Abstract
Pancreatic cancer is a leading cause of cancer-related mortality and often has a poor prognosis because of its late diagnosis, aggressive local invasion, early metastasis, and poor response to chemotherapy. The chemotherapeutic agent gemcitabine is effective for treating advanced pancreatic cancer, but its efficacy remains less than satisfactory. It is expected that further investigation of pancreatic cancer cell invasion and development of strategies to block this process should improve the disease prognosis. In this study, we tested our hypothesis that galectin-3 (gal-3), a multifunctional member of the β-galactoside-binding protein family, may regulate pancreatic cancer cell motility, and silencing of it inhibit cell motility. Previous studies demonstrated that this protein is associated with tumor cell adhesion, proliferation, differentiation, angiogenesis, apoptosis, and metastasis. Here, we used gal-3 small interfering RNA (siRNA) to silence its expression in various pancreatic cancer cell lines to determine whether gal-3 regulates cell proliferation, migration and invasion in vitro. We found that silencing gal-3 reduced cellular migration and invasion, but failed to affect proliferation. In gal-3 siRNA-transfected cells, we detected a decrease in β-catenin expression, an important signal for cancer cell invasion, which was caused by down-regulation of phosphorylated Akt and GSK-3β. We also found that matrix metalloproteinase (MMP)-2 expression was reduced by gal-3 silencing. These results indicate that gal-3-mediated invasion via MMP-2 regulated by β-catenin degradation is initiated by Akt phosphorylation in pancreatic cancer cells. Our results suggest that gal-3 can be a novel therapeutic target in pancreatic cancer.
Keywords: β-catenin, Galectin-3, Invasion, MMP-2, Pancreatic cancer, Wnt
Introduction
Pancreatic cancer is currently the fourth leading cause of cancer-related deaths in the Western world [1]; recent reports have estimated that over 36,000 patients will die from this disease in the United States by the end of 2009 [2]. This cancer has a very poor prognosis, principally because it is most commonly diagnosed during its later stages, and it is often characterized by aggressive local invasion, early metastasis and poor response to chemotherapy [3]. Gemcitabine, a nucleoside analog used for chemotherapeutic purposes, is a fairly effective drug for treating advanced and recurrent pancreatic cancer [4]. This agent has improved the survival of patients diagnosed with pancreatic cancer, but its efficiency remains limited [4, 5]. Unfortunately, no other agents have proven useful for treating advanced pancreatic cancer patients [4, 5]. Accordingly, researchers continue to investigate the development of novel treatments that can potentially improve the prognosis of pancreatic cancer. Most studies have focused on agents than can block or inhibit invasion and/or metastasis.
Galectins are a family of carbohydrate-binding proteins that bind β-galactoside moieties with both high affinity and specificity; to date, studies have identified 16 members of this family [6-9]. In particular, galectin-3 (gal-3) is one member that exhibits pleiotropic biological functions. Gal-3 is reportedly involved in several important processes, including cell–cell adhesion, cell–extracellular matrix (ECM) interactions, cell growth, differentiation, adhesion, migration, angiogenesis, malignant transformation, apoptosis, and cancer drug resistance [6–15]. Furthermore, there are several reports that have linked this protein to invasion and metastasis in several cancers. For instance, gal-3 was reported to regulate metastasis by increasing the detachment of breast and prostate cancer cells through binding to cell adhesion-related molecules and inhibiting cell–cell and cell–ECM interactions both in vitro and in vivo [16]. In addition, gal-3 overexpression has been shown to enhance cell motility and invasion of breast and lung carcinoma cells [17, 18]. One study showed that highly metastatic human breast cancer cells exhibit higher levels of gal-3 expression, which significantly increases adhesion to endothelial cells [19]. Another group revealed that gal-3 was overexpressed in malignant human gastric tissue compared with normal tissue, and suggested that increased cell motility resulted from an upregulation of fascin-1, an actin binding protein [20]. In contrast, downregulation of gal-3 decreased the motility of human colon cancer cells and human glioblastomas [21, 22]. Collectively, these results suggest that endogenous gal-3 regulates cell metastasis in a variety of cancers.
Although many studies have examined the role of gal-3 in various cancers, there are very few reports on this protein in the context of pancreatic cancer. To date, it has been demonstrated that gal-3 stimulates the infiltration of pancreatic cancer cells in vitro through interaction with pancreatic stellate cells, and that decreased gal-3 expression is associated with advanced pancreatic cancer stage, tumor de-differentiation, and metastasis based on immunohistochemical studies [23, 24]. However, many of the functions of gal-3 in pancreatic tumor progression, invasion, and metastasis remain unclear.
Recently, a number of studies have found a correlation between gal-3 and β-catenin expression in several cancers. It has been demonstrated that gal-3 binds β-catenin and regulates the Wnt/β-catenin signaling pathway in the human breast cancer cell line BT549 [25]. In addition, gal-3 mediates nuclear β-catenin accumulation and the Wnt signaling pathway by regulating glycogen synthase kinase-3β (GSK-3β) activity in human colon cancer cells [26]. Furthermore, gal-3 was reported to increase cell motility by upregulating fascin-1 expression via the Wnt signaling pathway in gastric cancer cell lines [20]. In the present study, to investigate how gal-3 regulates pancreatic cancer progression and/or metastasis, we examined various effects of transient gal-3 silencing by RNA interference on human pancreatic cancer cell lines. We found that silencing gal-3 decreased the cells’ abilities for migration and invasion, but not cell proliferation, and reduced the expression of β-catenin, phosphorylated Akt (pAkt) and GSK-3β (pGSK-3β). Furthermore, our results indicate that cell migration and invasion were reduced via decreased matrix metalloproteinase (MMP)-2 expression caused by suppression of the β-catenin/Wnt signaling pathway in pancreatic cancer cell lines.
Materials and methods
Cell culture and transfections
Human pancreatic cancer cell lines, PanC-1, AsPC-1, and BxPc-3, were purchased from American Type Culture Collection (ATCC; Manassas, VA). PanC-1 was maintained in Dulbecco’s modified Eagle’s medium (DMEM; Wako, Osaka, Japan), and AsPC-1 and BxPc-3 were maintained in RPMI-1640 (Wako) containing 1% penicillin-streptomycin antibiotics (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS). The Stealth™/siRNA duplex oligoribonucleotide for human gal-3 (gal-3-siRNA, 5′-GGGAAUGAUGUUGCCUUCCACUUUA-3′) and Stealth RNAi Negative Control Low GC Duplex were purchased from Invitrogen, and were transiently transfected in pancreatic cancer cell lines using the Lipofectamine RNAi MAX reagent (Invitrogen), following the manufacturer’s instructions. Cells were harvested after transfection for 48 h. Silencing of β-catenin was performed using SignalSilence® β-catenin-siRNA (Cell Signaling Technology, Beverly, MA).
