Abstract
The genera Aspergillus comprises species that produce mycotoxins such as aflatoxins, ochratoxins and patulin. These are cosmopolitan species, natural contaminants of agricultural products. In coffee grains, the most important Aspergillus species in terms of the risk of presenting mycotoxins belong to the genera Aspergillus Section Circumdati and Section Nigri. The purpose of this study was to assess the occurrence of isolated ochratoxigenic fungi of coffee grains from organic and conventional cultivation from the South of Minas Gerais, Brazil, as well as to evaluate which farming system presents higher contamination risk by ochratoxin A (OTA) produced by fungi. Thirty samples of coffee grains (Coffea arabica L.) were analysed, being 20 of them of conventional coffee grains and 10 of them organic. The microbiological analysis was done with the Direct Plating Technique in a Dichloran Rose Bengal Chloramphenicol Agar (DRBC) media. The identification was done based on the macro and micro morphological characteristics and on the toxigenic potential with the Plug Agar technique. From the 30 samples analysed, 480 filamentous fungi of the genera Aspergillus of the Circumdati and Nigri Sections were isolated. The ochratoxigenic species identified were: Aspergillus auricoumus, A. ochraceus, A. ostianus, A. niger and A. niger Aggregate. The most frequent species which produces ochratoxin A among the isolated ones was A. ochraceus, corresponding to 89.55%. There was no significant difference regarding the presence of ochratoxigenic A. ochreceus between the conventional and organic cultivation systems, which suggests that the contamination risk is similar for both cultivation systems.
Keywords: Aspergillus ochraceus, ochratoxin A, arabica coffee
Introduction
Like other agricultural products, coffee fruits and beans are subject to contamination by microorganisms during different development stages, from the crop field to storage (Batista et al., 2009). The presence of fungi in the coffee beans does not only affect quality in terms of flavor and aroma of the beverage, but also presents a safety risk for the final product, due to the production of toxic secondary metabolites, the mycotoxins, which can be harmful to consumers at certain concentrations (Bernnett and Klich, 2003, Vilela et al., 2010).
The contamination by ochratoxigenic fungi and production of ochratoxin A in the beans only occur in the presence of specific conditions like weather, plant susceptibility, environmental factors (such as temperature, damage caused by insects, pest attacks, contamination by other genera of fungi), chemical composition of beans, product cultivation and later handling, substrate nutrients, genetics of the microorganisms, and deficient storage (Paterson and Lima, 2010).
The difference between the conventional and organic cultivation for coffee production is mainly based on the products used during coffee growth. The organic coffee is produced without the utilization of high solubility pesticides and chemical fertilizers, which are replaced by sub-products originated from recycling plant and animal organic matter, animal dejects, bio-fertilizers, coffee pulp and husks, composting, worm humus, and so on (Theodoro and Guimarães, 2003).
Jestoi et al. (2004), analysing the levels of mycotoxins in cereal grains, have not found a significant difference for the concentration of the toxin between the conventional and organic cultivation systems, although the total mean concentration was slightly higher in the organic products. Since the organic products do not receive chemical supplies, the fruits and grains are exposed to fungi contamination, including potentially toxigenic fungi (Jestoi et al., 2004; Juan et al., 2008). The concentrations of ochratoxin A and ochratoxigenic Aspergillus species in grapes cultivated in the conventional and organic system were analysed by Ponsone et al. (2007). These authors observed that the presence of these fungi is not influenced by the cultivation system, but by the maturation stage of the fruits. In fact, most studies have concluded that more investigations are needed so that the safety of agriculture products can be assessed (Jestoi et al., 2004).
Several researches have been carried out to analyze the presence of ochratoxigenic fungi in coffee (Noonim et al., 2008; Silva et al., 2008; Batista et al., 2009, Vilela et al., 2010). The main ochratoxin A-producing species for beans coffee belong to the genera Aspergillus Section Circumdati and Section Nigri (Batista et al., 2003; Batista et al., 2009, Gil-Serna et al., 2011). The ochratoxin A-producing species of the genera Aspergillus are A. ochraceus (Taniwaki et al., 2003; Frisvad et al., 2004; Perrone et al., 2007; Batista et al., 2009; Gil-Serna et al., 2011;), A. niger (Taniwaki et al., 2003; Samson et al., 2004; Perrone et al., 2007), A. carbonarius (Taniwaki et al., 2003; Samson et al., 2004; Perrone et al., 2007), A. sulphureus (Batista et al., 2009), A. sclerotiorum (Batista et al., 2009), A. westerdijkiae (Frisvad et al., 2004, Gil-Serna et al., 2011). A. ochraceus is commonly found in coffee and is an important ochratoxin A producer (Suarez-Quiroz et al., 2004b; Batista et al., 2009; Vilela et al., 2011). A. carbonarius is common in grape and in robusta coffee. Its occurrence in coffee beans is not frequent in Brazil, unlike in Thailand, where this species is commonly isolated (Taniwaki et al., 2003; Noonim et al., 2008). Ochratoxin A is possibly carcinogenic to humans, as well as nephrotoxic, immunotoxic and teratogenic (IARC,1993).
