Abstract
IGFs and their binding proteins have been shown to exhibit both protective and deleterious effects in ocular disease. Recent studies have characterized the expression patterns of different IGFBPs in retinal layers and within the vitreous. IGFBP-3 has roles in vascular protection stimulating proliferation, migration, and differentiation of vascular progenitor cells to sites of injury. IGFBP-3 increases pericyte ensheathment and shows anti-inflammatory effects by reducing microglia activation in diabetes. IGFBP-5 has recently been linked to mediating fibrosis in proliferative vitreoretinopathy but also reduces neovascularization. Thus, the regulatory balance between IGF and IGFBPs can have profound impact on target tissues. This review discusses recent findings of IGF and IGFBP expression in the eye with relevance to different retinopathies.
Keywords: IGF, IGF binding protein, retinopathy, hypoxia, blood retinal barrier, vascular protection
Introduction
Insulin-like growth factors (IGFs) are peptides produced in the liver and throughout most tissues that stimulate mitogenic activity through their interaction with IGF receptors (IGFRs) [1]. Two forms have been identified: IGF-I and IGF-II; they are regulated by insulin-like growth factor binding proteins (IGFBPs) and IGFBP proteases to collectively form the IGF system. IGFBPs interact with a glycoprotein, the acid-labile subunit (ALS), and binds free IGF in serum to form a ternary complex and modulate IGF binding to IGFRs on endothelium [2]. Of the IGFBPs, IGFBP-3 is most abundant in postnatal serum and carries more than 75% of serum IGF-I and IGF-II in complexes [1,3]. Other IGFBPs bind a small proportion of IGF and less than 1% of IGFs are circulating freely [2]. The existence of IGFBPs was postulated in the 1960s but the definitive studies were carried in the mid 1980s until successful cloning and sequencing of six IGFBPs (IGFBP-1 to IGFBP-6) in the early 1990s [2,4–9]. Since then, nine IGFBP related proteins (IGFBP-rPs) sharing some homology have been identified. All bind to IGF although with lower affinity than IGFBPs [10–12].
Serum IGF-I is synthesized and released from the liver following activation of hepatic receptors via binding of growth hormone (GH), so IGF-I may be important for regulating growth [13,14]. A dual effector theory has been proposed suggesting that GH causes cell differentiation while IGF-I promotes cell proliferation [15]. Early studies in GH deficient children showed that IGF-I has a major role in regulating fetal growth, especially during the third trimester [16]. Recent pharmacokinetic studies have determined dosing parameters of IGF-I/IGFBP-3 to maintain IGF-I levels at normal physiologic range in preterm infants without significant changes to blood pressure, heart rate, or blood glucose levels [17]. Premature infants with insufficient IGF-I can be given exogenous IGF-I to promote normal vessel development and to prevent retinopathy of prematurity (ROP) [17,18].
Modulating IGFBP expression may have inhibitory or stimulatory effects depending on the microenvironment and cellular context [19–21]. IGF-I and IGF-II have been linked to atherosclerosis to stimulate vascular smooth muscle cell proliferation (VSMC) and maintain plaque stability [22,23]. Although VSMC proliferation may contribute to the development of plaques, it has also been suggested that reducing IGF-I levels below physiologic levels may lead to loss of VSMC, destabilize plaques, and thus increase in risk of thrombosis [24]. A reduction in circulating IGF-I levels has been shown to promote atherosclerosis in Apolipoprotein E-deficient mice [25]. Increased IGFBP-1 levels reduced plaque burden, lowers blood pressure, and confers protection from atherosclerosis in mice overexpressing IGFBP-1 [26]. Upon plaque inflammation, IGFBP-1 is activated to control SMC proliferation which may regulate fibroproliferative processes and subsequently plaque stability [27].
In prostate cancer, IGFBP-3 has been shown to mediate anti-growth signals, induce apoptosis in prostate cancer cells, and display antiangiogenic properties [28–32]. In breast cancer cells, IGFBP-3 appears to maintain cell survival under adverse microenvironments by binding to glucose-regulated protein 78 and stimulating autophagy [33]. IGFBP-3 can also bind to a cell death receptor, IGFBP-3R, that is expressed in M12 human prostate cancer cells and MDA231 breast cancer cells [34]. IGFBP-3/IGFBP-3R mRNA expression is reduced in invasive tissues compared to benign tissues [34]. Restoring the expression of IGFBP-3/IGFBP-3R enhanced tumor suppressive activity by activating Caspase-8 signaling [34].
Most tissues can synthesize IGF-I, therefore locally derived IGF-I may have more dominant roles in regulating the tissue microenvironment than serum IGF-I [15,35]. Within the eye, IGF-I receptors (IGF-IR) are present on retinal microvascular cells and their activation increases both DNA synthesis and promotes migration [15,36,37]. Vitreal IGF-I levels were found to be increased in diabetic patients [14,15,38]. Thus, IGF-I may be involved in retinal neovascularization, which is a primary determinant for retinopathy of prematurity (ROP) or proliferative diabetic retinopathy (PDR) [15]. Numerous studies have identified expression patterns of IGFBPs in the retina and vitreous humor when exposed to certain microenvironments. The focus of this review will examine recent findings of the IGF-IGFBP system and its role in retinopathy.
IGF/IGFBP expression in the eye
IGF-I and IGF-IR have been detected in retinal endothelial cells, lens epithelial cells, retinal pigment epithelium, cone photoreceptor cells, and Müller cells [39–43]. Using models of oxygen-induced retinopathy (OIR), microarrays determined differential global expression profiles between hypoxic and hyperoxic retinas. Retinas removed from hyperoxic chambers showed upregulation of genes associated with vasculogenesis, neurogenesis, and inflammation and included IGFBP-3 [44]. Alternatively, IGFBP-7, or IGFBP-rP1, was downregulated when compared to normoxic retinas [44].
Closer examination using laser capture microdissection identified localization and abundance of IGFs and IGFBPs [45]. IGF-IR expression was predominantly found in photoreceptor cells, which may be important for retinal vascular development since IGF-IR null mice showed decreased retinal vasculature [45,46]. IGFBP-2, IGFBP-4, and IGFBP-5 are expressed similarly between normoxic and hypoxic conditions. IGFBP-3 basal expression was lower, but was significantly induced to similar levels of other IGFBPs under hypoxia [45]. There were increased mRNA levels of IGFBP-3 and IGFBP-5 within neovascular tufts, however no functional protein levels were measured [45].
IGFBP levels in vitreous of the eye have been reported. Western blot analyses using biotinylated IGF-II revealed that IGFBP-2 and IGFBP-3 were predominant in the vitreous, but a small ~29kDa band confirmed by in vitro studies with IGFBP-3 protease indicated that IGFBP-3 existed in a cleaved form [47]. Schoen et al. found the cleaved form of IGFBP-3 to be more prominent in diabetic vitreous humor, suggesting a role of IGFBP-3 protease in regulating IGFBP-3 in the vitreous [48]. The functionality of the fragmented IGFBP-3 and significance of IGFBP-3 proteases in the vitreous has not been determined.
(mRen-2)27, a hypertensive rat model with elevated serum and ocular renin levels, was treated with streptozotocin to induce diabetes to evaluate expression of the IGF system [49]. In the diabetic state, overall abundance of IGFBP-5 was increased in the cornea and iris while IGFBP-6 was reduced [49]. IGFBP-1 was present in retinal layers with no change with diabetes whereas IGFBP-2, IGFBP-3, and IGFBP-4 were not detected by in situ hybridization [49]. Differences in consensus of mRNA expression of IGF system may be due to assay conditions and the particular animal model [49,50].
