Abstract
Introduction
Adherence to HIV antiretroviral therapy (ART) is a critical determinant of HIV-1 RNA viral suppression and health outcomes. It is generally accepted that HIV-related stigma is correlated with factors that may undermine ART adherence, but its relationship with ART adherence itself is not well established. We therefore undertook this review to systematically assess the relationship between HIV-related stigma and ART adherence.
Methods
We searched nine electronic databases for published and unpublished literature, with no language restrictions. First we screened the titles and abstracts for studies that potentially contained data on ART adherence. Then we reviewed the full text of these studies to identify articles that reported data on the relationship between ART adherence and either HIV-related stigma or serostatus disclosure. We used the method of meta-synthesis to summarize the findings from the qualitative studies.
Results
Our search protocol yielded 14,854 initial records. After eliminating duplicates and screening the titles and abstracts, we retrieved the full text of 960 journal articles, dissertations and unpublished conference abstracts for review. We included 75 studies conducted among 26,715 HIV-positive persons living in 32 countries worldwide, with less representation of work from Eastern Europe and Central Asia. Among the 34 qualitative studies, our meta-synthesis identified five distinct third-order labels through an inductive process that we categorized as themes and organized in a conceptual model spanning intrapersonal, interpersonal and structural levels. HIV-related stigma undermined ART adherence by compromising general psychological processes, such as adaptive coping and social support. We also identified psychological processes specific to HIV-positive persons driven by predominant stigmatizing attitudes and which undermined adherence, such as internalized stigma and concealment. Adaptive coping and social support were critical determinants of participants’ ability to overcome the structural and economic barriers associated with poverty in order to successfully adhere to ART. Among the 41 quantitative studies, 24 of 33 cross-sectional studies (71%) reported a positive finding between HIV stigma and ART non-adherence, while 6 of 7 longitudinal studies (86%) reported a null finding (Pearson's χ 2=7.7; p=0.005).
Conclusions
We found that HIV-related stigma compromised participants’ abilities to successfully adhere to ART. Interventions to reduce stigma should target multiple levels of influence (intrapersonal, interpersonal and structural) in order to have maximum effectiveness on improving ART adherence.
Keywords: HIV, stigma, disclosure, adherence, social support, poverty
Introduction
Adherence to HIV antiretroviral therapy (ART) is a critical determinant of HIV-1 RNA viral suppression and health outcomes [1–3]. Early studies of ART adherence focused primarily on cognitive processes that may affect adherence, such as forgetfulness and health literacy [4–6]. More recently, investigators have shown that ART adherence in resource-limited settings, where treatment is generally provided free of charge, may be contingent upon structural barriers, such as food insecurity [7–12] or geographic isolation and lack of resources to pay for transportation to clinic [13–17].
The stigma of HIV and AIDS is one social process that has been broadly assumed to adversely affect multiple facets of engagement in HIV-related care as well as other factors that may undermine ART adherence, including HIV serostatus disclosure [18–20], social support [18, 21] and mental wellbeing [21, 22]. Goffman [23] conceptualized stigma as an “attribute that is deeply discrediting” imposed by society that reduces someone “from a whole and usual person to a tainted, discounted one” (p. 3). When the attribute becomes linked to “discrediting dispositions” (e.g., negative evaluations or stereotypes), these may come to be widely believed in the community [24]. During the labelling process [25–27], persons with and without the stigmatized attribute are separated into “them” and “us” [28] and may be subjected to overt acts of hostility and discrimination (enacted stigma) [29]. To avoid the potentially unpleasant consequences of revealing their discredited status, stigmatized persons may elect to conceal their seropositivity from others [20, 30]. Stigmatized persons may also internalize the beliefs held in the community and develop self-defacing internal representations of themselves (internalized stigma) – possibly leading to demoralization, diminished self-efficacy and emotional distress [31, 32].
Despite substantive advances in our understanding of the stigma process, the mechanisms through which stigma compromises ART adherence are not well understood. From a public health perspective, this is an important gap in the literature because sustained adherence [33] is a critical step in the spectrum of engagement in HIV-related care [34, 35]. Although the “test-and-treat” approach [36] has achieved a great deal of popularity in a brief amount of time, observers have expressed concerns that persisting stigma may pose a major obstacle to its success [37]. Therefore, we undertook this review to systematically assess the relationship between HIV-related stigma and ART adherence.
Methods
Search strategy and study selection
Three study authors (AER, AGO, ACT) searched nine electronic databases for published and unpublished literature: BIOSIS Previews, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, the Educational Resources Information Center (ERIC), the Medical Literature Analysis and Retrieval System Online (MEDLINE), ProQuest Dissertations & Theses, PsycINFO, Web of Science (Science Citation Index Expanded, Social Sciences Citation Index, and Arts & Humanities Citation Index) and the World Health Organization African Index Medicus. In general, each set of search terms applied to these databases was oriented towards identifying studies of ART adherence among HIV-positive adults (Box S1). We conducted all searches in May 2011, with the exception of the ProQuest search, which was performed in June 2011. In February 2013, one study author (ACT) updated the MEDLINE search to identify more recent articles published since the study was initiated. We also consulted with experts in the field to identify additional studies that our systematic evidence search may have missed.
First we imported all records into EndNote reference management software (version X4.0.2, Thomson Reuters, Philadelphia, Penn.) and used the automated “Find Duplicates” function to exclude any duplicates. Then we screened the titles and abstracts of all records to identify studies that appeared to be potentially related to ART adherence among HIV-positive persons. We then obtained the full text of these articles for review, specifically to identify articles that reported either a quantitative estimate of association between a measure of stigma or disclosure and a measure of adherence, or qualitative findings about how stigma or lack of disclosure affected adherence. Although our review was focused on the relationship between stigma and adherence, we also chose to include studies examining the impacts of serostatus non-disclosure because it is a proximate consequence of stigma [19, 20]. Our goal in including qualitative studies as part of this systematic review was to inductively develop an in-depth understanding of persistent themes and assess the transferability of these themes across contexts [38]. Due to our interest in describing relationships between stigma and adherence across a wide range of countries, we chose not to exclude any study based on quality, country of origin or language.
Quality assessment
To assess the quality of the included qualitative studies, we adapted questions representing the three key conceptual domains described in the Critical Appraisal Skills Programme quality assessment tool [39, 40]. These domains also mapped onto prominent criteria employed by previous researchers as identified in the review of qualitative quality assessment tools by Tong et al. [41]. The criteria we used were as follows: (1) the role of the researcher was clearly described; (2) the sampling method was clearly described; (3) the method of data collection was clearly described; and (4) the method of analysis was clearly described. We found that the included qualitative studies consistently described the role of the research and the method of data collection, but many studies reported neither the sampling method nor the method of analysis. Overall, 15 studies were assessed to be at low risk of bias (Table S1).
To assess the quality of the included quantitative studies, we developed an assessment tool based on the six major conceptual domains identified by Sanderson et al. [42]. The criteria we used were as follows: (1) the study was based on a probability sample of participants; (2) the study used a validated self-report scale to measure stigma or disclosure; (3) the study used a validated self-report scale or objective count (e.g., pill count, pharmacy refill) to measure ART adherence; (4) the statistical analysis accounts for missingness at random (MAR) or missingness not at random (MNAR) (longitudinal studies only); (5) the study design or statistical analysis controls or adjusts for potential confounding; and (6) competing interests were declared. Overall, all studies except for one were assessed to be at risk of bias (Table S2).
Data synthesis
We organized studies by year of publication, country of origin, study design and types of measures employed. For the quantitative studies, due to substantial heterogeneity in the measures of stigma, serostatus disclosure and ART adherence that were employed, we did not attempt to summarize the data using meta-analysis. However, we examined patterns across studies with respect to the estimated associations and the precision of these estimates.
For the subset of qualitative studies, our goal was to generate new theoretical insights. Therefore, we used the iterative process of meta-synthesis proposed by Noblit and Hare [43] to identify themes that recurred frequently or were prominently featured throughout the data. Meta-synthesis (also described as meta-ethnography) is an interpretive approach to summarizing qualitative research that has been employed to understand vaginal practices in sub-Saharan Africa [44], delays in presentation for cancer care [45] and adherence to tuberculosis treatment [46]. Key themes and concepts were collected and peer-reviewed for inclusiveness. First-order findings (quotations) were used to support second-order interpretations (authors’ analyses) to gain new insight into the relationships between stigma and ART adherence. A summary definition of second-order constructs was generated for further clarification and then consolidated into a line of argument that led to a third-order analysis, which we describe below. Based upon the data set, we achieved theoretical saturation within the first 10 manuscripts, although basic elements for meta-themes were evident as early as six manuscripts. Variability within the data followed similar patterns, consistent with prior qualitative meta-synthesis research [47].
Results
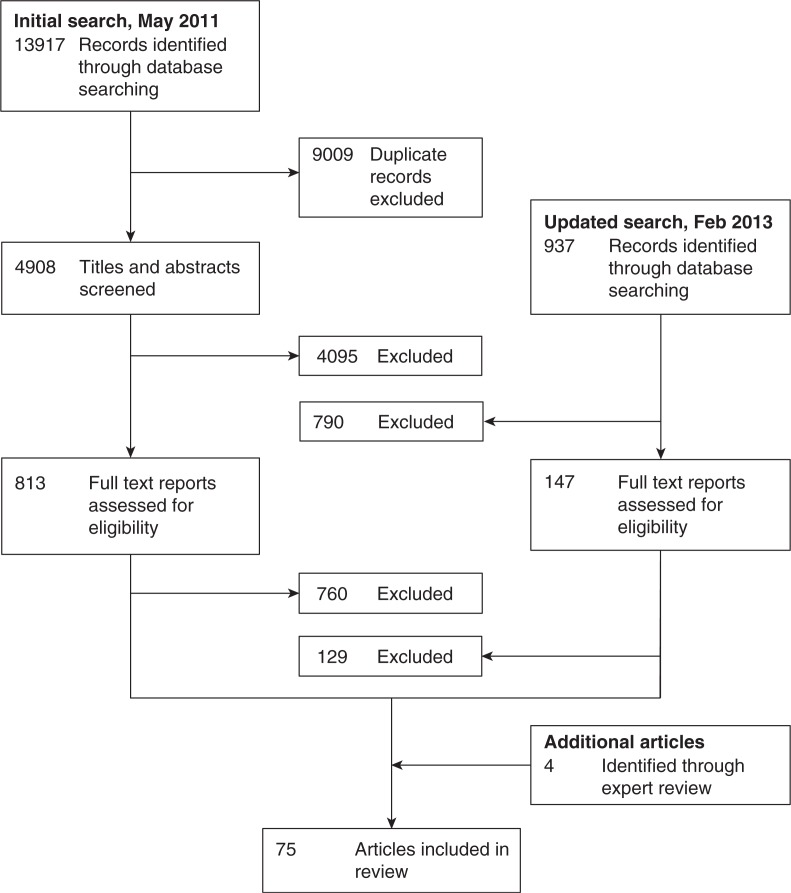
Our initial search yielded 14,854 records, of which 9009 were identified as duplicates through the use of automated software (Figure 1). After screening the titles and abstracts of the remaining 5845 records, we eliminated 4000 records that did not appear to contain relevant data on adherence or provided potentially relevant adherence data specific to a specialized population (e.g., children or pregnant women), eight unpublished conference abstracts or dissertations matched to subsequently published peer-reviewed journal articles in our database of records, 199 reviews that did not report original data, and 678 additional duplicates that had been misclassified as non-duplicates by the automated software. We retrieved 960 journal articles, unpublished dissertations and conference abstracts for full text review. Of these, 889 did not contain quantitative or qualitative data relating stigma or disclosure to ART adherence and were therefore excluded. Expert review suggested four additional articles for inclusion. The final sample included 75 studies: 34 qualitative studies and 41 quantitative studies.
Figure 1.
Flow diagram. We identified 14,854 records by searching nine electronic databases, yielding 34 qualitative studies and 41 quantitative studies.
Synthesis of qualitative studies
Thirty-four qualitative studies conducted during 1999–2013 were included in the review, including one written in French. Represented in these manuscripts were views from 1328 study participants in 26 countries. Of note, only one country from the UNAIDS Eastern Europe and Central Asia region was represented: Serbia and Montenegro. The median number of participants was 38 (interquartile range (IQR), 27 to 48; range, 6 to 118). Participants included adult men and women ranging in age from 18 years to over 60 years old, HIV-positive persons as well as providers of HIV care, single persons and those in intimate partnerships, and persons with and without children. Specific high-risk groups were well represented and included men who have sex with men, injection drug users and commercial sex workers.
After reviewing each of the qualitative studies in detail, we identified 24 second-order constructs, supported by original quotes, in multiple manuscripts. Second-order constructs relevant to ART adherence were identified, and key themes were generated into a line of argument that led to 15 third-order constructs. These were grouped into five distinct third-order labels that we categorized as themes, all of which are described in detail in Table 1.
Table 1.