The purpose of this study was to identify toxigenic fungi in organically and conventionally cultivated coffee beans in the South of Minas Gerais, Brazil, as well as to evaluate which farming system presents higher contamination risk by ochratoxin A produced by fungi.
Materials and Methods
Sampling
A total of 30 green coffee beans samples (Coffea arabic L.), harvest of the year 2009/2010, divided into 10-bean organic coffee sample and a 20-bean conventional coffee samples (Table 1). The samples were collected from the southern city of Minas Gerais - Brazil, (Poço Fundo: latitude −21°46′51″; longitude −45°57′54″; altitude 836 m); (Santo Antônio do Amparo: latitude −20°56′47″; longitude −44°55′08″; altitude 1013 m); (Lavras: latitude −21°14′43″; longitude −44°59′59″; altitude 919 m). These samples were analysed in the Laboratory of Food Microbiology - Mycology and Mycotoxins - Department of Food Science, Federal University of Lavras (Lavras, MG, Brazil).
Table 1.
Studied coffee samples.
| Samples | Cultivation system | Harvest | Locations |
|---|---|---|---|
| 5 | Organic | Harvest onto cloth | Poço Fundo |
| 3 | Organic | Swept from ground | Poço Fundo |
| 1 | Organic | Harvest onto cloth | Santo Antônio do Amparo |
| 1 | Organic | Swept from ground | Santo Antônio do Amparo |
| 4 | Conventional | Swept from ground | Poço Fundo |
| 4 | Conventional | Harvest onto cloth | Poço Fundo |
| 12 | Conventional | Harvest onto cloth | Lavras |
Mycological analysis
For isolation of fungi associated with green coffee beans, the direct plating technique was applied in DRBC medium - Dicloran Rose de Bengal Chloramphenicol (glucose 10.0 g; peptone 5.0 g; KH2PO4 1.0 g; MgSO4.7H2H 0.5 g; Agar 15.0 g; bengal rose 25.0 mg; dicloran 2.0 mg; chloramphenicol 100.0 mg; distilled water 1.0 L). A total of 100 coffee beans were plating directly without surface disinfection and 100 beans were plated with surface disinfection with 70% alcohol and 1% sodium hypochlorite according to Samson et al. (2000). The plates were incubated for 5–7 days at 25 °C. The overall percent contamination was expressed as the percentage of particles yielding visible growth of fungi.
Isolation and identification of fungi
The isolated fungi were purified and identified according to Klich (2002), Frisvad et al. (2004) and Samson et al. (2004).
The isolates were incubated in CYA medium - Czapek yeast Agar (K2HPO4 1.0 g; concentrate Czapek NaNO3 30.0 g; KCl 5.0 g; MgSO4.7H2O 5.0 g; FeSO4.7H2O 0.1 g; ZnSO4.7H2O 0.1 g CuSO4.5H2O 0.05 g; distilled water 100mL) in MEA - Malt Extract Agar (malt extract 20.0 g; peptone 1.0 g; glucose 30.0 g; Agar 20 g; distilled water 1 L) at 25 °C and CYA at 25 °C and 37 °C. After incubation for 7 days, the microscopic and macroscopic characteristics described by Klich (2002b) were observed.
Determination of OTA-producing fungi by the plug agar method
The isolates tested were inoculated in YES medium - Yeast Extract Sucrose Agar (yeast extract 20.0 g; sucrose 150.0 g; Agar 20.0 g; MgSO4.7H2O 0.5 g; distilled water 1 L) with metallic solution 1 mL (ZnSO4.7H2O 1%; CuSO4.5H2O 0.5%) for 7 days at 25 °C, according to Filtenborg and Frisvad (1980). The following was used: ochratoxin A standard (Sigma-Aldrich), thin layer chomatography plates (Merk-Silica Gel 60, 20×20) as mobile phase; toluene, ethyl acetate and formic acid 90% (60:30:10 v/v/v). After eluition, the plates were air dried. Mycotoxin production was confirmed by green fluorescence in ultraviolet light with λ = 366 nm in chromatovisor CAMAG (UF-BETRACHTER). The isolates considered as OTA producers presented an RF (refection factor) and a fluorescence spot similar to that of OTA standard.