The presence of IGFBPs both in the retina and in the vitreous to varying levels depending on the model suggest that they may have functional roles in regulating ocular physiology. Current evidence shows that IGFBPs play a key role in limiting free IGFs in circulation which can inhibit retinal angiogenesis growth and development. Since IGFBP-3 is the major protein that binds to free IGF, the predominantly cleaved form in the vitreous of diabetic eyes may lead to altered regulation of IGFs [47,48,51–53]. Further studies are necessary to understand the regulation of IGF axis expression and function as a protective or pathophysiological process.
Role of IGFBP-3 under hypoxia
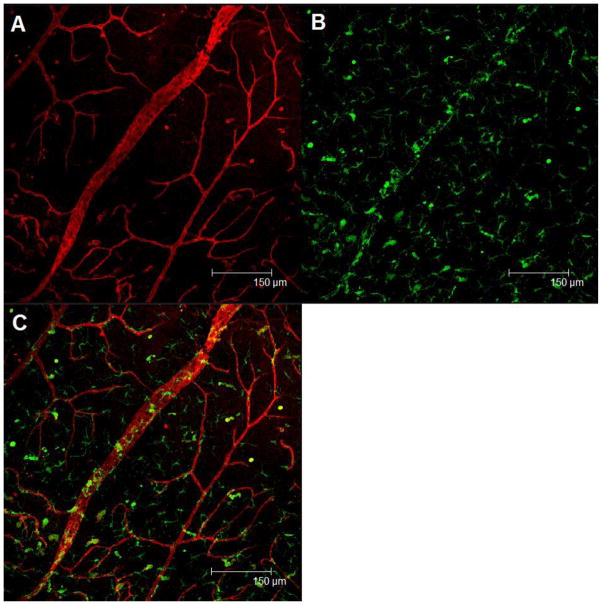
Retinopathy is associated with both dysfunctional repair and maintenance of the retinal vasculature. In diabetes associated retinal complications, endothelial progenitor cells (EPCs) displaying the CD34+ surface marker have reduced migratory, proliferative, and differentiation potential. CD34+ cells exposed with exogenous IGFBP-3 were able to migrate in a dose-dependent manner and increase endothelial nitric oxide synthase (eNOS) activity, a prominent factor in vasodilation, suggesting that IGFBP-3 can stimulate recruitment of precursor cells [3,54]. In vivo studies support this finding through increased bone marrow derived GFP+ cells in the retina in response to endothelial cell overexpression of IGFBP-3 (Figure 1).
Figure 1. Retinal vasculature in a GFP+ chimeric mouse (WT mouse undergoing bone marrow transplantation) that received within the vitreous liposomes containing a plasmid expressing IGFBP-3 under an endothelial specific promoter.
The mouse then was subjected to the retinal branch vein occlusion model. Three weeks after liposome injection, the mouse was sacrificed and a retinal flat mount was prepared. (A) The retinal vessels were labeled with rhodamine agglutinin (red) and imaged using confocal fluorescence microscopy. (B) Enhanced incorporation of GFP+ progenitor cells (green) into the retinal vessels is shown. (C) Merged channel. IGFBP-3’s protective and reparative effects on the vasculature may be, in part, the result of its ability to recruit endothelial progenitor cells, to sites of retinal injury.
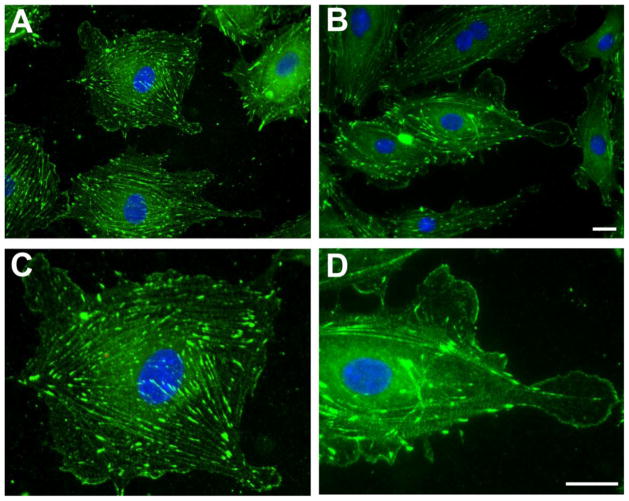
Migration of CD34+ progenitors represents a critical process for routine vascular maintenance as well as repair of injuries. We have previously shown that IGFBP-3 promotes retinal repair by stimulating bone marrow-derived cell homing following injury; many of these migrating cells are vascular progenitors [53]. Vasodilator-stimulated phosphoprotein (VASP) plays a pivotal role in cell migration and its activation is nitric oxide (NO)-dependent [55,56]. IGFBP-3 also induces eNOS activation and subsequent NO generation. We, therefore, investigated whether IGFBP-3 treatment affected VASP redistribution in human microvascular endothelial cells. Treatment with IGFBP-3 caused a rapid redistribution of VASP to the tip of lamellipodia promoting cell motility (Figure 2). IGFBP-3-mediated VASP redistribution was blocked by preincubation with by an NO scavenger [54].
Figure 2. IGFBP-3-mediated VASP redistribution in human microvascular endothelial cells from the lungs (HMVEC-L).
HMVEC-L, cultured on fibronectin-coated coverslips, were left untreated (A) or were treated with 100ng/ml IGFBP-3 for 15 minutes (B) and Vasodilator-stimulated phosphoprotein (VASP) biodistribution was detected by immunofluorescence. IGFBP-3 treatment caused the rapid redistribution of VASP to the cells’ periphery (A and B). C and D show, at higher magnification, a single cell in A and B, respectively. Note the uniform VASP distribution throughout the cytoplasm along the actin filaments in the untreated sample (C) and the presence of VASP-free areas together with increased VASP immunoreactivity along the plasmamembrane in the IGFBP-3- treated cell, (D). Representative results from three independent experiments are shown. Green: VASP; Blue: DAPI (for nuclear staining). (Scale bar = 25μm)
EPC numbers were also lower in the retina in IGFBP-3 knockout mice [57]. In vivo studies showed that overexpression of IGFBP-3, using a proliferating endothelial cell specific promoter, protected against vaso-obliteration in an OIR model and also reduced preretinal neovascularization in a model of branch vein occlusion (BVO) [3,58]. The laboratory of Lois Smith found similar results where low IGFBP-3 was correlated with vaso-obliteration [57]. The study implicates a role of IGFBP-3 in recruiting vascular progenitor cells to sites of injury following hypoxia and administration of IGFBP-3 may be a treatment strategy for revascularization and repair.
IGFBP-3 has additional roles aside from recruiting hematopoietic stem cells (HSCs) and progenitor cells to sites of retinal injury. While IGFBP-3 can promote HSC differentiation to endothelial cells, it can also regulate differentiation into pericytes and astrocytes to stabilize the vasculature [58]. Additionally, upon induction of retinal injury using laser photocoagulation injury, injection of an endothelial specific IGFBP-3 expressing plasmid showed increased pericyte ensheathment based on an observed increased in NG2+ immunoreactivity [58]. There was also a reduction in pericyte apoptosis based on less NG2+/TUNEL+ labeling compared to contralateral uninjected and control-vector injected eyes [58].
In hypoxic environments, inflammatory responses are recapitulated in the OIR model through the activation of resident microglia [58,59]. IGFBP-3 attenuates inflammatory responses by increasing microglia apoptosis and reducing the numbers of activated microglia [58]. The anti-inflammatory roles of IGFBP-3 have been observed in tissues other than the retina. IGFBP-3 has been shown to activate caspase activity in lungs of an asthma mouse model to degrade inhibitor of kappa B alpha and nuclear factor kappa B [60]. Similarly, administration of both IGF-I and IGFBP-3 in children with burn injuries reduced interleukin (IL)-6, IL-1β and tumor necrosis factor-alpha inflammatory markers [61,62].