Qualitative studies on stigma, disclosure and ART adherence (N=34)
| Third-order labels | Third-order constructs | Second-order constructs | Summary definition | First-order constructs | Source(s) |
|---|---|---|---|---|---|
| Social support | Intimate and familial relationships | Spousal, peer and familial support | Participants discussed support from spouses, peers and family as critical for overcoming stigma and maintaining adherence, as was having a sense of obligation to family |
Well, they encourage me, like my folks have [said] ‘you took your medication today?’ [55, p. 5] I am thankful to God for giving me such a good husband. He takes care of me well. I have given him a lot of trouble. He has spent so much money for my treatment. [54, p. 496] |
[48–70, 78, 79] |
| Context of male-dominated household decision-making | In cultures where men are typically heads of their households, women fear disclosing their serostatus as they fear social isolation and abandonment. Women may choose to have providers give the test information to their husbands by bringing them in for testing. In addition, in some cultures, women cannot travel alone to clinic to pick up their medications. | [After testing positive] I went back home and first kept quiet for two days. I asked myself, how can I approach him to tell him? One day when he came back, I told him, they checked my blood but they refused to give me the results until I take my spouse in for testing. I convinced him and he accompanied me. [57, p. S88] | [49, 52, 54, 57, 64, 68, 72] | ||
| Healthy children reducing stigma | Clinical response to ART in children of HIV-positive mothers reduces stigma and often re-establishes mother's role in family |
Then when she saw that since giving birth, my baby was not falling sick (the other children used to be sickly), that my baby was looking nice, did not have a rash, and was growing fast she said ‘I used to think you were infected. I had taken you out of all of my plans.’ I responded that, ‘I am not infected, don't you see my baby?’ So that's where I ended her suspicions about my being sick. Now she knows that I am not infected, which is not true. [57, p. S88] |
[57] | ||
| Compromised relationships | Physical manifestations of HIV and AIDS leads to social isolation | Physical signs of ill health may lead to abandonment or to the belief that the HIV-positive person is already dead | These days when people come to know that you have AIDS they don't want to come near you, as if you are an abominable thing (‘bakwenyinyala’). You cannot feel free. Wherever you go they start talking, ‘See that one, she is sick.’ [57, p. S88]) | [55–57, 64–67, 69, 71, 72] | |
| Complex regimens with large numbers of medications | Complex regimens characterized by a large pill burden that required undesired disclosure in order to adhere | … things got messed up, like my schedule, wherever you go, you got to bring the medicine pack, it's even upsetting to open a bunch of medicines. [53, p. 3] Our guests were at my home; I didn't feel comfortable pulling out my drug boxes, then I forgot and missed my drugs. [74, p. 467] | [12, 52, 53, 55, 59–61, 63–65, 69, 72–74] | ||
| Social rejection | Participants adopted strategies of concealment because they feared ridicule or discrimination if they disclosed their HIV status or if they were seen taking their medications | My company made it hard. You know, because I felt like I had to hide my medicine, you know? All, you know, for shame. [55, p. 5] Ordinary public thinks that if they mingle along with the patient means they will get HIV. [48, p. 532] | [12, 48, 49, 54–56, 59, 64–72, 76, 77] | ||
| Treatment side-effects | Observable side-effects of medications (e.g., dysmorphic body changes) carried stigma | It wasn't hard for me to take my medicines; it was the things that people would say … [55, p. 5] The medications compounded the way I felt, how badly I felt, but I kept taking them because I knew it was temporary. [74, p. 466] | [12, 53, 55, 56, 60, 61, 63–66, 68, 71, 73, 74, 76] | ||
| Negotiating disclosure to a child | Stigma associated with a child's HIV status | Maternal shame and stigma related to perinatal acquisition of HIV kept them from informing HIV-positive children about their seropositivity, with attendant challenges in ART adherence | The thing that disturbs me is that I always think what will I tell my child when he grows to a level of understanding and he asks me why he is taking drugs. Because even now he asks me, ‘Mummy I no longer cough but why am I still taking drugs every day?’ What will I tell the child?’ [57, p. S88] | [48, 53, 57, 64] | |
| Self-Identity | Race/minority status | Outsider status based on race | HIV-positive persons who belonged to racial minority groups felt further stigmatized and socially isolated | [49, 55] | |
| Sexual orientation/relationship status | Impact of social norms on stigma and willingness to disclose | Social norms further stigmatized HIV-positive persons if the mode of acquisition was not regarded as socially acceptable behavior | In the gay community, I can't go to somebody and say, ‘I'm HIV.’ People avoid the subject. They do not disclose it. [51, p. 906] | [50, 51, 54, 61–63, 71–74, 76, 77] | |
| Substance abuse | Social marginalization of injection drug use intensified for HIV-positive users | Participants who actively used illicit substances discussed being unable to establish relationships with HIV-negative persons or non-injection drug users, and feeling socially isolated | Drug users, it's a group that right now everyone in society hates. Including myself, I hate myself. But the problem is [that] there is nothing I can do. [77, p. 1244] | [51, 77] | |
| Redefining healthy living | Self-perception as pro-active/choosing to be healthy | Participants described knowing friends who died from AIDS and not wanting to be like them; the notion of “choosing to live” [74, p. 466] | Then I had some friends die of full-blown AIDS, and I looked around and seen what a horrible death that was … And so I know I wanted to live, and I wouldn't want to send my family through that. So I knew I had to take my medicine. [55, p .4] I didn't want to start drugs, but I had seen two AIDS patients dead. They hadn't used drugs. [74, p. 466] | [52–56, 58, 59, 61, 66, 70, 72–74] | |
| Acceptance of status | Self-identifying as someone who is HIV-positive | Participants who had accepted their status found it easier to adhere vs. those who had difficulty taking medications because it reminded them of their seropositivity | The thing is it's my life, you know. I don't see it much if somebody comes to me and tells me that, ‘you've got HIV – you are HIV’. I don't have a problem with that because that's not his problem, that's my problem you know. As long as I know how I manage it, I don't give a damn about any other person. [56, p. 303] | [50, 56, 67, 69, 70, 73, 74] | |
| Poverty | Economic implications of HIV | Mutually reinforcing relationship between poverty and stigma | HIV-related illness and perceived economic inadequacy leading to social exclusion | They see it as useless to assist someone who has a shorter time to live. It's like wasting money. Why assist someone who is going to die? [67, p. 1311] There is no need to waste any more money on her, give me this lady and I will put her in the car and take her to her rural home with her children. [72, p. 875] With ART, I have returned to work and earn money; friends who avoided me in the past are now more accepting of me … If I do not take this medicine as I am told, I will get sick and | [54, 56, 67, 72] |
| won't be able to work again. People will also begin to avoid me again. [72, p. 877] | |||||
| Economic insecurity resulting from HIV-related stigma | “I thought that people would know my HIV status when I have illnesses regularly and am out of the office several times.” [67, p. 1311] | [54, 67, 72] | |||
| Costs associated with treatment | Costs associated with purchasing medications or with travel to the treatment centre (along with loss of wages) made even free ART prohibitively expensive for some, leading to treatment interruptions | Even if I go for work I get Rs 100 in which 60 goes for tablets. So in the rest I have to manage the other expenses, which is very difficult. Medicines for HIV infection should be like other general medicines where everyone can afford to buy. Now I am not sure I can continue the treatment for a long time. [48, p. 529] | [12, 48, 54, 60, 61, 64, 67, 68, 70, 72, 76, 77] | ||
| Coping | Maladaptive strategies | Anger at diagnosis | Inability to accept diagnosis and anger at diagnosis, with associated inability to engage in HIV care and adhere to ART | I was mad, and I was upset, and I was in denial. And it took me five years to tell anybody that was close to me. So I kept that to myself for a long time, and I was very angry. Right now, I still don't take [the medicines] like I should. [55, p. 4] | [55, 72] |
| Substance use and abuse | Consumption of alcohol and use of drugs provided a temporary refuge but also made ART adherence more difficult | … I began to skip the medication. I said to myself, ‘Well, today I'm not taking it, ‘cause I'm gonna party … [drink] Come on, I was born to party … [53, p. 3] | [52, 53, 59, 73, 78] | ||
| Fear that drugs are dangerous and/or that HIV is a curse fuelled by stigma | Participants expressed concerns about taking medications feared to be dangerous or toxic | Rural people do still not believe this medicine [ART] works for HIV patients. HIV people will die eventually either taking or not taking ART. Why should I die by taking these malicious pills? [68, p. 3] | [12, 68, 71, 72] | ||
| Acceptance | Knowledge that taking medications will provide benefits | Acceptance of the diagnosis counter-balanced stigma, as participants described moving on a continuum from willingness to take medications, to engagement in pro-active healthy lifestyle changes | This is your own responsibility. You know what you got. You know you got medicine to take. No matter what nobody else say or how peoples feel about it, you got to take care of yourself first. [55, p. 4] | [54–56, 58, 59, 66, 67, 69, 70, 72–74] | |
| During [the] last 5 years, taking medications showed me its benefits. My CD4 cells [sic] count was 80, with high viral loads, but now I am okay. They actually helped and gave me more longevity. [74, p. 467] | |||||
| Mental wellbeing | Treatment of depression and anxiety related to diagnosis | Treatment of depression resulting from HIV diagnosis could ameliorate stigma and social isolation | [49, 57, 65, 67, 69, 72, 73, 77] | ||
| Morality and spirituality | Notion of God's will | Participants discussed relinquishing control of their lives to God and putting their faith in a higher power to help them overcome adversity | I just want to be a living witness, that God has all power. He can do all things, and I put my faith and trust in Him. [55, p. 4–5] I believe in the power of prayers – I believe in my church. It's got hope for me … because I have a feeling that God loves us … God is the person that gave you that disease, and God is the person who can take it out from you … You have to have faith in that. [56, p. 305] | [12, 52, 54–61, 67, 69, 72] | |
| Health systems | Importance placed in clinical support staff | Nursing and physician support to gain trust and overcome social isolation associated with stigma | Programs supporting social support and building trust with the adherence nurse or doctor were described as essential for people who reported stigma as a barrier to ART adherence |
I felt so alone. It's nice to know that somebody does understand what it is all about and you can depend on that person. [75, p. 117] I trust the doctors and nurses. Therefore I started the drugs. [74, p. 466] |
[50, 55, 58–60, 62, 63, 67, 69, 70, 72–75, 80] |
| Support in designing tolerable combination of medications that are easily available | Participants felt it was easiest to adhere if they were on tolerable medications and if providers were available in the event of adverse side effects vs. those who feared taking medications because of potential side effects or complications. It was also important to ensure that there were no stock-outs and that medications were easily available. |
I didn't know the advantages of medications, I feared the complications; therefore, I started it very late. Actually, it was [a] wasting of my time. [74, p. 466] We can't have any plan, because we don't know when supplies will fail. Some people can get medicine and some can't. [80, p. 317] |
[55, 58–60, 73, 74, 80] | ||
| Family-driven treatment | Establishing treatment for all members of the household | Treatment to all HIV-positive members of a family (including spouse and children) provided support to overcome stigma and improve medication adherence | [54, 57] |
Theme 1: social support
The most commonly cited theme related to ART adherence was the role of social support. Specifically, participants described spousal or familial support as being critical for enabling them to overcome enactments of HIV-related stigma and other obstacles to care and successfully adhere to treatment [48–70]. As noted by one 45 year-old HIV-positive rice dealer in Chennai, India,
A person without a family is like a single tree struggling for life. My children and my wife are my backbone. Now I have brought changes in myself and want to achieve many things. [54, p. 496]
Compromised relationships could result from either HIV illness or HIV treatment. Many participants described being socially isolated due to the physical manifestations of HIV-related illness [55–57, 64–67, 69, 71, 72] . As described by one HIV-positive mother in Kampala, Uganda,
These days when people come to know that you have AIDS they don't want to come near you, as if you are an abominable thing (‘bakwenyinyala’). You cannot feel free. Wherever you go they start talking, ‘See that one, she is sick’. [57, p. S88]
On the other hand, HIV treatment could also undermine social relationships. Unintended disclosure was viewed as a consequence of being on complex regimens that often needed to be taken multiple times per day [12, 52, 53, 55, 59–61, 63–65, 69, 72–74]. This was commonly discussed in some of the older studies, which were conducted during a time when pill burden was high and participants reported difficulty in understanding when and how to take their medications [12, 50, 52, 58, 60, 61, 64, 67, 68, 70, 74, 75]. Attempts at concealment, such as by hiding medications or furtively taking medications, were described as contributing to treatment interruptions [12, 48, 49, 54–56, 64–72, 76, 77].
In addition, some participants felt that the medications themselves were associated with side effects that had unwelcome physical manifestations:
[ART] has given more side-effects for me such as vomiting, herpes/zoster, and skin rashes. I have lost my sight in my right eye and my left eye also has poor vision.
– HIV-positive woman from far western Nepal [68, p. 7]
Desire to avoid these physical stigmas, or fear of “the thing [sic] that people would say” [55, p. 102], motivated some participants to avoid taking medications and evade detection.
A more circumscribed discussion in the literature related to norms about gender roles, particularly in patriarchal cultures. Byakika-Tusiime et al. [57] explained how HIV-positive women were better able to adhere to ART when others did not identify them as being infected with HIV. An HIV-positive mother could evade detection by giving birth to an uninfected child and establishing her role as a caretaker. This was discussed by an HIV-positive mother in Kampala, Uganda, who described how giving birth to a healthy baby changed her family's assumptions about the inevitability of her death:
When [my sister] saw that since giving birth, my baby was not falling sick (the other children used to be sickly), that my baby was looking nice, did not have a rash, and was growing fast she said ‘I used to think you were infected. I had taken you out of all my plans.’ I responded that ‘I am not infected, don't you see my baby?’ So that's where I ended her suspicions about my being sick. Now she knows that I am not infected, which is not true. [57, p. S88]
Other authors mentioned the importance of women being able to hide their seropositivity in settings where men dominated household decision-making, so as to avoid social isolation and/or abandonment [49, 52, 54, 64, 68, 72]. In these settings, some women reported relying on healthcare providers to inform their sexual partners of their HIV status rather than informing their partners directly themselves.
Women who gave birth to an HIV-positive child experienced feelings of shame and social rejection, both within and outside of the family. Participants in these studies discussed the difficulty associated with disclosing the status of an HIV-positive child, particularly in communities where HIV was highly stigmatized and where appearing ill often led to abandonment by one's family and community [48, 53, 55–57, 64–67, 69, 71, 72] .
The thing that disturbs me is that I always think what will I tell my child when he grows to a level of understanding and he asks me why he is taking drugs. Because even now he asks me, ‘Mummy, I no longer cough but why am I still taking drugs every day?’ What will I tell the child?’
– HIV-positive mother from Kampala, Uganda [57, p. S88]
Theme 2: self-identity
Self-identity was another prominent theme identified in these studies. Multiple studies elaborated on how social norms intensified the stigma of HIV and undercut participants’ willingness to disclose to others [50, 51, 54, 61–63, 71–74, 76, 77] . In many settings, study participants described HIV-related stigma as being layered on top of pre-existing inequalities, such as those related to gender, race or sexual minority status:
I often hear my friends speak negatively about people being HIV-positive. They always have degrading or negative remarks to make. What I dislike most is when they call people names (e.g., fagot, whore, and junkie). Whenever I go out with them or they come over to visit, I don't take my medications. I could never let them know I'm positive.
– HIV-positive African-American woman living in Baltimore, U.S. [49, p. 684]
Konkle-Parker et al. [55] and Edwards [49] both discussed the difficulty that persons in a minority group experienced when self-identifying as HIV-positive, since it often led to further enactments of stigma, including overt discrimination and/or acts of hostility. In such a setting (and consistent with Theme 1), many participants opted not to take their medications for fear of disclosure. Ware et al. [51] and Sabin et al. [77] described the added burden and social isolation that accompanied an HIV diagnosis among participants who actively used illicit substances. In these cases, self-efficacy was often low, and the lifestyle modifications required to achieve consistent adherence proved to be challenging for participants.
Drug users, it's a group that right now everyone in society hates. Including myself, I hate myself. But the problem is [that] there is nothing I can do.
– 40-year-old, injection drug using, HIV-positive married man living in Old Dali, Yunnan Province, China [77, p. 1244]
The experiences of persons who had internalized the stigma of HIV was contrasted with reports of persons who had accepted their HIV status and who had successfully cultivated a self-perception of being pro-active and “choosing to live” [74, p. 466]. These participants were able to successfully adhere to their ART regimens [52–56, 58, 59, 61, 66, 72–74] . In these studies, participants described how the deaths of HIV-positive friends motivated them to take responsibility for their own treatment. Some participants also described feeling strong enough to continue to work and provide for their families.
