely abandoned – the context for such waxing and waning of enthusiasm for specific historical approaches may be relevant for current clinical efforts. Similarly, the history of tES development reflects parallel developments in pharmacology including narcotics – which again may provide perspective on current clinical trials (Brunoni et al., 2012).
Our intention is that this historical dose analysis of tES, with requisite clarification and definition of dose terminology, will provide context on current approaches and facilitate rational investigation and adoption. To this end, we conclude this review with a proposal on five recent clinical and technical developments that may be leveraged toward broader adoption of tES in medicine namely: (1) Recognition of limitations in drugs that tES may overcome; (2) Advancement in the understanding of mechanisms and biomarkers of responsiveness; (3) Integration with cognitive and physical therapy supporting “functional targeting”; (4) Advanced montages supported by computational models; (5) General advancements in electronics and communication integrated into tES.
Unless otherwise specified we follow the following naming convention: Direct Current (DC) is used here to refer to uninterrupted unidirectional current flow. Either monophasic (unidirectional ramp up and down) or biphasic (bidirectional) pulses are referred to as pulsed current (PC). Alternating Current (AC) is used here to refer to sinusoidal waveforms.
2. Historical development
Our historical review initiates circa 1900 – though evidently the history of electrical stimulation is longer, several “streams” of tES we identified initiate around this period, (Robinovitch, 1914), including Electrosleep (ES) and Electroanesthesia (EA). These two approaches may in fact have been developed together with distinct terminology referring to the intended indication for use. Some of the earliest (if incomplete) documentation of tES devices also dates from this period, which in turn allows us to better specify dose. Notably, in 1914, Louise Robinovitch summarizes:
“Nothing but storage batteries of large capacity, 100 to 200 amperes, should be used. The current used for the patient and the motor should come from two separate sources. The current to be used for the patient is connected with the inlet binding posts of a graphite rheostat. A wire potentiometer should not be used because it brings troublesome inductance into the circuit…The current is interrupted on the negative pole; this pole is connected as follows: a wire connects the outlet binding post of the potentiometer (negative pole) with the wheel interrupter, a milliampere meter, a switch, and finally a resistance box (Wheatstone bridge or any graded resistance). The other binding post of the potentiometer (positive) is connected directly with the bridge. The resistance put into the circuit is from 300 to 500 ohms. …The circuit is then closed by means of the switch. A voltmeter is connected in shunt; all the other instruments are in series. The wheel interrupter is put in such a position as to allow the passage of the direct current without interrupting it (the wheel is not revolving). The voltmeter indicates, say, 40 volts; the milliamperemeter indicates, say, 20 milliamperes. Now the wheel interrupter is made to revolve by means of the motor, say, 1,500 to 2,000 times per minute. Whatever the amperage is while the wheel is interrupting the direct current, it is the aim to regulate the period of the passage of the current so as to have it pass only 1/10 of the time, 9/10 being lost. This is accomplished by changing the position of the adjustable contact lever in relation to the fixed lever on the wheel, while the latter is revolving. Keep on adjusting the movable lever until the milliampere meter that registered 20 milliamperes when the wheel was stationary now registers only 2 milliamperes while it is revolving. The period of the passage of the current is now 1/10…Now reduce the current to zero with the potentiometer, break the circuit by opening the switch, take out the bridge and substitute for it an animal or a patient. The wheel is made so that it can interrupt the current from 6,000 to 12,000 times per minute, according to the speed of its rotation”
From this description we may surmise this particular device was voltage controlled (~40 V) but that current was calibrated (e.g. 20 mA) under a temporary resistive load, with monophasic stimulation at >100 Hz with 10% duty cycle (1 ms pulse width).
2.1. Developments from Electrosleep to Cranial Electrotherapy Stimulation
Electrosleep (ES), in short, is the name for tPCS methods by which the brain was stimulated in order to induce a sleep-like state in the subject. The first studies on Electrosleep initiated in 1902 (Robinovitch, 1914; Gilula and Kirsch, 2005), however, the first clinical report of Electrosleep was published 12 years later by Robinovitch (Robinovitch, 1914; Brown, 1975). Apparently, much of the research regarding Electrosleep was conducted in Russia up until 1953, when clinical use of Electrosleep began in Europe (Smith, 2006). New approaches were developed mostly in Europe, such as changing electrode position from covering the eyes to locations around the eyes, presumably to “… reduce optic nerve irritation” (Brown, 1975; Obrosow, 1959). Electrosleep dose waveform was typically pulsed at 30–100 Hz, but at least one (unsuccessful) case of use of DC current was documented (Brown, 1975). After 1963, an increased use of Electrosleep in the United States was noted. Three years later, the first symposium on Electrosleep and Electroanesthesia was held in Graz, Austria (Knutson, 1967; Smith, 2006). At this symposium it was reasoned that Electrosleep does not actually induce sleep, rather it is an indirect side effect of the relaxing effects of stimulation. Therefore, the name of Electrosleep was changed to Cranial Electrostimulation Therapy (CET) (Knutson, 1967). This was the first of several changes of the name of Electrosleep over the next few decades, often with notable changes in dose. Some devices that were used during this time were: Jungbluth CET-1, Tritronics 100, Somatron 500, Lafayette 72000, Lafayette 72200, General Medical Industry 1-1007-1, Vreeland Oscillator, and the Leduc Stimulator (Brown, 1975; Perera, 2013; Fig. 2). There was also a recent study done in 2009 where a device called the Pulsatilla 1000 was utilized to treat pain in patients (Gabis et al., 2009).
Fig. 2.
The images shown in the figure above are devices that have been used since 1900 for the purpose of transcranial stimulation. Each row represents a different period of stimulation in regards to terminology. For the lack of a device image, a schematic of the Vreeland Oscillator was used due to the fact that it is a pure AC stimulator that was used in early ES/EA applications. All images have been approved for use by the companies or persons who hold the copyright to the images.
In 1969, Transcerebral Electrotherapy (TCET) was proposed as another alternative name, which was adopted by some authors (Brown, 1975). In 1977, Electrosleep and its derivatives went under review by the FDA and in 1978 were classified as a Class III device for the treatment of Anxiety, Insomnia, and Depression (FDA Executive Summary, 2012) under a nominally temporary “grandfather” clause. However, such devices were re-named as Cranial Electrotherapy Stimulation (CES) (Kirsch, 2010). The FDA status of CES remains debated and not fully addressed to the present day (FDA Executive Summary, 2012).
In 1972 a new method and device of Electrosleep called NeuroElectric Therapy (NET) (Patterson, 1976, 1979) was developed in England. Devices for NET include the Neurotone 804 and 901 (Fig. 2) however, they are not related to the Neurotone 101. Though NET preceded many modern CES devices (see below) it may have influenced the doses they used decades later. Another notable device, produced after the name change to CET, was the Neurotone 101, which was based on a Russian ES device brought to the United States. Although the Neurotone 101 is no longer in production, it was the first device to be approved by the FDA as a CES device (Kirsch, 2010) and all subsequent CES devices, such as the Alphastim, the Oasis Pro and the Fisher-Wallace Stimulator (Fig. 2), approved by the FDA were through a 510k process claiming equivalency, either direct or descendent, to the Neurotone 101. This ‘administrative’ equivalency is not reflected in identical dose of current CES devices, which in fact are often marketed to provide a novel (“exclusive”) dose. Given the variation of CES device outputs, we note these devices are not equivalent in regards to dose, and that safety/efficacy evidence from one device cannot support performance of other CES devices or, for that matter, a consensus on performance by a regulatory agency such as the FDA.
Modern Cranial Electrotherapy Stimulation (CES) is thus a historical descendant of Electrosleep even though dose and indications have continuously evolved. The FDA sanctioned CES in 1978 through a “grandfather clause” and since then has not advanced a regulatory consensus, even as more variants were developed.
2.2. Developments from Electroanesthesia to Limoge current and other related methods
Electroanesthesia (EA), in short, was intended to induce anesthesia in the subject using high frequency stimulation so that chemicals did not have to be used pre-surgery. Electroanesthesia studies started in 1903 but were first known as Electronarcosis (EN) (Robinovitch, 1914; Brown, 1975; Limoge et al., 1999). Russian scientists used the term “Electroanesthsia” to describe local anesthesia while “Electronarcosis” described general anesthesia (Brown, 1975). However, Electroanesthesia stopped being referred to as local, applied to the periphery, and began to be known as general anesthesia, now applied to the brain. Therefore, in this review, EA will refer to general anesthesia. One of the earliest published claims of success in regards to EA during surgery was made in 1914 by Leduc (Brown, 1975; Smith, 1971).