Hypoxia and ischemic injury can be attenuated with vasodilatory responses. We showed that IGFBP-3 can affect vasodilation in rat posterior cerebral arteries. IGFBP-3 administration displayed a dose-dependent decrease in artery constriction placed under intraluminal pressure [54]. The vasodilatory effect was lost in the presence of inhibitors to eNOS [54,63]. IGFBP-3 stimulated NO release in intact arteries that is independent of calcium mediated NO release [54]. IGFBP-3 by promoting NO generation and vasodilation may be important for ROP and for counteracting the progression of non-proliferative diabetic retinopathy (NPDR) to proliferative diabetic retinopathy (PDR). Further studies to determine reperfusion capability by IGFBP-3 through NO production in the retina should be examined.
IGFBP-3 and blood retinal barrier integrity
Blood retinal barrier (BRB) breakdown is strongly associated with the development of ocular disease [64–66]. Emerging evidence indicates that IGFBP-3 may have roles that maintain and restore the integrity of the BRB following injury. We isolated a mutant IGFBP-3 that does not bind to IGF-I (IGFBP-3NB) and found it improved vascular barrier protection and maintained claudin-5 and vascular endothelial-cadherin expression upon exposure to vascular endothelial growth factor (VEGF) [63,65]. Under normal physiological conditions, IGFBP-3 can counteract the activation of VEGF by binding IGF-I, however these results show an IGF-I independent role of IGFBP-3 in protecting the BRB [63,65]. Additionally, IGFBP-3 may protect the BRB by modulating inflammation. IGFBP-3NB was shown to reduce proinflammatory sphingomyelinase levels in the retina [65].
Vascular protective signaling mechanism of IGFBP-3
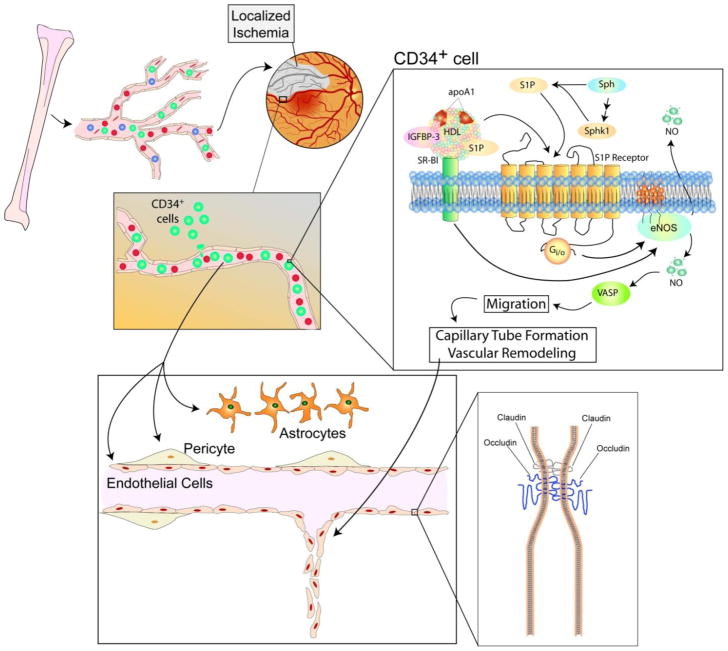
The induction of recruitment of CD34+ cells to sites of vascular injury involves a cascade of signaling mechanisms. IGFBP-3 binds to scavenger receptor class B, type1 (SR-B1), a receptor for high-density lipoprotein (HDL) [67,68]. NO is generated upon activation of SR-B1 by IGFBP-3 and subsequently by stimulation of eNOS activity [63,69]. A later study found IGFBP-3 activates PI3K/Akt pathway through SR-B1 [69]. IGFBP-3 also can stimulate sphingosine kinase (SphK)-1 activity which phosphorylates sphingosine to generate sphingosine-1-phosphate (S1P), a proangiogenic factor [63,69]. Mechanisms are summarized in Figure 3.
Figure 3. General mechanism of vascular repair by IGFBP-3.
CD34+ cells are recruited from the bone marrow by increased IGFBP-3 levels in the vasculature. IGFBP-3 binds to SR-B1, thereby leading to phosphorylation of eNOS and generating NO. IGFBP-3 can also mediate S1P generation by phosphorylating SphK-1, where S1P acts on its receptor to also stimulate phosphorylation of eNOS. NO stimulates a signaling cascade leading to VASP phosphorylation which is redistributed to polar ends of the cell and induces cell migration. Cells migrate to sites of injury to initiate capillary tube formation and vascular remodeling. IGFBP-3 can maintain tight and adherens junctions to maintain endothelium integrity as observed in the BRB.
IGF axis in diabetes-induced retinopathy
Chronic hyperglycemia can influence cellular responses in the presence of IGF-I. Bovine retinal endothelial cells (RECs) exposed to low and high levels of glucose showed enhanced proliferation in the presence of IGF-I [66,70]. Integrins may have roles in PDR progression [71]. IGF-I can activate αVβ3 integrin activation and maintain the signaling pathways that stimulate cell proliferation [66]. IGF-I protected human RECs (HRECs) from apoptosis when exposed to high glucose and serum starvation [72]. Exogenous IGFBP-3 induced a dose-dependent inhibition of HREC proliferation and at very high doses (1 mg/mL) increased apoptosis [72]. Alternatively, high glucose has also been shown to increase expression of IGFBP-3 in proximal tubular epithelial cells and induce apoptosis [73]. In prostate cancer, tumor cell proliferation was increased by over 3-fold in IGFBP-3 knockout lines [31].
VEGF-A is a major growth factor involved in ocular angiogenesis in retinopathy and its levels are correlated with neovascularization [74]. IGFBP-4 and IGFBP-5 have been found to counteract neovascularization in response to pro-angiogenic growth factors. Overexpression of IGFBP-5 inhibited VEGF-induced angiogenesis and inhibited both cell proliferation in human umbilical vein endothelial cells (HUVECs) and endothelial tube formation [75]. IGFBP-5 also suppressed phosphorylation of eNOS, thereby inhibiting endothelial vasodilation suggesting an antagonistic effect when compared with IGFBP-3 [75]. IGFBP-4 on the other hand inhibited fibroblast growth factor-2 and IGF-I induced angiogenesis in endothelial cells but had no effect in response to VEGF [76].
IGF-I has been shown to protect HRECs from apoptosis and enhance proliferation, therefore abnormally high levels observed in the vitreous humor may drive the progression of PDR [70]. Overexpression of IGF-I impaired functional recovery of acute ex vivo ischemic insult [77]. Our previous study disrupted IGF-IR and IGF-I binding using an IGF-IR ribozyme to investigate the interaction of IGF-I and retinopathy [43]. This ribozyme, driven by a proliferating endothelial cell specific promoter, reduced pre-retinal neovascularization in both OIR and BVO models [43]. Widespread IGF-IR disruption through loss of norepinephrine, however, reduced IGF-IR phosphorylation and signaling which led to increased apoptosis in the inner nuclear layers of the retina [78]. Similarly, long term diabetes have revealed increased IGFBP-3 expression in human tears which can reduce IGF-IR phosphorylation and may be implicated in the pathogenesis of ocular surface complications of the cornea [79].