Then I had some friends die of full-blown AIDS, and I looked around and seen what a horrible death that was … And so I know I wanted to live, and I wouldn't want to send my family through that. So I knew I had to take my medicine and … I know I wants to live
– HIV-positive African-American study participant from Mississippi [55, p. 4]
Theme 3: poverty
In several studies, participants also described how poverty and stigma were intertwined in a reciprocal and mutually reinforcing relationship (Figure 2). Participants spoke of being viewed as weak, unproductive members of society and of being excluded from informal networks of mutual aid:
They see it as useless to assist someone who has a shorter time to live. It's like wasting money. Why assist someone who is going to die?
– HIV-positive person living in Dar es Salaam, Tanzania [67, p. 1311]
Thus, conditions of poverty worsened stigma by emphasizing one's economic worth (or lack thereof) to the community. In resource-limited settings where social networks serve as a form of informal risk-sharing (consistent with Theme 1), and where neighbours often live in close proximity to each other, participants reported feeling ashamed and ultimately more stigmatized by the public nature of unwanted disclosures:
I used to have a neighbour … who knew my status. At times, I used to get porridge from KENWA and bring it home. She had a child who was my kid's friend and age mate. One day, I gave the porridge to her child and [she] was furious and shouted at the little girl; ‘where did you get that porridge? Take it back! You are taking porridge from people with AIDS,’ she was shouting outside and I was in the house.
– HIV-positive woman living in a slum community in Nairobi, Kenya [72, p. 874]
Conversely, stigma was also found to exacerbate the economic impacts of HIV. Economic insecurity resulting from stigma and social isolation was particularly challenging for widowed women who had lost their husbands to AIDS. Tarakeshwar et al. [54] described 9 out of 10 widowed women living in Chennai, India, who were discriminated against, experienced housing insecurity and were isolated by their in-laws after their husbands’ deaths. Stigma was also cited as leading to embarrassment at work, and ultimately causing participants to stop working in order to avoid disclosure, leading to further economic insecurity:
I was on 5 days leave [when I came to test for HIV] and I stayed another week. They were looking for me at work … I was staying [away] because I was sort of embarrassed by my own things. I was embarrassed by my own fate.
– 39-year-old HIV-positive unmarried man living in Gaborone, Botswana [56, p. 304]
Lastly, for participants in resource-limited settings, financial burdens posed a significant barrier to adherence due to costs of the medications themselves, the costs of transportation to pick up free medications from clinic, or wages foregone when attending clinic [12, 48, 54, 60, 61, 64, 67, 68, 70, 72, 76, 77]. These treatment interruptions further compromised participants’ health, reinforcing their status as unproductive members of the community.
Figure 2.
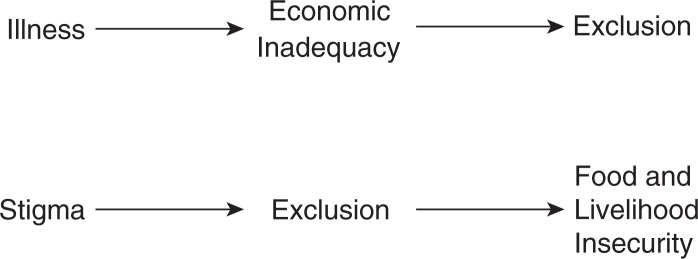
Reciprocal relationships between poverty and stigma. HIV-associated illness reinforces the perceived economic inadequacy of HIV-positive persons, who are excluded from networks of mutual aid. Stigmatized persons are excluded from the community, undermining their social support and worsening economic insecurity.
Theme 4: coping
Coping emerged as a means by which participants attempted to manage stigma and adhere to ART. At times, these coping strategies were maladaptive and detrimental to health. Many participants reported low self-esteem, depressed mood or anger related to their diagnosis, citing their inability to cope with their HIV status as the reason they failed to take their medications [49, 55, 57, 65, 67, 69, 72, 73, 77]:
I was mad, and I was upset, and I was in denial. And it took me five years to tell anybody that was close to me. So I kept that to myself for a long time, and I was very angry. Right now, I still don't take [the medicines] like I should.
– HIV-positive study participant recruited from a large public infectious disease clinic in Mississippi [55, p. 4]
In addition, ART misconceptions (e.g., “Why should I die by taking these malicious pills?”[68, p. 3]) and HIV conspiracy beliefs that were often fuelled by stigma led to ART non-adherence [12, 68, 71, 72]. Participants who lacked the internal resources to cope adaptively described how they self-medicated with alcohol or illicit substances, but these behaviours further compromised their abilities to consistently adhere to treatment [52, 53, 73].
Adaptive coping strategies included those that supported adequate treatment for depression and anxiety, along with acceptance of one's diagnosis. These strategies appeared to provide a protective buffer against stigma and promote acceptance of lifelong treatment [12, 54–56, 58, 61, 67, 69, 72–74] , particularly for those who were able to incorporate these into their new self-identities (consistent with Theme 2). Likewise, spirituality and faith in God enabled some participants to overcome adversity associated with disclosure and HIV-related stigma and to consistently take their medications [12, 52, 54–56, 61, 67, 69, 72] :
I am a Christian and a believer, I know that God exists but those medicines also were inspired by God. God is the one who gave inspiration to doctors to make those medicines for us.
– 59-year-old man on ART, from the Democratic Republic of Congo [12, p. 4]
Theme 5: health systems
A theme common to several studies was that different aspects of the health system could help to moderate the impacts of HIV-related stigma on ART adherence. Specifically, compassionate human capital elements could establish a supportive clinical environment for patients, while certain clinical programs could be designed to address care for the entire family. As noted by one HIV-positive participant in Connecticut,
[The nurses] take care of me, I love the people, they go to your home, like they're my friends. Every time they say, how are you doing? Do you need anything? [75, p. 117].
Doctors and nurses engaged in patient-centred care could help to establish bonds of trust and empower patients to overcome the stigma associated with taking medications [50, 55, 60, 62, 63, 67–70, 72–75, 80]. Some participants described how medication regimens optimized for tolerability, with the fewest side effects and lowest pill burden, allowed them to minimize the possibility that others in the community might recognize their HIV status; this, in turn, decreased stigma and increased participants’ chances of successfully adhering to treatment [55, 58, 60, 73, 74, 80]. Lastly, family-driven treatment programs designed to bring all HIV-positive members of the family into care were thought of as cultivating greater social support, reducing stigma and improving ART adherence [54, 57].
Synthesis of quantitative studies
Data from the quantitative studies were consistent with these lines of inquiry. Our systematic search protocol identified 34 cross-sectional and seven longitudinal studies conducted between 1997 and 2009 that examined the association between either stigma or disclosure and ART adherence (Table 2). These studies included data from 25,387 participants living in 18 different countries, with the largest proportion of studies (15/41 (37%)) based on data collected in the United States. The median number of participants was 300 (IQR, 201–439; range, 65–5760). Twenty-three studies (56%) measured HIV-related stigma, while 21 studies (51%) measured disclosure of seropositivity and three studies (7%) included a measure of both. Most of the studies examining the effect of HIV-related stigma (18/23 (78%)) on ART adherence employed a scale for which some evidence of reliability and/or validity had previously been obtained. In five studies, a multifactor scale was used (28%), while in others specific aspects of HIV-related stigma were measured, including enacted stigma (2/18 (11%)), disclosure concerns (3/18 (16%)), perceived stigma (3/18 (16%)) and internalized stigma (11/18 (61%)) (total percentage exceeds 100% as some studies administered more than one scale). Of the 18 studies that used a formal scale for measuring stigma, only three studies (17%) were conducted in a sub-Saharan African setting, and each of these used a newly developed stigma scale. The most widely used scale, administered in six studies, was the four-factor HIV Stigma Scale developed by Berger et al. [81]. To measure ART adherence, most studies used self-report (30/41 (73%)). Of these, slightly more than half (16/30 (53%)) employed a scale with previously demonstrated evidence of reliability or validity; the AIDS Clinical Trials Group measure developed by Chesney et al. [94] was the most frequently used among these (10/16 (63%)).
Table 2.
Studies reporting a quantitative measure of association between stigma or disclosure and ART adherence (N=41)
| Citation | Study design and population | Study period | Primary stigma or disclosure measure | Primary adherence measure | Findings |
|---|---|---|---|---|---|
| Birbeck et al. [82] | Cross-sectional study of 255 outpatients from 3 clinics in rural Zambia | 2005–06 | Disclosure of HIV seropositivity to spouse, family, friend, or no one | “Good adherence” was defined as (a) attendance at all ART clinic visits, (b) no lapse in drug collection, and (c) no clinic documentation indicating adherence problems | Of those who had not disclosed to anyone, only 17% had good adherence, whereas 50–66% of those who had disclosed to a spouse, family member or friend had good adherence (p=0.047) |
| Adeyemi et al. [83] | Cross-sectional study of 320 outpatients on ART for at least 12 months, recruited in 2 cities in Nigeria | 2009 | Unclear measure (“stigma and discrimination”) | Greater than one week delay in ART refill, as determined by comparison of date of scheduled appointment and date of actual refill | “Stigma and discrimination” was associated with increased odds of delayed ART refill (AOR=1.4; 95% CI=1.1–1.7), after adjusting for distance to clinic and occupation |
| Boyer et al. [84] | Cross-sectional study of 2381 inpatients in 27 national, provincial and district hospitals throughout Cameroon | 2006–07 | Personal experience of HIV-related stigma from partner or close family members | Self-reported ART adherence based on a 14-item scale related to dose-taking and dosing schedule [85], with “non-adherent” persons defined as those who had taken<100% of prescribed doses in the past four weeks but did not report any treatment interruptions lasting>2 consecutive days | Experience of discriminatory behaviours was associated with increased odds of non-adherence (AOR=1.74, 95% CI=1.14–2.65), after adjusting for household income, binge drinking, food insecurity, social support and healthcare supply-related factors |
| Cardarelli et al. [86] | Cross-sectional study of 103 outpatients at a preventive medicine clinic for low-income persons in Texas | 2008a | 40-item HIV stigma scale [81] | Non-adherence was defined as a positive screen on the simplified medication adherence questionnaire, a modified version of the Morisky scale, which contains 6 items related to forgetfulness or carelessness about ART dose taking behavior [87, 88] | The stigma score did not have a statistically significant association with non-adherence (AOR=1.01; 95% CI=0.98–1.03), after adjusting for race, education, racial discrimination, social support, perceived stress or sense of control |
| Carlucci et al. [89] | Cross-sectional study of 424 outpatients at a mission hospital in rural Zambia | 2006 | Single-item question about perceived stigma | Pill count adherence measured over a median of 84 days (interquartile range, 56–98 days), with optimal adherence defined as≥95% doses taken | Perceived stigma did not have a statistically significant association with adherence (AOR=1.1; 95% CI=0.55-2.1), after adjusting for travel time and transportation cost |
| Charurat et al. [90] | Cross-sectional study of 5760 persons initiating ART at five university teaching hospitals in urban Nigeria | 2005–06 | HIV disclosure to spouse or family members | Pharmacy refill adherence rate (days of medication dispensed divided by days between visits), with poor refill adherence defined as<95% adherence | Disclosure was associated with decreased odds of low adherence (AOR=0.85; 95% CI=0.75–0.97), after adjusting for education, employment, distance to clinic |
| and time on ART. There was no univariable association with loss to follow up (OR=0.96; 95% CI=0.82–1.12) | |||||
| Colbert [91] | Cross-sectional analysis of baseline data on 335 persons participating in a 5-year randomized clinical trial conducted in clinics and HIV service organizations in western Pennsylvania and northeast Ohio | 2003–07 | 40-item HIV stigma scale [81] | 30-day adherence as measured with electronic event monitoring, with poor adherence defined as<85% adherence | Neither personalized stigma (AOR=0.98; 95% CI=0.95-1.02) nor negative self-image (AOR=1.00; 95% CI=0.94–1.06) had a statistically significant association with poor adherence, after adjusting for mental health, self-efficacy and health literacy |
| Diiorio et al. [92] | Cross sectional study of 236 outpatients (32% women) from an HIV clinic in Atlanta | 2001–03 | Four items related to internalized stigma from the Perceived Stigma of HIV and AIDS Scale [93] | Five items related to logistical adherence barriers from the ACTG Adherence Instrument [94] | In a structural equation model, stigma had an indirect negative association with adherence: stigma was found to erode self-efficacy, which in turn was directly associated with adherence |
| Dlamini et al. [95] | Longitudinal study of 698 persons (72.3% on ART for more than 1 year) enrolled in a larger cohort in Lesotho, Malawi, South Africa, Swaziland and Tanzania | 2006–07 | 33-item HIV and AIDS Stigma Instrument-PLWA [96] | ACTG Adherence Instrument [94] | Persons who did not report any missing doses experienced a steeper decline in mean stigma over time, after adjusting for education, employment, food insecurity, social support and years since diagnosis |
| Do et al. [97] | Cross-sectional study of 300 outpatients from the largest ART clinic in Botswana | 2005 | Disclosure of seropositivity to a partner | Adherence defined as no missed doses with four-day and one-month recall, and no missed refill visits with 90-day recall | Non-disclosure was associated with an increased odds of non-adherence (p<0.02; AOR not shown), after adjusting for education, employment, travel time, duration of ART, depression, alcohol use and household size |
| Franke et al. [98] | 2-year longitudinal study of 134 adults initiating ART in urban Peru | 2005–09 | 40-item HIV stigma scale [81] | 30-day self-report, with “suboptimal” adherence defined as<95% [94] | On univariable analysis, perceived HIV stigma was not associated with suboptimal adherence (OR=1.03, 95% CI 0.94–1.12) and was not included in the final multivariable model |
| Goldman et al. [99] | Longitudinal study of 913 treatment-naïve adults initiating ART in urban Zambia | 2006–07 | Disclosure of HIV status to partner or spouse | Medication possession ratio based on cumulative days late for pharmacy refill visits, with≥95% defined as optimal adherence | Disclosure did not have a statistically significant association with optimal adherence (estimates not reported) |
| Kalichman et al. [100] | Cross-sectional study of 81 adults recruited from HIV clinical and community support services in Atlanta | 2005a | 4-item self-efficacy for disclosure decisions scale | 6-item standard medication adherence self-efficacy scale [101] | Self-efficacy for disclosure had a statistically significant correlation with self-efficacy for engaging in care (r=0.24, p<0.05) but not with self-efficacy for medication adherence (r=0.19, p>0.05) |