Protein extraction and western blot analysis
All cells were harvested after indicated control- and gal-3-siRNA treatments. Lysates were prepared in buffer [20 mM Tris-HCl at pH 7.6, 1 mM ethylenediamine tetraacetic acid, 140 mM NaCl, 1% Nonidet P-40, 1% aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 1 mM sodium vanadate]. We determined the protein concentration with a BCA Protein Assay Kit (Pierce, Rockford, IL) using bovine serum albumin (BSA) as a standard. Protein (40 μg) was resolved on a 5–20% gradient ReadyGel (Bio-Rad, Tokyo, Japan) and transferred to a Hybond-enhanced nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). β-actin was used as a loading control. Bands were detected using an enhanced chemiluminescence detection system (Amersham). Band density was measured by densitometry, quantified using “gel plotting macros” with ImageJ software, version 1.62 (National Institutes of Health, Bethesda, MD), and normalized to an indicated sample in the identical membrane.
Antibodies and primers
The following antibodies were used: anti-gal-3 (TIB166; ATCC), anti-β-catenin, anti-Akt, anti-pAkt, ant i-GSK-3β, anti-pGSK-3β, MMP-2 (Cell Signaling Technology). We also used anti-β-actin antibody (Sig ma, St. Louis, MO) as a control. The following primer sets were used: gal-3, 5′-GGCCACTGATTGT GCCTTAT-3′ (forward) and 5′-TGCAACCTTGAAGTGGTCAG-3′ (reverse); interleukin (IL)-8, 5′-ATG ACTTCCAAGCTGGCCG-3′ (forward) and 5′-CTCAGCCCTCTCTTCAAAAACTT-3′ (reverse); MMP-2, 5′-TGTCCCTACCGAGTCTCTTCTC-3′ (forward) and 5′-CCCTTGTCTAGGCTCTGCTAAA-3′ (reverse); fascin-1, 5′-ACCTGTCTGCCAATCAGGAC-3′ (forward) and 5′-CCCATTCTTCTTGGAGGTC A-3′ (reverse); β-catenin, 5′-GCCGGCTATTGTAGAAGCTG-3′ (forward) and 5′-GAGTCCCAAGGAGA CCTTCC-3′ (reverse); and β-actin, 5′-CTCCTCCTGAGCGCAAGTACTC-3′ (forward) and 5′-TCCTGCT TGCTGATCCACATC-3′ (reverse).
Cell proliferation assay
Cell proliferation was measured by an MTT tetrazolium assay. Briefly, human pancreatic cancer cells (2.5 × 103 cells/well) transfected with either control- or gal-3-siRNA were cultured in 96-well microtiter plates in a total volume of 100 μl/well. After the initial cell seeding, the cells were analyzed using a Cell Counting Kit (Dojindo Laboratories, Tokyo, Japan). Briefly, 10 μl of the cell counting solution was added and incubated under a humidified 5% CO2 atmosphere at 37°C for 2 h. The formazan produced was dissolved in 1 N HCl (100 μl/well), and the absorbance of each well was determined at 450 nm using an Emax-precision microtiter plate reader (Molecular Devices, Inc., Sunnyvale, CA). All experiments were performed in triplicate.
Wound-healing assay
We examined the migration of control- and gal-3-siRNA-transfected cells using an in vitro wound-healing assay. Briefly, the transfected cell lines were each grown on 3.5-cm plates with their respective culture media. After the growing cell layers had reached confluence, we inflicted a uniform wound (i.e., in a straight line) in each plate using a pipette tip, and washed the wounded layers with PBS to remove all cell debris. The cells were cultured in 5% CO2 at 37°C, and we subsequently evaluated the closure or filling in of the wounds at 48 h using bright-field microscopy (Nikon TMS; Nikon Corp., Tokyo, Japan) at ×40 magnification. All experiments were performed in triplicate.
In vitro migration and invasion assays
Cell motility was measured using 48-well BioCoat Cell Culture Inserts (BD Biosciences Inc., Bedford, MA). Briefly, fibronectin (5 μg/ml) in serum-free medium was placed in each lower chamber, which was separated from the upper chamber by a membrane with 8-μm pores. A single-cell suspension of human pancreatic cancer cells (5 × 104) in serum-free medium was placed in each upper chamber. After incubation for 72 h at 37°C, the cells were fixed with 70% ethanol and stained with hematoxylin and eosin. We subsequently counted the cells that migrated through the pores to the lower surface of each filter under a microscope and evaluated based on the mean values from five fields of view at ×200 magnification. Invasion assays were performed with Matrigel-coated chambers from a BioCoat Matrigel Invasion Chamber Kit (BD Biosciences, Inc.) using the same method as described above for the migration assays.
RNA isolation and cDNA synthesis
Total RNA was extracted from control- and gal-3-siRNA-transfected cells using an RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The quantity of isolated RNA was measured using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Template cDNA was synthesized from 13.5 μg of total RNA with an Omniscript Reverse Transcriptase Kit (Qiagen), random primers (hexadeoxyribonucleotide mixture) (Takara, Shiga, Japan), and a ribonuclease inhibitor (Porcine liver, Takara). Total RNA was reverse-transcribed with 4 U of Omniscript reverse transcriptase in a reaction volume of 20 μl (60 min at 37°C, 5 min at 93°C, and finally on ice). The resultant cDNA samples were stored at –30°C until analysis. All experiments were performed in triplicate.
Reverse-transcriptional polymerase chain reaction (RT-PCR) analysis
One microliter of each of the cDNA samples was mixed with 19 μl of mix solution [2 μl of 10× PCR Buffer II (Applied Biosystems), 0.05 mmol MgCl2, 4 μmol dNTP, 40 pmol of the forward primer, 40 pmol of the reverse primer, and 1 U of AmpliTag Gold (Applied Biosystems)]. PCR for gal-3 was performed for 30 cycles with denaturation at 94°C for 30 sec, annealing at 58°C for 30 sec, and extension at 72°C for 30 sec. Polymerase chain reaction (PCR) for IL-8 was performed for 35 cycles with denaturation at 94°C for 60 s, annealing at 65°C for 45 s, and extension at 72°C for 60 s. PCR for MMP-2 was performed for 35 cycles with denaturation at 94°C for 30 s, annealing at 30°C for 30 s, and extension at 72°C for 30 s. PCR for β-catenin was performed for 35 cycles with denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. PCR for fascin-1 was performed for 35 cycles with denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. PCR for β-actin was performed for 28 cycles with denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. The resultant amplified DNA fragments were resolved by electrophoresis on 2% agarose gels containing ethidium bromide. After equalizing β-actin of each sample by diluting the cDNAs with distilled water, we compared the expression of mRNA levels of the samples. All experiments were performed in triplicate.