Studies have established that PDR and accelerated neovascularization is preceded by elevated IGF levels, however serum levels correlating to the pathogenesis of PDR remain controversial [38,80–83]. Serum levels of IGF-I was found to be decreased in retinopathy, nephropathy, and neuropathy [84–91]. However, another study examining patients with either NPDR or PDR found no association of serum IGF-I in either insulin-dependent or insulin-independent diabetes [92]. The differences in these results may be due to sample size or patient characteristics and the methodology used to detect IGF-I [92]. Furthermore serum levels may not be as relevant as localized increases in IGFs and IGFBPs due to increased BRB permeability may be more relevant in PDR [52].
IGFBPs and proliferative vitreoretinopathy
Vascular injury or inflammation can lead to retinal detachment from the basement membranes of the eye. Proliferative vitreoretinopathy (PVR) is a fibrous scarring complication that is the leading cause in failing to treat rhegmatogenous retinal detachment [93–95]. Retinal tearing leads to fibrocellular scar formation at the vitreoretinal surface which contracts, pulling the retina away from the retinal pigment epithelium (RPE) [96]. Numerous studies have attributed RPE-derived cells in the role of retinal detachment as they are exposed to cytokines localized to the vitreous [97–99]. Human RPE cells exhibited increased proliferation when exposed to basic fibroblast growth factor or epidermal growth factor, and the effect is enhanced under hypoxic conditions [100]. One study examined whether IGFBP-5 could inhibit N-(4-hydroxyphenyl)retinamide (4HPR) induced neuronal differentiation of human retinal pigment epithelial cells (ARPE-19). IGFBP-5 did not inhibit transdifferentiation of ARPE-19, however, exogenous addition of recombinant IGFBP-5 showed increased proliferation of RPE cells [21]. Thus, the expression of IGFBP-5 may stimulate proliferation and migration of RPE cells that can be fibrotic, leading to progression of PVR [101].
Mukherjee et al. recently reported IGFBP expression patterns present in several RPE progressive phenotypes: normal, early reactive, and myofibroblastic [102]. IGFBP-1 was not detected, IGFBP-2 and IGFBP-4 were expressed only in normal RPE, while IGFBP-3, IGFBP-5, and IGFBP-6 were found in all three phenotypes, with IGFBP-5 being predominant in the myofibroblastic phenotype [102]. IGFBP-2 has been shown to inhibit IGF-induced responses, therefore the loss of IGFBP-2 in the early reactive phenotype may lead to increased growth factor activity and IGF mediated tractional force generation [99,103]. IGFBP-5 has also been shown to induce migration of human lung fibroblasts and induction of skin fibrosis, where migration of lung fibroblasts was via IGF independent mechanisms [104,105]. Similarly, inflammation has been linked to the pathogenesis of PVR, and IGFBP-5 has been shown to induce migration of mononuclear cells [104,106]. The presence of IGFBP-5 in a fibrotic state in different cell types may suggest a function of IGFBP-5 in mediating ocular fibrosis.
Hypertensive retinopathy
Long-term hypertension can potentially have similar retinal vascular pathology as observed in diabetic retinopathy. Association studies have found that uncontrolled hypertensive patients with no diabetic complications to be at higher risk of developing retinopathy [107]. 50–70% of hypertensive patients were more likely to have retinal hemorrhages [107,108]. Plasma levels of IGF-I were found to be inversely associated with hypertension and both in vitro and in vivo experiments showed that IGF-I decreases vascular resistance [109–111]. Groups have proposed a correlation of blood pressure to retinal microvascular changes, however the correlation decreases with age [107,109,112,113].
The regulation of IGFBP-3 levels may be influenced by standard treatments for hypertension. Basic and clinical studies to date suggest IGFBP-3 to be vascular protective, however its effect on hypertensive retinopathy is unclear. The ilSIRENTE study showed that older adults on angiotensin converting enzyme (ACE) inhibitors were found to have significantly increased IGFBP-3 levels in serum, however there was no significant association of ACE inhibitors with free IGF-I levels [114,115]. It may be that ACE inhibitor mechanism of action does not directly stimulate IGF-I activity to reduce vascular resistance, but rather works via increases in IGFBP-3 levels.
Retinal macroaneurysms can develop with complications of atherosclerosis and hypertension in up to 75% patients and manifests generally in elderly [116,117]. IGFBP-7 has recently been linked to familial retinal arterial macroaneurysm (FRAM) [118]. FRAM may be due to reduced mechanical integrity of the arterial wall and individuals with FRAM were found to have homozygous mutations in IGFBP-7 [118]. In situ hybridization of retinal whole-mounts indicated that IGFBP-7 expression was specific to the lens and retina in mouse embryos, however the expression of IGFBP-7 has not been carried out in human eyes [118]. Additionally, hypertension has not yet been associated with influencing expression of IGFBP-7, however IGFBP-7 has been linked to vascular function and endothelial-dependent vasodilation in high-ferritin insulin-dependent diabetes [119]. These findings reveal previously unknown roles of the low affinity binding proteins to IGFs and in disease. One can speculate that IGFBP-7 may have other potential functions in the pathogenesis of other retinopathies.
Conclusion
Efforts in studying IGFBPs in recent years have identified an increasing role of IGFs and IGFBPs in ocular complications. IGFBP-3 appears to have protective functions in the retina and BRB through stimulation and recruitment of CD34+ cells, pericyte stability, and reducing inflammation. However, the tissue microenvironment influences whether IGFBPs have stimulatory or inhibitory properties. The roles of IGFBP-5 in the RPE in PVR and IGFBP-7 in FRAM have opened up the field to further investigation. Future work should ascertain other regulatory mechanisms of the IGF system in ocular physiology and pathology.