| Kalichman et al. [102] | Cross-sectional study of 145 adults recruited from HIV clinical and community support services in Atlanta | 2008a | 6-item Internalized AIDS-Related Stigma Scale [103] | Monthly unannounced pill count conducted by telephone, averaged over four months, with adherence defined as ≥85% of doses taken | Internalized stigma had no statistically significant association with adherence (AOR=0.99, 95% CI 0.87–1.13) |
| Li et al. [104] | Cross-sectional study of 386 adults (23.9% of whom were treatment-naïve), recruited from four district hospitals throughout Thailand | 2007 | 8-item scale assessing serostatus disclosure to various social ties [105] and 9-item internalized stigma scale [106, 107] | 30-day self-reported adherence, with good adherence defined as no missed doses | Good adherence had a statistically significant association with disclosure (AOR=1.70; 95% CI=1.07–2.70) but not internalized stigma (AOR=0.83; 95% CI=0.51–1.36), after adjusting for education, employment, instrumental social support, depression symptom severity, family functioning and years since diagnosis |
| Li et al. [108] | Cross-sectional study of 202 outpatients enrolled in the Chinese national free ART program, selected from six HIV treatment sites in Hunan Province, China | 2009 | 34-item, five-factor HIV-related stigma scale [109] | Seven-day self-reported ART adherence as measured on a 5-point Likert scale [110] | Stigma was associated with a reduced odds of good adherence (AOR=0.96; 95% CI=0.93–0.98), after adjusting for education, family income, years since diagnosis and recent drug use |
| Lucero et al. [111] | Cross-sectional study of 65 persons aged >50 years recruited from two hospitals in New York City | 2001a | Disclosure of HIV seropositivity to family and friends | Self-report, rated on a 4-point Likert-type scale, with good adherence defined as “taking medication all of the time” | Disclosure was associated with better adherence (estimates not shown) |
| Martinez et al. [112] | Longitudinal study of 178 girls and women aged 15-24 years recruited from 5 cities throughout the U.S. | 2003–05 | The disclosure concerns and negative self-image subscales of the HIV stigma scale [81] | 12-item scale to measure self-reported dosing and scheduling adherence with a two-day recall | Baseline stigma did not have a statistically significant association with complete adherence at 12-month follow-up (b=−0.012, p>0.50). |
| Mo and Mak [113] | Cross-sectional study of 102 adults recruited from an outpatient clinic in Hong Kong | 2009a | 22-item self-stigma scale [114] | ACTG Adherence Instrument [94], with participants classified as “adherers,” “unintentional non-adherers,” or “intentional non-adherers” | Intentional non-adherers had greater self-stigma (4.11, SD 0.74) than adherers (3.78, SD 0.96) and unintentional non-adherers (3.22, SD 0.92) F[1,100]=7.58, p<0.001) |
| Molassiotis et al. [115] | Cross sectional study of 136 adults recruited from an outpatient clinic in Hong Kong | 2002a | HIV disclosure to others, including spouses or partners | ACTG Adherence Instrument [94], with good adherence defined as≥95% adherence | Disclosure did not have a statistically significant association with adherence (estimates not shown) |
| Muyingo et al. [116] | Secondary analysis of data from a randomized trial of 2957 treatment-naïve adults initiating ART at two treatment centres in Uganda and one in Zimbabwe | 2003–04 | Disclosure of HIV serostatus | Drug possession ratio, with complete adherence defined as 100% adherence | Disclosure did not have a statistically significant association with complete adherence (estimates not shown), after adjusting for education and duration of current partnership |
| Nachega et al. [117] | Cross-sectional study of 66 outpatients at an HIV clinic in South Africa | 2002 | Fear of stigma from partner | ACTG Adherence Instrument [94] | On univariable analysis, fear of stigma from partner was associated with reduced odds of >95% adherence (OR=0.13; 95% CI=0.02–0.70) |
| Olowookere et al. [118] | Cross sectional study of 318 adults on ART for at least three months, recruited from a university hospital HIV clinic in Nigeria | 2007 | Disclosure of HIV serostatus | Seven-day self-reported adherence, with non-adherence defined as<95% doses taken | Non-disclosure was associated with increased odds of non-adherence (AOR=1.7; 95% CI=1.0–2.8), after adjusting for transportation costs |
| Peltzer et al. [119] | Cross-sectional study of 735 adults newly initiating ART at one of 3 public hospitals in KwaZulu-Natal, South Africa | 2007–08 | 7-item version of the AIDS-Related Stigma Scale [120], modified to reflect internalized stigma; 7-item AIDS-related discrimination scale | ACTG Adherence Instrument [94] and 30-day visual analogue scale [121], with partial or full adherence defined as ≥95% adherence | Partial or full VAS adherence was associated with AIDS-related discrimination (AOR=0.60; 95% CI=0.46–0.78) but not internalized stigma (OR=1.11; 95% CI=0.97–1.27), after adjusting for alcohol use and social support; use of the ACTG Adherence Instrument yielded similar results |
| Penniman [122] | Secondary analysis of baseline data on 259 women enrolled in a larger cohort study in Los Angeles | 2005–06 | Disclosure of HIV serostatus to child | 3-item self-reported dose-taking and timing adherence with two-day recall | Non-disclosure was associated with reduced odds of adherence (AOR=0.46; 95% CI=0.24–0.88), after adjusting for stress, family functioning and depression symptom severity |
| Peretti-Watel et al. [123] | Cross-sectional study of 2932 adults recruited from 102 hospitals in France | 2003 | Disclosure of HIV serostatus to friends and family; HIV-related discrimination by friends or family | Self-reported measure based on dose and timing adherence with one-week recall, with “high adherence” defined as no doses missed or mistimed | Poor adherence was associated with HIV-related discrimination (AOR=1.68; 95% CI=1.00–2.82) but not selective disclosure to significant others (AOR=0.73; 95% CI=0.28–1.94), after adjustment for alcohol and drug use |
| Rao et al. [124] | Cross-sectional study of 720 outpatients from a university HIV clinic in Seattle | 2009 | Summated rating scale of 4 items related to internalized and enacted stigma, from the 24-item Stigma Scale for Chronic Illness [125] | 3 items from the ACTG Adherence Instrument [94], a one-item rating response measure [126] and a 30-day VAS [121] | In a structural equation model, stigma was associated with reduced adherence (b=–0.21, p<0.01); the authors concluded that the effect was mediated by depression symptom severity |
| Rintamaki et al. [127] | Cross-sectional study of 204 outpatients at two urban academic medical centre clinics in Illinois and Louisiana | 2001 | Summated rating scale of 3 items from the Patient Medication Adherence Questionnaire (PMAQ) [128, 129] related to internalized stigma and disclosure concerns | Non-adherence defined as any missed doses in the prior four days, assessed using the PMAQ | High stigma was associated with greater odds of non-adherence (AOR=3.3; 95% CI=1.4–8.1), after adjusting for race & education |
| Rotheram-Borus et al. [130] | Secondary analysis of baseline data from a randomized controlled trial of 409 adults recruited from 4 district hospitals in northern Thailand | 2009a | 7-item summative rating scale assessing extent of HIV serostatus disclosure to social network ties | Self-reported lifetime adherence, with good adherence defined as never having missed a dose | Disclosure had a statistically significant association with adherence (b=0.11, p<0.05); the authors concluded that disclosure operates primarily through its effect on family functioning |
| Rougemont et al. [131] | Longitudinal study of 312 treatment-naïve adults initiating ART in Yaoundé, Cameroun | 2006–07 | Disclosure of HIV serostatus to family | Pharmacy refill, with “non-adherers” defined as “renewal of prescriptions of later than two weeks” | Non-disclosure did not have a statistically significant association with non-adherence (AOR=0.98; 95% CI=0.81–1.18), after adjustment for income, education and distance to clinic |
| Sayles et al. [132] | Cross-sectional study of 202 adults recruited from 5 community organizations and 2 HIV clinic sites in Los Angeles | 2007 | 28-item internalized stigma scale [133] | Seven-day self-reported ART adherence as measured on a 5-point Likert scale [110], with suboptimal adherence as defined as any response other than “all of the time” | A high level of internalized stigma was not associated with suboptimal adherence (AOR=2.09; 95% CI=0.81–5.39), after adjusting for mental health, race, education, income, insurance and years since diagnosis |
| Spire et al. [134] | Longitudinal study of 445 treatment-naïve adults initiating ART, recruited from 47 hospitals across France | 1997 | Disclosure of HIV serostatus to a family member | Self-reported adherence over prior four days, with “adherent” defined as 100% adherence | 71% of participants who had disclosed to a family member at baseline were classified as adherent four months later, compared to 76% of those who had not disclosed (p=0.26) |
| Stirratt et al. [135] | Cross-sectional study of 215 adults recruited from 2 outpatient HIV clinics in New York City | 2000–04 | Disclosure of HIV serostatus to up to 15 family members and 15 personal contacts [136] | 14-day ART adherence as measured by electronic event monitoring | Percentage of informed family members had a statistically significant association with ART adherence (b=0.21, p<0.05) |
| after adjusting for self-efficacy, motivation and outcome expectancies | |||||
| Sumari-de Boer et al. [137] | Cross-sectional study of 201 outpatients at an academic medical centre HIV clinic in Amsterdam, the Netherlands | 2008–09 | Personalized stigma and disclosure concerns sub-scales of the HIV stigma scale [81] | 30-day pharmacy refill adherence, with non-adherence defined as<100% adherence | Non-adherence had a statistically significant association with disclosure concerns (AOR=1.1; 95% CI=1.01–1.2) but not personalized stigma (AOR not reported), after adjusting for years since diagnosis, quality of life and depression symptom severity |
| Van Dyk [138] | Cross-sectional study of 439 adults recruited from public health HIV clinics and hospitals in Pretoria, South Africa | 2008 | Disclosure of HIV serostatus to partner | 30-day self-reported adherence as elicited through a visual assessment scale [121], with optimum adherence defined as >90% adherence | 41% of participants who had disclosed to partners reported optimum adherence, compared to 21% of participants who had not disclosed (p=0.006) |
| Vanable et al. [139] | Cross sectional study of 221 outpatients in central New York state | 2001 | Five-item frequency of stigma-related experiences scale | Summary self-reported adherence measure averaged across 4 items based on a seven-day recall period | Stigma-related experiences had a negative association with self-reported adherence (b=−0.20, p<0.01), after adjusting for income, employment status and time since diagnosis |
| Waite et al. [140] | Cross-sectional study of 204 outpatients at two urban academic medical centre clinics in Illinois and Louisiana | 2001 | Summated rating scale of 3 items from the Patient Medication Adherence Questionnaire (PMAQ) [128, 129] related to internalized stigma and disclosure concerns | Non-adherence defined as any missed doses in the prior four days, assessed using a modified version of the PMAQ | A high level of stigma was associated with increased odds of non-adherence (AOR=3.1; 95% CI=1.3–7.7), after adjusting for insurance coverage, employment, mental disorder and history of alcohol or drug treatment |
| Wang et al. [141] | Cross-sectional study of 308 adults recruited from seven treatment sites in China | 2006 | Disclosure of HIV serostatus | Seven-day self-reported adherence, with good adherence defined as>90% of doses taken | Disclosure did not have a statistically significant association with adherence (estimates not shown) |
| Watt [142] | Cross sectional study of 340 persons in Tanzania | 2007a | 10-item perceived stigma scale [143], and number of social network ties to whom the participant had disclosed his or her seropositivity | Self-reported missed doses in the prior four days [94], and 30-day self-reported adherence using a modified visual analogue scale [121], with optimal adherence defined as≥95% adherence on both instruments | On univariable analysis, neither stigma nor disclosure had statistically significant associations with optimal adherence (estimates not shown) |
| Weiser et al. [144] | Cross-sectional study of 109 persons recruited from three private clinics in Botswana | 2000 | Disclosure of HIV serostatus | 12-month self-reported adherence [94], with good adherence defined as≥95% of doses taken | On univariable analysis, disclosure did not have a statistically significant association with good adherence (OR=3.55; 95% CI=0.91–13.92) |
| Wolitski et al. [145] | Cross-sectional study of 637 homeless or unstably housed persons in three U.S. cities | 2004 | Modified 6-item internalized and 6-item perceived HIV stigma scales [81] | Self-reported missed doses in the prior two days and seven days | Perceived stigma, but not internalized stigma, was associated with increased odds of missed doses in the past two days (AOR=1.40; 95% CI=1.00–1.95) and past seven days (AOR=1.41; 95% CI=1.05–1.89), after adjusting for housing status, education, and years since HIV diagnosis |
Refers to date of publication, as dates of data collection were not clearly described.
Among the 41 studies, 25 (61%) reported a positive finding (i.e., showing that stigma was associated with reduced ART adherence or that disclosure was associated with improved adherence) while 16 (39%) reported a null finding. No studies reported that better ART adherence was paradoxically associated with greater intensity of stigma or less disclosure. A roughly equal proportion of studies conducted outside of the United States reported a positive finding compared to US-based studies (16/26 (62%) vs. 9/15 (60%); Pearson's χ 2=0.01, p=0.92).
When the studies were disaggregated by study design, most of the cross-sectional studies (24/34 (71%)) reported a positive finding, while most of the longitudinal studies (6/7 (86%)) reported a null finding (Pearson's χ 2=7.7; p=0.005). When disaggregated by exposure, these differences were slightly attenuated. Among studies examining the impact of a stigma variable on adherence, 15/20 (75%) cross-sectional studies vs. 1/3 (33%) longitudinal studies reported a positive finding (Pearson's χ 2=2.14; p=0.14). Among studies examining the impact of disclosure on adherence, 11/17 (65%) cross-sectional studies vs. 0/4 (0%) longitudinal studies reported a positive finding (Pearson's χ 2=5.4; p=0.02).
In three cross-sectional studies, the authors fit structural equation models to investigate the relationships between study variables. Diiorio et al. [92] concluded that the association between stigma and ART adherence was mediated by self-efficacy: perceived stigma eroded one's confidence about adhering to a treatment regimen, which in turn undermined treatment adherence. Rao et al. [124] did not measure self-efficacy but concluded that internalized stigma worsened symptoms of depression, like fatigue and concentration difficulties, which in turn compromised one's ability to adhere to a complex treatment regimen. In the study by Rotheram-Borus et al. [130], disclosure had a statistically significant association with ART adherence; the authors concluded that the effect was mediated principally by improvements in family function.