Statistical analysis
To correlate the levels of OTA contamination with the different coffee samples, simple correspondence analysis was used, as described by Greenacre (1993). This technique consists of applying the main components such as the contingency table, in this case, a table displaying the ochratoxigenic fungi frequency in one line and the coffee sample in columns. The proportion of the coffee sample totals corresponds to the profile of the variables displayed in the column. Analogously, the profile for the variables lines is produced. For the comparison between the species Aspergillus Section Circumdati and Section Nigri in the two different cultivation systems, a simple descriptive analysis of media was carried out.
Results and Discussion
From the samples analysed, 100% were contaminated by filamentous fungi and approximately 50% contamination with yeasts. The main genera found in this study (Table 2) have also been reported in coffee beans in other researches carried out in Brazil and in other countries (Joosten et al., 2001; Pardo et al., 2004; Bokhari, 2007; Leong et al., 2007; Silva et al., 2008; Batista et al., 2009; Vilela et al., 2010).
Table 2.
Percentage of fungi found in conventional and organic cultivated system of coffee beans after plating with or without surface disinfection.
| Fungi | Organic system with disinfection | Organic system without disinfection | Conventional system with disinfection | Conventional system without disinfection |
|---|---|---|---|---|
| Aspergillus sp | 3.6 | 49.8 | 7.6 | 62.3 |
| Cladosporium sp | 0.9 | 0.08 | 6.9 | 5.0 |
| Eurotium sp | 0.24 | 0 | 0.4 | 1.8 |
| Fusarium sp | 53.1 | 33.3 | 28.5 | 24.1 |
| Mucor sp | 0.5 | 0 | 0 | 0.9 |
| Penicillium sp | 5.3 | 11.8 | 3.9 | 4.9 |
| Rhizopus sp | 0 | 0.3 | 0.7 | 0.2 |
| Tricoderma sp | 0 | 0 | 0 | 0.16 |
Analysing the samples plated without disinfection, the genera Aspergillus was found in about 62.3% of the samples. The Aspergillus genera was found in an incidence of 93% of coffee beans (Pardo et al., 2004), and was predominant in coffee beans in the Saudi Arabia (Bokhari, 2007).
Aspergillus Section Circumdati and Nigri corresponded to 56.54% of the total of contaminant fungi of coffee beans. This result was similar to the of Batista et al. (2003), who observed 54.78% for the same sections of the genera in Brazilian coffee beans. These two sections was responsible for 75% of the Vietnamese green coffee beans contamination (Leong et al., 2007). In this work, fungi of the genera Aspergillus Section Circumdati corresponded to 27.29% and Section Nigri to 29.25% of the contaminant fungi of the beans. In a study carried out by Batista et al. (2009), 41% of the isolated identified belonged to Section Circumdati and 25% to Section Nigri. Pardo et al. (2004) found 67.40% of beans, from different origins, infected with Aspergillus Section Nigri. In the present study, we observed a uniform distribution of filamentous fungi of the genera Aspergillus Section Circumdati and Section Nigri among organic and conventional coffee samples (Figures 1 and 2; Tables 3 and 4). The uniform distribution of the filamentous fungi in coffee samples from different regions have also reported for Batista et al. (2003). The presence of these fungi in coffee beans in Brazil is due to the geographical distribution of this genus, including latitudes 26° – 35° Klich (2002a), as well as environmental conditions and type of coffee processing.
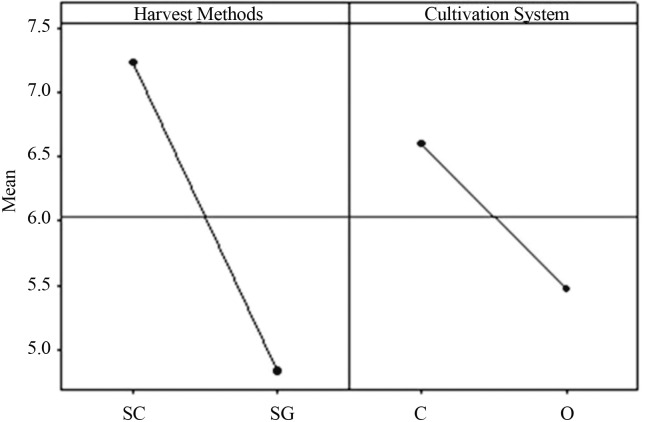
Figure 1.
Graphic of the main effects of the harvest method and type of coffee factors averages for the fungi of genus Aspergillus Section Nigri (data transformed by the square root). SC - harvest onto cloth SG- swept from ground. C - conventional. O - organic.
