Abstract
The endophytic strain Zong1 isolated from root nodules of the legume Sophora alopecuroides was characterized by conducting physiological and biochemical tests employing gfp-marking, observing their plant growth promoting characteristics (PGPC) and detecting plant growth parameters of inoculation assays under greenhouse conditions. Results showed that strain Zong1 had an effective growth at 28 ºC after placed at 4–60 ºC for 15 min, had a wide range pH tolerance of 6.0–11.0 and salt tolerance up to 5% of NaCl. Zong1 was resistant to the following antibiotics (μg/mL): Phosphonomycin (100), Penicillin (100) and Ampicillin (100). It could grow in the medium supplemented with 1.2 mmol/L Cu, 0.1% (w/v) methylene blue and 0.1–0.2% (w/v) methyl red, respectively. Zong1 is closely related to Pseudomonas chlororaphis based on analysis the sequence of 16S rRNA gene. Its expression of the gfp gene indicated that strain Zong1 may colonize in root or root nodules and verified by microscopic observation. Furthermore, co-inoculation with Zong1 and SQ1 (Mesorhizobium sp.) showed significant effects compared to single inoculation for the following PGPC parameters: siderophore production, phosphate solubilization, organic acid production, IAA production and antifungal activity in vitro. These results suggest strains P. chlororaphi Zong1 and Mesorhizobium sp. SQ1 have better synergistic or addictive effect. It was noteworthy that each growth index of co-inoculated Zong1+SQ1 in growth assays under greenhouse conditions is higher than those of single inoculation, and showed a significant difference (p < 0.05) when compared to a negative control. Therefore, as an endophyte P. chlororaphis Zong1 may play important roles as a potential plant-growth promoting agent.
Keywords: PGPC, endophyte, the gpf-marker, colonization, co-inoculation
Introduction
Sophora alopecuroides is a wild perennial herb of the xerophyte species and is widely distributed in northwestern China. However, most of northwestern China belongs to arid and semi-arid areas. S. alopecuroides shows excellent performance in drought and alkaline tolerance as well as sandstorm resistance due to its well-developed root system. In addition, S. alopecuroides plays a vital role in environmental protection in northwest of China (Zhao et al., 2010). It also has promising prospects through its utilization in pharmaceutics and pesticides, as a source of livestock feed, and its role as a natural windbreaker and nectar source. As a traditional Chinese medicine, this plant also is used to treat fever and diarrhea; some studies even suggest it has the potential to inhibit cancer cells (Song et al., 1999).
Endophytic bacteria live inside the plant tissues and do not cause visible damage or morphological changes to their hosts. In the last few decades, endophytic bacteria have attracted more and more attention as novel resources in the biocontrol of plant diseases and in the promotion of plant growth (Lin et al., 2009). They can benefit the host plants in a variety of ways, such as producing IAA (indole acetic acid), fixing nitrogen, dissolve phosphates, producing siderophores, suppressing phytopathogens by competition in the invasion sites and by secreting antibiotic compounds (Ryan et al., 2008), and by helping the symbiotic rhizobia to form nodules with unspecific hosts (Liu et al., 2010).
Endophytes, like Pseudomonas, Agrobacterium, Bacillus, Burkholderia and Enterobacteria, have been isolated from root nodules in various leguminous plants including alfalfa, clover, soybean pigeon pea, etc (Geetha et al., 2008) since 1902 (Zakhia et al., 2006; Kan et al., 2007; Li et al., 2008). Available reports indicated improved plant yield and health under greenhouse conditions (measured as an increase in root wet weight and nodulation) when co-inoculated with nodule endophytes compared to inoculation with rhizobia alone (Bai et al., 2003). Plant growth promoting bacteria (PGPB) have been co-inoculated with rhizobia which include strains of the following well-known rhizobacteria: Pseudomonas (Chandra et al., 2010; Chanway et al., 1989), Bacillus (Geetha et al., 2008), Azospirillum (Yahalom et al., 1988), and Azotobacter (Burns et al., 1981). For example, co-inoculation of some Pseudomonas and Bacillus strains along with effective Rhizobium spp. is shown to stimulated chickpea growth and nodulation, stimulate nitrogen fixation (Parmar et al., 1999), and increase growth and yield compared to single inoculation (Geetha et al., 2008). However, up to now, special nodule endophytes of S. alopecuroides have not yet been studied.
In a recent study, we collected and characterized nodule endophytic bacteria from legume plant S. alopecuroides (Zhao et al., 2010). The aims of this experiment are (i) to examine the colonization of gfp-tagged endophytic Pseudomonas chlororaphis strain Zong1, and (ii) to determine their plant growth promoting characterization (PGPC) in a single and combined inoculation test.
Materials and Methods
Isolation of nodule endophytic bacteria and nodulation verification
Thirty healthy nodules from fifteen S. alopecuroides plants were collected and carefully washed with sterile distilled water to remove nodule surface soil particles, surface sterilized with 95% alcohol for 30 s and with 3% NaClO (w/v) for 3 min, and finally rinsed 8 times to thoroughly eliminate NaClO with sterile distilled water. The surface-sterilized nodules were crushed and streaked on yeast-extract-mannitol agar (YEMA) plates for the isolation of endophytic bacteria with the standard methods described previously (Vincent, 1970). The plates were incubated at 28 ºC and single colonies were further purified by repeatedly streaking on the same medium and by microscopic examination. In order to verify surface sterilization, the surface sterilized nodules were rolled on the Nutrient Agar (NA) medium and the aliquots of water from final rinse solutions were plated onto NA plates as controls to detect possible contaminants. Plates without any contaminants were considered effectively surface sterilized and their corresponding YEMA plates were used for the isolation of endophytes. Nodulation capability was verified for nodule isolates by inoculating on surface sterilized and pre-germinated seeds.