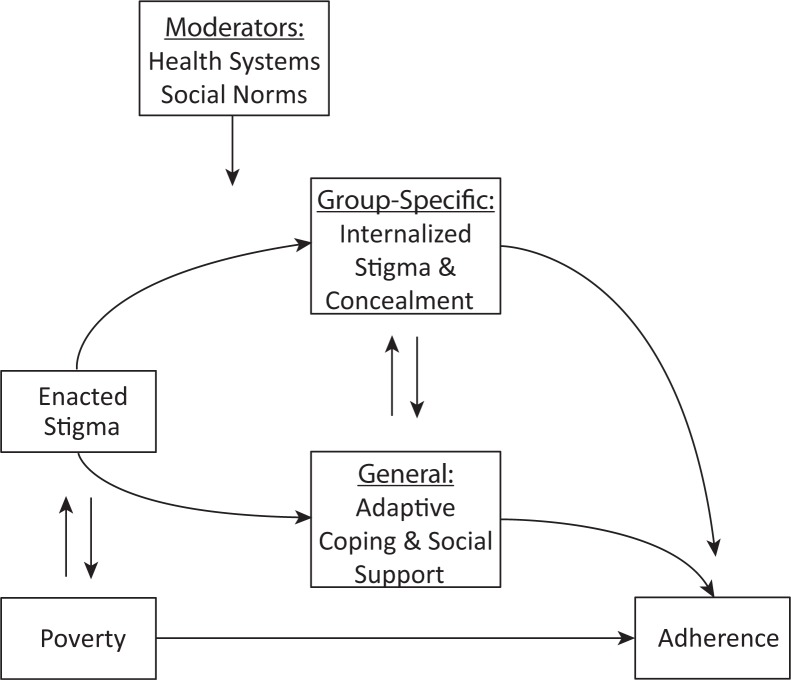
Conceptual model
To integrate our core findings from the qualitative and quantitative studies, we propose a conceptual model described in Figure 3, citing areas of congruence between our empirically derived themes and theoretical frameworks previously published by others. In our model, structural and economic barriers associated with poverty undermine ART adherence. Enacted stigma undermines ART adherence through psychological processes specific to HIV-positive persons as well as through general psychological processes that are common to HIV-positive and HIV-negative persons alike. Stigma and poverty have mutually reinforcing relationships with each other, particularly in resource-limited settings [146]: stigma and social isolation have adverse economic impacts and, conversely, poverty worsens stigma by highlighting the economic aspects of HIV's perceived association with premature morbidity and mortality.
Figure 3.
Conceptual model. This figure summarizes the findings of our meta-synthesis of 34 qualitative studies and analysis of 41 quantitative studies. The stigma of HIV was found to compromise ART adherence through general as well as group-specific psychological processes. Adaptive coping and social support were critical determinants of participants’ ability to overcome structural and economic barriers associated with poverty to successfully adhere to ART.
Internalized stigma may result when HIV-positive persons accept as valid the stigmatizing beliefs of the majority group. Because HIV infection is a potentially concealable stigma, HIV-positive persons may attempt to delay disclosure until disease progression renders further concealment impossible [147]. As elaborated in the stress process model [148, 149] and as described by the participants in the studies summarized in this review, HIV-positive persons draw on adaptive coping and social support to minimize the harmful effects of life stressors.
Adaptive coping and social support partially moderate the harmful effects of poverty on adherence and are represented in the diagram as effect modifiers: in the presence of adaptive coping or strong social support networks, the negative impacts of poverty on adherence are reduced. In this regard our synthesis is consistent with the social support model described by Ware et al. [150], who found that HIV-positive persons in Nigeria, Tanzania and Uganda relied heavily on social support to overcome structural and economic barriers to care. The authors concluded that the stigma of HIV was feared specifically because it weakened relationships that proved to be critical for everyday survival. In addition, as supported by both the qualitative and the quantitative studies summarized in this review, these general and group-specific psychological processes can directly benefit or undermine ART adherence. For example, in the setting of enacted stigma, many HIV-positive participants adopted strategies of concealment, which led directly to treatment interruptions.
The qualitative studies we identified also suggested a number of extensions to the model, namely that certain factors can moderate the severity of enacted stigma and their ultimate impacts on ART adherence. One such factor is the health system, which can be configured to support patients and minimize the harmful influences of stigma on ART adherence. Although resistance to stigma has been described [151], in countries with fragile healthcare systems resistance to stigma can be weakened as HIV-positive persons struggle with the anxieties of uncertain and unstable access to treatment [80]. Another factor involves social norms, which were described by participants in the qualitative studies as potentially intensifying the harmful influences of stigma. HIV-positive persons who belonged to sexual minority groups or who had acquired HIV through socially unacceptable means, in particular, experienced greater stigma because their self-identities and behaviours were defined by the majority as being inconsistent with social norms.
Discussion
In this systematic review of both qualitative and quantitative studies conducted among 26,715 HIV-positive persons living in 32 countries worldwide, we found that HIV-related stigma compromised ART adherence, primarily by undermining social support and adaptive coping. Our analysis is consistent with prior work demonstrating the importance of social ties in promoting adherence, particularly in resource-limited settings [33, 152], and reflects the centrality of social integration to the experience of HIV-positive persons engaged in treatment. These themes are all the more prominent in settings of extreme poverty where treatment barriers are highly prevalent [8, 14, 153] and where social ties may be essential for survival [72, 154, 155]. Our findings have implications for public health strategies now being explored in high-HIV prevalence regions, such as universal voluntary testing with immediate treatment [36]. The evidence search protocol was not designed to identify studies examining the influences of stigma on HIV testing [156, 157], pre-ART linkage to care [158, 159], ART refusal [160], or other treatment- and care-related behaviours along the entire continuum of engagement in care [35]. However, HIV-related stigma has been shown to adversely affect these treatment- and care-related behaviours in a wide range of settings [35, 161–166]. Optimization of the entire continuum of care is needed to maximize the public health impact of test-and-treat [34], thereby underscoring the importance of our findings.
Several limitations are important to consider when assessing this systematic review. First, it is well known that qualitative studies can be difficult to locate using conventional search strategies [167]. Although we adopted a purposefully broad search protocol that involved the full text review of 960 journal articles, unpublished dissertations and conference abstracts, we cannot exclude the possibility that we may have missed some relevant studies. Second, and related to the previous, we only identified one (qualitative) study from the UNAIDS Eastern Europe and Central Asia region. The HIV epidemic follows a different pattern in these countries, with concentrated epidemics most notably driven by injection drug use but also by prison overcrowding and unprotected sexual intercourse among men who have sex with men and sex workers [168–170]. For people belonging to these already marginalized subgroups, the stigma of their HIV serostatus is layered upon these pre-existing inequalities, thereby displacing them further downward in the status hierarchy. If we had been able to identify more studies from this region, it is possible that different themes could have been identified in the qualitative synthesis or that an even stronger association between stigma and ART adherence would have been described. Third, heterogeneity in the types of exposures and outcomes used in the quantitative studies precluded a formal meta-analysis. The vote counting-styled procedures we employed to synthesize their findings could not generate effect size estimates, are characterized by low statistical power [171] and cannot assess the magnitude of the purported relationship. As the field converges on the use of standardized and validated measures of stigma, disclosure and adherence, we expect that the methods of meta-analysis can be increasingly applied. Fourth, a greater proportion of longitudinal studies reported a null association between ART adherence and either stigma or disclosure. The difference appeared to be driven by studies examining the impact of disclosure on adherence. The single longitudinal study that documented a positive finding employed validated instruments to measure both stigma and self-reported ART adherence, but in general the relatively small number of longitudinal studies limited our ability to draw strong conclusions. Fifth, the majority of studies included in this review were assessed to be at risk of bias. A key reporting deficiency in the qualitative studies was lack of detail on the method of analysis. The majority of quantitative studies did not use validated exposure and outcome measures. Although these factors could exert unpredictable biases, we acknowledge they could have biased the qualitative and quantitative findings towards the null, with attendant effects on our conceptual model.
These caveats aside, the conceptual model that emerged from our synthesis of the literature has several important implications for programming and policy. At the individual level, interventions focused on enhancing social support by activating [172] or strengthening existing ties [173, 174], or facilitating either of these through the encouragement of serostatus disclosure [175–177], may be expected to improve ART adherence. These behaviours may in turn yield health and mental health dividends. Although our meta-synthesis highlighted positive self-identity as an important factor related to greater adherence, more research is needed to understand the conditions under which HIV-related outcomes are better than expected despite the experiences of HIV- and stigma-related adversity (which can be thought of as being related to the concept of resilience [178–180]). It should be acknowledged here that social ties are not uniformly beneficial. This was observed in our data showing that all relationships were not necessarily described as supportive and that some study participants’ experiences suggested positive benefits from concealment. There have been few intervention studies where disclosure was emphasized as a primary outcome [181], but the outcomes of HIV serostatus disclosure are not unambiguously positive. Due to HIV-related stigma, significant others may react in negative ways after learning about a loved one's seropositivity [182–184]. In order to avoid these undesirable outcomes, interventions targeting disclosure behaviours should be sensitive to these potential negative consequences.
At the structural level, our model suggests that structural interventions (which target the context in which people live, including social ties, resources and institutions [185]) to enhance the capacity of health systems for providing quality care may help to minimize the adverse effects of HIV-related stigma on ART adherence. Structural interventions that strengthen the livelihoods of HIV-positive persons may also be a promising avenue for subverting HIV-related stigma, particularly in resource-limited settings where contributing to local solidarity networks is a core social function [186] and where the economic impacts of HIV and AIDS have exacerbated both the instrumental and symbolic aspects of stigma attached to HIV [187]. Castro and Farmer [188] advanced the argument that “structural violence determines, in large part, who suffers from AIDS-related stigma and discrimination” (p. 55). Although some observers have speculated that economic strengthening or livelihood interventions may play a role in reducing HIV-related stigma [146], to our knowledge these hypotheses have not been formally tested [189, 190]. Related work suggests that these may spark a “virtuous” cycle: as stigma-related barriers are levelled and as HIV testing, treatment and other care-related behaviours become more widespread, the stigma of HIV and AIDS can be reduced [188, 191–195].
Notably, our conceptual model also suggests several promising points of intervention to improve ART adherence that have not consistently yielded benefits when tested for their impacts on ART adherence. For example, several studies described how effective treatment of depression could potentially improve treatment adherence, consistent with the positive prevention model elaborated by Sikkema et al. [196]. However, depression intervention studies have yielded mixed findings to date with regards to HIV treatment adherence outcomes [197–199]. Likewise social support interventions should also be expected to improve adherence, but these have also proved inconclusive [200–203]. The lack of consistent findings may potentially be explained by the fact that interventions targeting intrapersonal or interpersonal processes fail to address the larger social forces undermining adherence to HIV treatment. We emphasize here that the concepts embedded in our conceptual model span multiple levels of analysis [204, 205], ranging from intrapersonal processes (self-identity, coping), to interpersonal processes (social support, concealment), to structural factors (health systems, poverty, stigma). We therefore expect that interventions spanning multiple levels would yield the greatest impacts on reducing stigma [206], but these approaches have been rarely employed.
Conclusions
In this review of both qualitative and quantitative studies, we found that HIV-related stigma compromises ART adherence through general as well as group-specific psychological processes. Adaptive coping and social support were critical determinants of participants’ ability to overcome structural and economic barriers associated with poverty to successfully adhere to ART. Our conceptual model, which integrates the results of both quantitative and qualitative studies, suggests that the effects of stigma operate at multiple levels (intrapersonal, interpersonal and structural). Interventions to reduce stigma should target these multiple levels of influence in order to have maximum effectiveness on improving ART adherence.
Acknowledgements and funding
This study was funded in part by a Seed Grant from the Robert Wood Johnson Foundation Health and Society Scholars Program to ACT. The authors also acknowledge salary support from U.S. National Institutes of Health K23MH097667 (ITK), K23MH096651 (CP), K23MH079713 (SDW), K24MH087227 (DRB), and K23MH096620 (ACT). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
To access the supplementary material to this article please see Supplementary Files under Article Tools online.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ACT conceived the study. AER, AGO, and ACT acquired the data. ITK and ACT analyzed the data and prepared the initial draft of the manuscript. All authors assisted in interpretation of the data, revised the manuscript for important intellectual content, and approved the final version of the manuscript.