Louise Robinovitch, a scientist that worked with Leduc, summarizes: “In 1890 d’Arsonval found that high frequency currents above 3500 and not over 10,000 periods per second caused a certain degree of anesthesia. In 1892, or earlier, Hutchinson found that induction currents, frequently interrupted with the ribbon vibrator that he had invented, caused anesthesia. In 1901 Mile Pompilian produced anesthesia in frogs by subjecting them to induction currents frequently interrupted by means of a revolving wheel with 12 insulated segments designed by herself. In 1902 Leduc and Rouxeau experimented with direct currents interrupted by means of a revolving wheel designed by them” (Robinovitch, 1914).
Louise Robinovitch also worked with a stimulator that produced alternating currents called the Vreeland Oscillator (Robinovitch, 1914). The Vreeland Oscillator worked using a tube filled partly by mercury with three anodes, two resistors, two choke coils, two inductive coils and a variable condenser (Aardal, 1920). This system, when started by one of the anodes, would cause two arcs to appear and for the currents to be generated, the magnetic fields from the inductive coils would cause the arcs to oscillate causing one arc to become bigger than the other in an alternating fashion. The frequency of the device could be calibrated using a vibrating tuning fork (Aardal, 1920).
Safety and tolerability concerns, and the development of early chemical anesthetics, may have contributed to quelling interest in EA. In the 1940s research on EA focused on chemical primers being used in conjunction with EA (Brown, 1975; Knutson et al., 1956). Soon after, research appeared to largely halt again presumably due to severe side effects. For example, severe side effects such as cardiac arrest, respiratory arrest and apoplexy were observed (Knutson et al., 1956; Smith et al., 1967). A third wave of research in EA initiated after a study was published in 1960, proposing a new EA approach to reduce side effects: “… a combination of pulsed and direct currents…the very slow increase of current levels…and…the use of a generator that minimized changes in electrode impedance resulting from polarization” (Brown, 1975; Smith et al., 1966).
Research into EA dosage continued and the term Transcutaneous Cranial Electrical Stimulation (TCES) was adopted around 1960–1963, with the intended use to “potentiate some drug effects, especially opiates and neuroleptics, during anesthetic clinical procedures … [with the goal of] drastic reduction in pharmacologic anesthetic agent and reducing post-operative complications” (Limoge et al., 1999). Even though the term TCES was not adopted until the early 1960s, similar protocols were used as early as 1902 by Leduc (Limoge et al., 1999). In 1951, Denier proposed that high frequency trains of 90 kHz could be used to avoid muscular contraction (Limoge et al., 1999). Three years later, Knutson (1954) claimed that alternating currents at 700 Hz should be applied, but this was abandoned in 1958 due to cardiovascular complications (Limoge et al., 1999). In 1957, investigators in the Soviet Union attempted to add a DC component to Leduc’s currents but, as claimed by an American scientist Robert Smith, it resulted in a collection of undesirable side effects (Smith et al., 1966). In 1963, Aimé Limoge modified the TCES dose and called it Limoge Current (Limoge et al., 1999). In 1964, a study claimed pulsating currents are more effective than direct currents for the induction of EA (Brown, 1975). Another study suggested that the use of pure DC for EA required high intensity of approximately 40 mA (Brown, 1975).
In 1965, Interferential Stimulation (IS) was proposed by Russian scientists (Brown, 1975). Interferential Stimulation consisted of having two pairs of electrodes energized with sine waves of slightly shifted frequencies. The intention of this approach was that through pulsation the higher frequencies would create a lower frequency where the two frequencies intersect. This was clinically desired as low frequencies were presumed more efficacious in inducing EA whereas higher frequencies were more desirable for tolerability (i.e. reduced skin pain, sensation, etc.) (Brown, 1975; Smith, 1971). We note, however, that under the assumption that the time-constant in neuronal membranes effectively filters out all high frequency signals (>100 Hz; Bikson et al., 2004) then regardless of how they are combined and modulated, these signals would be neurophysiologically inactive (even though power is modulation). Aspects of combining low and high frequencies, perhaps analogous to Interferential Stimulation but with a single electrode, are suggested by manufacturers as relevant for some later CES technologies.
In the development of EA fading has two different meanings: decrease in anesthetic state (Smith et al., 1968) or increase in tolerability. In the first case, fading indicated a decrease in the subjects’ anesthetic state while the dosage was kept steady (Smith et al., 1968). Maintenance of anesthetic state was accomplished by either reduction of frequency or increase of current (Smith et al., 1968) consistent with modern neurophysiological analysis indicated increasing intensity or decreasing frequency increases polarization (Bikson et al., 2004). Fading, more recently, has been used to increase tolerability by incremental increase to the maximum dosage under the premise that sensation at the skin adapts to current flow. Indeed, fading is a common method used in many contemporary tES approaches such as tDCS. TCES has been studied to reduce post-operative analgesic requirements (Nekhendzy et al., 2010), as are other contemporary tES approaches (Borckardt et al., 2011).
While modern tES is concerned with the treatment of a broad range of pain disorders (Zaghi et al., 2011; Brunoni et al., 2012, 2013) and have been tested on acute (experimental) pain (Mylius et al., 2012; Borckardt et al., 2012) and post-operative (Borckardt et al., 2013), the clinical goal of modern tES is typically chronic pain relief and thus effects that, if less dramatic, outlast stimulation. Historical EA/TCES used current intensities that were typically well above those used in contemporary tES. None-the-less, the reported profound effects EA/TCES and approaches to enhance tolerability may be of relevance.
2.3. DC stimulation
Direct current stimulation has been used intermittently as a component in both ES and EA. In 1957, a DC bias was added to ES, which is traditionally applied using only AC or PC. The advent of TCES, around 1960–1963, in the third resurgence of EA research, also incorporated a DC bias. In 1969, pure direct current stimulation was investigated for inducing anesthesia (Brown, 1975). However, it was not until 1964 that preliminary studies heralding modern transcranial Direct Current Stimulation (tDCS) were published (Redfearn et al., 1964).
In 1964, Redfearn and Lippold investigated polarizing current for treatment of neuropsychiatric diseases (Redfearn et al., 1964), their use of prolonged (minutes) stimulation was motivated by animal studies showing that prolonged direct current stimulation could produce lasting changes in excitability (Bindman et al., 1964). While further open pilot studies and clinical observations suggested efficacy (Baker, 1970; Nias and Shapiro, 1974; Ramsay and Schlagenhauf, 1966), a following negative controlled trial (Arfai et al., 1970) seems to have halted investigation (at least as published in western journals) for several decades.
The neurophysiological basis of neuromodulation using short duration tDCS was investigated by Priori and colleagues in 1998 (Priori et al., 1998). Shortly after, Nitsche and Paulus established that prolonged (minutes) tDCS could produce lasting and polarity specific changes in cortical excitability (Nitsche and Paulus, 2000) followed by pilot clinical studies (Bolognini et al., 2009) for indications spanning depression (Datta et al., 2008), pain (Datta et al., 2008), epilepsy (Datta et al., 2008), and a broad range of neuropsychiatric disorders (Datta et al., 2008). TDCS is further explored for rehabilitation including after stroke (Datta et al., 2008). Moreover, due to the perceived safety of tDCS it was initially validated for neurophysiological changes in healthy subjects and continues to be investigated in healthy individuals for changes in behavior and cognitive performance (Datta et al., 2008).
In 2007, High-Definition transcranial Direct Current Stimulation (HD-tDCS) was proposed as a focalized form of tDCS (Datta et al., 2008, 2009). HD-tDCS electrodes were designed for increased charge-passage capacity through smaller a contact area (Minhas et al., 2010), arranged in arrays that can be optimized per indication (Dmochowski et al., 2011). HD-tDCS montages tested include the 4 × 1 configuration (Edwards et al., 2013) as well as and individually optimized arrays (Dmochowski et al., 2013). The focalization of current with HD-tDCS is an improvement upon tDCS where previously a broad area would be stimulated and now specific targets can be stimulated. Some of the devices that have been used are the Schneider (tDCS), Soterix Medical 1 × 1 (tDCS) and the Soterix Medical 4 × 1 (HD-tDCS) (Fig. 2).
Transcranial Micropolarization is a technique investigated in Russia which is a modified version of tDCS using small electrodes instead of pads as well as currents up to 1 mA that are claimed to be “weak” (Shelyakin and Preobrazhenskaya, 2009). Galvanic Vestibular Stimulation (GVS) is being investigated for effects on ocular and postural movement (Watson and Colebatch, 1997). Alongside GVS, Caloric Vestibular Stimulation (CVS) is under investigation due to similar areas being targeted by stimulation. However, CVS does not utilize electricity, rather irrigation of the ear canal using cold or warm water (Miller and Ngo, 2007).