Acknowledgments
This work was supported by the National Institutes of Health, R01 EY007739, R01 EY12601, and R01 DK090730.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferry RJ, Cerri RW, Cohen P. Insulin-like growth factor binding proteins: new proteins, new functions. Hormone Research. 1999;51:53–67. doi: 10.1159/000023315. [DOI] [PubMed] [Google Scholar]
- 2.Baxter RC, Martin JL. Binding proteins for the insulin-like growth factors: structure, regulation and function. Progress in Growth Factor Research. 1989;1:49–68. doi: 10.1016/0955-2235(89)90041-0. [DOI] [PubMed] [Google Scholar]
- 3.Chang KH, Chan-Ling T, McFarland EL, Afzal A, Pan H, Baxter LC, et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10595–600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6) Progress in Growth Factor Research. 1991;3:243–66. doi: 10.1016/0955-2235(91)90003-m. [DOI] [PubMed] [Google Scholar]
- 5.Rechler MM. Insulin-like growth factor binding proteins. Vitamins and Hormones. 1993;47:1–114. doi: 10.1016/s0083-6729(08)60444-6. [DOI] [PubMed] [Google Scholar]
- 6.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine Reviews. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld RG, Lamson G, Pham H, Oh Y, Conover C, De Leon DD, et al. Insulinlike growth factor-binding proteins. Recent Progress in Hormone Research. 1990;46:99–159. doi: 10.1016/b978-0-12-571146-3.50009-2. discussion 159–63. [DOI] [PubMed] [Google Scholar]
- 8.Zapf J. Physiological role of the insulin-like growth factor binding proteins. European Journal of Endocrinology/European Federation of Endocrine Societies. 1995;132:645–54. doi: 10.1530/eje.0.1320645. [DOI] [PubMed] [Google Scholar]
- 9.Lamson G, Giudice LC, Rosenfeld RG. Insulin-like growth factor binding proteins: structural and molecular relationships. Growth Factors (Chur, Switzerland) 1991;5:19–28. doi: 10.3109/08977199109000268. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers BD, Roalson EH, Thompson C. Phylogenetic analysis of the insulin-like growth factor binding protein (IGFBP) and IGFBP-related protein gene families. General and Comparative Endocrinology. 2008;155:201–7. doi: 10.1016/j.ygcen.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan W, Lai M. Insulin-like growth factor binding protein: a possible marker for the metabolic syndrome? Acta Diabetologica. 2010;47:5–14. doi: 10.1007/s00592-009-0142-3. [DOI] [PubMed] [Google Scholar]
- 12.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocrine Reviews. 2002;23:824–54. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. The Journals of Gerontology. Series A Biological Sciences and Medical Sciences. 2012;67:626–39. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant MB, Schmetz I, Russell B, Harwood HJ, Silverstein J, Merimee TJ. Changes in insulin-like growth factors I and II and their binding protein after a single intramuscular injection of growth hormone. The Journal of Clinical Endocrinology and Metabolism. 1986;63:981–4. doi: 10.1210/jcem-63-4-981. [DOI] [PubMed] [Google Scholar]
- 15.Schultz GS, Grant MB. Neovascular growth factors. Eye (London, England) 1991;5(Pt 2):170–80. doi: 10.1038/eye.1991.31. [DOI] [PubMed] [Google Scholar]
- 16.Gluckman PD. Clinical review 68: The endocrine regulation of fetal growth in late gestation: the role of insulin-like growth factors. The Journal of Clinical Endocrinology and Metabolism. 1995;80:1047–50. doi: 10.1210/jcem.80.4.7714063. [DOI] [PubMed] [Google Scholar]
- 17.Löfqvist C, Niklasson A, Engström E, Friberg LE, Camacho-Hübner C, Ley D, et al. A pharmacokinetic and dosing study of intravenous insulin-like growth factor-I and IGF-binding protein-3 complex to preterm infants. Pediatric Research. 2009;65:574–9. doi: 10.1203/PDR.0b013e31819d9e8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellström A, Engström E, Hård AL, Albertsson-Wikland K, Carlsson B, Niklasson A, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–20. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 19.Cobb LJ, Salih DAM, Gonzalez I, Tripathi G, Carter EJ, Lovett F, et al. Partitioning of IGFBP-5 actions in myogenesis: IGF-independent anti-apoptotic function. Journal of Cell Science. 2004;117:1737–46. doi: 10.1242/jcs.01028. [DOI] [PubMed] [Google Scholar]
- 20.Salih DAM, Tripathi G, Holding C, Szestak TAM, Gonzalez MI, Carter EJ, et al. Insulin-like growth factor-binding protein 5 (Igfbp5) compromises survival, growth, muscle development, and fertility in mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4314–9. doi: 10.1073/pnas.0400230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samuel W, Kutty RK, Vijayasarathy C, Pascual I, Duncan T, Redmond TM. Decreased expression of insulin-like growth factor binding protein-5 during N-(4-hydroxyphenyl)retinamide-induced neuronal differentiation of ARPE-19 human retinal pigment epithelial cells: regulation by CCAAT/enhancer-binding protein. Journal of Cellular Physiology. 2010;224:827–36. doi: 10.1002/jcp.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:435–44. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 23.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe−/− mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1916–24. doi: 10.1161/ATVBAHA.110.210831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libby P, Sasiela W. Plaque stabilization: Can we turn theory into evidence? The American Journal of Cardiology. 2006;98:26P–33P. doi: 10.1016/j.amjcard.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P. Low circulating insulin-like growth factor I increases atherosclerosis in ApoE-deficient mice. American Journal of Physiology Heart and Circulatory Physiology. 2011;300:H1898–906. doi: 10.1152/ajpheart.01081.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajwani A, Ezzat V, Smith J, Yuldasheva NY, Duncan ER, Gage M, et al. Increasing circulating IGFBP1 levels improves insulin sensitivity, promotes nitric oxide production, lowers blood pressure, and protects against atherosclerosis. Diabetes. 2012;61:915–24. doi: 10.2337/db11-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Razuvaev A, Folkersen L, Hedin E, Roy J, Brismar K, et al. The expression of IGFs and IGF binding proteins in human carotid atherosclerosis, and the possible role of IGF binding protein-1 in the regulation of smooth muscle cell proliferation. Atherosclerosis. 2012;220:102–9. doi: 10.1016/j.atherosclerosis.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. The Journal of Biological Chemistry. 1997;272:12181–8. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 29.Harms KL, Chen X. The C terminus of p53 family proteins is a cell fate determinant. Molecular and Cellular Biology. 2005;25:2014–30. doi: 10.1128/MCB.25.5.2014-2030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KW, Ma L, Yan X, Liu B, Zhang X, Cohen P. Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. The Journal of Biological Chemistry. 2005;280:16942–8. doi: 10.1074/jbc.M412757200. [DOI] [PubMed] [Google Scholar]
- 31.Mehta HH, Gao Q, Galet C, Paharkova V, Wan J, Said J, et al. IGFBP-3 is a metastasis suppression gene in prostate cancer. Cancer Research. 2011;71:5154–63. doi: 10.1158/0008-5472.CAN-10-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Lee KW, Anzo M, Zhang B, Zi X, Tao Y, et al. Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene. 2007;26:1811–9. doi: 10.1038/sj.onc.1209977. [DOI] [PubMed] [Google Scholar]
- 33.Grkovic S, O’Reilly VC, Han S, Hong M, Baxter RC, Firth SM. IGFBP-3 binds GRP78, stimulates autophagy and promotes the survival of breast cancer cells exposed to adverse microenvironments. Oncogene. 2012:1–9. doi: 10.1038/onc.2012.264. [DOI] [PubMed] [Google Scholar]