Immunofluorescence staining and confocal laser microscopy
Cells were seeded onto four-chamber slides, fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X for 20 min, and blocked with 3% BSA for 30 min. Cells were subsequently incubated with primary antibody for 1 h at 37°C. Furthermore, Alexa Fluor®-conjugated secondary antibodies (Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 594 rabbit anti-rat; Invitrogen) were added at 1:50 dilutions, and the cells were incubated for 40 min at 37°C. Nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI; Invitrogen) for 5 min at room temperature. Actin filaments were stained with Alexa Fluor-conjugated phalloidin (Invitrogen) for 1 h at room temperature.
Statistical analysis
Data were analyzed and expressed as mean ± standard deviation (SD) for continuous variables. The significant differences of values between control- and siRNA transfected cells were determined using Student’s t tests and Mann-Whitney’s U test. Data for gal-3, pAkt, pGSK-3beta and beta-catenin expression levels in three groups were analyzed with ANOVA (Figure 3). When the results of the ANOVA were significant, Dunnett’s tests were used to assess the differences in these expression levels among each group. All differences were statistically significant at the level of p < 0.05. Statistical analyses were performed using the JMP 5.01 for Windows software package (SAS Institute, Cary, NC).
Fig. 3.
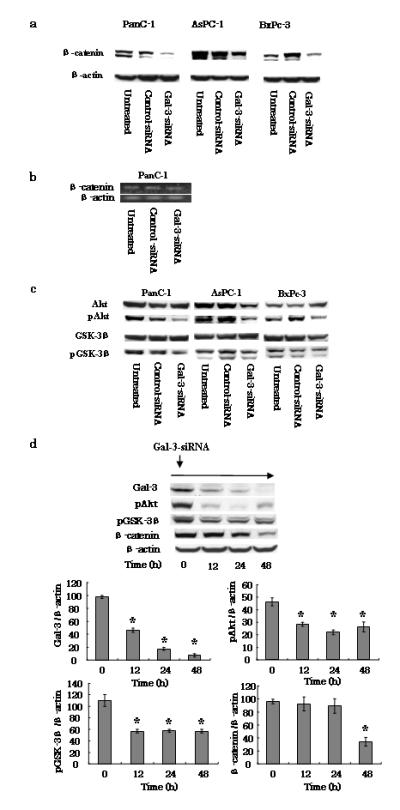
Effects of gal-3 silencing on the β-catenin/Wnt pathway. a Expression of β-catenin in gal-3-silenced cells. After transfection of cells with either control- or gal-3-siRNA for 48 h (or cells were left untreated), the cells were harvested and maintained for 72 h with DMEM containing 10% FBS. Total cell lysates were isolated and β-catenin expression levels were detected by western blotting using an anti-human β-catenin antibody. β-actin was used as a loading control. b Representative RT-PCR amplification of β-catenin. PanC-1 cells transfected with either control- or gal-3-siRNA using the same conditions as described above (a) were harvested, total RNA was extracted, and the β-catenin and β-actin (control) mRNA levels were detected by RT-PCR. c Effect of gal-3 silencing on expression of Akt and GSK-3β. Total and phosphorylated (p) Akt and GSK-3β expression levels in control- or gal-3-siRNA-transfected cells were detected by western blotting. d PanC-1 cells transfected with gal-3-siRNA and harvested at 0, 12, 24, and 48 h. Western blotting revealed time-dependent changes in the phosphorylation of both Akt and GSK-3β, and in β-catenin, following gal-3 silencing. Densitometry (mean ± SD) revealed significant decreases in the levels of these proteins in gal-3-siRNA-transfected cells (n = 3). Data of densitometry were analyzed by using ANOVA. Significant differences were detected in gal-3, pAkt, pGSK-3β and β-catenin expression levels. Dunnett’s tests were used to assess the differences in these expression levels compared with 0 hour. *P < 0.05 versus 0 h.
Results
Gal-3 silencing reduces migration and invasion of human pancreatic cancer cells
A number of previous studies have indicated that gal-3 is associated with proliferation, migration, and invasion in several cancers [6–15]. To elucidate the role of gal-3 in these processes of pancreatic cancer cells, we inhibited gal-3 expression in three pancreatic cancer cell lines by RNA interference and subsequently examined their behaviors. We used PanC-1, AsPC-1, and BxPC-3 cell lines in this study because we determined by RT-PCR (data not shown) and western blotting (Fig. 1a) that these cell lines expressed high levels of gal-3. To determine the temporal pattern of gal-3 gene silencing, we transfected each cell line with either gal-3- or control-siRNA for comparison. Nearly complete silencing was observed in PanC-1 and BxPc-3 cells by 48 h after transfection, which was maintained for up to 7 days thereafter (Fig. 1a and data not shown). As shown in this figure, gal-3 silencing achieved over 80% knockdown at 48 h after transfection in all three cell lines.
Fig. 1.
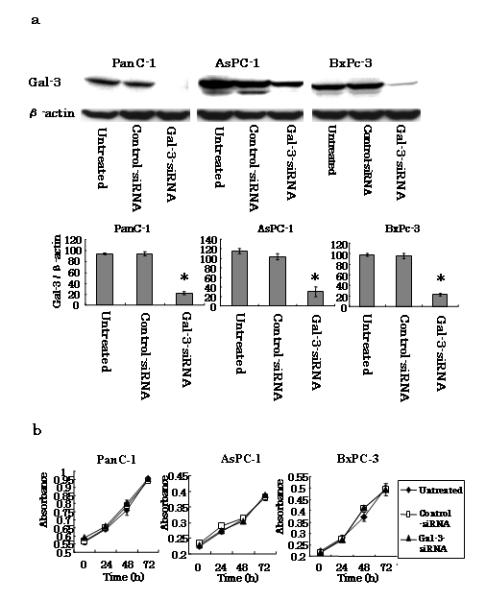
Effects of gal-3 silencing on PanC-1, AsPC-1 and BxPc-3 cell proliferation. a Gal-3 expression in human pancreatic cancer cell lines. Human pancreatic cancer cells were transfected with either gal-3 siRNA or control-siRNA, or were left untreated. Total cell lysates were isolated after 48 h of transfection and gal-3 expression levels were detected by western blotting using an anti-human gal-3 antibody. β-actin was also used as a loading control. Densitometry (mean ± SD) revealed over 80% knockdown of gal-3 in gal-3-siRNA-transfected cells (n = 3). *P < 0.05 versus control-siRNA. b Proliferation assays. The mean ± SD absorbance (n = 3) levels of the pancreatic cancer cells are shown. PanC-1, AsPC-1, and BxPc-3 cells were separately cultured in 96-well microplates, the indicated reagent (untreated, control-siRNA, or Gal-3-siRNA) was injected onto cells after 0, 24, 48, or 72 h of culture, and the plates were incubated for an additional 2 h. The absorbance levels were detected using a microplate reader.