2.4. ECT
Initially developed circa1933, Electroconvulsive Therapy (ECT) (Gilula and Kirsch, 2005; Abrams, 2002), used repetitive high-intensity pulses to trigger seizures. A common moniker used for ECT is Electroshock Therapy (EST). ECT was cleared by the FDA for Depression in 1976 as a “pre-amendment device” (“grandfathered” similar to the process CES). In 2011 the FDA summarized: “The ECT procedure was first conducted in 1938 (Rudorfer et al., 1997). Two Italian physicians, Ugo Cerletti and Lucio Bini, guided by a theory holding an antagonistic relationship between seizures and psychosis, became the first to use electricity to induce a therapeutic seizure in humans (Faedda et al., 2010). They reported on the first treatment of a patient using this method in 1939 (Bini, 1995). Joining a number of other somatic-based therapies of the era (prior to the advent of modern pharmacotherapy), ECT became a popular intervention for psychiatric conditions. Since that time, the use of ECT has [fluctuated]. In the 1950s and 60s, with the development of drug therapies for psychiatric conditions, and due to concern for serious device related adverse events, the use of ECT in the U.S. declined (Lisanby, 2007). However, in recent years, interest in and use of, ECT has experienced a resurgence; ECT use in the U.S. has been estimated at 100,000 individuals receiving this treatment annually (Hermann et al., 1995). Reflecting the greater proportion of women who suffer from major depression, two-thirds of patients who receive ECT are women (Olfson et al., 1998). In clinical practice, ECT is generally considered after failure of one or more antidepressant medication trials, or when there is need for a rapid and definitive response. ECT has been used to treat a variety of psychiatric disorders. These disorders include: Depression (unipolar and bipolar), Schizophrenia, Bipolar manic (and mixed) states, Catatonia, [and] Schizoaffective disorder. The evidence supporting the effectiveness of ECT for each of these indications is variable.” A controversial topic in ECT research is if clinical changes require the induction of a seizure, or if seizures are not always mechanistically pivotal such that the generation of high-intensity electrical fields can lead directly to changes (e.g. raising the possibility take the “convulsive” out of ECT).
Whereas the development of drug therapies may have arrested the development of other tES treatments, it is notable that even “after the advent of antidepressant medications and other pharmacologic treatments, only electroconvulsive therapy (ECT) remained as a nonsurgical and non-pharmacological tool originating in those early years still in routine use over seven decades later” (Rosa and Lisanby, 2012), though the authors continue, “Today we are experiencing a re-emergence of non-pharmacological somatic treatments, possibly because of limitations of medications for a significant percentage of patients…and because engineering advances have enabled previously unprecedented tools for non-invasive neuromodulation” (see Section 4).
2.5. Contemporary approaches
Two contemporary forms of tES are transcranial Alternating Current Stimulation (tACS) and transcranial Random Noise Stimulation (tRNS) (Paulus, 2011). Both tACS and tRNS use relativity low-intensity current and are being investigated for therapeutic effects (Paulus, 2011). A modified protocol for tACS is transcranial Sinusoidal Direct Current Stimulation (tSDCS) (Antal et al., 2008) where the stimulation is monophasic due to a DC bias added to the sinusoid.
Another form of tES that was used by Marshall et al. (2006) consisted of monophasic trapezoidal pulses with a DC bias, frequency of 0.75 Hz. The pulses used by Lisa Marshall and colleagues were investigated for their effects on learning. The subject would learn the task before sleeping, and be tested on the task the next morning. The stimulation would occur 4 min after stage 2 sleep occurred for the first time, without reversion to stage 1, and stimulation continued at 5 min intervals with a 1 min break throughout the night (Marshall et al., 2006).
2.6. TES
The first mention of “TES” was 1980 in a study by Morton and Merton (Merton and Morton, 1980). “TES” uses single (isolated) high-intensity pulses to typically activate motor cortex and stimulate motor response. This early use of “TES” resulted in many contemporary investigators associating “TES” with only supra-threshold, short-duration pulses delivered randomly, with low frequency. In this review, we use tES in the broader sense and “TES” (quotes and capitals) to specify the use of supra-threshold low frequency pulses. “TES” technique can be painful and was not investigated for therapeutic applications, but remains used for diagnostic purposes under anesthesia (Zentner et al., 1989; Macdonald, 2002; Kalkman et al., 1992). For the purposes of experimental stimulation in awake subjects, with low-frequency supra-threshold intensity, contemporary investigators often use Transcranial Magnetic Stimulation (TMS) instead, as it is more tolerated for these purposes. “TES” continues to be used for intra-operative evaluation in anesthetized subjects and “TES” was first “cleared” by the FDA in 2002 for this purpose. “TES” thus stands apart from other tES approaches in that it is intended use it for neurophysiological evaluation rather than treatment, and as such there is little debate over its efficacy which is manifest in triggering evoked muscle responses.
2.7. Non-cranial therapies
Non-cranial electrical therapies are mentioned here only in context of historical relevance to cranial therapies. Given the similarities in waveform and timing, the advent of Limoge Currents apparently informed the development of Transcutaneous Electrical Nerve Stimulation (TENS) in 1974, which was however applied to the periphery for varied indications including pain, neuromuscular, and orthopedic. The relevance of the vast literature on TENS (including healing of damaged tissues) back to the central nervous system remains unaddressed and compelling. Microcurrent Electrical Therapy (MET) was developed approximately in 1984 and was incorporated into CES devices such as the Alpha-stim 100 (Limoge et al., 1999; Kirsch, 2010). Joseph Ventura commented on hurdles to broad and consistent technology acceptance that is prescient for current concerns with cranial therapies (also see size rules below): “At the same time this was happening, the market was flooded with inexpensive imitation devices from overseas. These devices did not use the Wing parameters [the same dose as original devices] and did not work like the clinical units. But because they were labeled as Microcurrent, customers thought they were using the real thing but didn’t get real results. Microcurrent began its long, slow descent into irrelevancy.”
Another non-cranial therapy, ElectroAcupuncture, is indicated for local anesthesia in combination with anesthetic primers and combines EA (in this case local EA) and acupuncture (Christensen et al., 1993). Iontophoresis is an FDA approved technology using sustained direct current to facilitate transdermal transport of ions or drugs, typically limited to the periphery.
3. Dosage
This section aims to further clarify the stimulation dose associated with select approaches. It is noteworthy that even in early transcranial electrical stimulation development it was recognized that: (1) Stimulation waveform along with electrode positions (stimulation dose, Peterchev et al., 2012) can be varied to change efficacy and safety; (2) the value of current controlled stimulation in contrast to voltage controlled stimulation; and (3) that electrode design including the use of a fluid/gel (electrolyte) buffer between the metal electrode and skin increases skin tolerability (Merrill et al., 2005). None-the-less, ad hoc and often poorly documented variations in dose are coming in the literature, a matter that remains of concern to this date (Peterchev et al., 2012). Unless otherwise stated, we presume that stimulation was current controlled.
Though we divide dose by category below, certain over-arching developments can be noted for both electrode design and waveform. “Active” and “return” terminology for electrodes reflect only the brain target of interest with “active” located nearer the target; evidently both electrodes will affect brain function and indeed the position of the return determines “active” current flow (Bikson et al., 2010). The early approach to tES involved two “active” electrodes placed directly over the eyes with two “return” electrodes, presumably to facilitate active current delivery through the optic foramina. Active electrode positions around the eye (e.g. supraorbital) were explored, as well as reducing the number of active electrodes (e.g. single electrode on the forehead) or using just one return electrode. After 1970, approaches using electrodes on or around the ears were explored (though much earlier examples of ear electrodes are noted), with presumed current flow to deeper brain structures (Datta et al., 2013). In the 1980s, approaches using tES showed that current could be delivered focally using small closely spaced electrodes on the scalp (for example as indicated by motor responses). After 2000, contemporary approaches (e.g. tDCS, tACS) used reduced currents and large-sponge electrodes (Nitsche and Paulus, 2000) with an “active” electrode placed over the nominal target. Though the use of larger electrodes and distant electrodes precludes focal stimulation (Datta et al., 2009) of cortex or avoidance of deep brain structures (DaSilva et al., 2012), functional effects of tDCS may be shaped (Nitsche et al., 2007). Recent generations of technologies using arrays of small High-Definition (HD) electrodes are intended to allow focal brain stimulation and more precise control of and how deeper structures are targeted (Datta et al., 2008, 2009; Dmochowski et al., 2011, 2013; Edwards et al., 2013; Minhas et al., 2010). Though explored initially for delivering DC waveforms (HD-tDCS; Borckardt et al., 2012; Caparelli-Dagquer et al., 2012; Kuo et al., 2013; Minhas et al., 2010), stimulation with HD-tES arrays may be leveraged to focalize delivery of any waveform (e.g. HD-tACS, HD-tRNS, HD-tPCS, etc.).
