Abstract
Background:
Myelotoxicity during initial cycles of chemotherapy for Hodgkin lymphoma is associated with better outcome, supporting the concept of individualised dosing based on pharmacodynamic end points to optimise results. This study was performed to identify the maximum tolerated dose (MTD) of doxorubicin within cycles 1–3 ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine). Circulating biomarkers of response (nucleosomal DNA, nDNA) and epithelial toxicity (Cytokeratin 18, CK18) were also measured.
Methods:
Dose escalation of doxorubicin in cycles 1–3 ABVD supported by pegfilgrastim was performed on a six-patient cohort basis (35, 45 and 55 mg m–2) with doxorubicin reduced to 25 mg m–2 or omitted in cycles 4–6 to maintain cumulative exposure of 103–130% standard ABVD. BVD was given at standard doses throughout. Six additional subjects were recruited at the MTD.
Results:
Twenty-four subjects were recruited. Dose-limiting toxicities (DLTs) of grade 3 neuropathy, pneumonitis, palmar-plantar erythema and neutropenic infection were observed at 55 mg m–2, so 45 mg m–2 was declared the MTD. In patients who subsequently experienced DLT at any time, large increases in CK18 were seen on day 3 of cycle 1 ABVD.
Conclusion:
Escalated ABVD incorporating doxorubicin at 45 mg m–2 in cycles 1–3 can be delivered safely with pegfilgrastim support. Circulating cell death biomarkers may assist in the development of future individualised dosing strategies.
Keywords: Hodgkin lymphoma, ABVD chemotherapy, cell death biomarkers, phase I
The aim of treatment for advanced stage Hodgkin lymphoma (HL) is to maximise the cure rate, while minimising short- and long-term toxicity (Bauer et al, 2011). ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) is a commonly used drug combination (Cheson, 2007) and although more intensive regimens can result in improved freedom from treatment failure (FFTF; Engert et al, 2009) these have not yet been shown to translate into improved overall survival. Furthermore, increased short-term (infection) and long-term (secondary AML and infertility) toxicity (Bauer et al, 2011) can result and so dose intensification may only be appropriate for patients with a predicted poorer prognosis.
The German Hodgkin Study Group (GHSG) demonstrated in studies involving over 4000 uniformly treated patients that grade 3/4 leukopenia was strongly associated with better FFTF (Klimm et al, 2005; Bauer et al, 2011). These data show that dosing based on body surface area results in variable toxicity between individuals, and increased toxicity correlates with better outcomes. Both observations suggest that pharmacodynamic dosing, based on delivering a dose capable of producing a certain level of toxicity, may be superior to standard dosing. The pharmacokinetics of doxorubicin differs markedly between individuals (Joerger et al, 2007; Bains et al, 2010; Elis et al, 2010), which may partially explain differences in toxicity and tumour response (Elis et al, 2010) and underlines the attraction of individualised dosing.
The GHSG data suggest that the biological effects of the first three cycles of chemotherapy are critical in terms of disease control (Klimm et al, 2005). Individualised dose escalation is therefore predicted to be most effective when applied during these first three cycles. Doxorubicin given at 25 mg m–2 every 2 weeks remains a key component of ABVD. This drug has been given as a single agent at doses of 150 mg m–2 every 2 weeks with growth factor support (Bronchud et al, 1989) giving scope for increasing the doxorubicin dose within ABVD.
Dose escalation may be associated with improved outcome but should not be at the cost of unacceptable toxicity. Validated biomarkers that objectively measure tumour response and host toxicity may allow dose individualisation to optimise tumour kill and minimise toxicity. Studies exploring early dose escalation in HL with prospective measurement of pharmacodynamic end points are therefore needed. This study integrated prospective evaluation of circulating cell death biomarkers as potential biomarkers of response and epithelial toxicity.
During apoptotic cell death, DNA is cleaved by caspase-activated endonucleases to generate nucleosomal DNA (nDNA; Oberhammer et al, 1993). Circulating nDNA levels are elevated in patients with cancer, including lymphoma, compared with healthy controls and have demonstrated prognostic and pharmacodynamic utility in studies both in solid tumours and in lymphoma (Holdenrieder et al, 2008; Greystoke et al, 2011). In patients with lung cancer, both lower baseline values and larger reductions in nDNA during cycle 1 are associated with subsequent response to chemotherapy (Holdenrieder et al, 2008). Previous studies have suggested that nDNA levels decrease following therapy for HL (Greystoke et al, 2011), although this study was not powered to determine associations with subsequent response.