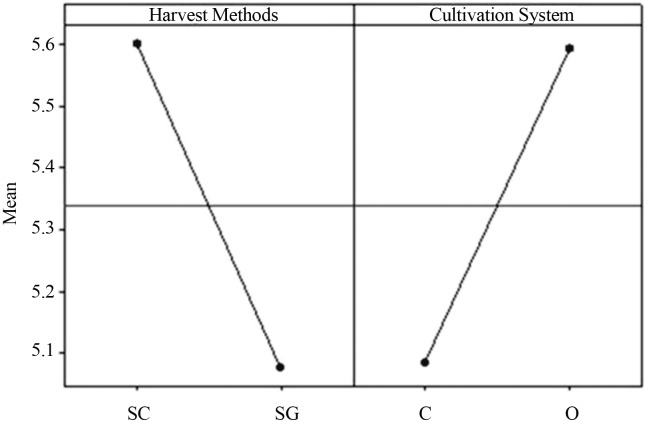
Figure 2.
Graphic of the main effects of type of coffee and harvest method factors averages for fungi of the genus Aspergillus Section Circumdati (data transformed by the square root). SC - Harvest onto cloth SG- swept from ground. C - conventional. O - organic.
Table 3.
Variance analysis for the calculation of filamentous fungi of the Genus Aspergillus Section Nigri.
| VSa | FDb | SSc | MSd | Fe | p-valuef |
|---|---|---|---|---|---|
| Harvest | 1 | 22.342 | 22.342 | 4.69 | 0.049 |
| Coffee | 1 | 4.935 | 4.935 | 1.04 | 0.329 |
| Coffee harvest* | 1 | 0.009 | 0.009 | 0.00 | 0.967 |
| Error | 12 | 57.136 | 4.761 | ||
| Total | 15 | 82.191 |
OBS: Data transformed by the square root.
VS - Variation source.
FD - Freedom degree.
SS - Sum of squares.
MS - Mean square.
F - Test of F.
p-value - level of significance.
Table 4.
Variance analysis for the calculation of filamentous fungi of the genus Aspergillus Section Circumdati.
| VSa | FDb | SSc | MSd | Fe | p-valuef |
|---|---|---|---|---|---|
| Harvest | 1 | 1.071 | 1.071 | 0.19 | 0.672 |
| Coffee | 1 | 1.016 | 1.016 | 0.18 | 0.680 |
| Coffee harvest* | 1 | 0.007 | 0.007 | 0.00 | 0.973 |
| Error | 12 | 68.377 | 68.377 | ||
| Total | 15 | 70.811 | 70.811 |
OBS: Data transformed by the square root.
VS - Variation source.
FD - Freedom degree.
SS - Sum of the squares.
MS - Mean square.
F - Test of F.
p-value - Significance level.
Figures 1 and 2 and Tables 3 and 4 shows the results of contaminations by genera Aspergillus Section Circumdati and Section Nigri in coffee bean samples regarding the cultivation system (conventional and organic). It was possible to observe that there were no significant differences. Regarding the harvesting type, the harvest on cloth presented a significant difference for fungi of the genera Aspergillus Section Nigri. The harvesting system directly affects the incidence of fungi in fruit and coffee beans (Batista et al., 2009). The coffee berries harvest onto a cloth are fruits that were in the tree, which can be ripe, unripe or dried on the tree (Batista et al., 2009); these fruits are exposed to more insulation. Aspergillus Section Nigri are more resistant to UV light due to the spore colors, which confers a larger capacity of competition for the substrate, and can justify the presence of fungi of the genera Aspergillus Section Nigri onto the cloth (Abarca et al., 2003; Romero et al., 2005; Duarte et al., 2010). The cloth used for harvest could also be contaminated with fungal spores of the genus Aspergillus Section Nigri, and favors the contamination of coffee fruits and beans.
Of all the samples analysed, 480 isolates of the genus Aspergillus were obtained and identified, based on the morphological characteristics. Of these isolates, 277 were of the genera Aspergillus Section Nigri and 203 Section Circumdati. Of the Section Circumdati, the most common species, both for conventional and organic cultivation coffee was A. ochraceus (n = 169) representing 35.20% of the total of isolates, a similar proportion as the ones reported in other studies with coffee fruits and beans (Batista et al., 2003; Suarez-Quiroz et al., 2004b; Silva et al., 2008). Due to the special relevance of this species for coffee quality and safety, in function of its large distribution and toxigenic potential, A. ochraceus has been the target of many researches with coffee beans and fruits (Silva et al., 2000; Batista et al., 2003; Taniwaki et al., 2003; Suarez-Quiroz et al., 2004a; Vilela et al., 2010).