In the context of waveform, a notable overarching progression was: (1) from basic waveforms (often limited to existing stimulation hardware), to increasingly complex and customized waveforms motivated by the perception that increased efficacy, safety, or tolerability was needed; (2) increasing complexity and (proprietary) uniqueness especially developed in commercial devices (e.g. CES); (3) leading to a reversion to the most basic waveform after 2000, associated with a resurgence of clinical interest using standardized and defined approaches. Early intended uses focused on short-term effects motivated investigators to explore increased intensities (e.g. sleep, anesthesia), while interest in chronic diseases (e.g. depression) is consistent with efforts using reduced (well tolerated) current intensities and increasingly prolonged (repeated session) use.
3.1. Electrosleep and derivative techniques
The dosage for Electrosleep has evolved since it first was investigated in 1902 (Gilula and Kirsch, 2005). Dosage used for Electrosleep consisted of electrode placement over each eye and a return electrode over the mastoid, with a waveform consisting of 100 Hz monophasic (Robinovitch, 1914) pulses between 2 and 25 mA (Robinovitch, 1914; Knutson, 1967). The pulse width was between 0.3 and 0.6 ms and stimulation duration lasted from 20 to 60 min (Robinovitch, 1914; Knutson, 1967). There is at least one case in which Electrosleep was induced where one electrode was placed on the forehead and the other on the subjects’ right palm (Robinovitch, 1914). In 1966, the name changed to CET and shortly afterward a new dosage was developed. Due to subject discomfort and the changing perception that penetration of current in to the brain (including deep brain structures) did not require placement of electrodes directly on top of the eyes (Brown, 1975; von Richthofen and Mellor, 1979). Under this CET electrode montage, the stimulation waveform was pulsed at 30–100 Hz, pulse width of 1–2 ms, at 0.1–0.5 mA (von Richthofen and Mellor, 1979). TCET was proposed as a new name for ES/CET but under this new nomenclature the dose for TCET was unchanged in regards to electrode placement or waveform (Brown, 1975).
A notable change in dosage occurred with the advent of NET and CES after 1970. In NET and CES, the number of electrodes was reduced from 3 to 2 (Net Device Corp.; Kirsch, 2010; Liss Body Stimulator). The electrode placement for NET was in the subjects’ ears (NET Device Corp. Information) – an approach later adopted by some CES devices with electrodes clipped onto the ears (Kirsch, 2010). The waveform used in NET, and also in some later CES devices, was 0.5–100 Hz stimulation at up to 600 μA over a period of 20 min (Kirsch, 2010; NET Device Corp. Information). According to the manufacturer of the NET device model 804, cartridges (shown in Fig. 2) were used to deliver specific dosages for different drug addictions. The other variant for CES devices uses 2 electrodes placed on top of the forehead. The waveform for this variant of CES uses 15, 500 or 15,000 Hz at 4 V with 50 ms pulses and ‘off’ periods of 16.7 ms (Datta et al., 2013; Liss Body Stimulator Manual(M); Liss Body Stimulator Manual(B)).
3.2. Electroanesthesia and derivative techniques
The dose for Electroanesthesia evolved since the early 1900s. An early electrode placement for EA/EN consists of 4 electrodes with either 2 electrodes applied to each temple or to the bilateral frontal and occipital areas (Brown, 1975) however, there were other electrode placement styles such as having the return electrodes placed on the subjects hand (Robinovitch, 1914). There are a wide range of frequencies and current intensities that were evaluated. As noted, EA has been tested with pure DC requiring current approximately 40 mA to induce EA (Brown, 1975; Robinovitch, 1914; Sances et al., 1969). Under AC-only conditions, the frequency ranged from 10 to 20 kHz with intensities approximately 10 mA (Geddes et al., 1964); higher current intensities were claimed to be needed with higher frequencies and currents of 500 mA and frequencies around 200 kHz have been used. When biased by DC, AC frequencies typically remained in the same range with the AC component ranging from 2.5 to 5 mA with the DC component also ranging from 2.5 to 5 mA (Brown, 1975). In some instances waveforms with a high frequency of ‘ON’ periods were incorporated into TCES. TCES uses three electrodes rather than the four in EA; the electrodes are positioned with a single electrode between the eyebrows and two return electrodes on the retro-mastoid region (Brown, 1975; Limoge et al., 1999). TCES waveform consists of frequency trains. The high frequency portion of the train is ‘ON’ for 3–4 ms at 130–167 kHz and ‘OFF’ for 8 ms periods. The low frequency portion (‘ON’/’OFF’) was ~77–100 Hz and the overall waveform uses 200–350 mA with 30–35 V (Limoge et al., 1999).
3.3. Transcranial Direct Current Stimulation/transcranial Random Noise Stimulation/transcranial Alternating Current Stimulation
Developed over the last decade, transcranial Direct Current Stimulation (tDCS), transcranial Random Noise Stimulation (tDCS), and transcranial Alternating Current Stimulation (tACS) are three different distinct forms of “contemporary” tES as far as waveform, but all typically share the same approach in regards to electrode montage (number and shape). In addition, though each applies a distinct waveform, in all cases the duration of stimulation is typically 10–20 min with a peak current of a 1–2 mA (Paulus, 2011). Conventionally, two electrodes are used with one positioned “over” the target region and the other elsewhere on the scalp (often the contra-lateral supraorbital region) or elsewhere on the body in at an extra-cephalic location (Paulus, 2011; Antal et al., 2008; Zaehle et al., 2010; DaSilva et al., 2011). Electrodes are typically saline soaked sponge material wrapped around a conductive rubber electrode, though gel may also be used. In tDCS the (positive) anode and (negative) cathode are distinguished for their actions on cortical excitability, and 1–2 mA is typically applied over 5–20 min (Paulus, 2011). For tACS, a single sinusoid at 10–40 Hz with a peak intensity of 0.4–1 mA has been tested (Antal et al., 2008; Paulus, 2011; Zaehle et al., 2010). The waveform parameter for tRNS includes: “a frequency spectrum between 0.1 and 640 Hz… [and]…a normally distributed random level of current generated for every sample at a sampling rate of 1280 samples per second with no overall DC offset.” (Paulus, 2011; Chaieb et al., 2009). One can speculate if the increasingly complex waveforms used in CES approach noise-like action on the brain.
3.4. Transcranial Electrical Stimulation
“TES” uses high-intensity pulses (150–1840 V, presumed to be voltage controlled) lasting between 13 and 48 μs at an intermittent frequency of 1–3 s or much lower when used for monitoring purposes (Rossini et al., 1985; Zentner et al., 1989; Rothwell et al., 1994; Kalkman et al., 1992). Typically stimulation is applied using a bifocal (and bipolar) montage, but a “unifocal” montage has also been explored with an active electrode over the target and a “ring” of return electrodes, either as a single band or 12 separate electrodes, around the width of the scalp (Rossini et al., 1985; Rothwell et al., 1994; Kalkman et al., 1992). These techniques are typically not with therapeutic intent, but rather to examine excitability for monitoring or experimental purposes – though we are not aware of studies excluding any lasting effects.
3.5. Electroconvulsive therapy
The waveforms for ECT are high-intensity, ~800 mA, with trains (AC or pulsed bursts) lasting 1–6 s per cycle. The electrodes are placed either unilaterally or bilaterally on the cranium and current intensity is typically increased by varying the number of pulses per train, pulse duration, or intensity until a seizure is triggered (Gilula and Kirsch, 2005; Sackeim et al., 2000). Modern efforts to refine dose has focused on minimized memory loss for example through focused stimulation, reviewed elsewhere (Spellman et al., 2009; Datta et al., 2008; Rosa and Lisanby, 2012).