We observed cell proliferation of the cell lines for 72 h, and detected no differences between control-and gal-3-siRNA-transfected cells for any of the cell lines (Fig. 1b). In contrast and as shown in Fig. 2a, gal-3 silencing induced an approximate 2-fold decrease in the migration of each of the three cell lines according to chamber-based assays (P < 0.05). Moreover, wound-healing assays confirmed the inhibitory effect of gal-3 silencing on cell migration; we found that the time required for wound closure of gal-3-silenced PanC-1 cells was significantly longer than that required for the corresponding control cells (Fig. 2b). We also examined the difference between the morphologies of the control- and gal-3-siRNA-transfected PanC-1 cells by immunofluorescence staining and confocal microscopy. After 48 h of transfection, cells were harvested, seeded for 6 h, and stained with phalloidin to label their actin filaments. Confocal microscopy revealed that control-siRNA transfected cells were more protruding than cells transfected with gal-3-siRNA (Fig. 2c).
Fig. 2.
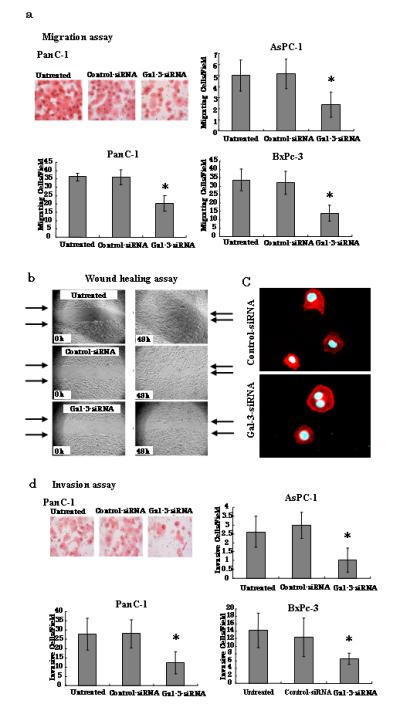
Effects of gal-3 silencing on PanC-1, AsPC-1 and BxPc-3 cell motility. a Migration assays. Cells that migrated through the pores to the lower surface of each chamber are shown. After transfection with either control- or gal-3-siRNA for 48 h (or cells were left untreated), the PanC-1, AsPC-1, and BxPc-3 cells were tested for migration using a modified Boyden chamber method, and were evaluated on the basis of the mean values from five fields of view at ×200 magnification for each treatment. Bars are represented as the mean ± SD. (n=5) *P < 0.05 versus control-siRNA. b Representative examples of wounding experiment results from PanC-1 cells. PanC-1 cells transfected with either control- or gal-3-siRNA were wounded (time 0) and maintained for 48 h in DMEM with 10% FBS. Arrows point to the edges of the wounds. Wound closure time (measured after 48 h) was slower in cells transfected with gal-3-siRNA than in those transfected with control-siRNA. c Immunofluorescence staining revealed by confocal microscopy. PanC-1 cells transfected with either control- or gal-3-siRNA were seeded for 6 h, fixed, and stained with Alexa Fluor-conjugated Phalloidin (red) to indicate actin filaments. Nuclei were stained with DAPI (blue). Cells were imaged with confocal microscopy. d Invasion assays. Cells that invaded through the pores to the lower surface of Matrigel-coated chambers are shown. Invaded cells transfected with control- or gal-3-siRNA were evaluated on the basis of the mean values from five fields of view at ×200 magnification for each treatment. Data for each treatment are represented as the mean ± SD. (n= 5) *P < 0.05 versus control-siRNA.
Furthermore, we determined that gal-3 silencing affected cell invasion similarly to migration of the pancreatic cancer lines. Specifically, we found that cell invasion was decreased by approximately 2-fold in all three gal-3-silenced pancreatic cancer cell lines, as compared with their corresponding control cells (P < 0.05), although the number of cells that invaded was somewhat less than the number that migrated in the earlier chamber-based assay (Fig. 2d). Collectively, these results indicate that silencing gal-3 reduces pancreatic cancer cell migration and invasion, but not proliferation, at least in vitro in our experimental systems. We showed summary of results from these functional assays in three cell lines in supplemental file.
Gal-3 silencing induced degradation of β-catenin and suppresses phosphorylation of both Akt and GSK-3β
It has been reported that gal-3 plays a role in Wnt signaling in breast, colon, and gastric cancer cells by interacting with β-catenin [20, 25–27]. Accordingly, we investigated the signaling pathway that was mediated by gal-3 and that was associated with cell motility. Specifically, we first confirmed whether gal-3 modulates β-catenin expression in human pancreatic cells by western blotting. Based on the expression level of each control-siRNA-transfected cell line, we determined that β-catenin expression correlated well with total gal-3 levels in all three lines (Fig. 3a). In addition, gal-3 silencing drastically reduced β-catenin expression in transfected PanC-1, AsPC-1, and BxPc-3 cells (Fig. 3a). To determine whether β-catenin is regulated at the transcriptional level, we also investigated the RNA level of β-catenin in gal-3-silenced PanC-1 cells. However, we found that while the protein level of β-catenin was decreased in gal-3-silenced cells, its RNA level did not change (Fig. 3b).
To further investigate the signaling pathway that mediated β-catenin expression, we assessed the expression of total and phosphorylated Akt and GSK-3β in the three cells lines transfected with either control-or gal-3-siRNA by western blotting. We examined these proteins in particular because previous studies have shown that downregulation of their phosphorylated forms is an important requisite for cancer cell invasion [13, 26]. We found that the expression levels of both proteins remained unchanged after silencing gal-3, whereas the phosphorylated forms of the proteins were strongly reduced by this transfection (Fig. 3c). In contrast, cells transfected with control-siRNA exhibited little or no change in pAkt or pGSK-β3. In addition, we examined the time-dependent changes (across 48 h) in the expression of these phosphorylated proteins and of β-catenin in gal-3-siRNA-transfected PanC-1 cells. Interestingly, we found that, after initiating gal-3-siRNA transfection of the cells, decreases in both pAkt and pGSK-3β occurred within 12 h and a subsequent decrease in β-catenin expression was detected at 48 h (Fig. 3d). At the indicated times, densitometric analysis of the time-lapse western blots showed significant (P < 0.05) reductions in gal-3, pAkt, pGSK-3β and β-catenin protein expression compared with the corresponding levels in untreated cells (at 0 h). These results indicate that phosphorylation of Akt and GSK-3β occurs before the downregulation of β-catenin during gal-3 silencing in pancreatic cancer cells.