References
- 1.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–66. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 3.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–3. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Squier C, Sivek C, Wagener M, Nguyen MH, Yu VL. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8(3):261–9. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- 5.Mehta S, Moore RD, Graham NM. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11(14):1665–70. doi: 10.1097/00002030-199714000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Maggiolo F, Ripamonti D, Arici C, Gregis G, Quinzan G, Camacho GA, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin Trials. 2002;3(5):371–8. doi: 10.1310/98b3-pwg8-pmyw-w5bp. [DOI] [PubMed] [Google Scholar]
- 7.Au JT, Kayitenkore K, Shutes E, Karita E, Peters PJ, Tichacek A, et al. Access to adequate nutrition is a major potential obstacle to antiretroviral adherence among HIV-infected individuals in Rwanda. AIDS. 2006;20(16):2116–8. doi: 10.1097/01.aids.0000247580.16073.1b. [DOI] [PubMed] [Google Scholar]
- 8.Weiser SD, Tuller DM, Frongillo EA, Senkungu J, Mukiibi N, Bangsberg DR. Food insecurity as a barrier to sustained antiretroviral therapy adherence in Uganda. PLoS One. 2010;5(4):e10340. doi: 10.1371/journal.pone.0010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalofonos IA. “All I eat is ARVs”: the paradox of AIDS treatment interventions in central Mozambique. Med Anthropol Q. 2010;24(3):363–80. doi: 10.1111/j.1548-1387.2010.01109.x. [DOI] [PubMed] [Google Scholar]
- 10.Nagata JM, Magerenge RO, Young SL, Oguta JO, Weiser SD, Cohen CR. Social determinants, lived experiences, and consequences of household food insecurity among persons living with HIV/AIDS on the shore of Lake Victoria, Kenya. AIDS Care. 2012;24(6):728–36. doi: 10.1080/09540121.2011.630358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiser SD, Palar K, Frongillo EA, Tsai AC, Kumbakumba E, dePee S, et al. Longitudinal assessment of associations between food insecurity, antiretroviral adherence and HIV treatment outcomes in rural Uganda. AIDS. Aug 9; doi: 10.1097/01.aids.0000433238.93986.35. Forthcoming 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musumari PM, Feldman MD, Techasrivichien T, Wouters E, Ono-Kihara M, Kihara M. “If I have nothing to eat, I get angry and push the pills bottle away from me”: A qualitative study of patient determinants of adherence to antiretroviral therapy in the Democratic Republic of Congo. AIDS Care. 2013;25(10):1271–7. doi: 10.1080/09540121.2013.764391. [DOI] [PubMed] [Google Scholar]
- 13.Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–65. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 14.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav. 2010;14(4):778–84. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taiwo BO, Idoko JA, Welty LJ, Otoh I, Job G, Iyaji PG, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr. 2010;54(1):85–92. doi: 10.1097/01.qai.0000371678.25873.1c. [DOI] [PubMed] [Google Scholar]
- 16.Pyne-Mercier LD, John-Stewart GC, Richardson BA, Kagondu NL, Thiga J, Noshy H, et al. The consequences of post-election violence on antiretroviral HIV therapy in Kenya. AIDS Care. 2011;23(5):562–8. doi: 10.1080/09540121.2010.525615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siedner MJ, Lankowski A, Tsai AC, Muzoora C, Martin JN, Hunt PW, et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS. 2013;27(9):1503–8. doi: 10.1097/QAD.0b013e32835fd873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith R, Rossetto K, Peterson BL. A meta-analysis of disclosure of one's HIV-positive status, stigma and social support. AIDS Care. 2008;20(10):1266–75. doi: 10.1080/09540120801926977. [DOI] [PubMed] [Google Scholar]
- 19.Steward WT, Herek GM, Ramakrishna J, Bharat S, Chandy S, Wrubel J, et al. HIV-related stigma: adapting a theoretical framework for use in India. Soc Sci Med. 2008;67(8):1225–35. doi: 10.1016/j.socscimed.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai AC, Bangsberg DR, Kegeles SM, Katz IT, Haberer JE, Muzoora C, et al. Internalized stigma, social distance, and disclosure of HIV seropositivity in rural Uganda. Ann Behav Med. May 21; doi: 10.1007/s12160-013-9514-6. Forthcoming 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logie C, Gadalla TM. Meta-analysis of health and demographic correlates of stigma towards people living with HIV. AIDS Care. 2009;21(6):742–53. doi: 10.1080/09540120802511877. [DOI] [PubMed] [Google Scholar]
- 22.Mak WW, Poon CY, Pun LY, Cheung SF. Meta-analysis of stigma and mental health. Soc Sci Med. 2007;65(2):245–61. doi: 10.1016/j.socscimed.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Goffman E. Stigma: notes on the management of spoiled identity. Englewood Cliffs: Prentice-Hall; 1963. [Google Scholar]
- 24.Jones EE, Farina A, Hastorf AH, Markus H, Miller DT, Scott RA. Social stigma: the psychology of marked relationships. New York: W.H. Freeman; 1984. [Google Scholar]
- 25.Scheff TJ. Being mentally ill: a sociological theory. Chicago: Aldine; 1966. [Google Scholar]
- 26.Scambler G, Hopkins A. Being epileptic: coming to terms with stigma. Sociol Health Illn. 1986;8(1):26–43. [Google Scholar]
- 27.Link BG, Cullen FT, Struening E, Shrout PE. A modified labeling theory approach to mental disorders: an empirical assessment. Am Sociol Rev. 1989;54(3):400–23. [Google Scholar]
- 28.Devine PG, Plant EA, Harrison K. The problem of ‘us’ versus ‘them’ and AIDS stigma. Am Behav Sci. 1999;42(7):1212–28. [Google Scholar]
- 29.Allport GW. The nature of prejudice. Reading: Addison-Wesley; 1954. [Google Scholar]
- 30.Pennebaker JW. Confession, inhibition, and disease. In: Berkowitz L, editor. Advances in experimental social psychology. Vol. 22. Orlando: Academic Press; 1989. pp. 211–44. [Google Scholar]
- 31.Simbayi LC, Kalichman S, Strebel A, Cloete A, Henda N, Mqeketo A. Internalized stigma, discrimination, and depression among men and women living with HIV/AIDS in Cape Town, South Africa. Soc Sci Med. 2007;64(9):1823–31. doi: 10.1016/j.socscimed.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai AC, Bangsberg DR, Frongillo EA, Hunt PW, Muzoora C, Martin JN, et al. Food insecurity, depression and the modifying role of social support among people living with HIV/AIDS in rural Uganda. Soc Sci Med. 2012;74(12):2012–19. doi: 10.1016/j.socscimed.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai AC, Bangsberg DR. The importance of social ties in sustaining medication adherence in resource-limited settings. J Gen Intern Med. 2011;26(12):1391–3. doi: 10.1007/s11606-011-1841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 37.Jurgens R, Cohen J, Tarantola D, Heywood M, Carr R. Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet. 2009;373(9669):1079. doi: 10.1016/S0140-6736(09)60644-9. author reply 80–1. [DOI] [PubMed] [Google Scholar]
- 38.Dixon-Woods M, Fitzpatrick R. Qualitative research in systematic reviews. Has established a place for itself. BMJ. 2001;323(7316):765–6. doi: 10.1136/bmj.323.7316.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National CASP Collaboration for Qualitative Methodologies. 10 questions to help you make sense of qualitative research; Milton Keynes: Milton Keynes Primary Care Trust; 2006. [Google Scholar]
- 40.Atkins S, Lewin S, Smith H, Engel M, Fretheim A, Volmink J. Conducting a meta-ethnography of qualitative literature: lessons learnt. BMC Med Res Methodol. 2008;8:21. doi: 10.1186/1471-2288-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 42.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–76. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 43.Noblit GW, Hare RD. Meta-ethnography: synthesizing qualitative studies. Newbury Park: Sage; 1988. [Google Scholar]
- 44.Martin Hilber A, Kenter E, Redmond S, Merten S, Bagnol B, Low N, et al. Vaginal practices as women's agency in sub-Saharan Africa: a synthesis of meaning and motivation through meta-ethnography. Soc Sci Med. 2012;74(9):1311–23. doi: 10.1016/j.socscimed.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 45.Smith LK, Pope C, Botha JL. Patients’ help-seeking experiences and delay in cancer presentation: a qualitative synthesis. Lancet. 2005;366(9488):825–31. doi: 10.1016/S0140-6736(05)67030-4. [DOI] [PubMed] [Google Scholar]
- 46.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4(7):e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Method. 2006;18(1):59–82. [Google Scholar]
- 48.Kumarasamy N, Safren SA, Raminani SR, Pickard R, James R, Krishnan AK, et al. Barriers and facilitators to antiretroviral medication adherence among patients with HIV in Chennai, India: a qualitative study. AIDS Patient Care STDs. 2005;19(8):526–37. doi: 10.1089/apc.2005.19.526. [DOI] [PubMed] [Google Scholar]
- 49.Edwards LV. Perceived social support and HIV/AIDS medication adherence among African American women. Qual Health Res. 2006;16(5):679–91. doi: 10.1177/1049732305281597. [DOI] [PubMed] [Google Scholar]
- 50.Nachega JB, Knowlton AR, Deluca A, Schoeman JH, Watkinson L, Efron A, et al. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. A qualitative study. J Acquir Immune Defic Syndr. 2006;1(43 Suppl):S127–33. doi: 10.1097/01.qai.0000248349.25630.3d. [DOI] [PubMed] [Google Scholar]
- 51.Ware NC, Wyatt MA, Tugenberg T. Social relationships, stigma and adherence to antiretroviral therapy for HIV/AIDS. AIDS Care. 2006;18(8):904–10. doi: 10.1080/09540120500330554. [DOI] [PubMed] [Google Scholar]
- 52.Skhosana NL, Struthers H, Gray GE, McIntyre JA. HIV disclosure and other factors that impact on adherence to antiretroviral therapy: the case of Soweto, South Africa. Afr J AIDS Res. 2006;5(1):17–26. doi: 10.2989/16085900609490363. [DOI] [PubMed] [Google Scholar]
- 53.Melchior R, Nemes MI, Alencar TM, Buchalla CM. Desafios da adesão ao tratamento de pessoas vivendo com HIV/AIDS no Brasil. Rev Saúde Pública. 2007;41(Suppl 2):87–93. doi: 10.1590/s0034-89102007000900014. [DOI] [PubMed] [Google Scholar]
- 54.Tarakeshwar N, Srikrishnan AK, Johnson S, Vasu C, Solomon S, Merson M, et al. A social cognitive model of health for HIV-positive adults receiving care in India. AIDS Behav. 2007;11(3):491–504. doi: 10.1007/s10461-006-9161-z. [DOI] [PubMed] [Google Scholar]
- 55.Konkle-Parker DJ, Erlen JA, Dubbert PM. Barriers and facilitators to medication adherence in a southern minority population with HIV disease. J Assoc Nurses AIDS Care. 2008;19(2):98–104. doi: 10.1016/j.jana.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nam SL, Fielding K, Avalos A, Dickinson D, Gaolathe T, Geissler PW. The relationship of acceptance or denial of HIV-status to antiretroviral adherence among adult HIV patients in urban Botswana. Soc Sci Med. 2008;67(2):301–10. doi: 10.1016/j.socscimed.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 57.Byakika-Tusiime J, Crane J, Oyugi JH, Ragland K, Kawuma A, Musoke P, et al. Longitudinal antiretroviral adherence in HIV+ Ugandan parents and their children initiating HAART in the MTCT-Plus family treatment model: role of depression in declining adherence over time. AIDS Behav. 2009;13(Suppl 1):82–91. doi: 10.1007/s10461-009-9546-x. [DOI] [PubMed] [Google Scholar]
- 58.Watt MH, Maman S, Earp JA, Eng E, Setel PW, Golin CE, et al. “It's all the time in my mind”: facilitators of adherence to antiretroviral therapy in a Tanzanian setting. Soc Sci Med. 2009;68(10):1793–800. doi: 10.1016/j.socscimed.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalanzi DJN. Adherence behavior and the impact of HAART on quality of life of Ugandan adults [Ph.D. dissertation] Denton: Texas Woman's University; 2009. [Google Scholar]
- 60.Ruanjahn G, Roberts D, Monterosso L. An exploration of factors influencing adherence to highly active anti-retroviral therapy (HAART) among people living with HIV/AIDS in Northern Thailand. AIDS Care. 2010;22(12):1555–61. doi: 10.1080/09540121003759901. [DOI] [PubMed] [Google Scholar]
- 61.Badahdah AM, Pedersen DE. “I want to stand on my own legs”: a qualitative study of antiretroviral therapy adherence among HIV-positive women in Egypt. AIDS Care. 2011;23(6):700–4. doi: 10.1080/09540121.2010.534431. [DOI] [PubMed] [Google Scholar]
- 62.Gusdal AK, Obua C, Andualem T, Wahlstrom R, Chalker J, Fochsen G. Peer counselors’ role in supporting patients’ adherence to ART in Ethiopia and Uganda. AIDS Care. 2011;23(6):657–62. doi: 10.1080/09540121.2010.532531. [DOI] [PubMed] [Google Scholar]
- 63.Van Tam V, Pharris A, Thorson A, Alfven T, Larsson M. “It is not that I forget, it's just that I don't want other people to know”: barriers to and strategies for adherence to antiretroviral therapy among HIV patients in Northern Vietnam. AIDS Care. 2011;23(2):139–45. doi: 10.1080/09540121.2010.507741. [DOI] [PubMed] [Google Scholar]
- 64.Daftary A, Padayatchi N. Social constraints to TB/HIV healthcare: accounts from coinfected patients in South Africa. AIDS Care. 2012;24(12):1480–6. doi: 10.1080/09540121.2012.672719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matovu SN, La cour K, Hemmingsson H. Narratives of Ugandan women adhering to HIV/AIDS medication. Occup Ther Int. 2012;19(4):176–84. doi: 10.1002/oti.1330. [DOI] [PubMed] [Google Scholar]
- 66.Nyanzi-Wakholi B, Lara AM, Munderi P, Gilks C. The charms and challenges of antiretroviral therapy in Uganda: the DART experience. AIDS Care. 2012;24(2):137–42. doi: 10.1080/09540121.2011.596518. [DOI] [PubMed] [Google Scholar]
- 67.O'Laughlin KN, Wyatt MA, Kaaya S, Bangsberg DR, Ware NC. How treatment partners help: social analysis of an African adherence support intervention. AIDS Behav. 2012;16(5):1308–15. doi: 10.1007/s10461-011-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wasti SP, Simkhada P, Randall J, Freeman JV, van Teijlingen E. Factors influencing adherence to antiretroviral treatment in Nepal: a mixed-methods study. PLoS One. 2012;7(5):35547. doi: 10.1371/journal.pone.0035547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okoror TA, Falade CO, Olorunlana A, Walker EM, Okareh OT. Exploring the cultural context of HIV stigma on antiretroviral therapy adherence among people living with HIV/AIDS in southwest Nigeria. AIDS Patient Care STDs. 2013;27(1):55–64. doi: 10.1089/apc.2012.0150. [DOI] [PubMed] [Google Scholar]
- 70.Portelli MS, Tenni B, Kounnavong S, Chanthivilay P. Barriers to and facilitators of adherence to antiretroviral therapy among people living with HIV in Lao PDR: a qualitative study. Asia Pac J Public Health. Apr 24; doi: 10.1177/1010539512442082. Forthcoming 2012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71.Murray LK, Semrau K, McCurley E, Thea DM, Scott N, Mwiya M, et al. Barriers to acceptance and adherence of antiretroviral therapy in urban Zambian women: a qualitative study. AIDS Care. 2009;21(1):78–86. doi: 10.1080/09540120802032643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Izugbara CO, Wekesa E. Beliefs and practices about antiretroviral medication: a study of poor urban Kenyans living with HIV/AIDS. Sociol Health Illn. 2011;33(6):869–83. doi: 10.1111/j.1467-9566.2010.01328.x. [DOI] [PubMed] [Google Scholar]