3.6. High-Definition transcranial Direct Current Stimulation
HD-tDCS shares the same waveform with tDCS, 1–2 mA for 5–20 min, however the large sponge electrodes used for tDCS (as for tACS/tRNS) are replaced with an array of smaller electrodes. The electrode montage is then optimized for brain targeting for example the 4 × 1-Ring montage uses a center electrode which determines the polarity of stimulation (anode or cathode) and four return electrodes at ~4–7 cm radius. More broadly, High-Definition transcranial Electrical Stimulation (HD-tES) spans all efforts to focalize prior diffuse tES protocols by using arrays of HD electrodes to rationally guide current flow (Dmochowski et al., 2011).
4. Present outlook and summary – six rules for success of tES technology
The present review, the first to our knowledge to attempt to comprehensively characterize tES technology across a century, presents a historical narrative with two overarching features: (A) techniques incrementally evolved through five “streams” (Fig. 1), and (B) individual approaches fluctuate in popularity over time. Against this historical narrative of tES is the broader backdrop of medical research that, despite false starts and setbacks, did establish a myriad of drug and surgery based approaches as standards of neuropsychiatric care. The central question thus appears: why given a century of research in tES does it remain marginalized compared to other approaches to treat neuropsychiatric diseases and neurological disorders (Edelmuth et al., 2010)? More specifically, why did tES approaches continuously evolve during this period without any given technique gaining traction and stability? Though historically pointed, this question seems key in informing ongoing tES approaches and the ever-expanding array of techniques and indications.
Historical tES publications, like all medical research, include an inherent positive bias, and provide minimal clues as why a technique with presumably promising efficacy would be abandoned (even by its existing users). But careful reading, including of conference notes, indicates that concerns about side effects and complications seem to have motivated changes in early tES approaches which generally employed relatively high currents (>10 mA). Parallel pharmaceutical developments (e.g. antidepressants; Rosa and Lisanby, 2012) may have further arrested tES development in regard to specific indications. But, these apparent setbacks in tES are qualified by: (1) increasing appreciation of the side-effects and limitations of drugs (Rush et al., 2006); (2) recognition that therapeutic effects may require prolonged (repetitive) treatment (early approach may have anticipated immediate effects), correct dosage, as well as proper inclusion criteria (e.g. disease etiology); (3) recognition that tES may be especially suited for integration with cognitive or physical therapy for “functional targeting” (Cano et al., 2013) whereas much historical tES was applied in isolation (Rosa and Lisanby, 2012); (4) enhancement in anatomical targeting through computational model driven electrode montages including High-Definition approaches where much historical tES used montages producing diffuse current flow, (5) lack of standardization (qualification, documentation, rational regulation) of tES approaches (Edelmuth et al., 2010) since the precise dose of tES determines outcome; (6) understanding the success of any medical treatment is guided by not simply medical factors but also dissemination and cost (and even societal stigma).
In the following six areas, ongoing (if early) successes with modern tES are emerging:
Cost/benefit: Modern tES treatment is researched as a “non-drug” alternative, in cases with evaluation of efficacy against drugs (Brunoni et al., 2013). As with many investigational treatments, clinical trial inclusion in tES trials of individuals typically enroll patients who either did not respond to drugs or could not tolerate the side effects – but the marketing of tES as a non-drug alternative specifically points to emerging concerns about drug medication (Jensen et al., 2013; Rosa and Lisanby, 2012). tES leaves no apparent “electrical residue” such that any behavioral changes must result from lasting (therapeutic) changes in brain function, as opposed to an apparent prolonged chemical presence with some drugs. In many indications the flat-line in the percent of drug refractory patients (despite ever increasing number of marketed drugs), the reliance on off-label drug prescriptions (where regulatory standards are ambiguous), increased prescription drug abuse, and the need to curtail costs, all indirectly encourage new directions in treatment. Thus patients, clinicians, and payers are anxious for new approaches, and few medical techniques have favorable factors and momentum of tES.
Mechanism and biomarkers: Increased sophistication in (pre-clinical) biomarkers of response (DosSantos et al., 2012; Marshall et al., 2011; Antal and Paulus, 2012; Zaehle et al., 2010; Schutter and Hortensius, 2011) with an associated emphasis on mechanism (Nitsche and Paulus, 2011; Rahman et al., 2013; Bikson et al., 2004; Radman et al., 2009; Spezia Adachi et al., 2012; Laste et al., 2012; Marquez-Ruis et al., 2012), recognition of potential to titrate individual dose (Datta et al., 2012), and increased duration of treatments-contemporary approaches use more tolerated (<2 mA) approaches often applied over extended period of time (e.g. weeks or months). Moreover, effects of tES were historically clinical, which have less sensitivity than current measures such as transcranial magnetic stimulation (Cortes et al., 2012) – better biomarkers allow for direction of more subtle (pre-clinical) changes that can then inform stimulation patterns/dose.
-
Integration with other therapies: Especially with contemporary tES approaches that are well tolerated (including tDCS, tACS, and tRNS) it is convenient to combine stimulation with various forms of directed physical and/or cognitive training. This form of training ranges from motor physical therapy (including using robots; Edwards et al., 2009; Nair et al., 2011), speech therapy (Torres et al., 2013; Baker et al., 2010), to training on higher cognitive functions (Turkeltaub et al., 2012). Thus in cases where physical or cognitive training has (moderate but established) therapeutic benefit, tES is applied as an adjunct to “boost” the effects of therapy (Martin et al., 2013). In some cases the integration of low-intensity tES with training seems obvious, as in conjunction with post-stroke rehabilitation, and in other cases creative, such as pairing with visual illusions in neuropathic pain (Kumru et al., 2013; D’Urso et al., 2013) or with behavioral therapy to target cognitive impairments associated with depression. Generally, it remains to be investigated if tES is best applied before (e.g. priming), during, and/or after (e.g. consolidation) other therapies. The scientific rational includes reinforcement of electrical stimulation effects with tES (Fritsch et al., 2010; Bikson et al., 2004). In addition, combining other targeted therapies with tES allows for “functional targeting” where those processes (e.g. synapses, networks) activated during tES are thus specifically modulated.
Indeed, the effects of tES can be profoundly altered by brain state. The most obvious example of this is if the subject observes, imagines, or performs voluntary movement, where the evoked muscle response to brain stimulation (“TES” or TMS) is immediately stronger. However, sustained tES with neuromodulatory intent has historically been delivered with the subject at rest. Having the brain functionally engaged during or around the time of neuromodulatory tES application has only recently been explored. The theoretical rationale for combining techniques is unclear and may have started with tDCS and motor learning (Antal et al., 2004). Since both motor practice and tDCS could separately enhance voluntary performance (tDCS transiently) it may have seemed logical that the combination could lead to a summed effect or enhancement. Alternately, tDCS may have been an appealing method to augment the physiological effects of voluntary practice and learning, such as placing synapses in a more permissive state for LTP/LTD induction, or facilitating consolidation that occurs in the post-practice period. Indeed physiologic studies from around a decade ago, using non-invasive electric (tDCS) as well as magnetic stimulation (TMS) show that there is a strong physiologic interaction of the static field combined with TMS-synaptic activity (Lang et al., 2004). The characteristics of the stimulation (such as timing and dose) can change the observed excitability modulation – augment, reduce, or even reverse the effect. Combining the NIBS with behavior should systematically test parameters of stimulation and behavior, such that the combination is physiologically complementary (Edwards et al., 2009), and can lead to a desired functional outcome (Giacobbe et al., 2013). The relationship to brain excitability (MEP) and activity levels (EEG, fMRI, cMRI) have been previously described, but remain incompletely understood, particularly in relation to function and voluntary behavior changes. Furthermore, change in behavior may not always be as expected with tES neuromodulation when combined with behavourial/functional brain activity, and in some cases may lead to a transformation of the effect rather than simply a scaling (Giacobbe et al., 2013). The observed effects may critically depend on the time of assessment in relation to the intervention (e.g. immediately post, next day, etc.).
Finally, the combined interventions in patients are likely to be influenced by disease-specific pathophysiology, concurrent pharmacotherapy, altered biological response ceiling – that can predispose for better or worse susceptibility of response, as well as the stimulation waveform and montage. By testing the various combinations incrementally, it is likely that defined interventions begin to show robust results across individuals and move therapies towards becoming optimal.