We also examined the subcellular distribution of β-catenin in both control- and gal-3-siRNA-transfected PanC-1 cells by immunofluorescence staining and confocal microscopy. These experiments showed that β-catenin was distributed in the cytoplasm and nuclei of control-siRNA transfected cells, and in the cytoplasm and membranes of gal-3-siRNA-transfected cells (Fig. 4). Furthermore, the markers for gal-3 and β-catenin were colocalized in both cell types. Collectively, these results indicate that there was increased degradation of β-catenin in the cytoplasm of gal-3-silenced cells, which is consistent with results from previous reports [26, 27].
Fig. 4.
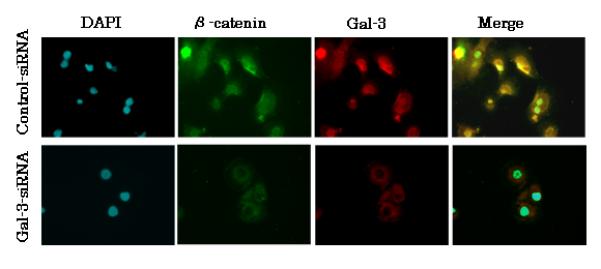
Subcellular localization of β-catenin in control-siRNA and gal-3-siRNA cells. Immunofluorescence was performed on PanC-1 cells transfected with either control- or gal-3-siRNA using anti-gal-3 (red) or anti-β-catenin (green) antibodies, followed by DAPI nuclear counter staining (blue). The merged image showing all markers is also shown (colocalization of markers is yellow).
Silencing β-catenin suppresses proliferation and invasion of PanC-1 cells
To further investigate the role of β-catenin in the aggressiveness (i.e., proliferation and invasion) of pancreatic cancer cells, we silenced β-catenin in PanC-1 cells by siRNA transfection, and determined whether β-catenin was correlated with either cell proliferation or invasion. After cells were treated with β-catenin-siRNA for 48 h, we confirmed complete knock down of β-catenin by western blotting (Fig. 5a). To determine the effect of β-catenin expression on cell proliferation, we determined the proliferation rates of both control- and β-catenin-siRNA PanC-1 cells using an MTT assay. We detected a significant decrease in the proliferation rate of cells transfected with β-catenin-siRNA compared with the rate of the control cells (P < 0.05) (Fig. 5b). Furthermore, we performed a chamber-based invasion assay on PanC-1 cells transfected with either control- or β-catenin-siRNA, and found that β-catenin silencing caused a significant decrease in cell invasion compared with the activity of the control cells (P < 0.05) (Fig. 5c). These results indicate that a decrease in β-catenin expression in pancreatic cancer cells is associated with decreases in cell proliferation and invasion.
Fig. 5.
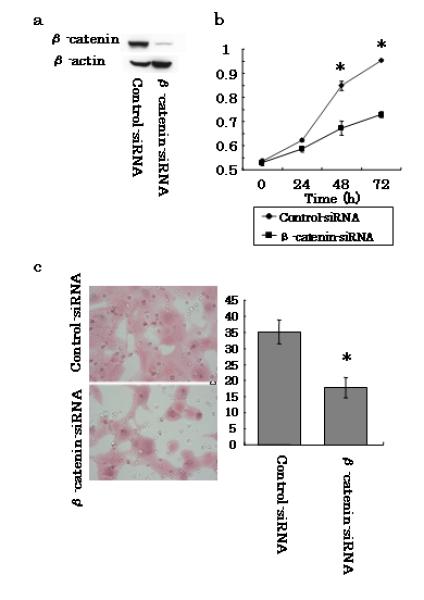
Effects of β-catenin silencing on PanC-1 cells. a Human pancreatic cancer cells were transfected with either control- or β-catenin-siRNA. Total cell lysates were isolated after transfection for 48 h and gal-3 expression levels were detected by western blotting using an anti-human β-catenin antibody. β-actin was used as a loading control. b Proliferation assays. PanC-1 cells transfected with control- or β-catenin-siRNA were cultured in 96-well microplates, and the indicated reagent was injected after 0, 24, 48, or 72 h of culture and the cells were incubated for an additional 2 h. The absorbance was detected using a microplate reader. Data represent the mean ± SD of each treatment (n = 3). c Invasion assays. Cells that invaded through the pores to the lower surface of Matrigel-coated chambers are shown. Invaded cells transfected with control- or β-catenin-siRNA were evaluated on the basis of the mean values from five fields of view at ×200 magnification for each. Data are represented as the mean±SD of each treatment (n= 3). *P < 0.05 versus the control-siRNA.
MMP-2 is mediated by gal-3 through the Wnt signaling pathway and its expression is correlated with cell invasion
Because it was previously demonstrated that gal-3 binding to β-catenin regulates the Wnt/β-catenin signaling pathway in cancer cells and that gal-3 mediates a number of important molecules, including IL-8, MMP-2 and fascin-1 [20, 28], we examined these molecules in this study. We found notable decreases in both MMP-2 and IL-8 expression, but not fascin-1 expression, in gal-3-silenced PanC-1 cells (Fig. 6a). RT-PCR for MMP-2 and IL-8 was performed with both transfected cell types to confirm whether these molecules were regulated by the β-catenin/Wnt signal. RT-PCR data revealed decreased MMP-2 expression in β-catenin-silenced cells compared with that in control cells, but no change of IL-8 was detected (Fig. 6b). MMP-2 expression was reconfirmed at the protein level in gal-3-silenced PanC-1 cells by western blotting (Fig. 6c). Densitometric analysis showed a significant (P < 0.05) reduction in MMP-2 expression in gal-3-siRNA-transfected cells. These results indicate that silencing gal-3 in pancreatic cancer cells reduced MMP-2 expression via the Wnt signaling pathway.
Fig. 6.
