Abstract
Plasmodium falciparum is the major causative agent of malaria, a disease of worldwide importance. Resistance to current drugs such as chloroquine and mefloquine is spreading at an alarming rate, and our antimalarial armamentarium is almost depleted. The malarial parasite encodes two homologous aspartic proteases, plasmepsins I and II, which are essential components of its hemoglobin-degradation pathway and are novel targets for antimalarial drug development. We have determined the crystal structure of recombinant plasmepsin II complexed with pepstatin A. This represents the first reported crystal structure of a protein from P. falciparum. The crystals contain molecules in two different conformations, revealing a remarkable degree of interdomain flexibility of the enzyme. The structure was used to design a series of selective low molecular weight compounds that inhibit both plasmepsin II and the growth of P. falciparum in culture.
Full text
PDF

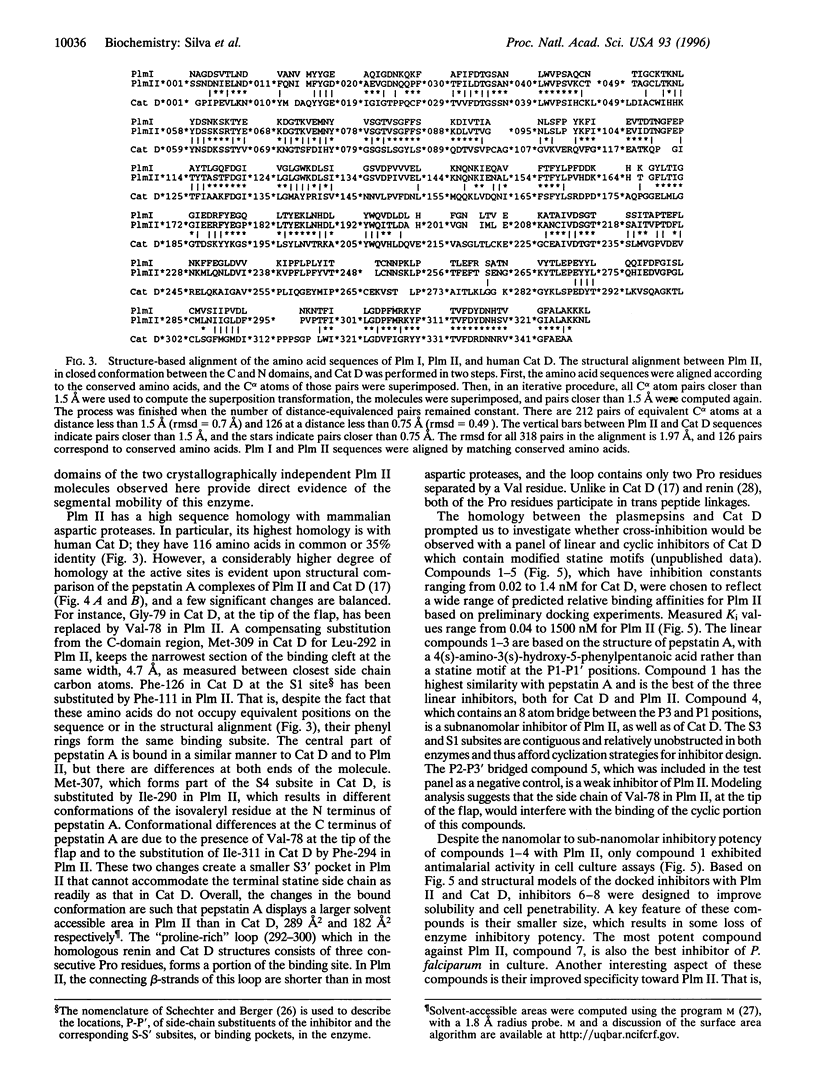
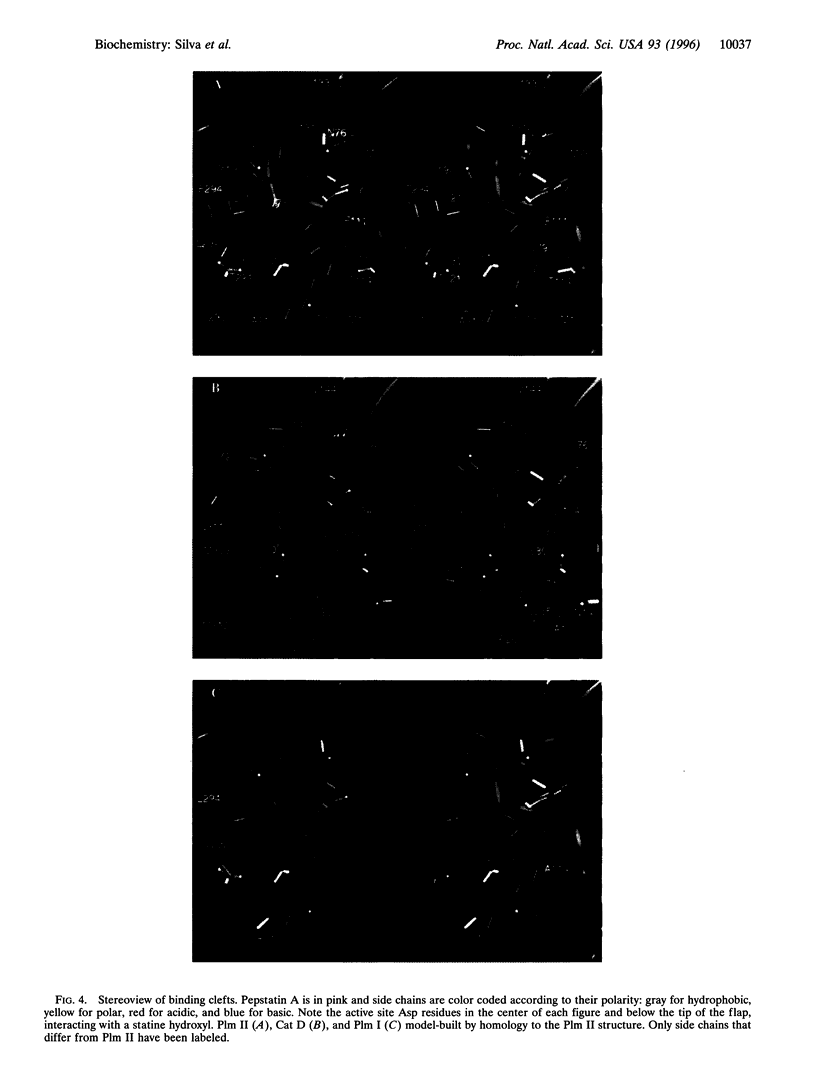
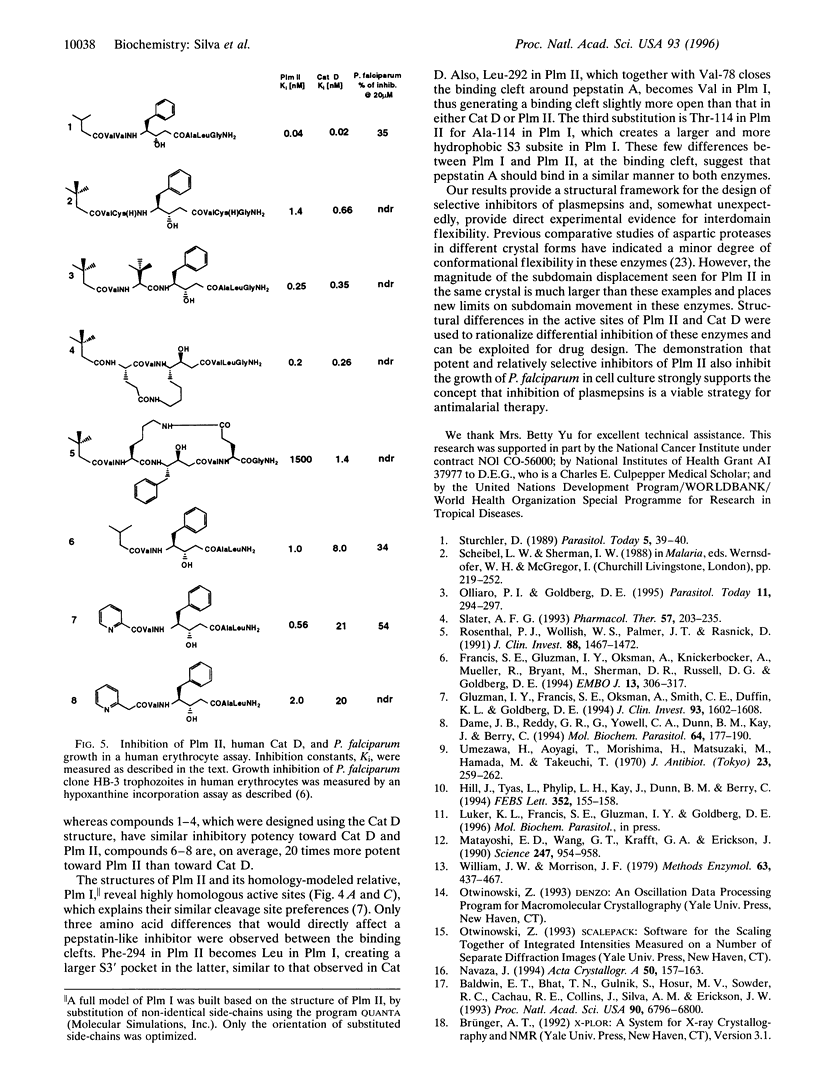

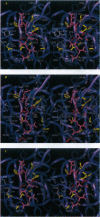
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad-Zapatero C., Rydel T. J., Erickson J. Revised 2.3 A structure of porcine pepsin: evidence for a flexible subdomain. Proteins. 1990;8(1):62–81. doi: 10.1002/prot.340080109. [DOI] [PubMed] [Google Scholar]
- Baldwin E. T., Bhat T. N., Gulnik S., Hosur M. V., Sowder R. C., 2nd, Cachau R. E., Collins J., Silva A. M., Erickson J. W. Crystal structures of native and inhibited forms of human cathepsin D: implications for lysosomal targeting and drug design. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6796–6800. doi: 10.1073/pnas.90.14.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystricky S., Szu S. C. O-acetylation affects the binding properties of the carboxyl groups on the Vi bacterial polysaccharide. Biophys Chem. 1994 Jul;51(1):1–7. doi: 10.1016/0301-4622(94)00002-6. [DOI] [PubMed] [Google Scholar]