Targeting of brain regions: The relationship between tES dose (e.g. electrode montage and waveform controlled by the operator) and underlying brain current flow is often not trivial and computational “forward” models of tES are designed precisely to make this connection – to predict which brain regions are stimulated for any given montage and so allow rational selection of a preferred task-specific montage. Early models were generated analytically (Rush and Driscoll, 1968) but advancement in numerical approaches (Wagner et al., 2007), leading to the introduction by our group of MRI-derived individual models with gyri-specific resolution (Datta et al., 2009) heralded a renaissance in the use and precision of these techniques (Datta et al., 2013; Bai et al., 2013; Bikson et al., 2012) and indeed the generation of new montages such as the 4 × 1 High-Definition montage (Edwards et al., 2013; Kuo et al., 2013) and automatic targeting (Dmochowski et al., 2011). As noted above, the ability to titrate dose to individuals (e.g. presence of stroke or TBI; Datta et al., 2010, 2011) or populations (e.g. obese or children; Truong et al., 2012) is supported by these same models. Combined with increased sophistication in “functional targeting”, better understanding of anatomical targeting may fundamentally enhance tES efficacy and safety.
-
Qualification and Regulation: Changing tES dose is analogous to changing the chemical composition of a drug (Peterchev et al., 2012), such that lack of control and reproducibility will impair the constructive development of tES (Edelmuth et al., 2010). With the development of fully electronic controlled waveforms and current controlled stimulation, inherent variability in device function was removed but, especially in the CES stream of development, with time there was an increased variety in devices and device settings. The turn of the millennium marked a reversion to simplified and better-documented tES waveforms (e.g. tDCS, tACS) and devices providing standardized waveforms (rather than a proprietary and secretive blend), which provided a substrate for rational reproduction and testing by multiple clinical teams. We argue that the resurgence of research in precisely these contemporary “simplified” approaches is directly related to the establishment and control of dose. Only by reproducing tES dose can mechanistic and clinical trials build incrementally and increase rigor. Yet, in spite of this noted progress with contemporary approaches (or maybe because of the resulting excitement/press), qualification and regulation remains a fundamental deficiency for tES advancement in general.
There is wide dissatisfaction with the (lack) of regulation in modern tES. Even for those who oppose any government restrictions, the lack of precise device qualifications (e.g. what is tDCS) generates confusion and risk. FDA Quality Systems production standards and CE mark provide one bar for rigor in hardware design but involve little medical science. The US FDA regulation of tES is driven by commercial entities seeking marketing approval (i.e. only company applications are considered) and so seems unlikely to provide general guidance on none-proprietary techniques like tDCS. The FDA further appears reluctant to either formally approve or ban devices “cleared” (grand-fathered) over 30 years ago without compete medical review. While at the same time the FDA clears more “me-too” (510k) products with a wide range of dose parameters. General electrical safety standards from agencies such as IEC and UL are of limited use as they provide limited and incomplete scientific/medical justification.
We propose the first and non-controversial hurdle is qualification, which requires precise classification of tES by dose as well as inclusion criterion. This in turn allows correct comparison across tES efforts and rational progress. The alternative is an undocumented drift in tES approaches and confusion about efficacy and safety. Indeed, this review is an attempt to provide clarity on historical and contemporary methods, though we stop short of suggesting specific testing methods for qualification. The anecdotal experience with microcurrent therapy (discussed above) suggests lack of qualification, leading to confusion in clinic and market place, can destroy and otherwise promising approach. We reiterate a basic principle often ignored: devices producing a different, in any aspect, tES dose (waveform, electrode, duration, etc.) are distinct, just like drugs with different chemical composition are not the same.
Dose compliance monitoring: Leveraging rapid advances in electronics and communication allowing development of devices that are simple to use, and provide more consistent stimulation (including though targeting software; Dmochowski et al., 2011) including control of compliance by limiting and recording stimulation application timing and dose. Historically, poor control and/or documentation of tES dose may have hampered identification of real effects. Interestingly, despite available technological complexity (e.g. microcontroller driven devices) the notable transition circa 2000 from increasingly complicated tES waveforms (e.g. proprietary commercial “blends”) to simpler waveforms (e.g. DC, one frequency sinusoidal AC) may enhance reproducibility and rational progress.
With increasing medical and technological sophistication, and with recognition of these six rules, we anticipate a much delayed “coming-of-age” for tES, leading to broad medical adoption and acceptance over the next decade.
Highlights.
Cranial Electrotherapy Stimulation is a descendant of Electrosleep.
Transcutaneous Cranial Electrical Stimulation is a descendant of Electroanesthesia.
There is a need for better reporting of dosages and devices used.
There is a shift to basic dosages in contemporary approaches.
References
- Aardal AA. A study of the Vreeland Oscillator. University of Wisconsin; 1920. [Google Scholar]
- Abrams R. Electroconvulsive therapy. USA: Oxford University Press; 2002. [Google Scholar]
- Antal A, Nitsche MA, Kincses TZ, Kruse W, Hoffmann K, Paulus W. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. European Journal of Neuroscience. 2004;19:2888–92. doi: 10.1111/j.1460-9568.2004.03367.x. [DOI] [PubMed] [Google Scholar]
- Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimulation. 2008;1:97–105. doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Antal A, Paulus W. Clinical EEG and Neuroscience. 2012. Investigating neurplastic changes in the human brain induced by transcranial direct (tDCS) and alternating current (tACS) stimulation methods; p. 43. [DOI] [PubMed] [Google Scholar]
- Arfai E, Theano G, Montagu JD, Robin AA. A controlled study of polarization in depression. British Journal of Psychiatry. 1970;116:433–4. doi: 10.1192/bjp.116.533.433. [DOI] [PubMed] [Google Scholar]
- Auri-stim Medical and Manual. Auri-stim Medical Manual NET-1000 technical specifications. 2013 http://www.net1device.com/specs.htm.
- Bai S, Loo C, Dokos S. A review of computational models of transcranial electrical stimulation. Critical Reviews in Biomedical Engineering. 2013;41:21–35. doi: 10.1615/critrevbiomedeng.2013007163. [DOI] [PubMed] [Google Scholar]
- Baker AP. Brain stem polarization in the treatment of depression. South African Medical Journal. 1970;44:473–5. [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229–36. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. Journal of Physiology. 2004;558:175–90. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Datta A, Rahman A, Scaturro J. Electrode Montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode’s position and size. Clinical Neurophysiology. 2010;121:1976–8. doi: 10.1016/j.clinph.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Rahman A, Datta A. Computational models of transcranial direct current sitmulation. Clinical EEG and Neuroscience. 2012;43:179–83. doi: 10.1177/1550059412445138. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. Journal of Physiology. 1964;172:369–82. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini L. Professor Bini’s notes on the first electro-shock experiment. Convulsive Therapy. 1995;11:260–1. [PubMed] [Google Scholar]
- Brown CC. Electroanesthesia and electrosleep. The American Psychologist. 1975;30:402–10. doi: 10.1037//0003-066x.30.3.402. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. Journal of NeuroEngineering and Rehabilitation. 2009;6:1–13. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borckardt JJ, Romagnuolo J, Reeves ST, Madan A, Frohman H, Beam W, et al. Feasibility, safety, and effectiveness of transcranial direct current stimulation for decreasing post-ERCP pain: a randomized, sham-controlled, pilot study. Gastrointestinal Endoscopy. 2011;73:1158–64. doi: 10.1016/j.gie.2011.01.050. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, et al. A pilot study for the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. Journal of Pain. 2012;13:112–20. doi: 10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Reeves ST, Robinson SM, May JT, Epperson TI, Gunselman RJ, et al. Transcranial Direct Current Stimulation (tDCS) reduces postsurgical opioid consumption in Total Knee Arthroplasty (TKA) Clinical Journal of Pain. 2013 doi: 10.1097/AJP.0b013e31827e32be. [in press] [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Ferruci R, Bortolomasi M, Scelzo E, Boggio PS, Fregni F, et al. Interactions between transcranial direct current stimulation (tDCS) and pharmacological interventions in the Major Depressive Episode: findings from a naturalistic study. European Psychiatry. 2012;S0924–9338:114–9. doi: 10.1016/j.eurpsy.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–91. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Kerwin L, Feusner J. A pilot study of cranial electrotherapy stimulation for generalized anxiety disorder. Journal of Clinical Psychiatry. 2008;69:412–7. doi: 10.4088/jcp.v69n0311. [DOI] [PubMed] [Google Scholar]