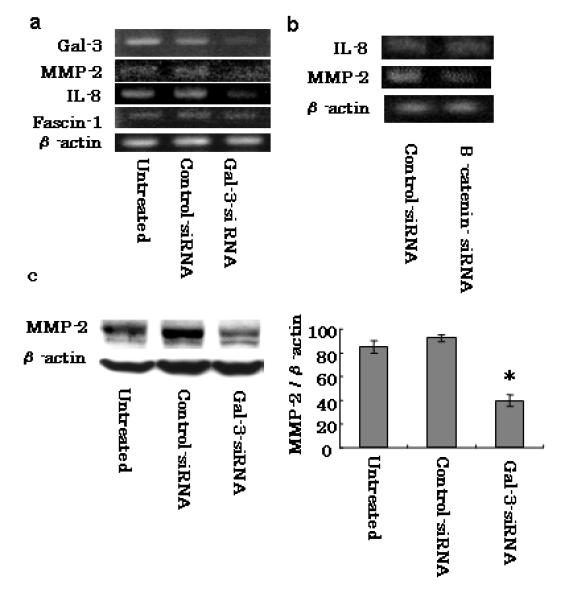
Factors mediated by gal-3 in pancreatic cancer cell lines. a Representative RT-PCR amplification of MMP-2, IL-8, and fascin-1 in PanC-1 cells transfected with either control- or gal-3-siRNA for 48 h. Transfected cells were harvested, total RNA was extracted, and MMP-2, IL-8, and fascin-1 mRNA levels were detected by RT-PCR. b Effect of β-catenin silencing on MMP-2 and IL-8 expression of PanC-1 cells. Cells transfected with either control- or β-catenin- siRNA for 48 h were harvested, total RNA was extracted from each, and MMP-2 and IL-8 mRNA levels were detected by RT-PCR. c Expression of MMP-2 was confirmed at the protein level. Total cell lysates were isolated after control- or gal-3-siRNA transfection for 48 h and MMP-2 expression levels were detected by western blotting. Densitometry (mean ± SD, n=3) revealed a significant decrease in MMP-2 expression in gal-3-siRNA-transfected cells (representative data are shown). *P < 0.05 versus control-siRNA.
Discussion
In this study, we have demonstrated that transient silencing of gal-3 reduced cell migration and invasion of three human pancreatic cancer cell lines (PanC-1, AsPC-1, and BxPc-3) in vitro. A number of previous studies showed that gal-3 expression is correlated with cell motility in several cancers, and suggested that gal-3 inhibited cell–cell and cell–ECM interactions, causing morphological changes, modulating the surface expression of some integrins, engaging NG2-proteoglycans, facilitating adhesion to endothelial cells, and regulating focal adhesion dynamics [14–16, 27–29]. However, the detailed function of gal-3 in cell motility has remained unclear. Some studies of gal-3 have indicated that its expression is associated with proliferation, migration, invasion and prognosis of pancreatic cancer. For instance, Jiang et al. reported that gal-3 expression and pancreatic stellate cells were involved in the proliferation and infiltration process of SW1990 pancreatic cancer cells in vitro, and that a gal-3 antibody inhibited cell proliferation [24]. However, we found that gal-3-siRNA transfection had little effect on the proliferation of PanC-1, AsPC-1, and BxPc-3 cells. This difference is likely due to the differences in the cell lines and experimental systems used. In addition, gal-3-overexpressing pancreatic cancer cells were reported to exhibit morphological changes, including becoming rounded and protrusive in shape [28, 30]. In agreement with this, we found that silencing gal-3 affected cell morphology by suppressing protrusion of cells. Furthermore, Shimamura et al. reported that decreased gal-3 expression was associated with advanced disease stage, tumor de-differentiation, and metastasis based on examination of resected patient tissues [23]. Collectively, these findings indicate that gal-3 expression plays important roles in determination of cell morphology and the levels of cell migration, invasion, and possibly proliferation of pancreatic cancer cells, and that this protein may have prognostic implications for pancreatic cancer patients.
Here, we also examined the key factors and signaling pathways that are mediated by gal-3 and that are associated with pancreatic cancer cell motility. A number of recent studies have demonstrated a correlation between gal-3 and β-catenin in several cancer cell lines [19, 25–27], and have implicated this association in cancer invasion. Accordingly, in this study we examined whether gal-3 silencing affected β-catenin expression in human pancreatic cancer cell lines PanC-1, AsPC-1, and BxPc-3. We found that gal-3-siRNA-transfected cells from all lines had drastically reduced β-catenin levels compared with their corresponding control cells. In addition, PanC-1 cells transfected with gal-3-siRNA exhibited increased degradation of β-catenin in their cytoplasm. Of note, we also attempted to exemplify a direct interaction between gal-3 and β-catenin [or T-cell factor (TCF)-4] by coimmunoprecipitation in the cell lines, but were unable to obtain clear results.
The β-catenin/Wnt pathway plays a key role in development, tissue homeostasis, and cancer susceptibility. It has been demonstrated that unphosphorylated β-catenin binds to the TCF/Lef (lymphoid enhancer factor) family of transcription factors and activates their specific target genes, including those that encode c-Myc, cyclin D1, cyclooxgenase-2, and MMP-7 [32–34]. One study has also shown that fascin-1, an actin bundling protein, is upregulated by gal-3 via the Wnt pathway and that its expression is correlated with the level of cell motility in gastric cancer [20]. It has also been shown in a breast cancer cell line that the phosphorylation of gal-3 was mediated by the phosphorylation of GSK-3β, and resulted in the regulation of β-catenin/Wnt signaling [25, 27]. In addition, it has been reported that phosphoinositide 3-kinase (PI3K)-activated Akt may phosphorylate GSK-3β at Ser9, thereby inactivating GSK-3β and augmenting TCF4 transcriptional activity [35]. Furthermore, it has been demonstrated that gal-3 regulates Akt phosphorylation in breast and thyroid cancers [13, 36]. In this study, using a time-lapse western blotting experiment, we determined that the levels of pAkt and pGSK-3β decreased before the decrease of β-catenin expression. Thus, we conclude that Akt phosphorylation is the first step that occurs upon gal-3 silencing, which subsequently downregulates GSK-3β phosphorylation and induces β-catenin degradation. This proposed pathway matches the pathway previously reported for colon cancer cells [26].
We also assessed specific molecules from the Wnt signaling pathway that reportedly mediate gal-3 and affect cancer cell invasion, including IL-8, MMP-2, and fascin-1 [20, 28]. We detected decreased MMP-2 and IL-8 expression in gal-3-silenced PanC-1 cells compared with untreated or control-siRNA-transfected PanC-1 cells, but saw little change in fascin-1 expression. In addition, we also examined MMP-2 and IL-8 expression of β-catenin-silenced PanC-1 cells to determine whether the β-catenin/Wnt signaling pathway was associated with pancreatic cancer cell invasion. First, we established that both cellular proliferation and invasion levels were decreased in β-catenin-silenced cells. This decreased proliferation was previously thought to occur because of a halted cell cycle via downregulation of cyclin D1 and c-myc, but our results indicate that it may be due to other signals that are altered as a result of changes in cyclin D1 and c-myc expression. Second, we determined that MMP-2 expression, but not IL-8 expression, was decreased in β-catenin-silenced PanC-1 cells. Therefore, we conclude that the β-catenin/Wnt signaling pathway regulates MMP-2 in PanC-1 cells. MMPs are a family of zinc-containing proteolytic enzymes that play an important role in tumor cell invasion and metastasis by breaking down ECM proteins to facilitate cancer cell motility and penetration [37]. MMP-2 is often overexpressed in pancreatic carcinoma tissue, and MMP-2 activation has been strongly implicated in tumor invasion and metastasis in pancreatic cancer [38, 39]. These findings, together with our own data, indicate that MMP-2 plays an important role in pancreatic cancer cell progression.