Considering all the samples analysed, the most common species of the genus Aspergillus Section Nigri in the conventional cultivation system was A. tubingensis with 42.70% of the isolates. In the organic cultivation system, the main species was A. foetidus with 35.80%. Noonim et al. (2008) isolated, identified and evaluated the ochratoxigenic potential of Aspergillus species in coffee beans in two regions of Thailand, and they also found the non-ochratoxigenic species A. foetidus and A. tubingensis in the beans analysed. However, similar works with green coffee beans, only in the conventional cultivation system, were carried out in Brazil (Taniwaki et al., 2003) and in other countries, like Saudi Arabia (Bokhari, 2007), and Vietnam (Ilic et al., 2007; Leong et al., 2007). In all these studies A. niger predominated. A. niger is largely distributed in the environment (Urbano et al., 2001) and is isolated not only from coffee beans, but also from other foods like grape and derivatives (Magnoli et al., 2003; Perrone et al., 2007). The concentration of ochratoxin A and ochratoxigenic Aspergillus species in grapes cultivated in conventional and organic system were analysed by Ponsone et al. (2007). These authors observed that the presence of these fungi is not influenced by the cultivation system, but by the maturation stage of the fruits.
The other species found in this work have also been identified in other coffee researches, such as A. auricomus (19 isolates) and A. sulphureus (8 isolated), which have also been reported by Batista et al. (2009), A. niger and A. niger Aggregate reported by Silva et al. (2008) and Batista et al. (2009).
All the 480 isolated were tested for the capacity of producing ochratoxin A with the Plug Agar technique. Considering the 30 coffee bean samples, the species A. ochraceus was the main producer of this mycotoxin. Similar results were found by Batista et al. (2003).
Analysing the eight samples of coffee from the conventional cultivation and the eight samples of coffee from the organic cultivation, provenient from the Poço Fundo, it was possible to observe that of the 107 isolates of the genus Aspergillus Section Circumdati, 62.62% (n = 67) produced ochratoxin A (Table 5).
Table 5.
Identification of potentially toxigenic species and isolates. Samples from the Poço Fundo.
| Genus Aspergillus (number of same) | N. of isolates identified | N. of potentially toxigenica isolates |
|---|---|---|
| Organic coffee (8) | ||
| Section Circumdati | ||
| A. auricomus | 7 | 4 |
| A. ochraceus | 49 | 32 |
| A.ostianus | 1 | 1 |
| A. sulphureus | 2 | ND |
| Section Nigri | ||
| A. foetidus | 22 | ND |
| A. niger | 7 | ND |
| A. niger Aggregate | 10 | 2 |
| A. tubingensis | 12 | ND |
| Conventional coffee (8) | ||
| Section Circumdati | ||
| A. auricomus | ND | ND |
| A. ochraceus | 45 | 28 |
| A. ostianus | 2 | 2 |
| A. sulphureus | 1 | ND |
| Section Nigri | ||
| A. foetidus | 20 | ND |
| A. niger | 33 | 4 |
| A. niger Aggregate | 15 | ND |
| A. tubingensis | 13 | ND |
ND - not detected by the Plug Agar method.
The values obtained in this study were close to the ones obtained by Batista et al. (2003), who identified 74.6% of the Section Circumdati species as ochratoxin A-producing, in conventional coffee beans. Of the ochratoxin A-producing fungi, the major producer species was A. ochraceus 89.55% (Table 5). In the species of A. auricomus and A. ostianus we observed isolates capable of producing mycotoxin (Table 5) these species as potentially ochratoxigenic also were reported by Batista et al. (2003, 2009).
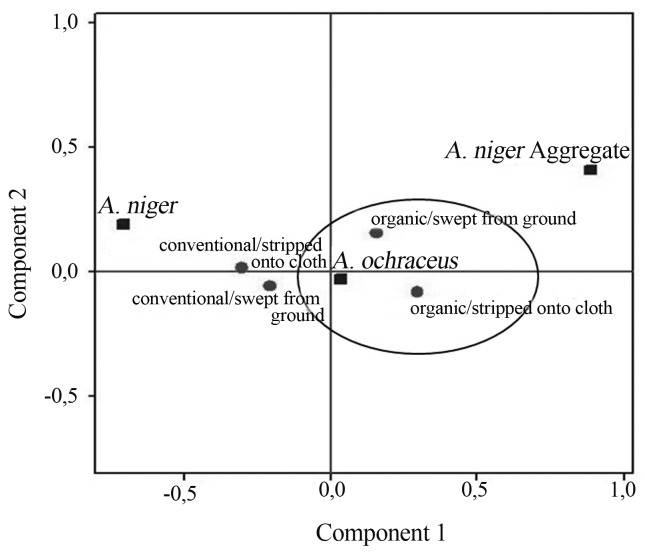
Although the correspondence analysis does not establish the statistical significance of the associations and does not evaluate the independent effect of each characteristic, this analysis combines the advantages of non-linear and multi-dimensional methods, which allows the description of the characteristics that occur simultaneously in the coffee culture, and the identification of the different profiles of the ochratoxigenic species found. With the map (Figure 3) generated from the correspondence analysis, it was possible to observe in the coffee samples from the Poço Fundo, that A. ochraceus was the toxin-producing species found more often in both cultivation systems. The similarity of frequency of this species was notorious in both cultivation systems evaluated, as well as the harvest methods (harvest onto cloth and swept from the ground). Regarding the other species (A. niger and A. niger Aggregate), we verified that no cultivation system or type of harvesting was characterized according to the presence of the isolate.