- Cano T, Morales-Quezada JL, Bikson M, Fregni F. Methods to focalize noninvasive electrical brain stimulation: principles and future clinical development for the treatment of pain. Expert Review of Neurotherapeutics. 2013;13:465–7. doi: 10.1586/ern.13.41. [DOI] [PubMed] [Google Scholar]
- Caparelli-Dagquer EM, Zimmermann TJ, Mooshagian E, Parra LC, Rice JK, Datta A, et al. A pilot study on effects of 4 × 1 High-Definition tDCS on motor cortex excitability. Conf proc IEEE eng med biol soc. 2012:735–8. doi: 10.1109/EMBC.2012.6346036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaieb L, Kovacs G, Cziraki C, Greenlee M, Paulus W, Antal A. Short-duration trans-cranial random noise stimulation induces blood oxygenation level dependent response attenuation in the human motor cortex. Experimental Brain Research. 2009;198:439–44. doi: 10.1007/s00221-009-1938-7. [DOI] [PubMed] [Google Scholar]
- Christensen PA, Rotne M, Vedelsdal R, Jensen R, Jacobsen K, Husted C. Electroacupuncture in anesthesia for hysterectomy. British Journal of Anesthesia. 1993;71:835–8. doi: 10.1093/bja/71.6.835. [DOI] [PubMed] [Google Scholar]
- Cortes M, Black-Schaffer RM, Edwards DJ. Transcranial magnetic stimulation as an investigative tool for motor dysfunction and recovery in stroke: an overview for neurorehabilitation clinicians. Neuromodulation. 2012;15:316–25. doi: 10.1111/j.1525-1403.2012.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in trans-cranial direct current stimulation. Journal of Visualized Experiments. 2011:51. doi: 10.3791/2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012;52:1283–95. doi: 10.1111/j.1526-4610.2012.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Elwassif M, Battaglia F, Bikson M. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. Journal of Neural Engineering. 2008;5:163–74. doi: 10.1088/1741-2560/5/2/007. [DOI] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation. 2009;2:201–7. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bikson M, Fregni F. Transcranial direct current stimulation in patients with skull defects and skull plates: high-resolution computational FEM study of factors altering cortical current flow. Neuroimage. 2010;52:1268–78. doi: 10.1016/j.neuroimage.2010.04.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimulation. 2011;4:169–74. doi: 10.1016/j.brs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-Individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Frontiers in Psychiatry. 2012;3:1–8. doi: 10.3389/fpsyt.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Dmochowski JP, Guleyupoglu B, Bikson M, Fregni F. Cranial electrotherapy stimulation and transcranial pulsed current stimulation: a computer based high-resolution modeling study. Neuroimage. 2013;65:280–7. doi: 10.1016/j.neuroimage.2012.09.062. [DOI] [PubMed] [Google Scholar]
- Dimitrov DTz, Ralev ND. Signals and systems for electrosleep. Electronics and Electrical Engineering. 2009;5:95–8. [Google Scholar]
- Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. Journal of Neural Engineering. 2011;8:1–16. doi: 10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- Dmochowski JP, Datta A, Huang Y, Richardson JD, Bikson M, Fridriksson J, et al. Targeted transcranial direct current stimulation for rehabilitation after stroke. Neuroimage. 2013;75:12–9. doi: 10.1016/j.neuroimage.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DosSantos MF, Love TM, Martikainen IK, Nascimento TD, Fregni F, Cummiford C, et al. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Frontiers in Psychiatry. 2012;3:1–6. doi: 10.3389/fpsyt.2012.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Urso G, Mantovani A, Micillo M, Priori A, Muscettola G. Transcranial direct current stimulation and cognitive-behavioral therapy: evidence of a synergistic effect in treatment-resistant depression. Brain Stimulation. 2013;6:465–7. doi: 10.1016/j.brs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Edelmuth RC, Nitsche MA, Battistella L, Fregni F. Why do some promising brain-stimulation devices fail the next steps of clinical development? Expert Review of Medical Devices. 2010;7:67–97. doi: 10.1586/erd.09.64. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restorative Neurology and Neuroscience. 2009;27:199–207. doi: 10.3233/RNN-2009-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–75. doi: 10.1016/j.neuroimage.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faedda GL, Becker I, Baroni A, Tondo L, Aspland E, Koukopoulos A. The origins of electroconvulsive therapy: Prof. Bini’s first report on ECT. Journal of Affective Disorders. 2010;120:12–5. doi: 10.1016/j.jad.2009.01.023. [DOI] [PubMed] [Google Scholar]
- FDA Executive Summary. Prepared for the January 27–28, 2011 meeting of the neurological panel meeting to discuss the classification of electroconvulsive therapy devices; 2012. [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabis L, Shklar B, Baruch YK, Raz R, Gabis E, Geva D. Pain reduction using transcranial electrostimulation: a double-blind “active placebo” controlled trial. Journal of Rehabilitation Medicine. 2009;41:256–61. doi: 10.2340/16501977-0315. [DOI] [PubMed] [Google Scholar]
- Geddes LA, Hoft HE, Voss C. Cardiovascular respiratory studies during electronarcosis in the dog. Cardiovascular Research Center Bulletin. 1964;3:38–47. [PubMed] [Google Scholar]
- Giacobbe V, Krebs HI, Volpe BT, Pascual-Leone A, Rykman A, Zeirati G, et al. Transcranial direct current stimulation (tDCS) and robotic practice in chronic stroke: the dimension of timing. Neurorehabilitation. 2013 doi: 10.3233/NRE-130927. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula MF, Kirsch DL. Cranial electrotherapy stimulation review: a safer alternative to psychopharmaceuticals in the treatment of Depression. Journal of Neurotherapy. 2005;9:7–26. [Google Scholar]
- Hermann RC, Dorwart RA, Hoover CW, Brody J. Variation in ECT use in the United States. American Journal of Psychiatry. 1995;152:869–75. doi: 10.1176/ajp.152.6.869. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Sherlin LH, Askew RL, Fregni F, Witkop G, Gianas A, et al. Effects of non-pharmacological pain treatments on brain states. Clinical Neurophysiology. 2013 doi: 10.1016/j.clinph.2013.04.009. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman CJ, Drummond JC, Kennelly NA, Patel PM, Partridge BL. Intraoperative monitoring of tibialis anterior muscle motor evoked responses to transcranial electrical stimulation during partial neuromuscular blockade. Anesthesia and Analgesia. 1992;75:584–9. doi: 10.1213/00000539-199210000-00021. [DOI] [PubMed] [Google Scholar]
- Kirsch DL. Low level brain stimulation for anxiety: a review of 50 years of research and supporting data. 2010. [Google Scholar]
- Knutson RC, Tichy FY, Reitman JR. Use of electrical current as an anesthetic agent. Anesthesiology. 1956;17:815. doi: 10.1097/00000542-195611000-00013. [DOI] [PubMed] [Google Scholar]
- Knutson RC. Experiments in electronarcosis: a preliminary study. Anesthesiology. 1954;17:815–25. doi: 10.1097/00000542-195409000-00014. [DOI] [PubMed] [Google Scholar]
- Knutson RC. First international symposium on electrosleep therapy and electroanesthesia. Anesthesia and Analgesia. 1967;46:333–9. [Google Scholar]
- Kumru H, Soler D, Vidal J, Navarro X, Tormos JM, Pascual-Leone A, et al. The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: an evoked potentials and quantitative thermal testing study. European Journal of Pain. 2013;17:55–66. doi: 10.1002/j.1532-2149.2012.00167.x. [DOI] [PubMed] [Google Scholar]
- Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimulation. 2013;6:644–8. doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning with transcranial Direct Current Stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after effects. Biological Psychiatry. 2004;56:634–9. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Laste G, Caumo W, Adachi LN, Rozisky JR, de Macedo IC, Filho PR, et al. After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Experimental Brain Research. 2012;221:75–83. doi: 10.1007/s00221-012-3149-x. [DOI] [PubMed] [Google Scholar]
- Li F, Tsien JZ. Memory and the NMDA receptors. New England Journal of Medicine. 2009;361:302–3. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoge A, Robert C, Stanley TH. Transcutaneous cranial electrical stimulation (TCES): a review 1998. Neuroscience and Biobehavioral Reviews. 1999;23:529–38. doi: 10.1016/s0149-7634(98)00048-7. [DOI] [PubMed] [Google Scholar]
- Lisanby SH. Electroconvulsive therapy for depression. New England Journal of Medicine. 2007;357:1939–45. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- Liss Body Stimulator Manual(B), Bipolar Model No. SBL-502-B Manual 480.
- Liss Body Stimulator Manual(M), Monopolar Model No. SBL-501-M Manual.
- Liss Stimulators Application Notes – Monopolar/Bipolar; 1991. p. 1–3.