- 34.Ingermann AR, Yang YF, Han J, Mikami A, Garza AE, Mohanraj L, et al. Identification of a novel cell death receptor mediating IGFBP-3-induced anti-tumor effects in breast and prostate cancer. The Journal of Biological Chemistry. 2010;285:30233–46. doi: 10.1074/jbc.M110.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Ercole AJ, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:935–9. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King GL, Goodman AD, Buzney S, Moses A, Kahn CR. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. The Journal of Clinical Investigation. 1985;75:1028–36. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant M, Jerdan J, Merimee TJ. Insulin-like growth factor-I modulates endothelial cell chemotaxis. The Journal of Clinical Endocrinology and Metabolism. 1987;65:370–1. doi: 10.1210/jcem-65-2-370. [DOI] [PubMed] [Google Scholar]
- 38.Grant M, Russell B, Fitzgerald C, Merimee TJ. Insulin-like growth factors in vitreous. Studies in control and diabetic subjects with neovascularization. Diabetes. 1986;35:416–20. doi: 10.2337/diab.35.4.416. [DOI] [PubMed] [Google Scholar]
- 39.Walker JL, Zhang L, Zhou J, Woolkalis MJ, Menko AS. Role for alpha 6 integrin during lens development: Evidence for signaling through IGF-1R and ERK. Developmental Dynamics : an Official Publication of the American Association of Anatomists. 2002;223:273–84. doi: 10.1002/dvdy.10050. [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal R, Wohlleben H, Malek G, Schlichting L, Thieme H, Bowes Rickman C, et al. Insulin-like growth factor-1 contributes to neovascularization in age-related macular degeneration. Biochemical and Biophysical Research Communications. 2004;323:1203–8. doi: 10.1016/j.bbrc.2004.08.219. [DOI] [PubMed] [Google Scholar]
- 41.Zygar CA, Colbert S, Yang D, Fernald RD. IGF-1 produced by cone photoreceptors regulates rod progenitor proliferation in the teleost retina. Brain Research Developmental Brain Research. 2005;154:91–100. doi: 10.1016/j.devbrainres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Li F, Cao W, Steinberg RH, LaVail MM. Basic FGF-induced down-regulation of IGF-I mRNA in cultured rat Müller cells. Experimental Eye Research. 1999;68:19–27. doi: 10.1006/exer.1998.0572. [DOI] [PubMed] [Google Scholar]
- 43.Shaw LC, Pan H, Afzal A, Calzi SL, Spoerri PE, Sullivan SM, et al. Proliferating endothelial cell-specific expression of IGF-I receptor ribozyme inhibits retinal neovascularization. Gene Therapy. 2006;13:752–60. doi: 10.1038/sj.gt.3302718. [DOI] [PubMed] [Google Scholar]
- 44.Ishikawa K, Yoshida S, Kadota K, Nakamura T, Niiro H, Arakawa S, et al. Gene expression profile of hyperoxic and hypoxic retinas in a mouse model of oxygen-induced retinopathy. Investigative Ophthalmology & Visual Science. 2010;51:4307–19. doi: 10.1167/iovs.09-4605. [DOI] [PubMed] [Google Scholar]
- 45.Lofqvist C, Willett KL, Aspegren O, Smith ACH, Aderman CM, Connor KM, et al. Quantification and localization of the IGF/insulin system expression in retinal blood vessels and neurons during oxygen-induced retinopathy in mice. Investigative Ophthalmology & Visual Science. 2009;50:1831–7. doi: 10.1167/iovs.08-2903. [DOI] [PubMed] [Google Scholar]
- 46.Kondo T, Vicent D, Suzuma K, Yanagisawa M, King GL, Holzenberger M, et al. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. The Journal of Clinical Investigation. 2003;111:1835–42. doi: 10.1172/JCI17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guidry C, King JL. Isolation and characterization of vitreous insulin-like growth factor binding proteins. Investigative Ophthalmology & Visual Science. 2011;52:303–9. doi: 10.1167/iovs.10-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoen TJ, Waldbillig RJ, Searcy G, Gaudet SJ, Jones BE, Chader GJ, et al. Identification and partial characterization of a proteinase specific for insulin-like growth factor binding protein-3 in aqueous and vitreous humors. Current Eye Research. 1995;14:127–35. doi: 10.3109/02713689508999924. [DOI] [PubMed] [Google Scholar]
- 49.Bergman PB, Moravski CJ, Edmondson SR, Russo VC, Bach La, Wilkinson-Berka JL, et al. Expression of the IGF system in normal and diabetic transgenic (mRen-2)27 rat eye. Investigative Ophthalmology & Visual Science. 2005;46:2708–15. doi: 10.1167/iovs.04-0921. [DOI] [PubMed] [Google Scholar]
- 50.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–4. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 51.Meyer-Schwickerath R, Pfeiffer A, Blum WF, Freyberger H, Klein M, Lösche C, et al. Vitreous levels of the insulin-like growth factors I and II, and the insulin-like growth factor binding proteins 2 and 3, increase in neovascular eye disease. Studies in nondiabetic and diabetic subjects. The Journal of Clinical Investigation. 1993;92:2620–5. doi: 10.1172/JCI116877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spranger J, Bühnen J, Jansen V, Krieg M, Meyer-Schwickerath R, Blum WF, et al. Systemic levels contribute significantly to increased intraocular IGF-I, IGF-II and IGF-BP3 [correction of IFG-BP3] in proliferative diabetic retinopathy. Hormone and Metabolic Research = Hormon-Und Stoffwechselforschung = Hormones Et Métabolisme. 2000;32:196–200. doi: 10.1055/s-2007-978621. [DOI] [PubMed] [Google Scholar]
- 53.Spranger J, Möhlig M, Osterhoff M, Bühnen J, Blum WF, Pfeiffer AF. Retinal photocoagulation does not influence intraocular levels of IGF-I, IGF-II and IGF-BP3 in proliferative diabetic retinopathy-evidence for combined treatment of PDR with somatostatin analogues and retinal photocoagulation? Hormone and Metabolic Research = Hormon-Und Stoffwechselforschung = Hormones Et Métabolisme. 2001;33:312–6. doi: 10.1055/s-2001-15283. [DOI] [PubMed] [Google Scholar]
- 54.Kielczewski JL, Jarajapu YPR, McFarland EL, Cai J, Afzal A, Li Calzi S, et al. Insulin-like growth factor binding protein-3 mediates vascular repair by enhancing nitric oxide generation. Circulation Research. 2009;105:897–905. doi: 10.1161/CIRCRESAHA.109.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–9. [PubMed] [Google Scholar]
- 56.Li Calzi S, Purich DL, Chang KH, Afzal A, Nakagawa T, Busik JV, et al. Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation: evidence for blunted responsiveness in diabetes. Diabetes. 2008;57:2488–94. doi: 10.2337/db08-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lofqvist C, Chen J, Connor KM, Smith ACH, Aderman CM, Liu N, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10589–94. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kielczewski JL, Hu P, Shaw LC, Li Calzi S, Mames RN, Gardiner Ta, et al. Novel protective properties of IGFBP-3 result in enhanced pericyte ensheathment, reduced microglial activation, increased microglial apoptosis, and neuronal protection after ischemic retinal injury. The American Journal of Pathology. 2011;178:1517–28. doi: 10.1016/j.ajpath.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies MH, Eubanks JP, Powers MR. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Molecular Vision. 2006;12:467–77. [PubMed] [Google Scholar]