Figure 3.
Perception map of the associations between filamentous fungi and the harvest method of the sample coffee from Poço Fundo.
Regarding the ochratoxin A-producing species reported by other authors, Batista et al. (2003) found A. ochraceus and A. sulphureus as the main species, while Pardo et al. (2004) found 23.1% of ochratoxin A-producing Aspergillus ochraceus. Urbano et al. (2001) obtained the same results as the present work, with 88.1% of A. ochraceus producers of the toxin. Batista et al. (2009) analysed ochratoxigenic fungi in coffee beans conventionally processed by dry and wet methods; of fungi of the genera Aspergillus Section Circumdati, 92.69% were identified as A. ochraceus and of these, 95% produced ochratoxin A. Taniwaki et al. (2003) found 75% of these species with capacity of producing this toxin.
Besides these species reported as ochratoxin A-producing, recent studies also show that other species may be potentially producers of mycotoxin in coffee, like A. elegans, A. steynii and A. westerdijkiae (Batista et al., 2009; Gil-Serna et al., 2011;Prado et al., 2006). Gil-Serna et al. (2011) stated that the species of A. steynii and A. westerdijkiae can represent a higher risk of producing ochratoxin A, due to their capacity of producing a great amount of it and the diversity of foods that these species may contaminate. However, these authors do not ignore the possibility that A. elegans and A. ochraceus produce the same toxin.
Comparing the results of the coffee beans samples from the Poço Fundo, of the 132 isolates of the genera Aspergillus Section Nigri, 4.54% were capable of producing ochratoxin A (Table 5). Pardo et al. (2004) found 7.3% of isolates of the Section Nigri as ochratoxin A producers, while Urbano et al. (2001) found 11.5% in a similar study. In contrast with these results, Batista et al. (2003; 2009) have not found any ochratoxigenic isolates of the Section Nigri.
Of the ochratoxin A-producing isolates belonging to Section Nigri analysed in this work (Table 5), 10% were A. niger (n = 4) and 8% A. niger Aggregate (n = 2). This result agrees with the one reported by Noonim et al. (2008), who found only 13% of the isolates of A. niger as ochratoxin A producers.
This research did not find A. carbonarius, which is described by other researchers as an important ochratoxin A-producing species. A. carbonarius is the species of the genus Aspergillus Section Nigri with the highest ochratoxin A production potential (Prado et al., 2004). However, it is not a common species of arabica coffee beans as A. niger (Noonim et al., 2008; Palacios-Cabeira et al., 2005). This statement was reinforced by the studies of Taniwaki et al. (2003), who found 62.95% of A. niger and 6.19% of A. carbonarius in coffee beans and identified as ochratoxigenic only 3% of the isolates of A. niger while in the isolates of A. carbonarius this proportion was of 77%. That the contamination with A. carbonarius is slightly higher in robusta coffee than in arabica coffee (Pardo et al., 2004; Noonim et al., 2008).
The eventual divergence about ochratoxin A production by the genus Aspergillus suggests that the synthesis of ochratoxin A is dependent on the interaction of several environmental factors (Mühlencoert et al., 2004), more than the simple fact of growth, that is, the inability to produce ochratoxin A in determined conditions does not justify any conclusion about the general ability to produce the mycotoxin (Bakutis et al., 2006). The production of secondary metabolites such as mycotoxins are not essential for the microorganism. The environmental factors will regulate the genes and enzymes involved in the production of ochratoxin A (Mühlencoert et al., 2004).
Based on the results it was possible to observe that the organic and conventional coffee cultivation systems did not differ in terms of the contamination of beans by species of the genera Aspergillus Section Nigri and Section Circumdati. Likewise, there was no difference in terms of the occurrence of A. ochraceus, which was the main ochratoxigenic species found, which allows us to affirm that there is no influence of the cultivation system on the contamination by Aspergillus species, neither on the occurrence of ochratoxigenic species that belong to this genus. Both cultivation systems present the same risks of contamination. Not all the isolates of the A. ochraceus species were ochratoxin A producers; however, just the presence of this ochratoxigenic species does not imply that the production of the mycotoxin will necessarily occur.
Acknowledgments
To Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the financial support (Project n. CBB-APQ - 00781-08).