- Chu A. H., Ackers G. K. Mutual effects of protons, NaCl, and oxygen on the dimer-tetramer assembly of human hemoglobin. The dimer Bohr effect. J Biol Chem. 1981 Feb 10;256(3):1199–1205. [PubMed] [Google Scholar]
- Davies D. R. The structure and function of the aspartic proteinases. Annu Rev Biophys Biophys Chem. 1990;19:189–215. doi: 10.1146/annurev.bb.19.060190.001201. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Baldwin E. T., Bhat T. N., Gulnik S. Structure of human cathepsin D: comparison of inhibitor binding and subdomain displacement with other aspartic proteases. Adv Exp Med Biol. 1995;362:181–192. doi: 10.1007/978-1-4615-1871-6_22. [DOI] [PubMed] [Google Scholar]
- Francis S. E., Gluzman I. Y., Oksman A., Knickerbocker A., Mueller R., Bryant M. L., Sherman D. R., Russell D. G., Goldberg D. E. Molecular characterization and inhibition of a Plasmodium falciparum aspartic hemoglobinase. EMBO J. 1994 Jan 15;13(2):306–317. doi: 10.1002/j.1460-2075.1994.tb06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein M., Lesk A. M., Chothia C. Structural mechanisms for domain movements in proteins. Biochemistry. 1994 Jun 7;33(22):6739–6749. doi: 10.1021/bi00188a001. [DOI] [PubMed] [Google Scholar]
- Gluzman I. Y., Francis S. E., Oksman A., Smith C. E., Duffin K. L., Goldberg D. E. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J Clin Invest. 1994 Apr;93(4):1602–1608. doi: 10.1172/JCI117140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J., Tyas L., Phylip L. H., Kay J., Dunn B. M., Berry C. High level expression and characterisation of Plasmepsin II, an aspartic proteinase from Plasmodium falciparum. FEBS Lett. 1994 Sep 26;352(2):155–158. doi: 10.1016/0014-5793(94)00940-6. [DOI] [PubMed] [Google Scholar]