Cytokeratin 18 (CK18), a major component of the epithelial cytoskeleton, is not expressed in cells of lymphoid origin. Consequently, in patients with lymphoma elevated circulating CK18 levels reflect epithelial toxicity. In a pilot study of patients with lymphoma receiving cytotoxic chemotherapy, larger increases in CK18 by day 3 were associated with subsequent epithelial toxicity (Greystoke et al, 2011). The ability of CK18 to anticipate toxicity early enough to change treatment was therefore also evaluated in this study.
Materials and methods
Aims
The primary end point was to identify the maximum tolerated dose (MTD) of doxorubicin escalated in cycles 1–3 ABVD in patients with advanced HL. Secondary end points were response rates, survival and toxicity including changes in cardiac function. Exploratory end points included the association of changes in circulating cell death biomarkers with response and toxicity.
Study design
The protocol was approved by the Manchester Research Ethics Committee and run according to Good Clinical Practice with all participating subjects giving informed, written consent before study entry. A dose escalation phase 1 design was used to define MTD with six patients enrolled per cohort. Dose-limiting toxicity (DLT) was defined as grade 4 haematological toxicity lasting ⩾5 days, or grade 3 non-haematological toxicity (excepting nausea, vomiting), occurring at any time during treatment. Provided that ⩽2 subjects experienced DLT dose escalation could occur as described in Table 1. If ⩾3 subjects experienced DLT that dose was considered intolerable and the previously assessed dose level was declared the MTD. An additional six patients were enrolled at the MTD to further evaluate toxicity, efficacy and biomarker data at this dose.
Table 1. Planned doses of Dox by cohort delivered in the phase 1 study of dose-escalated Dox in cycles 1–3 ABVD.
| |
Dose of Dox in mg m–2 in cycles: | |
||||
|---|---|---|---|---|---|---|
| Cohort | 1–3 | 4 | 5 | 6 | Total dose Dox (% of standard ABVD) | Dose intensity Dox cycles 1–3 as % of standard ABVD |
| 1 |
35 |
25 |
25 |
0 |
310 mg m–2 (103) |
140 |
| 2 |
45 |
25 |
0 |
0 |
330 mg m–2 (110) |
180 |
| 3 |
55 |
0 |
0 |
0 |
330 mg m–2 (110) |
220 |
| 4 | 65 | 0 | 0 | 0 | 390 mg m–2 (130) | 260 |
Abbreviations: ABVD=doxorubicin, bleomycin, vinblastine, dacarbazine; Dox=doxorubicin.
Dose intensity in cycles 1–3 and cumulative dose over 6 cycles relative to standard ABVD are also shown.
Inclusion and exclusion criteria
Patients aged 18–60 with a histological diagnosis of previously untreated advanced HL (stage II with B symptoms and/or mediastinal bulk, III or IV) were eligible. Patients had normal renal function as determined by serum creatinine (unless abnormalities were considered to be due to HL), normal serum bilirubin and normal left ventricular ejection fraction (LVEF). Patients with reproductive potential were required to use contraception during chemotherapy and for 6 months following completion.
Baseline assessments
Consenting patients had full blood count, urea and electrolytes and liver function tests assessed before therapy along with baseline assessment of cardiac function by MUGA or echocardiogram. Stage was determined by contrast CT, PET-CT and bone marrow biopsy. The International Prognostic Score was also calculated and recorded. (Hasenclever and Diehl, 1998).
Treatment details
The dose of doxorubicin in cycles 1–3 ABVD was escalated as shown in Table 1. Standard doses of bleomycin, vinblastine and dacarbazine were given in all cohorts. The planned cumulative dose of doxorubicin was maintained at between 310 and 390 mg m–2 by giving standard dose (25 mg m–2) or omitting doxorubicin altogether in later cycles. With this design, the dose intensity of doxorubicin in cycles 1–3 of planned cohort 4 was increased to 260% of baseline, whereas the cumulative dose over 6 cycles was restricted to 390 mg m–2 (130% of baseline), well within the ceiling dose of 500 mg m–2 (Table 1).