Previous reports have shown that IL-8 is directly mediated by Wnt signaling [40, 41]; however, our experiments indicated that IL-8 is not mediated by β-catenin/Wnt signaling. IL-8, which is also known as CXCL8, is a proinflammatory CXC chemokine that reported plays a role in cell metabolism, proliferation, invasion, angiogenesis, and survival [42-46]. Of note, in the present study, IL-8 expression was decreased in gal-3-silenced PanC-1 cells. Therefore, we performed an ELISA using culture medium from control- and gal-3-siRNA-transfected PanC-1, AsPC-1, and BxPC-3 cells, and found that IL-8 secretion was significantly decreased in gal-3-silenced cells for all lines (data not shown). We also applied anti-IL-8 antibody treatment to PanC-1 cells at various concentrations and subsequently detected a dose-dependent decrease of invasion (data not shown).
In this study, we also determined that Akt phosphorylation regulated by gal-3 is an important factor for regulating GSK-3β. We found that gal-3-regulated Akt phosphorylation is associated with gemcitabine- and cisplatin-induced apoptosis in pancreatic cancer both in vitro and in vivo (data not shown). Accordingly, we believe that one of the most important roles of gal-3 is regulation of Akt in pancreatic cancer. However, gal-3-mediated cell invasion occurs via, not only Akt, but also other factors that are mediated by gal-3 through the β-catenin/Wnt signaling pathway.
Based on our results, several signals that are regulated by gal-3 are correlated with levels of cell invasion and contribute to pancreatic cancer cell malignancy in vitro. However, a previous immunohistochemistry study reported that high gal-3 expression was associated with good prognosis [23]. We also performed immunohistochemical examination of gal-3 in invasive ductal carcinoma samples from the pancreas, and obtained results similar to those shown in a previous report (unpublished observations) [23]. Because we thought that the tumor interacts with the stroma and affects the surrounding environment, we believe that gal-3 expression is mediated by many factors in clinical samples.
In conclusion, in this study we show for the first time a novel and possibly clinically significant role of gal-3 in pancreatic cancer cell migration and invasion by regulating Akt, which in turn regulates GSK-3β phosphorylation and β-catenin degradation. However, this study revealed only some of the key factors and signals that mediate gal-3-regulated pancreatic cancer cell invasion and obtained results similar to those reported elsewhere [20, 25–27]. We believe that these results provide a basis for further, more detailed pre-clinical studies, and suggest that gal-3 may ultimately offer a target for agents designed to treat patients with advanced pancreatic cancer. To deliver the gal-3-siRNA to the tumor selectively, it may inhibit cancer cell invasion and metastasis in advanced pancreatic cancer with high gal-3 expression, and it can be a new treatment which assists for present treatment.
Supplementary Material
Acknowledgments
We thank Dr. Tomoharu Fukumori for supplying the pGEX-Gal-3 plasmid. We also thank Hayato Yamauchi and Naritaka Tanaka for their technical assistance and advice. This work was supported in part by the grant 3R37-CA46120-21 from the National Institutes for Health/National Cancer Institute (awarded to AR).
Abbreviations
- ATCC
American Type Culture Collection
- BSA
bovine serum albumin
- DAPI
4′,6-diamino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- ECM
extracellular matrix
- FBS
fetal bovine serum
- gal-3
galectin-3
- GSK-3β
glycogen synthase kinase-3β
- IL
interleukin
- MMP
matrix metalloproteinase
- pAkt
phosphorylated Akt
- PCR
polymerase chain reaction
- pGSK-3β
phosphorylated glycogen synthase kinase-3β
- PI3K
phosphoinositide 3-kinase
- RT-PCR
reverse-transcription polymerase chain reaction
- SD
standard deviation
- siRNA
small interfering RNA
- TCF
t-cell factor
References
- 1.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10:58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Niedergethmann M, Alves F, Neff JK, Heidrich B, Aramin N, Li L, Pilarsky C, Grutzmann R, Allgayer H, Post S, Gretz N. Gene expression profiling of liver metastases and tumour invasion in pancreatic cancer using an orthotopic SCID mouse model. Br J Cancer. 2007;97:1432–40. doi: 10.1038/sj.bjc.6604031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, Haddock MG, Schaefer P, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. Jama. 2008;299:1019–26. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 6.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Felzi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, Leffler H, Liu FT, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–8. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 7.Gray CA, Adelson DL, Bazer FW, Burghardt RC, Meeusen EN, Spencer TE. Discovery and characterization of an epithelial-specific galectin in the endometrium that forms crystals in the trophectoderm. Proc Natl Acad Sci U S A. 2004;101:7982–7. doi: 10.1073/pnas.0402669101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaoka K, Ohno S, Kawasaki H, Suzuki K. Overexpression of a beta-galactoside binding protein causes transformation of BALB3T3 fibroblast cells. Biochem Biophys Res Commun. 1991;179:272–9. doi: 10.1016/0006-291x(91)91365-j. [DOI] [PubMed] [Google Scholar]
- 9.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–10. [PubMed] [Google Scholar]
- 10.Nangia-Makker P, Nakahara S, Hogan V, Raz A. Galectin-3 in apoptosis, a novel therapeutic target. J Bioenerg Biomembr. 2007;39:79–84. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akahani S, Nangia-Makker P, Inohara H, Kim HR, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–6. [PubMed] [Google Scholar]
- 12.Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resist Updat. 2007;10:101–8. doi: 10.1016/j.drup.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazurek N, Sun YJ, Liu KF, Gilcrease MZ, Schober W, Nangia-Makker P, Raz A, Bresalier RS. Phosphorylated galectin-3 mediates tumor necrosis factor-related apoptosis-inducing ligand signaling by regulating phosphatase and tensin homologue deleted on chromosome 10 in human breast carcinoma cells. J Biol Chem. 2007;282:21337–48. doi: 10.1074/jbc.M608810200. [DOI] [PubMed] [Google Scholar]
- 14.Fukumori T, Oka N, Takenaka Y, Nangia-Makker P, Elsamman E, Kasai T, Shono M, Kanayama HO, Ellerhorst J, Lotan R, Raz A. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res. 2006;66:3114–9. doi: 10.1158/0008-5472.CAN-05-3750. [DOI] [PubMed] [Google Scholar]