- 60.Lee YC, Jogie-Brahim S, Lee DY, Han J, Harada A, Murphy LJ, et al. Insulin-like growth factor-binding protein-3 (IGFBP-3) blocks the effects of asthma by negatively regulating NF-κB signaling through IGFBP-3R-mediated activation of caspases. The Journal of Biological Chemistry. 2011;286:17898–909. doi: 10.1074/jbc.M111.231035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeschke MG, Barrow RE, Herndon DN. Insulinlike growth factor I plus insulinlike growth factor binding protein 3 attenuates the proinflammatory acute phase response in severely burned children. Annals of Surgery. 2000;231:246–52. doi: 10.1097/00000658-200002000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spies M, Wolf SE, Barrow RE, Jeschke MG, Herndon DN. Modulation of types I and II acute phase reactants with insulin-like growth factor-1/binding protein-3 complex in severely burned children. Critical Care Medicine. 2002;30:83–8. doi: 10.1097/00003246-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 63.Jarajapu YPR, Cai J, Yan Y, Li Calzi S, Kielczewski JL, Hu P, et al. Protection of blood retinal barrier and systemic vasculature by insulin-like growth factor binding protein-3. PloS One. 2012;7:e39398. doi: 10.1371/journal.pone.0039398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Bao S, Lai D, Rapkins RW, Gillies MC. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. 2008;57:1026–33. doi: 10.2337/db07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kielczewski JL, Li Calzi S, Shaw LC, Cai J, Qi X, Ruan Q, et al. Free insulin-like growth factor binding protein-3 (IGFBP-3) reduces retinal vascular permeability in association with a reduction of acid sphingomyelinase (ASMase) Investigative Ophthalmology & Visual Science. 2011;52:8278–86. doi: 10.1167/iovs.11-8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller EC, Capps BE, Sanghani RR, Clemmons DR, Maile La. Regulation of igf-I signaling in retinal endothelial cells by hyperglycemia. Investigative Ophthalmology & Visual Science. 2007;48:3878–87. doi: 10.1167/iovs.07-0014. [DOI] [PubMed] [Google Scholar]
- 67.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nature Medicine. 2001;7:853–7. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 68.Trigatti B, Rigotti A, Krieger M. The role of the high-density lipoprotein receptor SR-BI in cholesterol metabolism. Current Opinion in Lipidology. 2000;11:123–31. doi: 10.1097/00041433-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Nofer J-R, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. The Journal of Clinical Investigation. 2004;113:569–81. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson SH, Davis MI, Caballero S, Grant MB. Modulation of retinal endothelial cell behaviour by insulin-like growth factor I and somatostatin analogues: implications for diabetic retinopathy. Growth Hormone & IGF Research : Official Journal of the Growth Hormone Research Society and the International IGF Research Society. 2001;11(Suppl A):S53–9. doi: 10.1016/s1096-6374(01)80009-5. [DOI] [PubMed] [Google Scholar]
- 71.Ning A, Cui J, Maberley D, Ma P, Matsubara J. Expression of integrins in human proliferative diabetic retinopathy membranes. Canadian Journal of Ophthalmology Journal Canadien D’ophtalmologie. 2008;43:683–8. doi: 10.3129/i08-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spoerri PE, Caballero S, Wilson SH, Shaw LC, Grant MB. Expression of IGFBP-3 by human retinal endothelial cell cultures: IGFBP-3 involvement in growth inhibition and apoptosis. Investigative Ophthalmology & Visual Science. 2003;44:365–9. doi: 10.1167/iovs.02-0309. [DOI] [PubMed] [Google Scholar]
- 73.Yoo EG, Lee WJ, Kim JH, Chae HW, Hyun SE, Kim DH, et al. Insulin-like growth factor-binding protein-3 mediates high glucose-induced apoptosis by increasing oxidative stress in proximal tubular epithelial cells. Endocrinology. 2011;152:3135–42. doi: 10.1210/en.2010-1122. [DOI] [PubMed] [Google Scholar]
- 74.Crawford TN, Alfaro DV, Kerrison JB, Jablon EP. Diabetic retinopathy and angiogenesis. Current Diabetes Reviews. 2009;5:8–13. doi: 10.2174/157339909787314149. [DOI] [PubMed] [Google Scholar]
- 75.Rho SB, Dong SM, Kang S, Seo SS, Yoo CW, Lee DO, et al. Insulin-like growth factor-binding protein-5 (IGFBP-5) acts as a tumor suppressor by inhibiting angiogenesis. Carcinogenesis. 2008;29:2106–11. doi: 10.1093/carcin/bgn206. [DOI] [PubMed] [Google Scholar]
- 76.Contois LW, Nugent DP, Caron JM, Cretu A, Tweedie E, Akalu A, et al. Insulin-like growth factor binding protein-4 differentially inhibits growth factor-induced angiogenesis. The Journal of Biological Chemistry. 2012;287:1779–89. doi: 10.1074/jbc.M111.267732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prêle CM, Reichelt ME, Mutsaers SE, Davies M, Delbridge LM, Headrick JP, et al. Insulin-like growth factor-1 overexpression in cardiomyocytes diminishes ex vivo heart functional recovery after acute ischemia. Cardiovascular Pathology : the Official Journal of the Society for Cardiovascular Pathology. 2012;21:17–27. doi: 10.1016/j.carpath.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 78.Panjala SR, Thomas Sa, Steinle JJ. Effects of insulin-like growth factor-1 (IGF-1) receptor signaling on rates of apoptosis in retina of dopamine beta hydroxylase (Dbh−/−) knockout mice. Autonomic Neuroscience : Basic & Clinical. 2010;152:21–6. doi: 10.1016/j.autneu.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Y-C, Buckner BR, Zhu M, Cavanagh HD, Robertson DM. Elevated IGFBP3 levels in diabetic tears: a negative regulator of IGF-1 signaling in the corneal epithelium. The Ocular Surface. 2012;10:100–7. doi: 10.1016/j.jtos.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors. Studies in diabetics with and without retinopathy. The New England Journal of Medicine. 1983;309:527–30. doi: 10.1056/NEJM198309013090904. [DOI] [PubMed] [Google Scholar]
- 81.Dills DG, Moss SE, Klein R, Klein BE. Association of elevated IGF-I levels with increased retinopathy in late-onset diabetes. Diabetes. 1991;40:1725–30. doi: 10.2337/diab.40.12.1725. [DOI] [PubMed] [Google Scholar]
- 82.Hyer SL, Sharp PS, Brooks RA, Burrin JM, Kohner EM. A two-year follow-up study of serum insulinlike growth factor-I in diabetics with retinopathy. Metabolism: Clinical and Experimental. 1989;38:586–9. doi: 10.1016/0026-0495(89)90222-9. [DOI] [PubMed] [Google Scholar]
- 83.Chantelau E, Eggert H, Seppel T, Schönau E, Althaus C. Elevation of serum IGF-1 precedes proliferative diabetic retinopathy in Mauriac’s syndrome. The British Journal of Ophthalmology. 1997;81:169–70. doi: 10.1136/bjo.81.2.168b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feldmann B, Jehle PM, Mohan S, Lang GE, Lang GK, Brueckel J, et al. Diabetic retinopathy is associated with decreased serum levels of free IGF-I and changes of IGF-binding proteins. Growth Hormone & IGF Research : Official Journal of the Growth Hormone Research Society and the International IGF Research Society. 2000;10:53–9. doi: 10.1054/ghir.2000.0140. [DOI] [PubMed] [Google Scholar]
- 85.Frystyk J, Bek T, Flyvbjerg A, Skjaerbaek C, Ørskov H. The relationship between the circulating IGF system and the presence of retinopathy in Type 1 diabetic patients. Diabetic Medicine : a Journal of the British Diabetic Association. 2003;20:269–76. doi: 10.1046/j.1464-5491.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 86.Fujihara M, Uemasu J, Kawasaki H. Serum and urinary levels of insulin-like growth factor I in patients with chronic renal disease and diabetes mellitus: its clinical implication. Clinical Nephrology. 1996;45:372–8. [PubMed] [Google Scholar]
- 87.Wedrychowicz A, Dziatkowiak H, Nazim J, Sztefko K. Insulin-like growth factor-1 and its binding proteins, IGFBP-1 and IGFBP-3, in adolescents with type-1 diabetes mellitus and microalbuminuria. Hormone Research. 2005;63:245–51. doi: 10.1159/000085941. [DOI] [PubMed] [Google Scholar]
- 88.Bereket A, Lang CH, Wilson TA. Alterations in the growth hormone-insulin-like growth factor axis in insulin dependent diabetes mellitus. Hormone and Metabolic Research = Hormon- Und Stoffwechselforschung = Hormones Et Métabolisme. n.d;31:172–81. doi: 10.1055/s-2007-978716. [DOI] [PubMed] [Google Scholar]
- 89.Chiarelli F, Giannini C, Mohn A. Growth, growth factors and diabetes. European Journal of Endocrinology/European Federation of Endocrine Societies. 2004;151(Suppl):U109–17. doi: 10.1530/eje.0.151u109. [DOI] [PubMed] [Google Scholar]