Construction of gfp-marked P. chlororaphis Zong1 and examination of colonization
Since the plasmid pMP2444 harboring the green fluorescent protein (gfp) gene (Stuurman et al., 2000) was transformed into E. coli S17-1 as reported (Chen et al., 2003), the Escherichia coli S17-1 strain was used as the donor in a transformation test, was grown at 37 ºC Lysogeny broth (LB, 10 g NaCl/L) medium supplied with 30 mg/mL gentamycin (Stuurman et al., 2000). The transformed E. coli S17-1 resistant to gentamycin was used in electroporation with the re-isolated P. chlororaphis strain Zong1, which has been proven to be sensitive to gentamycin (30 μg/mL). The donor E. coli S17-1 with pMP2444 was added to the competent cell of strain Zong1, thawed on ice, and mixed quickly. The mixture was incubated on ice for 15 min, transferred into a sterile pre-chilled cuvette (interelectrode gap: 0.2 cm), and placed in a Gene Pulser II apparatus equipped with a Pulse Controller (BioRad Laboratories, Tokyo, Japan)(Kazunori et al., 2003). The electroporation unit was set at the following values: 12.5 kV/cm, 25 F and 200 Ω. Following the pulsing, the cells were immediately diluted with 1 mL of LB medium, transferred into a sterilized tube, and incubated at a 30 ºC for 3–4 h. From each tube, 100 μL was plated onto the LB medium supplied with 30 μg/mL gentamycin, placed at 30 ºC for 12 h. Bacterial colonies were exposed to blue light to check the expression of gfp (Stuurman et al., 2000). The stability of the plasmid pMP2444 in P. chlororaphis was analyzed by replica plating of the diluted samples grown on LB with or without antibiotic for 15 times under the laboratory conditions tested. A gfp-tagged derivative of P. chlororaphis Zong1 was grown and stored on TY or YMA medium containing 30 mg/mL gentamycin.
Seeds of S. alopecuroides were inoculated with 200 μL per seed by pipettor with a mixture of P. chlororaphis strain Zong1 marked with gfp and Mesorhizobium sp. SQ1 (1:1 v/v) at a density of about 108 cells/mL for each strain. Plants were grown under greenhouse conditions, the change of root surface and hair after 48 hours of inoculation, and developed well nodules after three weeks of inoculation could be detected, the existence of P. chlororaphis Zong1 in the root and nodules was examined by observing the green fluorescence under a confocal laser scanning microscope using a scanning wavelength of 488 nm.
Potential plant growth promoting characteristics of isolates
Examination of siderophore production
Bacterial cultures were multiplied in Lysogeny broth (LB, 10 g NaCl/L) for 72 h and aliquots of pure bacterial culture were inoculated in plates containing agar Chrome Azurol S (CAS) and incubated at 30 ºC. Each plate was observed daily for 7 days to detect the appearance of orange color around the colony (Schwyn and Neilands, 1987). Experiments were performed in triplicate.
Phosphate solubilization
Log phase LB pure bacterial cultures were spot inoculated on Pikovasky’s inorganic and Mongina organic culture plates, incubated at 30 ºC, and observed daily for 7 days for appearance of transparent halos (Katznelson and Bose, 1959). Experiments were performed in triplicate.
Antifungal activity
Spores of fungal cultures (Fusarium oxysporum, Magnaporthe grisea, Botrytis cinerea Pers., Valsa mali Miyabe et Yamada, Alternaria alternata) were grown on Potato Dextrose Agar (PDA) plates and a small block of agar with fungal growth was cut using sterile puncher (Ø = 4 mm) and placed in the centre of a fresh PDA plate. Tested strains were spot inoculated on the PDA plate’s edge about 25 mm from the centre, incubated at 30 ºC for 7 days and observed for zones of inhibition. Fungal mycelia cultivated for 7 days without spot inoculation were used as control (Zhao et al., 2011).
Organic acid production
Bacterial cultures were spot inoculated onto MM9 (Sambrook et al., 2001) agar medium, after incubation for 48 hours at 30 ºC, and observed for a drop in pH using methyl red as an indicator dye which changed from yellow to pink below pH 5.0. Isolates having the ability to produce organic acid showed a pink zone around the colony.
IAA production
Indole acetic acid (IAA) production was estimated by inoculating a bacterial suspension (1 × 108 cfu/mL) in 10 mL (LB) broth containing L-tryptophan (100 μg/mL), and shaken incubation for 72 h. IAA concentration in the culture supernatant was estimated using Salkowskis reagent (Gordon and Weber, 1951).
Plant inoculation experiment under greenhouse condition
S. alopecuroides seeds were treated with 98% sulphuric acid for 60 min, and subsequently rinsed 6 times with sterilized demineralized water (Zhao et al., 2010). The seeds were then surface sterilized by immersion in absolute alcohol for 1 min, immersed in 0.1% (w/v) HgCl2 for 2 min and rinsed 8 times with sterile distilled water. Surfaced sterile seeds were germinated axenically in Petri dishes filled with moist filter paper at 28 ºC for 72 h.
Inoculum of strains was prepared by growing cells in nutrient broth at 30 ºC, 120 rev/min until an exponential growth phase. Bcateria were then harvested by centrifugation (8000 rev/min for 10 min), washed twice in sterilized demineralized water and resuspended in the same demineralized water to a density of approx. 108–109 cfu/mL.