- 15.Akimoto Y, Kawakami H, Oda Y, Obinata A, Endo H, Kasai K, Hirano H. Changes in expression of the endogenous beta-galactoside-binding 14-kDa lectin of chick embryonic skin during epidermal differentiation. Exp Cell Res. 1992;199:297–304. doi: 10.1016/0014-4827(92)90438-e. [DOI] [PubMed] [Google Scholar]
- 16.Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–11. [PubMed] [Google Scholar]
- 17.Warfield PR, Makker PN, Raz A, Ochieng J. Adhesion of human breast carcinoma to extracellular matrix proteins is modulated by galectin-3. Invasion Metastasis. 1997;17:101–12. [PubMed] [Google Scholar]
- 18.O’Driscoll L, Linehan R, Liang YH, Joyce H, Oglesby I, Clynes M. Galectin-3 expression alters adhesion, motility and invasion in a lung cell line (DLKP), in vitro. Anticancer Res. 2002;22:3117–25. [PubMed] [Google Scholar]
- 19.Khaldoyanidi SK, Glinsky VV, Sikora L, Glinskii AB, Mossine VV, Quinn TP, Glinsky GV, Sriramarao P. MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by Thomsen-Friedenreich antigen-galectin-3 interactions. J Biol Chem. 2003;278:4127–34. doi: 10.1074/jbc.M209590200. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan R, Park SH, Chun KH. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology. 2010;138:1035–1045. e1031–2. doi: 10.1053/j.gastro.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 21.Hittelet A, Camby I, Nagy N, Legendre H, Bronckart Y, Decaestecker C, Kaltner H, Nifant’ev NE, Bovin NV, Pector JC, Salmon I, Gabius HJ, et al. Binding sites for Lewis antigens are expressed by human colon cancer cells and negatively affect their migration. Lab Invest. 2003;83:777–87. doi: 10.1097/01.lab.0000073129.62433.39. [DOI] [PubMed] [Google Scholar]
- 22.Debray C, Vereecken P, Belot N, Teillard P, Brion JP, Pandolfo M, Pochet R. Multifaceted role of galectin-3 on human glioblastoma cell motility. Biochem Biophys Res Commun. 2004;325:1393–8. doi: 10.1016/j.bbrc.2004.10.181. [DOI] [PubMed] [Google Scholar]
- 23.Shimamura T, Sakamoto M, Ino Y, Shimada K, Kosuge T, Sato Y, Tanaka K, Sekihara H, Hirohashi S. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2002;8:2570–5. [PubMed] [Google Scholar]
- 24.Jiang HB, Xu M, Wang XP. Pancreatic stellate cells promote proliferation and invasiveness of human pancreatic cancer cells via galectin-3. World J Gastroenterol. 2008;14:2023–8. doi: 10.3748/wjg.14.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64:6363–7. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- 26.Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, Hung MC, Bresalier RS. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343–9. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimura T, Takenaka Y, Fukumori T, Tsutsumi S, Okada K, Hogan V, Kikuchi A, Kuwano H, Raz A. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005;65:3535–7. doi: 10.1158/0008-5472.CAN-05-0104. [DOI] [PubMed] [Google Scholar]
- 28.Mourad-Zeidan AA, Melnikova VO, Wang H, Raz A, Bar-Eli M. Expression profiling of Galectin-3-depleted melanoma cells reveals its major role in melanoma cell plasticity and vasculogenic mimicry. Am J Pathol. 2008;173:1839–52. doi: 10.2353/ajpath.2008.080380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15:3580–90. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu DK, Chernyavsky AI, Chen HY, Yu L, Grando SA, Liu FT. Endogenous galectin-3 is localized in membrane lipid rafts and regulates migration of dendritic cells. J Invest Dermatol. 2009;129:573–83. doi: 10.1038/jid.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetz JG, Joshi B, Lajoie P, Strugnell SS, Scudamore T, Kojic LD, Nabi IR. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J Cell Biol. 2008;180:1261–75. doi: 10.1083/jcb.200709019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 33.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–91. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 35.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 36.Lin CI, Whang EE, Abramson MA, Donner DB, Bertagnolli MM, Moore FD, Jr, Ruan DT. Galectin-3 regulates apoptosis and doxorubicin chemoresistance in papillary thyroid cancer cells. Biochem Biophys Res Commun. 2009;379:626–31. doi: 10.1016/j.bbrc.2008.12.153. [DOI] [PubMed] [Google Scholar]
- 37.Kuniyasu H, Ellis LM, Evans DB, Abbruzzese JL, Fenoglio CJ, Bucana CD, Cleary KR, Tahara E, Fidler IJ. Relative expression of E-cadherin and type IV collagenase genes predicts disease outcome in patients with resectable pancreatic carcinoma. Clin Cancer Res. 1999;5:25–33. [PubMed] [Google Scholar]
- 38.Koshiba T, Hosotani R, Wada M, Miyamoto Y, Fujimoto K, Lee JU, Doi R, Arii S, Imamura M. Involvement of matrix metalloproteinase-2 activity in invasion and metastasis of pancreatic carcinoma. Cancer. 1998;82:642–50. doi: 10.1002/(sici)1097-0142(19980215)82:4<642::aid-cncr5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 39.Han F, Zhu HG. Caveolin-1 regulating the invasion and expression of matrix metalloproteinase (MMPs) in pancreatic carcinoma cells. J Surg Res. 2009;159:443–50. doi: 10.1016/j.jss.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 40.Levy L, Neuveut C, Renard CA, Charneau P, Branchereau S, Gauthier F, Van Nhieu JT, Cherqui D, Petit-Bertron AF, Mathieu D, Buendia MA. Transcriptional activation of interleukin-8 by beta-catenin-Tcf4. J Biol Chem. 2002;277:42386–93. doi: 10.1074/jbc.M207418200. [DOI] [PubMed] [Google Scholar]
- 41.Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 42.MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S, Purcell C, McGurk M, Johnston PG, Waugh DJ. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res. 2007;5:737–48. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- 43.Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU. Autocrine growth effect of IL-8 and GROalpha on a human pancreatic cancer cell line, Capan-1. Pancreas. 2000;21:52–6. doi: 10.1097/00006676-200007000-00051. [DOI] [PubMed] [Google Scholar]
- 44.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 45.Kamohara H, Takahashi M, Ishiko T, Ogawa M, Baba H. Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-alpha and leukemia inhibitory factor in pancreatic carcinoma cells: Impact of CXCL-8 as an autocrine growth factor. Int J Oncol. 2007;31:627–32. [PubMed] [Google Scholar]
- 46.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–91. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