- 90.Feldmann B, Lang GE, Arnavaz A, Jehle PM, Böhm BO, Lang GK. Decreased serum level of free bioavailable IGF-I in patients with diabetic retinopathy. Der Ophthalmologe : Zeitschrift Der Deutschen Ophthalmologischen Gesellschaft. 1999;96:300–5. doi: 10.1007/s003470050409. [DOI] [PubMed] [Google Scholar]
- 91.Capoluongo E, Pitocco D, Lulli P, Minucci A, Santonocito C, Manto A, et al. Inverse correlation between serum free IGF-I and IGFBP-3 levels and blood pressure in patients affected with type 1 diabetes. Cytokine. 2006;34:303–11. doi: 10.1016/j.cyto.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 92.Payne JF, Tangpricha V, Cleveland J, Lynn MJ, Ray R, Srivastava SK. Serum insulin-like growth factor-I in diabetic retinopathy. Molecular Vision. 2011;17:2318–24. [PMC free article] [PubMed] [Google Scholar]
- 93.Pastor JC, Universitario I, Aplicada DO. MAJOR REVIEW Proliferative Vitreoretinopathy : An Overview. 1998;43 doi: 10.1016/s0039-6257(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 94.Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye (London, England) 2002;16:369–74. doi: 10.1038/sj.eye.6700194. [DOI] [PubMed] [Google Scholar]
- 95.Pastor JC, de la Rúa ER, Martín F. Proliferative vitreoretinopathy: risk factors and pathobiology. Progress in Retinal and Eye Research. 2002;21:127–44. doi: 10.1016/s1350-9462(01)00023-4. [DOI] [PubMed] [Google Scholar]
- 96.Grierson I, Mazure A, Hogg P, Hiscott P, Sheridan C, Wong D. Non-vascular vitreoretinopathy: the cells and the cellular basis of contraction. Eye (London, England) 1996;10(Pt 6):671–84. doi: 10.1038/eye.1996.160. [DOI] [PubMed] [Google Scholar]
- 97.Peters MA, Burke JM, Clowry M, Abrams GW, Williams GA. Development of traction retinal detachments following intravitreal injections of retinal Muller and pigment epithelial cells. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Für Klinische Und Experimentelle Ophthalmologie. 1986;224:554–63. doi: 10.1007/BF02154745. [DOI] [PubMed] [Google Scholar]
- 98.Yang CH, Huang TF, Liu KR, Chen MS, Hung PT. Inhibition of retinal pigment epithelial cell-induced tractional retinal detachment by disintegrins, a group of Arg-Gly-Asp-containing peptides from viper venom. Investigative Ophthalmology & Visual Science. 1996;37:843–54. [PubMed] [Google Scholar]
- 99.Wong CA, Potter MJ, Cui JZ, Chang TS, Ma P, Maberley AL, et al. Induction of proliferative vitreoretinopathy by a unique line of human retinal pigment epithelial cells. Canadian Journal of Ophthalmology Journal Canadien D’ophtalmologie. 2002;37:211–20. doi: 10.1016/s0008-4182(02)80112-0. [DOI] [PubMed] [Google Scholar]
- 100.Khaliq A, Jarvis-Evans J, McLeod D, Boulton M. Oxygen modulates the response of the retinal pigment epithelium to basic fibroblast growth factor and epidermal growth factor by receptor regulation. Investigative Ophthalmology & Visual Science. 1996;37:436–43. [PubMed] [Google Scholar]
- 101.Hiscott P, Sheridan C, Magee RM, Grierson I. Matrix and the retinal pigment epithelium in proliferative retinal disease. Progress in Retinal and Eye Research. 1999;18:167–90. doi: 10.1016/s1350-9462(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 102.Mukherjee S, King JL, Guidry C. Phenotype-associated changes in retinal pigment epithelial cell expression of insulin-like growth factor binding proteins. Investigative Ophthalmology & Visual Science. 2009;50:5449–55. doi: 10.1167/iovs.09-3383. [DOI] [PubMed] [Google Scholar]
- 103.Mukherjee S, Guidry C. The insulin-like growth factor system modulates retinal pigment epithelial cell tractional force generation. Investigative Ophthalmology & Visual Science. 2007;48:1892–9. doi: 10.1167/iovs.06-1095. [DOI] [PubMed] [Google Scholar]
- 104.Sureshbabu A, Okajima H, Yamanaka D, Shastri S, Tonner E, Rae C, et al. IGFBP-5 induces epithelial and fibroblast responses consistent with the fibrotic response. Biochemical Society Transactions. 2009;37:882–5. doi: 10.1042/BST0370882. [DOI] [PubMed] [Google Scholar]
- 105.Yasuoka H, Yamaguchi Y, Feghali-Bostwick Ca. The pro-fibrotic factor IGFBP-5 induces lung fibroblast and mononuclear cell migration. American Journal of Respiratory Cell and Molecular Biology. 2009;41:179–88. doi: 10.1165/rcmb.2008-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moysidis SN, Thanos A, Vavvas DG. Mechanisms of inflammation in proliferative vitreoretinopathy: from bench to bedside. Mediators of Inflammation. 2012;2012:815937. doi: 10.1155/2012/815937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klein R, Klein BE, Moss SE, Wang Q. Hypertension and retinopathy, arteriolar narrowing, and arteriovenous nicking in a population. Archives of Ophthalmology. 1994;112:92–8. doi: 10.1001/archopht.1994.01090130102026. [DOI] [PubMed] [Google Scholar]
- 108.Katsi V, Marketou M, Vlachopoulos C, Tousoulis D, Souretis G, Papageorgiou N, et al. Impact of Arterial Hypertension on the Eye. Current Hypertension Reports. 2012 doi: 10.1007/s11906-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 109.Wong TY, Klein R, Klein BEK, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Investigative Ophthalmology & Visual Science. 2003;44:4644–50. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 110.Sowers JR. Insulin and insulin-like growth factor in normal and pathological cardiovascular physiology. Hypertension. 1997;29:691–9. doi: 10.1161/01.hyp.29.3.691. [DOI] [PubMed] [Google Scholar]
- 111.Zhang L, Curhan GC, Forman JP. Plasma insulin-like growth factor-1 level and risk of incident hypertension in nondiabetic women. Journal of Hypertension. 2011;29:229–35. doi: 10.1097/HJH.0b013e32834103bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 1999;150:263–70. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 113.Wong TY, Hubbard LD, Klein R, Marino EK, Kronmal R, Sharrett AR, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. The British Journal of Ophthalmology. 2002;86:1007–13. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Landi F, Russo A, Cesari M, Barillaro C, Onder G, Zamboni V, et al. The ilSIRENTE study: a prospective cohort study on persons aged 80 years and older living in a mountain community of Central Italy. Aging Clinical and Experimental Research. 2005;17:486–93. doi: 10.1007/BF03327416. [DOI] [PubMed] [Google Scholar]
- 115.Onder G, Liperoti R, Russo A, Capoluongo E, Minucci A, Lulli P, et al. Use of ACE inhibitors is associated with elevated levels of IGFBP-3 among hypertensive older adults: results from the IlSIRENTE study. European Journal of Clinical Pharmacology. 2007;63:389–95. doi: 10.1007/s00228-007-0262-z. [DOI] [PubMed] [Google Scholar]
- 116.Rabb MF, Gagliano DA, Teske MP. Retinal arterial macroaneurysms. Survey of Ophthalmology. n.d;33:73–96. doi: 10.1016/0039-6257(88)90160-9. [DOI] [PubMed] [Google Scholar]
- 117.DellaCroce JT, Vitale AT. Hypertension and the eye. Current Opinion in Ophthalmology. 2008;19:493–8. doi: 10.1097/ICU.0b013e3283129779. [DOI] [PubMed] [Google Scholar]
- 118.Abu-Safieh L, Abboud EB, Alkuraya H, Shamseldin H, Al-Enzi S, Al-Abdi L, et al. Mutation of IGFBP7 causes upregulation of BRAF/MEK/ERK pathway and familial retinal arterial macroaneurysms. American Journal of Human Genetics. 2011;89:313–9. doi: 10.1016/j.ajhg.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.López-Bermejo A, Khosravi J, Ricart W, Castro A, Hwa V, Pratt KL, et al. Insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1/MAC25) is linked to endothelial-dependent vasodilation in high-ferritin type 2 diabetes. Diabetes Care. 2007;30:1615–7. doi: 10.2337/dc06-1905. [DOI] [PubMed] [Google Scholar]