- Matayoshi E. D., Wang G. T., Krafft G. A., Erickson J. Novel fluorogenic substrates for assaying retroviral proteases by resonance energy transfer. Science. 1990 Feb 23;247(4945):954–958. doi: 10.1126/science.2106161. [DOI] [PubMed] [Google Scholar]
- Olliaro P. L., Goldberg D. E. The plasmodium digestive vacuole: metabolic headquarters and choice drug target. Parasitol Today. 1995 Aug;11(8):294–297. doi: 10.1016/0169-4758(95)80042-5. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. J., Wollish W. S., Palmer J. T., Rasnick D. Antimalarial effects of peptide inhibitors of a Plasmodium falciparum cysteine proteinase. J Clin Invest. 1991 Nov;88(5):1467–1472. doi: 10.1172/JCI115456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A., Veerapandian B., Cooper J. B., Foundling S. I., Hoover D. J., Blundell T. L. High-resolution X-ray diffraction study of the complex between endothiapepsin and an oligopeptide inhibitor: the analysis of the inhibitor binding and description of the rigid body shift in the enzyme. EMBO J. 1989 Aug;8(8):2179–2188. doi: 10.1002/j.1460-2075.1989.tb08340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Sielecki A. R., Hayakawa K., Fujinaga M., Murphy M. E., Fraser M., Muir A. K., Carilli C. T., Lewicki J. A., Baxter J. D., James M. N. Structure of recombinant human renin, a target for cardiovascular-active drugs, at 2.5 A resolution. Science. 1989 Mar 10;243(4896):1346–1351. doi: 10.1126/science.2493678. [DOI] [PubMed] [Google Scholar]
- Slater A. F. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol Ther. 1993 Feb-Mar;57(2-3):203–235. doi: 10.1016/0163-7258(93)90056-j. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Morishima H., Matsuzaki M., Hamada M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot (Tokyo) 1970 May;23(5):259–262. doi: 10.7164/antibiotics.23.259. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 1979;63:437–467. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]