Germinated seed were soaked in the bacterial suspension and control seeds were soaked in sterilized water at 30 ºC for 3 h. Seeds were transferred to plastic pots filled with sterilized perlite-vermiculite (1:1) moistened with nitrogen-free plant nutrient solution as described by Vincent et al. (1970). The inoculated seedlings were cultured under greenhouse condition, that is, programmed for a 14 h/d photoperiod at a constant temperature of 28 ºC during the day and 20 ºC during the night with about 60% relative humidity. All pot experiments were performed in five repeat with five seedlings per pot, and seedlings with Mesorhizobium sp. SQ1 alone were used as positive controls (PC); Seedlings without any bacteria were the negative control (NC); Seedlings with isolate Zong1 and seedlings co-inoculated with Zong1 and isolate Mesorhizobium sp. SQ1 (1:1 v/v) were used as the experimental systems. The plants were harvested after six weeks of inoculation when well developed nodules could be detected, and plant biomass such as dry and fresh weight, shoot and root length, nodule number per plant were measured and compared to control plants (NC).
Sequencing and phylogenetic analysis
The total genomic DNA was extracted from culture of nodule isolate Zong1 with the previous method (Moulin et al., 2004). The 16S rRNA gene was selectively amplified from the genomic DNA by PCR with the universal forward primer P1 (5′-CgggATCCAgAgTTTgATCCTggCTCAg AACgAACgCT-3′) and reverse primer P6 (5′-Cggg ATCCTACggCTACCTTgTTACgACTTCACCCC-3′) respectively corresponding to the positions of 8~37 bp and 1479~1506 bp in E. coli 16S rRNA gene (Van et al., 1996). An aliquot of PCR product of isolate Zong1 was directly sequenced by Sangon Biotech (Shanghai) Co., Ltd. using the same primers mentioned above. The acquired and related sequences were matched with ClustalX1.81 software, imported into Bioedit 4.8.4 and manually corrected. Phylogenetic tree were constructed using the Jukes-Cantor model and neighbor-joining method (Saitou and Nei, 1987) in TREECON package (Van and Y De, 1997), and computation of the similarity of each strain tested was done with the DNAMAN application (version 6.0.3.40, lynnon corporation). The 16S rRNA gene sequences obtained were submitted to NCBI GenBank (http://www.ncbi.nlm.nih.gov/) under the accession number HM241942.
Statistical analysis
The parameters of plant growth promoting characteristics and the parameters of growth and nodulation of S. alopecuroides were examined with ANOVA analysis using the SPSS 17.0 package (by the Data Theory Scaling System Group, Faculty of Social and Behavioral Sciences, Leiden University, The Netherlands).
Results
Isolation of endophytic bacteria and nodulation test
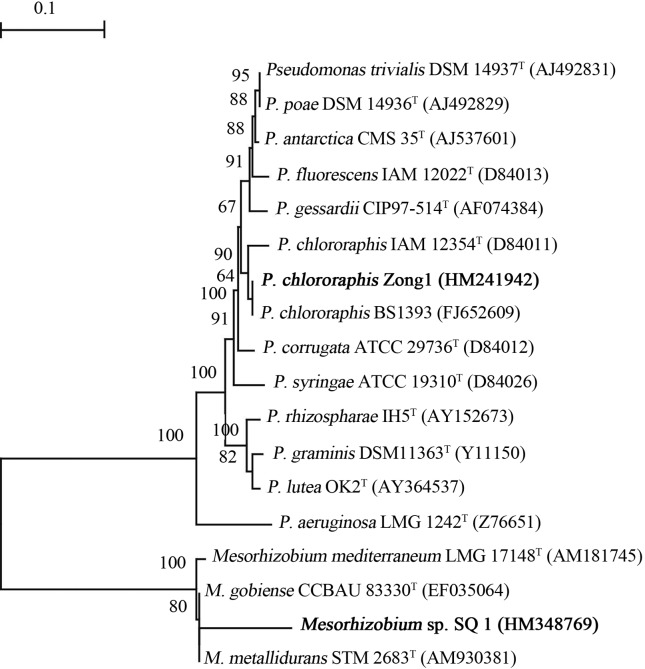
Twelve nodule bacteria were isolated from S. alopecuroides root nodules. Nodulation test results showed that eight nodule isolates could not form root nodules, which were defined as endophytes. Physiological and biochemical tests were conducted (Table S1), which included measurements such as temperature, pH values and salt tolerance, antibiotic sensitivity, metal susceptibility, and dye resistance. On the basis of these characteristics, a selected strain was termed Zong1. Sequencing of 16S rRNA gene and phylogenetic analysis (Figure 1) indicated that strain Zong1 is most related to Pseudomonas chlororaphis (GenBank accession number HM241942).
Figure 1.
Phylogenetic tree reconstructed with neighbour-Joining method based on alignment of nucleotide sequences of the 16S rRNA gene from representative strains (shown in bold) and reference strains. Accession number of GenBank database is presented in parentheses for each strain. Bootstrap values greater than 50% were indicated. Scale bar represents the number of substitutions per site.
Construction of gfp-marked P. chlororaphis strain Zong1 and colonization
Experiment results showed that when cells were transformed by electroporation with the pMP2444 plasmid, carrying the gfp gene, and cultured on LB medium supplied with 30 μg/mL gentamycin, bacterial colonies presented green color when they were exposed to blue light (255 nm wave length, portable ultraviolet lamp). Tansformant containing plasmid pMP2444, was chosen for further studies and compared to the wild-type strain during growth in LB and minimal medium (data not shown), indicating that the presence of the plasmid does not interfere in the transformant life cycle. Results suggested that fluorescence was still observed and the growth rates of strains were identical, suggesting that insertion of the plasmid did not interfere with normal cell growth.