- 73.Brion JM, Menke EM. Perspectives regarding adherence to prescribed treatment in highly adherent HIV-infected gay men. J Assoc Nurses AIDS Care. 2008;19(3):181–91. doi: 10.1016/j.jana.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Mohammadpour A, Yekta ZP, Nikbakht Nasrabadi AR. HIV-infected patients’ adherence to highly active antiretroviral therapy: a phenomenological study. Nurs Health Sci. 2010;12(4):464–9. doi: 10.1111/j.1442-2018.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- 75.Bontempi JM, Burleson L, Lopez MH. HIV medication adherence programs: the importance of social support. J Community Health Nurs. 2004;21(2):111–22. doi: 10.1207/s15327655jchn2102_05. [DOI] [PubMed] [Google Scholar]
- 76.Mouala C, Roux P, Okome M, Sentenac S, Okome F, Nziengui U, et al. Bilan de quelques études sur l'observance aux ARV en Afrique. Med Trop (Mars) 2006;66(6):610–4. [PubMed] [Google Scholar]
- 77.Sabin LL, Desilva MB, Hamer DH, Keyi X, Yue Y, Wen F, et al. Barriers to adherence to antiretroviral medications among patients living with HIV in southern China: a qualitative study. AIDS Care. 2008;20(10):1242–50. doi: 10.1080/09540120801918651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dahab M, Charalambous S, Hamilton R, Fielding K, Kielmann K, Churchyard GJ, et al. “That is why I stopped the ART”: patients’ & providers’ perspectives on barriers to and enablers of HIV treatment adherence in a South African workplace programme. BMC Public Health. 2008;8:63. doi: 10.1186/1471-2458-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilbert L, Walker L. ‘My biggest fear was that people would reject me once they knew my status …’: stigma as experienced by patients in an HIV/AIDS clinic in Johannesburg, South Africa. Health Soc Care Community. 2010;18(2):139–46. doi: 10.1111/j.1365-2524.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- 80.Bernays S, Rhodes T. Experiencing uncertain HIV treatment delivery in a transitional setting: qualitative study. AIDS Care. 2009;21(3):315–21. doi: 10.1080/09540120802183495. [DOI] [PubMed] [Google Scholar]
- 81.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24(6):518–29. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 82.Birbeck GL, Chomba E, Kvalsund M, Bradbury R, Mang'ombe C, Malama K, et al. Antiretroviral adherence in rural Zambia: the first year of treatment availability. Am J Trop Med Hyg. 2009;80(4):669–74. [PubMed] [Google Scholar]
- 83.Adeyemi A, Olubunmi F, Oluseyi A. Predictors of adherence for patients on highly active antiretroviral therapy in HIV treatment program; 12th Annual International Meeting of the Institute of Human Virology; Tropea, Calabria, Italy: 2010. [cited 2010 Oct 4–8] [Google Scholar]
- 84.Boyer S, Clerc I, Bonono C-R, Marcellin F, Bile P-C, Ventelou B. Non-adherence to antiretroviral treatment and unplanned treatment interruption among people living with HIV/AIDS in Cameroon: individual and healthcare supply-related factors. Soc Sci Med. 2011;72(8):1383–92. doi: 10.1016/j.socscimed.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 85.Carrieri P, Cailleton V, Le Moing V, Spire B, Dellamonica P, Bouvet E, et al. The dynamic of adherence to highly active antiretroviral therapy: results from the French National APROCO cohort. J Acquir Immune Defic Syndr. 2001;28(3):232–9. doi: 10.1097/00042560-200111010-00005. [DOI] [PubMed] [Google Scholar]
- 86.Cardarelli R, Weis S, Adams E, Radaford D, Vecino I, Munguia G, et al. General health status and adherence to antiretroviral therapy. J Int Assoc Physicians AIDS Care (Chic) 2008;7(3):123–9. doi: 10.1177/1545109708318526. [DOI] [PubMed] [Google Scholar]
- 87.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 88.Knobel H, Alonso J, Casado JL, Collazos J, Gonzalez J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS. 2002;16(4):605–13. doi: 10.1097/00002030-200203080-00012. [DOI] [PubMed] [Google Scholar]
- 89.Carlucci JG, Kamanga A, Sheneberger R, Shepherd BE, Jenkins CA, Spurrier J, et al. Predictors of adherence to antiretroviral therapy in rural Zambia. J Acquir Immune Defic Syndr. 2008;47(5):615–22. doi: 10.1097/QAI.0b013e318165dc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, Ele P, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS One. 2010;5(5):e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colbert AM. Functional health literacy, medication-taking self-efficacy and HIV medication adherence [Ph.D. dissertation] Pittsburgh: University of Pittsburgh; 2007. [Google Scholar]
- 92.Diiorio C, McCarty F, Depadilla L, Resnicow K, Holstad MM, Yeager K, et al. Adherence to antiretroviral medication regimens: a test of a psychosocial model. AIDS Behav. 2009;13(1):10–22. doi: 10.1007/s10461-007-9318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pequegnat W, Bauman LJ, Bray JH, DiClemente R, DiIorio C, Hoppe SK, et al. Measurement of the role of families in prevention and adaptation to HIV/AIDS. AIDS Behav. 2001;5(1):1–19. [Google Scholar]
- 94.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 95.Dlamini PS, Wantland D, Makoae LN, Chirwa M, Kohi TW, Greeff M, et al. HIV stigma and missed medications in HIV-positive people in five African countries. AIDS Patient Care STDs. 2009;23(5):377–87. doi: 10.1089/apc.2008.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holzemer WL, Uys LR, Chirwa ML, Greeff M, Makoae LN, Kohi TW, et al. Validation of the HIV/AIDS stigma instrument – PLWA (HASI-P) AIDS Care. 2007;19(8):1002–12. doi: 10.1080/09540120701245999. [DOI] [PubMed] [Google Scholar]
- 97.Do NT, Phiri K, Bussmann H, Gaolathe T, Marlink RG, Wester CW. Psychosocial factors affecting medication adherence among HIV-1 infected adults receiving combination antiretroviral therapy (cART) in Botswana. AIDS Res Hum Retroviruses. 2010;26(6):685–91. doi: 10.1089/aid.2009.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Franke MF, Murray MB, Muñoz M, Hernandez-Diaz S, Sebastian JL, Atwood S, et al. Food insufficiency is a risk factor for suboptimal antiretroviral therapy adherence among HIV-infected adults in urban Peru. AIDS Behav. 2011;15(7):1483–9. doi: 10.1007/s10461-010-9789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goldman JD, Cantrell RA, Mulenga LB, Tambatamba BC, Reid SE, Levy JW, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24(8):1031–5. doi: 10.1089/aid.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalichman SC, Cain D, Fuhrel A, Eaton L, Di Fonzo K, Ertl T. Assessing medication adherence self-efficacy among low-literacy patients: development of a pictographic visual analogue scale. Health Educ Res. 2005;20(1):24–35. doi: 10.1093/her/cyg106. [DOI] [PubMed] [Google Scholar]
- 101.Gifford AL, Lorig K, Chesney M, Laurent D, Gonzalez V. Patient education to improve health-related quality of life in HIV/AIDS: a pilot study. 11th International Conference on AIDS. Vancouver, British Columbia, Canada: 1996. [cited 1996 Jul 7–12] [Google Scholar]
- 102.Kalichman SC, Pope H, White D, Cherry C, Amaral CM, Swetzes C, et al. Association between health literacy and HIV treatment adherence: further evidence from objectively measured medication adherence. J Int Assoc Physicians AIDS Care (Chic) 2008;7(6):317–23. doi: 10.1177/1545109708328130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009;21(1):87–93. doi: 10.1080/09540120802032627. [DOI] [PubMed] [Google Scholar]
- 104.Li L, Lee SJ, Wen Y, Lin C, Wan D, Jiraphongsa C. Antiretroviral therapy adherence among patients living with HIV/AIDS in Thailand. Nurs Health Sci. 2010;12(2):212–20. doi: 10.1111/j.1442-2018.2010.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee SJ, Li L, Jiraphongsa C, Iamsirithaworn S, Khumtong S, Rotheram-Borus MJ. Regional variations in HIV disclosure in Thailand: implications for future interventions. Int J STD AIDS. 2010;21(3):161–5. doi: 10.1258/ijsa.2009.009008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herek GM, Capitanio JP. Public reactions to AIDS in the United States: a second decade of stigma. Am J Public Health. 1993;83(4):574–7. doi: 10.2105/ajph.83.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Apinundecha C, Laohasiriwong W, Cameron MP, Lim S. A community participation intervention to reduce HIV/AIDS stigma, Nakhon Ratchasima province, northeast Thailand. AIDS Care. 2007;19(9):1157–65. doi: 10.1080/09540120701335204. [DOI] [PubMed] [Google Scholar]
- 108.Li X, Huang L, Wang H, Fennie KP, He G, Williams AB. Stigma mediates the relationship between self-efficacy, medication adherence, and quality of life among people living with HIV/AIDS in China. AIDS Patient Care STDs. 2011;25(11):665–71. doi: 10.1089/apc.2011.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li X, He G, Wang H, Huang L, Liu L. Development and evaluation of HIV/AIDS-related stigma and discrimination scale. Chin J Nurs. 2010;45(6):496–9. [Google Scholar]
- 110.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34(8):1115–21. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 111.Lucero AF, Smith C, Ufford LJ, Leipzig RM. Poor adherence to HAART in older adults with HIV infection. J Am Geriatr Soc. 2001;49(4):S93–4. [Google Scholar]
- 112.Martinez J, Harper G, Carleton RA, Hosek S, Bojan K, Glum G, et al. The impact of stigma on medication adherence among HIV-positive adolescent and young adult females and the moderating effects of coping and satisfaction with health care. AIDS Patient Care STDs. 2012;26(2):108–15. doi: 10.1089/apc.2011.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mo PK, Mak WW. Intentionality of medication non-adherence among individuals living with HIV/AIDS in Hong Kong. AIDS Care. 2009;21(6):785–95. doi: 10.1080/09540120802511968. [DOI] [PubMed] [Google Scholar]
- 114.Mak WW, Cheung RY, Law RW, Woo J, Li PC, Chung RW. Examining attribution model of self-stigma on social support and psychological well-being among people with HIV+/AIDS. Soc Sci Med. 2007;64(8):1549–59. doi: 10.1016/j.socscimed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 115.Molassiotis A, Nahas-Lopez V, Chung WY, Lam SW, Li CK, Lau TF. Factors associated with adherence to antiretroviral medication in HIV-infected patients. Int J STD AIDS. 2002;13(5):301–10. doi: 10.1258/0956462021925117. [DOI] [PubMed] [Google Scholar]
- 116.Muyingo SK, Walker AS, Reid A, Munderi P, Gibb DM, Ssali F, et al. Patterns of individual and population-level adherence to antiretroviral therapy and risk factors for poor adherence in the first year of the DART trial in Uganda and Zimbabwe. J Acquir Immune Defic Syndr. 2008;48(4):468–75. doi: 10.1097/QAI.0b013e31817dc3fd. [DOI] [PubMed] [Google Scholar]
- 117.Nachega JB, Stein DM, Lehman DA, Hlatshwayo D, Mothopeng R, Chaisson RE, et al. Adherence to antiretroviral therapy in HIV-infected adults in Soweto, South Africa. AIDS Res Hum Retroviruses. 2004;20(10):1053–6. doi: 10.1089/aid.2004.20.1053. [DOI] [PubMed] [Google Scholar]
- 118.Olowookere SA, Fatiregun AA, Akinyemi JO, Bamgboye AE, Osagbemi GK. Prevalence and determinants of nonadherence to highly active antiretroviral therapy among people living with HIV/AIDS in Ibadan, Nigeria. J Infect Dev Countr. 2008;2(5):369–72. doi: 10.3855/jidc.199. [DOI] [PubMed] [Google Scholar]
- 119.Peltzer K, Friend-du Preez N, Ramlagan S, Anderson J. Antiretroviral treatment adherence among HIV patients in KwaZulu-Natal, South Africa. BMC Public Health. 2010;10:111. doi: 10.1186/1471-2458-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kalichman SC, Simbayi LC, Jooste S, Toefy Y, Cain D, Cherry C, et al. Development of a brief scale to measure AIDS-related stigma in South Africa. AIDS Behav. 2005;9(2):135–43. doi: 10.1007/s10461-005-3895-x. [DOI] [PubMed] [Google Scholar]
- 121.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269–77. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- 122.Penniman TV. The impact of family functioning on depression and medication adherence among mothers infected with HIV. Los Angeles: University of California at Los Angeles; 2010. [Ph.D. dissertation] [Google Scholar]
- 123.Peretti-Watel P, Spire B, Pierret J, Lert F, Obadia Y. Management of HIV-related stigma and adherence to HAART: evidence from a large representative sample of outpatients attending French hospitals (ANRS-EN12-VESPA 2003) AIDS Care. 2006;18(3):254–61. doi: 10.1080/09540120500456193. [DOI] [PubMed] [Google Scholar]
- 124.Rao D, Feldman BJ, Fredericksen RJ, Crane PK, Simoni JM, Kitahata MM, et al. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS Behav. 2012;16(3):711–6. doi: 10.1007/s10461-011-9915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rao D, Choi SW, Victorson D, Bode R, Peterman A, Heinemann A, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI) Qual Life Res. 2009;18(5):585–95. doi: 10.1007/s11136-009-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12(1):86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 127.Rintamaki LS, Davis TC, Skripkauskas S, Bennett CL, Wolf MS. Social stigma concerns and HIV medication adherence. AIDS Patient Care STDs. 2006;20(5):359–68. doi: 10.1089/apc.2006.20.359. [DOI] [PubMed] [Google Scholar]
- 128.DeMasi R, Tolson J, Pham S, Capuano G, Graham N, Fisher R, et al. Self-reported adherence to HAART and correlation with HIV RNA: initial results with the patient medication adherence questionnaire. Chicago: 1999. th Conference on Retroviruses and Opportunistic Infections, [cited 1999 Jan 31–Feb 4] [Google Scholar]
- 129.DeMasi RA, Graham NM, Tolson JM, Pham SV, Capuano GA, Fisher RL, et al. Correlation between self-reported adherence to highly active antiretroviral therapy (HAART) and virologic outcome. Adv Ther. 2001;18(4):163–73. doi: 10.1007/BF02850110. [DOI] [PubMed] [Google Scholar]
- 130.Rotheram-Borus MJ, Stein JA, Jiraphongsa C, Khumtong S, Lee SJ, Li L. Benefits of family and social relationships for Thai parents living with HIV. Prev Sci. 2010;11(3):298–307. doi: 10.1007/s11121-009-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rougemont M, Stoll BE, Elia N, Ngang P. Antiretroviral treatment adherence and its determinants in Sub-Saharan Africa: a prospective study at Yaounde Central Hospital, Cameroon. AIDS Res Ther. 2009;6:21. doi: 10.1186/1742-6405-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101–8. doi: 10.1007/s11606-009-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sayles JN, Hays RD, Sarkisian CA, Mahajan AP, Spritzer KL, Cunningham WE. Development and psychometric assessment of a multidimensional measure of internalized HIV stigma in a sample of HIV-positive adults. AIDS Behav. 2008;12(5):748–58. doi: 10.1007/s10461-008-9375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spire B, Duran S, Souville M, Leport C, Raffi F, Moatti JP. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: from a predictive to a dynamic approach. Soc Sci Med. 2002;54(10):1481–96. doi: 10.1016/s0277-9536(01)00125-3. [DOI] [PubMed] [Google Scholar]
- 135.Stirratt MJ, Remien RH, Smith A, Copeland OQ, Dolezal C, Krieger D. The role of HIV serostatus disclosure in antiretroviral medication adherence. AIDS Behav. 2006;10(5):483–93. doi: 10.1007/s10461-006-9106-6. [DOI] [PubMed] [Google Scholar]