References
- Abarca ML, Accensi F, Bragulat MR, Castellá G, Cabañes FJ. Aspergillus carbonarius as the main source of ochratoxin A contamination in dried vine fruits from the Spanish market. J of Food Prot. 2003;66:504–506. doi: 10.4315/0362-028x-66.3.504. [DOI] [PubMed] [Google Scholar]
- Bakutis B, Baliukoniene V, Lugauskas A. Factos predetermining the abundance of fungi and mycotoxins in grain from organic and conventional farms. Ekologija. 2006;3:122–127. [Google Scholar]
- Batista LR, Chalfoun SM, Prado G, Schwan RF, Whealsa E. Toxigenic fungi associated with processed (green) coffee beans (Coffea arabica L.) Int J Food Microbiol. 2003;85:293–300. doi: 10.1016/s0168-1605(02)00539-1. [DOI] [PubMed] [Google Scholar]
- Batista LR, Chalfoun SM, Silva CF, Cirillo M, Varga EA, Schwan RF. Ochratoxin A in coffee beans (Coffea arabica L.) processed by dry and wet methods. Food Control. 2009;20:784–790. [Google Scholar]
- Bernnett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhari FM. Mycotoxins and toxigenic fungi in arabic coffee beans in Saudi Arabia. Adv Biol Res. 2007;1:56–66. [Google Scholar]
- Duarte SC, Pena A, Lino CM. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010;27:187–198. doi: 10.1016/j.fm.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Filtenborg O, Frisvad JC. A simple screening method for toxigenic molds in pure cultures. LebensWissen Technol. 1980;13:128–130. [Google Scholar]
- Frisvad JC, Frank JM, Houbraken JAMP, Kuijpers AFA, Samson RA. New ochratoxin A producing species of Aspergillus section Circumdati. Stud Mycol. 2004;50:23–43. [Google Scholar]
- Gil-Serna J, Vázquez C, Sardiñas N, González-Jaén MT, Patiño B. Revision of ochratoxin a production capacity by the main species of Aspergillus Section Circumdati. Aspergillus steynii revealed as the main risk of OTA contamination. Food Control. 2011;22:343–345. [Google Scholar]
- Greenacre M. Correspondence Analysis in Practice. Academic Press; New York: 1993. [Google Scholar]
- IARC. Monographs on the evaluation of carcinogenic risks to humans, some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines, and mycotoxins. International Agency for Research on Cancer. 1993;56:489–521. [Google Scholar]
- Ilic Z, Bui T, Tran-Dinh N, Dang MHV, Kennedy I, Carter D. Survey of Vietnamese coffee beans for the presence of ochratoxigenic Aspergilli. Mycopathologia. 2007;163:177–182. doi: 10.1007/s11046-007-0099-0. [DOI] [PubMed] [Google Scholar]
- Jestoi M, Somma MV, Kouva M, Veijalainen P, Rizzo A, Ritieni A, Peltonen K. Levels of mycotoxins and sample cytotoxicity of selected organic and conventional grain-based products purchased from Finnish and Italian markets. Mol Nutr Food Res. 2004;48:229–307. doi: 10.1002/mnfr.200400026. [DOI] [PubMed] [Google Scholar]
- Joosten HMLJ, Goetz J, Pittet A, Schellenberg M, Bucheli P. Production of ochatoxin A by Aspergillus carbonarius on coffee cherries. Int J Food Microbiol. 2001;65:39–44. doi: 10.1016/s0168-1605(00)00506-7. [DOI] [PubMed] [Google Scholar]
- Juan C, Moltó JC, Lino CM, Mañes J. Determinaton of ochratoxin A in organic and non-organic cereals and cereal products from Spain and Portugal. Food Chem. 2008;107:525–530. [Google Scholar]
- Klich MA. Biogeography of Aspergillus species in soil and litter. Mycologia. 2002;94:21–27. [PubMed] [Google Scholar]
- Klich MA. Identification of Common Aspergillus species. Centraalbureau voor Schimmelautures; Netherlands: 2002. [Google Scholar]
- Leong SL, Hien LT, An TV, Trang NT, Hocking AD, Scott ES. Ochratoxin A-producing Aspergilli in Vietnamese green coffee beans. Lett Appl Microbiol. 2007;45:301–306. doi: 10.1111/j.1472-765X.2007.02189.x. [DOI] [PubMed] [Google Scholar]
- Magnoli C, Astoreca A, Ponsone L, Combina M, Palacio G, Rosa CA, Dalcero AM. Survey of mycoflora and ochratoxin A in dried vine fruits from Argentina markes. Lett Appl Microbiol. 2003;37:179–184. doi: 10.1111/j.1472-765X.2004.01583.x. [DOI] [PubMed] [Google Scholar]
- Mühlencoert E, Mayer I, Zapf MW, Vogel RF, Niessen L. Production of ochratoxin A by Aspergillus ochraceus. Eur J Plant Pathol. 2004;110:651–659. [Google Scholar]