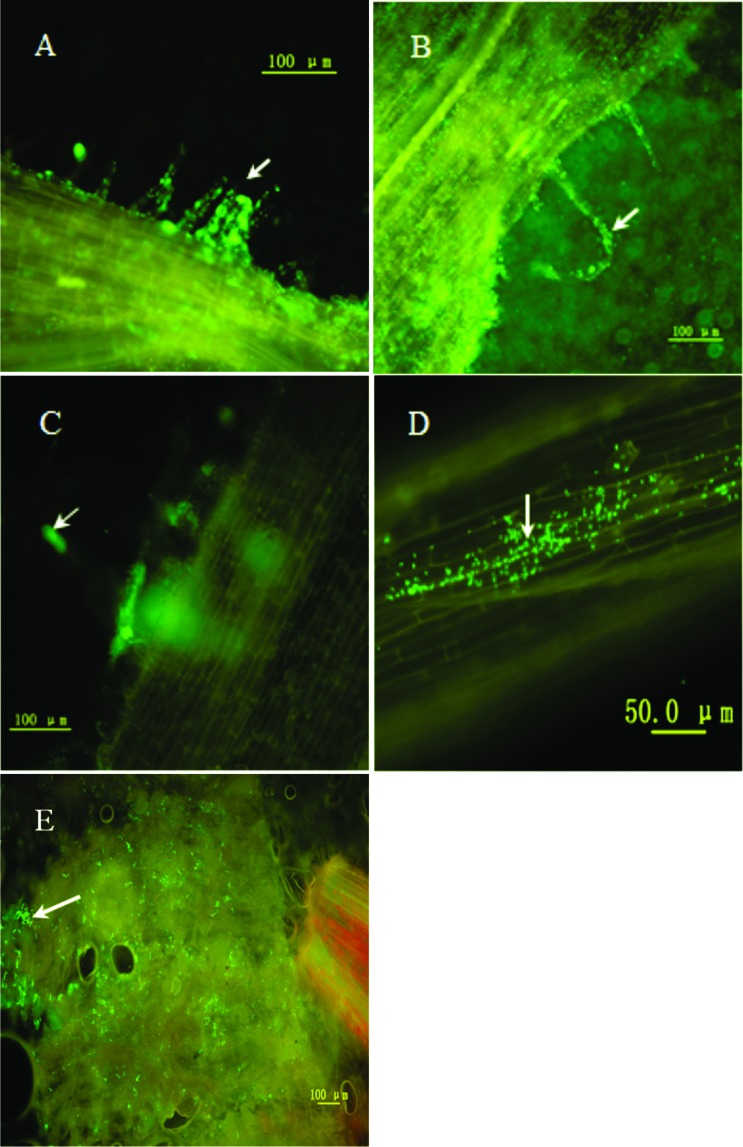
By inoculation and examination, results shown in Figure 2 (A–E) indicated that the process of adsorption, invasion, and colonization accompanied Mesorhizobium sp. SQ1 in root hairs, root woodiness, and in the root nodule.
Figure 2.
Visualization of S. alopecuroides rhizosphere colonization by GFP tagged P. chlororaphis Zong1 and Mesorhizobium sp. SQ1. White arrow indicates root hair adsorbed marked bacteria and root hair become curled (A–B) after inoculation for 48 h. Arrow in C indicates a forming infection thread after inoculation for 72 h, White arrows point to microcolonies in woodiness (D) and in root nodules (E) after inoculation for three weeks.
Potential plant growth promoting characteristics
All of three treatments Zong1+ SQ1, SQ1 and Zong1 (Table 1) gave a positive CAS assay showing that they all produced siderophores. Compared with the control, co-inoculation Zong1+SQ1 showed the strongest capability of producing siderophores, and Zong1 was second. As for the ability of phosphate solubilization (organic phosphate and inorganic phosphate), these strains were same as the case of produced siderophores, ie, co-inoculation Zong1+SQ1 showed the most significant phosphate solubilization with 3.46(D/d) and 3.68(D/d) for organic phosphate and inorganic phosphate, respectively. However, none of the isolates showed any production of organic acid. Three treatments all showed positive for IAA production, and co-inoculation with Zong1+SQ1 treatments was the most significant, measured at 63.07 mg/L. Another, three treatments showed different certain extent antifungal activity to plant pathogenic fungi, co-inoculation (Zong1+SQ1 treatments) was the most significant inhibition ratios, and Zong1 was second, while SQ1 has no effect to F. oxysporum, M. grisea, B. cinere Pers. All inhibition ratios of three treatments were compared to Control, specific data were showed in Table 1. As a whole, co-inoculation with Zong1+SQ1 showed best effects than inoculation alone in vitro. For example, inhibition ratios show 86.21%, 67.27%, 76.25%, 82.67% for Fusarium oxysporum, Magnaporthe grisea, Botrytis cinere Pers., Valsa mali Miyabe et Yamada, Alternaria alternate, respectively. Therefore, mixed inoculation (P. chlororaphi Zong1 and Mesorhizobium sp. SQ1) was more effective on PGP traits than observed when the strains were evaluated alone. But the definite mechanism of their interaction needs further study.
Table 1.