- 136.el-Bassel N, Cooper DK, Chen DR, Schilling RF. Personal social networks and HIV status among women on methadone. AIDS Care. 1998;10(6):735–49. doi: 10.1080/09540129848352. [DOI] [PubMed] [Google Scholar]
- 137.Sumari-de Boer IM, Sprangers MA, Prins JM, Nieuwkerk PT. HIV stigma and depressive symptoms are related to adherence and virological response to antiretroviral treatment among immigrant and indigenous HIV infected patients. AIDS Behav. 2012;16(6):1681–9. doi: 10.1007/s10461-011-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Dyk AC. Treatment adherence following national antiretroviral rollout in South Africa. Afr J AIDS Res. 2010;9(3):235–47. doi: 10.2989/16085906.2010.530177. [DOI] [PubMed] [Google Scholar]
- 139.Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10(5):473–82. doi: 10.1007/s10461-006-9099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Waite KR, Paasche-Orlow M, Rintamaki LS, Davis TC, Wolf MS. Literacy, social stigma, and HIV medication adherence. J Gen Intern Med. 2008;23(9):1367–72. doi: 10.1007/s11606-008-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang H, He G, Li X, Yang A, Chen X, Fennie KP, et al. Self-reported adherence to antiretroviral treatment among HIV-infected people in Central China. AIDS Patient Care STDs. 2008;22(1):71–80. doi: 10.1089/apc.2007.0047. [DOI] [PubMed] [Google Scholar]
- 142.Watt MH. Understanding patients' adherence to antiretroviral therapy: a mixed-methods study in Arusha, Tanzania. Chapel Hill: University of North Carolina at Chapel Hill; 2008. [Ph.D. dissertation] [Google Scholar]
- 143.Mbwambo J, Kilonzo G, Kopoka P, Nyblade L. Understanding HIV-related stigma in Tanzania. Dar es Salaam: Department of Psychiatry, Muhimbili University College of Health Sciences; 2003. [Google Scholar]
- 144.Weiser S, Wolfe W, Bangsberg D, Thior I, Gilbert P, Makhema J, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003;34(3):281–8. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 145.Wolitski RJ, Pals SL, Kidder DP, Courtenay-Quirk C, Holtgrave DR. The effects of HIV stigma on health, disclosure of HIV status, and risk behavior of homeless and unstably housed persons living with HIV. AIDS Behav. 2009;13(6):1222–32. doi: 10.1007/s10461-008-9455-4. [DOI] [PubMed] [Google Scholar]
- 146.Tsai AC, Bangsberg DR, Weiser SD. Harnessing poverty alleviation to subvert the stigma of HIV in sub-Saharan Africa. PLoS Med. doi: 10.1371/journal.pmed.1001557. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alonzo AA, Reynolds NR. Stigma, HIV and AIDS: an exploration and elaboration of a stigma trajectory. Soc Sci Med. 1995;41(3):303–15. doi: 10.1016/0277-9536(94)00384-6. [DOI] [PubMed] [Google Scholar]
- 148.Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22(4):337–56. [PubMed] [Google Scholar]
- 149.Pearlin LI. The sociological study of stress. J Health Soc Behav. 1989;30(3):241–56. [PubMed] [Google Scholar]
- 150.Ware NC, Idoko J, Kaaya S, Biraro IA, Wyatt MA, Agbaji O, et al. Explaining adherence success in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009;6(1):e11. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abrahams N, Jewkes R. Managing and resisting stigma: a qualitative study among people living with HIV in South Africa. J Int AIDS Soc. 2012;15(2):17330. doi: 10.7448/IAS.15.2.17330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bangsberg DR, Deeks SG. Spending more to save more: interventions to promote adherence. Ann Intern Med. 2010;152(1):54–6. doi: 10.1059/0003-4819-152-1-201001050-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsai AC, Bangsberg DR, Emenyonu N, Senkungu JK, Martin JN, Weiser SD. The social context of food insecurity among persons living with HIV/AIDS in rural Uganda. Soc Sci Med. 2011;73(12):1717–24. doi: 10.1016/j.socscimed.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Samuels FA, Rutenberg N. “Health regains but livelihoods lag”: findings from a study with people on ART in Zambia and Kenya. AIDS Care. 2011;23(6):748–54. doi: 10.1080/09540121.2010.532535. [DOI] [PubMed] [Google Scholar]
- 155.Gausset Q, Mogensen HO, Yameogo WM, Berthe A, Konate B. The ambivalence of stigma and the double-edged sword of HIV/AIDS intervention in Burkina Faso. Soc Sci Med. 2012;74(7):1037–44. doi: 10.1016/j.socscimed.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 156.Wong LH, Rooyen HV, Modiba P, Richter L, Gray G, McIntyre JA, et al. Test and tell: correlates and consequences of testing and disclosure of HIV status in South Africa (HPTN 043 Project Accept) J Acquir Immune Defic Syndr. 2009;50(2):215–22. doi: 10.1097/QAI.0b013e3181900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Young SD, Hlavka Z, Modiba P, Gray G, Van Rooyen H, Richter L, et al. HIV-related stigma, social norms, and HIV testing in Soweto and Vulindlela, South Africa: National Institutes of Mental Health Project Accept (HPTN 043) J Acquir Immune Defic Syndr. 2010;55(5):620–4. doi: 10.1097/QAI.0b013e3181fc6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bassett IV, Wang B, Chetty S, Mazibuko M, Bearnot B, Giddy J, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51(2):135–9. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP, et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5(3):9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25(17):2177–81. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Weiser SD, Heisler M, Leiter K, Percy-de Korte F, Tlou S, DeMonner S, et al. Routine HIV testing in Botswana: a population-based study on attitudes, practices, and human rights concerns. PLoS Med. 2006;3(7):261. doi: 10.1371/journal.pmed.0030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wolfe WR, Weiser SD, Bangsberg DR, Thior I, Makhema JM, Dickinson DB, et al. Effects of HIV-related stigma among an early sample of patients receiving antiretroviral therapy in Botswana. AIDS Care. 2006;18(8):931–3. doi: 10.1080/09540120500333558. [DOI] [PubMed] [Google Scholar]
- 163.Geng EH, Nash D, Kambugu A, Zhang Y, Braitstein P, Christopoulos KA, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7(4):234–44. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Musheke M, Bond V, Merten S. Individual and contextual factors influencing patient attrition from antiretroviral therapy care in an urban community of Lusaka, Zambia. J Int AIDS Soc. 2012;15(Suppl 1):1–9. doi: 10.7448/IAS.15.3.17366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bogart LM, Chetty S, Giddy J, Sypek A, Sticklor L, Walensky RP, et al. Barriers to care among people living with HIV in South Africa: contrasts between patient and healthcare provider perspectives. AIDS Care. 2013;25(7):843–53. doi: 10.1080/09540121.2012.729808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Musheke M, Ntalasha H, Gari S, McKenzie O, Bond V, Martin-Hilber A, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in sub-Saharan Africa. BMC Pub Health. 2013;13:220. doi: 10.1186/1471-2458-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically relevant qualitative studies in MEDLINE. Stud Health Technol Inform. 2004;107(Pt 1):311–6. [PubMed] [Google Scholar]
- 168.Bridge J, Lazarus JV, Atun R. HIV epidemics and prevention responses in Asia and Eastern Europe: lessons to be learned? AIDS. 2010;3(24 Suppl):S86–94. doi: 10.1097/01.aids.0000390094.91176.d8. [DOI] [PubMed] [Google Scholar]
- 169.Thorne C, Ferencic N, Malyuta R, Mimica J, Niemiec T. Central Asia: hotspot in the worldwide HIV epidemic. Lancet Infect Dis. 2010;10(7):479–88. doi: 10.1016/S1473-3099(10)70118-3. [DOI] [PubMed] [Google Scholar]
- 170.Jolley E, Rhodes T, Platt L, Hope V, Latypov A, Donoghoe M, et al. HIV among people who inject drugs in Central and Eastern Europe and Central Asia: a systematic review with implications for policy. BMJ Open. 2012;2(5):e001465. doi: 10.1136/bmjopen-2012-001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hedges LV, Olkin I. Vote-counting methods in research synthesis. Psychol Bull. 1980;88(2):359–69. [Google Scholar]
- 172.Lin N. Building a network theory of social capital. Connect (Tor) 1999;22(1):28–51. [Google Scholar]
- 173.The ENRICHD Investigators. Enhancing Recovery in Coronary Heart Disease (ENRICHD) study intervention: rationale and design. Psychosom Med. 2001;63(5) [PubMed] [Google Scholar]
- 174.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 175.Holt R, Court P, Vedhara K, Nott KH, Holmes J, Snow MH. The role of disclosure in coping with HIV infection. AIDS Care. 1998;10(1):49–60. doi: 10.1080/09540129850124578. [DOI] [PubMed] [Google Scholar]
- 176.Paxton S. The paradox of public HIV disclosure. AIDS Care. 2002;14(4):559–67. doi: 10.1080/09540120208629674. [DOI] [PubMed] [Google Scholar]
- 177.Kalichman SC, DiMarco M, Austin J, Luke W, DiFonzo K. Stress, social support, and HIV-status disclosure to family and friends among HIV-positive men and women. J Behav Med. 2003;26(4):315–32. doi: 10.1023/a:1024252926930. [DOI] [PubMed] [Google Scholar]
- 178.Garmezy N, Masten AS, Tellegen A. The study of stress and competence in children: a building block for developmental psychopathology. Child Dev. 1984;55(1):97–111. [PubMed] [Google Scholar]
- 179.Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57(3):316–31. doi: 10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed] [Google Scholar]
- 180.Masten AS, Garmezy N, Tellegen A, Pellegrini DS, Larkin K, Larsen A. Competence and stress in school children: the moderating effects of individual and family qualities. J Child Psychol Psychiatry. 1988;29(6):745–64. doi: 10.1111/j.1469-7610.1988.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 181.Wolitski RJ, Gomez CA, Parsons JT. Effects of a peer-led behavioral intervention to reduce HIV transmission and promote serostatus disclosure among HIV-seropositive gay and bisexual men. AIDS. 2005;1(19 Suppl):S99–109. doi: 10.1097/01.aids.0000167356.94664.59. [DOI] [PubMed] [Google Scholar]
- 182.Simoni JM, Mason HR, Marks G, Ruiz MS, Reed D, Richardson JL. Women's self-disclosure of HIV infection: rates, reasons, and reactions. J Consult Clin Psychol. 1995;63(3):474–8. doi: 10.1037//0022-006x.63.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mansergh G, Marks G, Simoni JM. Self-disclosure of HIV infection among men who vary in time since seropositive diagnosis and symptomatic status. AIDS. 1995;9(6):639–44. doi: 10.1097/00002030-199506000-00017. [DOI] [PubMed] [Google Scholar]
- 184.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82(4):299–307. [PMC free article] [PubMed] [Google Scholar]
- 185.Tsai AC. A typology of structural approaches to HIV prevention: a commentary on Roberts and Matthews. Soc Sci Med. 2012;75(9):1562–7. doi: 10.1016/j.socscimed.2012.06.033. discussion 1568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Seeley J, Russell S. Social rebirth and social transformation? Rebuilding social lives after ART in rural Uganda. AIDS Care. 2010;22(Suppl 1):44–50. doi: 10.1080/09540121003605054. [DOI] [PubMed] [Google Scholar]
- 187.Neuberg SL, Smith SM, Asther T. Why people stigmatize: toward a biocultural framework. In: Heatherton TF, Kleck RE, Hebl MR, Hull JG, editors. The social psychology of stigma. New York: The Guilford Press; 2000. pp. 31–61. [Google Scholar]
- 188.Castro A, Farmer P. Understanding and addressing AIDS-related stigma: from anthropological theory to clinical practice in Haiti. Am J Public Health. 2005;95(1):53–9. doi: 10.2105/AJPH.2003.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sengupta S, Banks B, Jonas D, Miles MS, Smith GC. HIV interventions to reduce HIV/AIDS stigma: a systematic review. AIDS Behav. 2011;15(6):1075–87. doi: 10.1007/s10461-010-9847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brown L, Macintyre K, Trujillo L. Interventions to reduce HIV/AIDS stigma: what have we learned? AIDS Educ Prev. 2003;15(1):49–69. doi: 10.1521/aeap.15.1.49.23844. [DOI] [PubMed] [Google Scholar]
- 191.Farmer P, Leandre F, Mukherjee JS, Claude M, Nevil P, Smith-Fawzi MC, et al. Community-based approaches to HIV treatment in resource-poor settings. Lancet. 2001;358(9279):404–9. doi: 10.1016/s0140-6736(01)05550-7. [DOI] [PubMed] [Google Scholar]
- 192.Farmer P, Leandre F, Mukherjee J, Gupta R, Tarter L, Kim JY. Community-based treatment of advanced HIV disease: introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy) Bull World Health Organ. 2001;79(12):1145–51. [PMC free article] [PubMed] [Google Scholar]
- 193.Wolfe WR, Weiser SD, Leiter K, Steward WT, Percy-de Korte F, Phaladze N, et al. The impact of universal access to antiretroviral therapy on HIV stigma in Botswana. Am J Public Health. 2008;98(10):1865–71. doi: 10.2105/AJPH.2007.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Baranov V, Bennett D, Kohler H-P. The indirect impact of antiretroviral therapy. Northeast Universities Development Consortium Conference; Hanover; 2012. [cited 2012 Nov 3–4] [Google Scholar]
- 195.Tsai AC, Bangsberg DR, Bwana M, Haberer JE, Frongillo EA, Muzoora C, et al. How does antiretroviral treatment attenuate the stigma of HIV? Evidence from a cohort study in rural Uganda. AIDS Behav. May 14; doi: 10.1007/s10461-013-0503-3. Forthcoming 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sikkema KJ, Watt MH, Drabkin AS, Meade CS, Hansen NB, Pence BW. Mental health treatment to reduce HIV transmission risk behavior: a positive prevention model. AIDS Behav. 2010;14(2):252–62. doi: 10.1007/s10461-009-9650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Safren SA, O'Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010;67(12):1282–90. doi: 10.1001/archgenpsychiatry.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tsai AC, Karasic DH, Hammer GP, Charlebois ED, Ragland K, Moss AR, et al. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV-positive adults: a randomized controlled trial. Am J Public Health. 2013;103(2):308–15. doi: 10.2105/AJPH.2011.300422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Simoni JM, Huh D, Frick PA, Pearson CR, Andrasik MP, Dunbar PJ, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52(4):465–73. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pearson CR, Micek MA, Simoni JM, Hoff PD, Matediana E, Martin DP, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007;46(2):238–44. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007;26(4):488–95. doi: 10.1037/0278-6133.26.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Decroo T, Telfer B, Biot M, Maikere J, Dezembro S, Cumba LI, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. J Acquir Immune Defic Syndr. 2011;56(2):e39–44. doi: 10.1097/QAI.0b013e3182055138. [DOI] [PubMed] [Google Scholar]
- 204.Krieger N. Proximal, distal, and the politics of causation: what's level got to do with it? Am J Public Health. 2008;98(2):221–30. doi: 10.2105/AJPH.2007.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health. 2012;102(5):936–44. doi: 10.2105/AJPH.2011.300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Link BG, Phelan JC. Conceptualizing stigma. Annu Rev Sociol. 2001;27(1):363–85. [Google Scholar]