- Noonim P, Mahakarnchanakul W, Nielsen KF, Frisvad JC, Samson RA. Isolation, identification and toxigenic potential of ochratoxin A- producing Aspergillus species from coffee beans grown in two regions of Thailand. Int J Food Microbiol. 2008;128:197–202. doi: 10.1016/j.ijfoodmicro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Palacios-Cabeira H, Taniwaki MH, Hashimoto JM, Menezes HC. Growth of Aspergillus ochraceus, A. carbonarius and A. niger on culture media at different water activities and temperatures. Braz J Microbiol. 2005;36:24–28. [Google Scholar]
- Pardo E, Marín S, Ramos AJ, Sanchis V. Occurrence of ochratoxigenic fungi and ochratoxin A in green coffee from diferent origins. Food Science Technol Int. 2004;10:45–50. [Google Scholar]
- Pardo E, Sanchis V, Ramos AJ, Marín S. Non-specificity of nutritional substrate for ochratoxin A production by isolates of Aspergillus ochraceus. Food Microbiol. 2006;23:351–358. doi: 10.1016/j.fm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Paterson RRM, Lima N. How will climate change affect mycotoxins in food? Food Res Int. 2010;43:1902–1914. [Google Scholar]
- Perrone G, Susca A, Cozzi G, Ehrlich J, Varga JC, Frisvad M, Meijer A, Noonim P, Mahakarnchanakul W, Samson RA. Biodiversity of Aspergillus species in some important agricultural products. Stud Mycol. 2007;59:53–66. doi: 10.3114/sim.2007.59.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsone ML, Combina M, Dalcero A, Chulze S. Ochratoxin A and ochratoxigenic Aspergillus species in Argentinean wine grapes cultivated under organic and non-organic systems. Int J Food Microbiol. 2007;114:131–135. doi: 10.1016/j.ijfoodmicro.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Romero SM, Comerio RM, Larumbe G, Ritieni A, Vaamonde G, Fernández Pinto V. Toxigenic fungi isolated from dried vine fruits in Argentina. Int J Food Microbiol. 2005;104:43–49. doi: 10.1016/j.ijfoodmicro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Samson RA, Hoekstra ES, Frisvad JC, Filtenborg O. Introduction to Food- and Airborne Fungi. Voor Schimmelcultures Baarn Delft, Centraalbureau; 2000. [Google Scholar]
- Samson RA, Houbraken JAMP, Kuijpers AFA, Frank M, Frisvad JC. New ochratoxin A or sclerotium producing species in Aspergillus Section Nigri. Stud Mycol. 2004;50:45–61. [Google Scholar]
- Silva CF, Schwan RF, Dias ES, Wheals AE. Microbial diversity during maturation and natural producessing of coffee cherries of Coffea arabica in Brazil. Int J Food Microbiol. 2000;60:251–260. doi: 10.1016/s0168-1605(00)00315-9. [DOI] [PubMed] [Google Scholar]
- Silva CF, Batista LB, Schwan RF. Incidence and distribution of filamentous fungi during fermentation, drying and storage of coffee (Coffea arabica L.) beans. Braz J Microbiol. 2008;39:521–526. doi: 10.1590/S1517-838220080003000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Quiroz M, Gonzáles-Rios O, Barel M, Guyot B, Schorr-Galindo S, Guiraud JP. a) Effect of chemical and environmental factors on Aspergillus ochraceus growth and toxigenesis in green coffee. Food Microbiol. 2004;21:629–634. [Google Scholar]
- Suarez-Quiroz M, Gonzáles-Rios O, Barel M, Guyot B, Schorr-Galindo S, Guiraud JP. b) Study of ochratoxin A-producing strains in coffee processing. Int J Food Sci Technol. 2004;39:501–507. [Google Scholar]
- Taniwaki MH, Pitt JI, Teixeira AA, Iamanaka BT. The source of ochratoxin A in Brazilian coffee and its formation in relation to processing methods. Int J Food Microbiol. 2003;82:173–179. doi: 10.1016/s0168-1605(02)00310-0. [DOI] [PubMed] [Google Scholar]
- Theodoro CGC, Guimarães JBC. Avaliação do estado nutricional de agrosistemas de café orgânico no estado de Minas Gerais. Ciênc Agrotec. 2003;27:1222–1230. [Google Scholar]
- Urbano GR, Taniwaki MH, Leitão MF, Vicentini MG. Occurrence of ochratoxin A-production fungi in raw Brazilian coffee. J Food Protect. 2001;64:1226–1230. doi: 10.4315/0362-028x-64.8.1226. [DOI] [PubMed] [Google Scholar]
- Vilela DM, Pereira GV, Silva CF, Batista R, Schwan RF. Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea arabica L.) Food Microbiol. 2010;27:1128–1135. doi: 10.1016/j.fm.2010.07.024. [DOI] [PubMed] [Google Scholar]