Plant growth promoting characteristics of SQ1, Zong1 isolated from root nodules in different treatments.
| Treatments | Plant growth promoting characterization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Siderophore (D/d#) | Phosphate solubilization | Organic acid | IAA (mg/L) | Antifungal activity(colony diameter/cm) | ||||||
|
|
|
|||||||||
| Organic phosphate (D/d) | Inorganic phosphate (D/d) | F. oxysporum | M. grisea | B. cinere Pers | V. mali Miyabe et Yamada | A. alternata | ||||
| Zong1+ SQ1 | 4.48 ± 0.11a§ | 3.46 ± 0.06 a | 3.68 ± 0.04 a | - | 63.07 ± 0.02 a | 0.8(86.21*) | 1.8(67.27) | 1.9(76.25) | 1.3(82.67) | 0.9(86.76) |
| SQ1 | 1.21 ± 0.06 b | 1.35 ± 0.08 b | 1.23 ± 0.03 c | - | 22.39 ± 0.03 b | - | - | - | 5.5(26.67) | 5.2(23.53) |
| Zong1 | 3.65 ± 0.04 c | 3.18 ± 0.02 c | 3.45 ± 0.03 b | - | 59.04 ± 0.01 c | 1.1(60.34) | 2.0(63.64) | 2.1(73.75) | 2.3(69.33) | 1.2(82.35) |
| Control | / | / | / | / | - | 5.8 | 5.5 | 8.0 | 7.5 | 6.8 |
D/d means the ability to produce Siderophore, Phosphate solubilization. D-Diameter of Colony and halo; d-Colony diameter.
- negative action./blank; Control for IAA assay was LB (10 g NaCl/L) without inoculated bacterial suspension under same incubation condition; Control for antifungal activity assays were fungal mycelia cultivated for 7 days on PDA plates without tested strains under the same incubation condition.
The same letter means no significant difference between treatments (p = 0.05). The data in columns are the mean ± SD of five repetitions.
Inhibition ratio (%)= (Control colony diameter-treatment colony diameter)100%/Control colony diameter.
Plant inoculation assay
To further confirm plant growth promoting characteristics, we performed plant inoculation assays. Results in Table 2 showed that each growth parameters of inoculated Zong1 were higher than those of negative control (NC) and SQ1 (PC) on different degrees. But it is noteworthy that each growth parameters of co-inoculated Zong1+SQ1 was higher than those of single inoculation (Zong1, PC, NC) and showed significant difference (p < 0.05).
Table 2.
Effect of P. chlororaphis Zong1 and Mesorhizobium sp. SQ1 on growth and nodulation of S. alopecuroides.
| Treatments | Shoot length (cm) | Root length (cm) | Shoot fresh weight (g/plant) | Root fresh weight (g/plant) | Shoot dry weight (g/plant) | Root dry weight (g/plant) | Nodule number/plant |
|---|---|---|---|---|---|---|---|
| SQ1+ Zong1 | 11.61 ± 0.48 a* | 17.21 ± 0.98 a | 0.229 ± 0.009 a | 0.218 ± 0.028 a | 0.049 ± 0.009 ab | 0.085 ± 0.004 a | 8.67 a |
| Zong1 | 9.4 ± 0.53 ab | 12.25 ± 0.30 b | 0.092 ± 0.004 b | 0.182 ± 0.009 ab | 0.031 ± 0.013 ab | 0.057 ± 0.006 b | 0 b |
| SQ1(PC) | 8.57 ± 0.28 bc | 11.34 ± 0.45 bc | 0.089 ± 0. 004 b | 0.116 ± 0.018 ab | 0.013 ± 0.002 a | 0.013 ± 0.001 c | 2.333 ± 0.33 b |
| NC | 6.42 ± 0.61 c | 9.17 ± 0.33 c | 0.058 ± 0.002 c | 0.154 ± 0.003 b | 0.004 ± 0.001 b | 0.008 ± 0.001 c | 0 b |
The letters a and b indicate different Tukey grouping. The same latter means no difference among treatments, while different letter means significant difference (p < 0.05).
The value in the column are the averages ± standard error (n = 3), and each set consisted of 10 plants.
PC-Seedlings inoculated with Mesorhizobium sp. SQ1 alone as positive control; NC-Seedlings inoculated without any bacteria as negative control.
SQ1+Zong1 indicate co-inoculated treatments with SQ1 and Zong1 (1:1 v/v).
Discussion
GFP is a useful biomarker for examining biological localization because the cell can be studied nondestructively and without the addition of confounding exogenous substrates or cofactors (Tombolini et al., 1997). In this study, we described the construction of new gfp-containing plasmids for use in P. chlororaphis strain Zong1, experiments have demonstrated gfp-marked cells can be used to simplify the detection and locate the position of an individual cell on roots or root nodules of Sophora alopecuroides with standard epifluorescence microscopes and filter sets (Figure 2 D, E). Additionally, once GFP is synthesized and properly folded, no energy source is required for its act, in contrast to the lux system, which requires ATP for activity (Stewart et al., 1992). As for the legume host plant S. alopecuroides, colonization of gfp-marked P. chlororaphis strain Zong1 in root tissue or root nodules were first detected and reported. Therefore, gfp-marking techniques will provide valuable information for a wide range of P. chlororaphis species associated with Sophora.