- Macdonald DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. Journal of Clinical Neurophysiology. 2002;19:416–29. doi: 10.1097/00004691-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Martin DM, Liu R, Alonzo A, Green M, Player MJ, Sachdev P, et al. Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. International Journal of Neuropsychopharmacology. 2013:1–10. doi: 10.1017/S1461145713000539. [in press] [DOI] [PubMed] [Google Scholar]
- Marquez-Ruis J, Leal-Campanario R, Sanchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6710–5. doi: 10.1073/pnas.1121147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Marshall L, Kirov R, Brade J, Molle M, Born J. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS ONE. 2011;6:1–10. doi: 10.1371/journal.pone.0016905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill D, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. Journal of Neuroscience Methods. 2005;141:171–98. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Miller SM, Ngo TT. Studies of Caloric Vestibular Stimulation: implications for the cognitive neurosciences, the clinical neurosciences and neurophysiology. Acta Neuropsychiatrica. 2007;19:183–203. doi: 10.1111/j.1601-5215.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, et al. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. Journal of Neuroscience Methods. 2010;190(2):188–97. doi: 10.1016/j.jneumeth.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain. 2012;153:1350–63. doi: 10.1016/j.pain.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Nair DG, Renga B, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restorative Neurology and Neuroscience. 2011;29:411–20. doi: 10.3233/RNN-2011-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekhendzy V, Lemmens HJ, Tingle M, Nekhendzy M, Angst MS. The analgesic and antihyperalgesic effects of transcranial electrostimulation with combined direct and alternating current in healthy volunteers. Anesthesia and Analgesia. 2010;111:1301–7. doi: 10.1213/ANE.0b013e3181e3697e. [DOI] [PubMed] [Google Scholar]
- NET Device Corp Information. AboutNET. 2013 http://www.netdevice.net/aboutnet.php.
- Nias DK, Shapiro MB. The effects of small electrical currents upon depressive symptoms. British Journal of Psychiatry. 1974;125:414–5. doi: 10.1192/bjp.125.4.414. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortexes by weak transcranial direct current stimulation. Journal of Physiology. 2000;527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. Journal of Neurophysiology. 2007;97:3109–17. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Transcranial direct current stimulation—update 2011. Restorative Neurology and Neuroscience. 2011;29:463–92. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- Obrosow AN. Electro-sleep therapy. In: Licht E, editor. Therapeutic electricity and ultraviolet radiation. New Haven, CT: Editor; 1959. [Google Scholar]
- Olfson M, Marcus S, Sackeim HA, Thompson J, Pincus HA. Use of ECT for the inpatient treatment of recurrent major depression. American Journal of Psychiatry. 1998;155:22–9. doi: 10.1176/ajp.155.1.22. [DOI] [PubMed] [Google Scholar]
- Patterson MA. Effects of Neuro-Electric Therapy (N.E.T) in drug addiction: interim report. Bulletin on Narcotics. 1976;28(4):55–62. [PubMed] [Google Scholar]
- Patterson MA. Electrotherapy: addictions and neuroelectric therapy. Nursing Times. 1979;75(48):2080–3. [PubMed] [Google Scholar]
- Paulus W. Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychological Rehabilitation. 2011;21:602–17. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- Perera T. Telegraph and scientific instrument museum. 2013 http://www.w1tp.com.
- Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection and reporting practices. Brain Stimulation. 2012;5:435–53. doi: 10.1016/j.brs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuro Report. 1998;9:2257–60. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimulation. 2009;2:215–28. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. Journal of Physiology. 2013;591:2563–78. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JC, Schlagenhauf G. Treatment of depression with low voltage direct current. Southern Medical Journal. 1966;59:932–4. doi: 10.1097/00007611-196608000-00013. [DOI] [PubMed] [Google Scholar]
- Redfearn JWT, Lippold OCJ, Costain R. Preliminary account of the clinical effects of polarizing the brain in certain psychiatric disorders. The British Journal of Psychiatry. 1964;110:773–85. doi: 10.1192/bjp.110.469.773. [DOI] [PubMed] [Google Scholar]
- von Richthofen CL, Mellor CS. Cerebral electrotherapy: methodological problems in assessing its therapeutic effectiveness. Psychological Bulletin. 1979;86:1264–71. [PubMed] [Google Scholar]
- Robinovitch LG. Electrical analgesia, sleep and resuscitation. In: Gwathmey JT, editor. Anesthesia. New York: Appleton; 1914. [Google Scholar]
- Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. Journal of Physiology. 1994;481:243–50. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37:102–16. doi: 10.1038/npp.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Marciani MG, Caramia M, Roma V, Zarola F. Nervous Propagation along ‘central’ motor pathways in intact man: characteristics of motor responses to ‘bifocal’ and ‘unifocal’ spine and scalp non-invasive stimulation. Electroencephalography and Clinical Neurophysiology. 1985;61:272–86. doi: 10.1016/0013-4694(85)91094-6. [DOI] [PubMed] [Google Scholar]
- Rudorfer MV, Henry ME, Sackeim HA. Electroconvulsive therapy. In: Tasman A, Kay J, Lieberman JA, editors. Psychiatry. Vol. 2. Philadelphia: W.B. Saunders Co; 1997. [Google Scholar]
- Rush S, Driscoll DA. Current distribution in the brain from surface electrodes. Anesthesia and Analgesia. 1968;47:717–23. [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STARD report. American Journal of Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A Prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Archives of General Psychiatry. 2000;57:425–34. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- Sances A, Larson SJ, Hause LL. A neuron model for electroanesthesia. Proceedings of the 2nd international symposium on electrosleep and electroanesthesia. 1969;2:306–14. [Google Scholar]
- Schutter DJ, Hortensius R. Brain oscillations and frequency-dependant modulation of cortical excitability. Brain Stimulation. 2011;4:97–103. doi: 10.1016/j.brs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Shelyakin A, Preobrazhenskaya I. Principles of the weak direct current therapy. 2009 https://sites.google.com/site/micropolarization/principles.
- Smith RB. Cranial electrotherapy stimulation: its first fifty years, plus three. 2006. [Google Scholar]
- Smith RH, Tatsuno J, Zouhar RL. Electroanesthesia: a review – 1966. Anesthesia and Analgesia. 1967;46:109–25. [PubMed] [Google Scholar]
- Smith RH, Andrew JH, Suzuki H, Tatsuno J. Electroanesthesia studies: a new current patter and control of fading. Anesthesia and Analgesia. 1968;47:627–32. [PubMed] [Google Scholar]
- Smith RH. Electroanesthesia (EA), 1971. Anesthesiology. 1971;34:60–72. doi: 10.1097/00000542-197101000-00018. [DOI] [PubMed] [Google Scholar]
- Spellman T, Peterchev AV, Lisanby SH. Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology. 2009;34:2002–10. doi: 10.1038/npp.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spezia Adachi LN, Canumo W, Laste G, Fernandes Medeiros L, Ripoll Rozisky J, de Souza A, et al. Reversal of chronic stress-induced pain by transcranial direct current stimulation (tDCS) in an animal model. Brain Research. 2012;1489:17–26. doi: 10.1016/j.brainres.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Torres J, Drebing D, Hamilton R. TMS and tDCS in post-stroke aphasia: integrating novel treatment approaches with mechanisms of plasticity. Restorative Neurology and Neuroscience. 2013;31:501–15. doi: 10.3233/RNN-130314. [DOI] [PubMed] [Google Scholar]
- Truong DQ, Magerowski G, Pascual-Leone A, Alonso-Alonso M, Bikson M. Finite element study of skin and fat delineation in an obese subject for transcranial Direct Current Stimulation. Conf proc IEEE eng med biol soc. 2012:6587–90. doi: 10.1109/EMBC.2012.6347504. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Benson J, Hamilton RH, Datta A, Bikson M, Coslett HB. Left lateralizing transcranial direct current stimulation improves reading efficiency. Brain Stimulation. 2012;5:201–7. doi: 10.1016/j.brs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SRD, Colebatch JG. EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroencephalography and clinical Neurophysiology. 1997;105:476–83. doi: 10.1016/s0924-980x(97)00044-1. [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007;35:1113–24. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Rach S, Herrmann CS. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS ONE. 2010;5:1–7. doi: 10.1371/journal.pone.0013766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghi S, Thiele B, Pimentel D, Pimentel T, Fregni F. Assessment and treatment of pain with non-invasive cortical stimulation. Restorative Neurology and Neuroscience. 2011;29:439–51. doi: 10.3233/RNN-2011-0615. [DOI] [PubMed] [Google Scholar]
- Zentner J, Kiss I, Ebner A. Influence of anesthetics—nitrous oxide in particular—on electromyographic response evoked by transcranial electrical stimulation of the cortex. Neurosurgery. 1989;24:253–6. doi: 10.1227/00006123-198902000-00016. [DOI] [PubMed] [Google Scholar]