Among Gram-negative soil bacteria, Pseudomonas is the most abundant genus in the rhizosphere (Bardas et al., 2009). Root-associated Pseudomonas spp. strains have long been known to be beneficial to plants attribute to their plant-growth promotion effect (PGPE) or their potential as biological control agents. Since these endophytes may directly stimulate plant growth by increasing nutrient uptake and enhancing plant biomass, producing phytohormones (IAA), siderophores, solublizating phosphorus (Lugtenberg and Kamilova, 2009), fixing nitrogen (Yan et al., 2010a) and decreasing heavy metal toxicity (Suranjana and Manas, 2009). In addition, endophytic Pseudomonas spp. can also indirectly induce PGPE by controlling phytopathogens or pathogenic fungi using mechanisms such as producing antibiotic factors (Jousset et al., 2010; Rochat et al., 2010; Vallet-Gely et al., 2010), enhancing competition for colonization sites (Wensing et al., 2010), and induction of systemic resistance (Matilla et al., 2010).
As for PGPC of strain P. chlororaphis Zong1, results showed that tested parameters (Table 1) present beneficial actions in vitro, such as the IAA production, siderophores and phosphorus solubilization. Another, it showed certain antifungal activity to plant pathogenic fungi. These indicated that strain Zong1 may stimulate and promote plant growth. Similar reports prove this viewpoint. Choong-Minei et al. (Choong-Min et al., 2007) reported that rhizosphere colonies in tobacco P. chlororaphis O6 can stimulate growth promotion and induce resistance against Cucumber mosaic virus, as well as protect cucumber plants against leaf spotting caused by Corynespora cassicola; Selin et al. (2010) reported that P. chlororaphis strain PA23 initially isolated from soybean root tips can protect canola from the devastating effects of stem rot caused by the fungus Sclerotinia sclerotiorum (Lib.), and antibiotic production is the primary mechanism of pathogen inhibition. It was previously demonstrated that strain P. chlororaphis MA 342 was a very effective and consistent biocontrol agent against seed-borne barley net blotch caused by Drechslera teres (Riccardo et al., 2009).
Comparing with PGPC of alone inoculation (Zong1 or SQ1 or control), we found that effects of co-inoculation Zong1+SQ1 have more significant than single treatments. Previous reports have shared this observation. For example, non-pathogenic P. putida WCS358 combined with Fusarium oxysporum Fo47 provided better suppression ofFusarium flax wilt than either alone, and also showed better PGPE (Whipps, 2001). Combined P. chlororaphis 30–84 with P. fluorescens Q2-87 provided greater suppression of take-all of wheat than either alone (Duffy et al., 1996), combination of P. chlororaphis PCL1391 and P. fluorescens WCS365 showed biocontrol ability against the tomato pathogen Fusarium oxysporum f. sp. radicis-lycopersici (Bardas et al., 2009). These cases all suggested that PGPE produced by rhizosphere bacteria co-inoculation indirectly of controls phytopathogens or pathogenic fungi, which was further supported by our experiment results.
It was noteworthy that each growth parameters of co-inoculation with Zong1+SQ1 in plant inoculation assays was higher than those of single innoculation (Zong1, PC, NC), and showed significant effects. Interestingly, PGPC of co-inoculated Zong1+SQ1 coincide with each growth parameters of co-inoculation in growth assays under greenhouse conditions. Similar reports proved our results. Jay et al. (2010) reported that combined P. strata with Rhizobium have shown significantly increased dry matter, nodulation, grain yield and phosphorus uptake over the non-inoculated control in legumes. Recent results of studies with PGPR and Rhizobium/Bradyrhizobium sp. have shown co-inoculation may increased root and shoot biomass, nodule dry matter, nitrogenase activity, N2-fixation and grain yield in chickpea and various legumes (Verma et al., 2010). In this experiment, co-inoculation of combination P. chlororaphis Zong1 with Mesorhizobium sp. SQ1 performed under greenhouse conditions showed that endophytes P. chlororaphis zong1 play important roles either in co-inoculation or inoculation alone, and is a potential biological control agent and plant-growth promoting agent. The effects brought by co-inoculation P. chlororaphis Zong1 and other rhizobial genera, both known and unknown, provide a basis for supporting future research in this area.
Supplementary Material
Table S1 - Physiological and biochemical tests results of twelve isolates obtained from S. alopecuroides root nodules.
Acknowledgments
This work was supported by projects from the 973 Project of China (2010CB126502), National Science Foundation of China (U1204301, 31125007 and 31070444), Henan Provincial Department of Science and Technology Research Project (112102110177), Henan Provincial Education Department of Science and Technology Research Key Research Project(12A210019) and Foundation for University Key Teacher by the Ministry of Education of Henan Province(2012GGJS166).
References
- Bai Y, Zhou X, Smith DL. Crop ecology, management and quality. Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci. 2003;43:1774–1781. [Google Scholar]
- Bardas GA, L Lagopodi A, Kadoglidou K, Tzavella-Klonari K. Biological control of three Colletotrichum lindemuthianum races using Pseudomonas chlororaphis PCL1391 and Pseudomonas fluorescens WCS365. Biol Control. 2009;2:139–145. [Google Scholar]
- Burns TA, Jr, Bishop PE, Israel DW. Enhanced nodulation of leguminous plant roots by mixed cultures of Azotobacter vinelandi and damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Appl Environ Microbiol. 1981;62:865–871. [Google Scholar]
- Chandra SN, Puneet SC, Sangeeta MD, Karishma S, Ajit V, William JS. Tripartite interactions among Paenibacillus lentimorbus NRRL B-30488, Piriformospora indica DSM 11827, and Cicer arietinum L. World J Microbiol Biotechnol. 2010;26:1393–1399. [Google Scholar]
- Chanway CP, Hynes RK, Nelson LM. Plant growth promoting rhizobacteria: Effects on growth and nitrogen fixation of lentil (Lensesculenta Moench) and pea (Pisum sativum L.) Soil Biol Biochem. 1989;21:511–517. [Google Scholar]
- Chen WM, James EK, Prescott AR, Kierans M, Sprent JI. Nodulation of Mimosa spp. by the β-Proteobacterium Ralstonia taiwanensis. Mol Plant-Microb Interact. 2003;16:1051–1061. doi: 10.1094/MPMI.2003.16.12.1051. [DOI] [PubMed] [Google Scholar]
- Choong-Min Ryu, Beom Ryong, Kang Song, Hee Han, Song Mi, Cho Joseph W, Kloepper Anne J, Anderson Young, Cheol Kim. Tobacco cultivars vary in induction of systemic resistance against Cucumber mosaic virus and growth promotion by Pseudomonas chlororaphis O6 and its gacS mutant. Eur J Plant Pathol. 2007;119:383–390. [Google Scholar]
- Duffy BK, Simon A, Weller DM. Combination of Trichoderma koningii with fluorescent pseudomonads for control of take-all on wheat. Phytopathology. 1996;86:188–194. [Google Scholar]
- Geetha R, Sing FJ, Desai A, Archana G. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Biores Technol. 2008;99:4544–4550. doi: 10.1016/j.biortech.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Gordon AS, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay PV, Janardan Y, Kavindra NT. Impact of plant growth promoting rhizobacteria on crop production. Int J Agric Res. 2010;11:954–983. [Google Scholar]
- Jousset A, Rochat L, Scheu S, Bonkowski M, Keel C. Predator-prey chemical warfare determines the expression of biocontrol genes by rhizosphere pseudomonads. Appl Environ Microbiol. 2010;62:552–563. doi: 10.1128/AEM.02941-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan FL, Chen ZY, Wang ET, Tian CF, Sui XH, Chen WX. Characterization of symbiotic and endophytic bacteria isolated from root nodules of herbaceous legumes grown in Qinghai-Tibet Plateau and in other zones of China. Arch Microbiol. 2007;188:103–115. doi: 10.1007/s00203-007-0211-3. [DOI] [PubMed] [Google Scholar]
- Katznelson H, Bose B. Metabolic activity and phosphate dissolving capability of bacterial isolates from wheat root, rhizosphere and non-rhizosphere soil. Can J Microbiol. 1959;5:79–85. doi: 10.1139/m59-010. [DOI] [PubMed] [Google Scholar]
- Kazunori T, Tasuka S, Muhammad Z, Joyce N, Yuichi S, Masao S, Takeo Y, Kiwamu M, Shocichiro A. Incorporation of a DNA sequence encoding green fluorescent protein (GFP) into endophytic diazotroph from sugarcane and sweet potato and the colonizing ability of these bacteria in Brassica oleracea. Microbes Environ. 2006;21:122–128. [Google Scholar]
- Li JH, Wang ET, Chen WF, Chen WX. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol Biochem. 2008;40:238–246. [Google Scholar]
- Lin L, Qiao YS, Ju ZY, Ma CW, Liu YH, Zhou YJ, Dong HS. Isolation and characterization of endophytic Bacillus subtilis Jaas ed1 antagonist of eggplant Verticillium wilt. Biosci Biotechnol Biochem. 2009;73:1489–1493. doi: 10.1271/bbb.80812. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang ET, Ren DW, Chen WX. Mixture of endophytic Agrobacterium and Sinorhizobium meliloti strains could induce nonspecific nodulation on some woody legumes. Arch Microbiol. 2010;192:229–234. doi: 10.1007/s00203-010-0543-2. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- Matilla MA, Ramos JL, Bakker PAHM, Doornbos R, Badri DV, Vivanco JM, Ramos-González MI. Pseudomonas putida KT2440 causes induced systemic resistance and changes in Arabidopsis root exudation. Environ Microbiol Rep. 2010;2:381–388. doi: 10.1111/j.1758-2229.2009.00091.x. [DOI] [PubMed] [Google Scholar]
- Moulin L, Béna G, Boivin-Masson C, St Pkowski T. Phylogenetic analyses of symbiotic nodulation genes support vertical and lateral gene co-transfer within the Bradyrhizobium genus. Mol Phylogenet Evol. 2004;30:720–732. doi: 10.1016/S1055-7903(03)00255-0. [DOI] [PubMed] [Google Scholar]
- Parmar N, Dadarwal KR. Stimulation of nitrogen fixation and induction of flavonoid-like compounds by rhizobacteria. J Appl Microbiol. 1999;86:36–64. [Google Scholar]
- Riccardo T, van der Gaag DJ, Gerhardson B, Jansson JK. Colonization pattern of the biocontrol strain Pseudomonas chlororaphis MA 342 on barley seeds visualized by using green fluorescent protein. Appl Environ Microbiol. 1999;8:3674–3680. doi: 10.1128/aem.65.8.3674-3680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat L, Péchy-Tarr M, Baehler E, Maurhofer M, Keel C. Combination of fluorescent reporters for simultaneous monitoring of root colonization and antifungal gene expression by a biocontrol pseudomonad on cereals with flow cytometry. Mol Plant-Microbe Interact. 2010;23:949–961. doi: 10.1094/MPMI-23-7-0949. [DOI] [PubMed] [Google Scholar]
- Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: Recent developments and applications. FEMS Microbiol Lett. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Vol. 1. Cold Spring Harbor; New York: 2001. [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophore. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Selin C, Habibian R, Poritsanos N, Athukorala NPS, Fernando D, R de Kievit T. Phenazines are not essential for Pseudomonas chlororaphis PA23 biocontrol of Sclerotinia sclerotiorum, but do playa role in biofilm formation. FEMS Microbiol Ecol. 2010;71:73–83. doi: 10.1111/j.1574-6941.2009.00792.x. [DOI] [PubMed] [Google Scholar]
- Song JZ, Xu HX, Tian SJ, But PP. Determination of quinolizidine alkaloids in traditional Chinese herbal drugs by nonaqueous capillary electrophoresis. J Chromatogr A. 1999;857:303–311. doi: 10.1016/s0021-9673(99)00758-x. [DOI] [PubMed] [Google Scholar]
- Stewart G, Williams P. lux genes and the applications of bacterial bioluminescence. J Gen Microbiol. 1992;138:1289–1300. doi: 10.1099/00221287-138-7-1289. [DOI] [PubMed] [Google Scholar]
- Stuurman N, Bras CP, Schlaman HRM, Wijfjes AHM, Bloemberg G, Spaink HP. Use of green fluorescent protein color variants expressed on stable broad-host-range vectors to visualize rhizobia interacting with plants. Mol Plant-Microb Interact. 2000;13:1163–1169. doi: 10.1094/MPMI.2000.13.11.1163. [DOI] [PubMed] [Google Scholar]
- Suranjana AR, Manas KR. Bioremediation of heavy metal toxicity-with special reference to chromium. Al Ame J Med Sci. 2009;2:57–63. [Google Scholar]
- Tombolini RA, Unge ME, Davey FJB, Jansson JK. Flow cytometric and microscopic analysis of GFP-tagged Pseudomonas fluorescens bacteria. FEMS Microbiol Ecol. 1997;22:17–28. [Google Scholar]
- Vallet-Gely I, Novikov A, Augusto L, Liehl P, Bolbach G, Péchy-Tarr M, Cosson P, Keel C, Caroff M, Lemaitre B. Association of hemolytic activity of Pseudomonas entomophila, a versatile soil bacterium, with cyclic lipopeptide production. Appl Environ Microbiol. 2010;76:910–921. doi: 10.1128/AEM.02112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkum P, Beyene D, Eardly BD. Phylogenetic relationships among Rhizobium species nodulating the common bean (Phaseolus vulgaris L.) Int J Syst Evol Microbiol. 1996;46:240–244. doi: 10.1099/00207713-46-1-240. [DOI] [PubMed] [Google Scholar]
- Van de Peer Y, De Wachter R. Construction of evolutionary distance trees with TREECON for Windows: Accounting for variation in nucleotide substitution rate among sites. Comput Appl Biosci. 1997;132:227–230. doi: 10.1093/bioinformatics/13.3.227. [DOI] [PubMed] [Google Scholar]
- Verma JP, Yadav J, Kavindra NT. Application of Rhizobium sp. BHURC01 and plant growth promoting Rhizobactria on nodulation, plant biomass and yields of Chickpea (Cicer arietinum L.) Int J Agric Res. 2010;3:148–156. [Google Scholar]
- Vincent JM. A Manual for the Practical Study of the Root-Nodule Bacteria. Blackwell Scientific; Oxford: 1970. The cultivation, isolation and maintenance of rhizobia; pp. 1–13. [Google Scholar]
- Wensing AD, Braun S, Büttner P, Expert D, Völksch B, S Ullrich M, Weingart H. Impact of siderophore production by Pseudomonas syringae pv. syringae 22d/93 on epiphytic fitness and biocontrol activity against Pseudomonas syringae pv. glycinea 1a/96. Appl Environ Microbiol. 2010;76:2704–2711. doi: 10.1128/AEM.02979-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Experiment Botany, Root Special issue. 2001:487–511. doi: 10.1093/jexbot/52.suppl_1.487. [DOI] [PubMed] [Google Scholar]
- Yahalom E, Okon Y, Dovrat A. Early nodulation in legumes inoculated with Azospirillum and Rhizobium. Symbiosis. 1988;6:69–80. [Google Scholar]
- Yan YL, Yang J, Dou YT, Chen M, Ping SZ, Peng JP, Lu W, Zhang W, Yao ZY, Li HQ, Liu W, He S, Geng LZ, Zhang XB, Yang F, Yu HY, Zhan YH, Li DH, Lin ZL, Wang YP, Elmerich C, Lin M, Jin Q. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. PNAS. 2010a;21:7564–7569. doi: 10.1073/pnas.0801093105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhia F, Jeder H, Willems A, Gillis M, Dreyfus B, De Lajudie P. Diverse bacteria associated with root nodules of spontaneous legumes in Tunisia and first report for nifH-like gene within the genera Microbacterium and Starkeya. Microbial Ecol. 2006;51:375–393. doi: 10.1007/s00248-006-9025-0. [DOI] [PubMed] [Google Scholar]
- Zhao LF, Deng ZS, Yang WQ, Cao Y, Wang ET, Wei GH. Diverse rhizobia associated with Sophora alopecuroides grown in different regions of Loess Plateau in China. Syst Appl Microbiol. 2010;33:468–477. doi: 10.1016/j.syapm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Zhao LF, Xu YJ, Sun R, Deng ZS, Yang WQ, Wei GH. Identification and characterization of the endophytic plant growth prompter Bacillus Cereus strain mq23 isolated from Sophora Alopecuroides root nodules. Braz J Microbiol. 2011;42:567–575. doi: 10.1590/S1517-838220110002000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 - Physiological and biochemical tests results of twelve isolates obtained from S. alopecuroides root nodules.