Pegfilgrastim (Neulasta, Amgen, Thousand Oaks, CA, USA) was given on days 2 and 16 of every cycle-escalated ABVD as primary prophylaxis. Other supportive medications were given at investigator discretion. Consolidation radiotherapy was given to residual radiological abnormalities after chemotherapy according to local guidelines. Treatment on relapse was at treating physician's discretion.
Tumour assessment
Response was assessed locally 2–4 weeks after completion of chemotherapy by CT, PET-CT and repeat bone marrow biopsy (if involved at baseline) according to the Revised Response Criteria for Malignant Lymphoma (Cheson et al, 2007).
Biomarker methodology
Blood samples were taken for biomarker analysis at days 1, 3, 8, 15, 18 and 22 of cycles 1–3 and processed as described previously (Greystoke et al, 2008). On treatment days samples were taken before chemotherapy. Serum was analysed for nDNA using the Cell Death Detection ELISA (Roche, Basel, Switzerland) and CK18 using the M65 ELISA (Peviva, Stockholm, Sweden). All analyses followed the manufacturer's instructions, conducted according to Good Clinical Practice for Laboratories. As nDNA is a quasi-quantative ELISA, data are expressed as optical density readings. The M65 ELISA is a semiquantative assay and data are expressed in U l–1.
Statistical analysis was performed using GraphPad Prism version 5.02 for Windows, (GraphPad Software, San Diego, CA, USA, www.graphpad.com) and probability values of P⩽0.05 were considered significant throughout. As the biomarker data were positively skewed, non-parametric tests were used to satisfy the assumptions of variance between sample groups. The area under the curve (AUC) was calculated using the trapezoid method.
Results
Determination of MTD
Twenty-four subjects (12 women and 12 men) were recruited at four participating sites in the UK between January 2009 and May 2010. Their median age was 36 years (range 18 to 57) and all had advanced disease with stages 2AX–4BX and a median International Prognostic Score of 2 (range 0–6; Hasenclever and Diehl, 1998; Table 2). All subjects had classical HL except one subject with stage IIB nodular lymphocyte predominant HL. Subject 08 in cohort 2 with assigned stage IIIB was entered into the study erroneously (elevated serum bilirubin) and this represents a protocol deviation. The bilirubin was between 39 and 67 mmol l–1 (grade 2 according to Common Toxicity Criteria for Adverse Events (CTCAE) version 3) in the 7 days before treatment but fell to within normal range by day 8 of cycle 1. This subject did not experience any DLT and is currently alive and disease free with a normal serum bilirubin.
Table 2. Characteristics of subjects enrolled in the phase 1 study of dose-escalated doxorubicin in cycles 1–3 ABVD.
| Dose level of doxorubicin | |||
|---|---|---|---|
| |
35 mg m–2 |
45 mg m–2 |
55 mg m–2 |
|
Number |
6 |
12 |
6 |
|
Age | |||
| Median | 42.5 | 37 | 30.5 |
| Range |
21–56 |
18–57 |
24–45 |
|
Gender | |||
| Male | 2 | 6 | 4 |
| Female |
4 |
6 |
2 |
| Stage |
IIxA–IVxB |
IIAa–IVxB |
IIIB–IVB |
|
Hasenclever score | |||
| Median | 1 | 2 | 3 |
| Range | 0–3 | 0–6 | 0–4 |
Abbreviation: ABVD=doxorubicin, bleomycin, vinblastine, dacarbazine.
Unsuitable for XRT, x signifies bulk disease.
There was one episode of DLT in the six subjects in cohort 1 (35 mg m–2). No DLTs were seen in the initial six subjects treated in cohort 2 (45 mg m–2) but in cohort 3 (55 mg m–2), three of six patients experienced a total of six episodes of DLT, and this dose was therefore considered intolerable. The MTD was declared to be 45 mg m–2 and as per protocol a further six patients were enrolled at this dose. There were two episodes of DLT in these six patients and so in total 2 of 12 subjects treated at 45 mg m–2 had DLT.
Description of DLT
There were nine episodes of DLT in six patients consisting of one episode of grade 3 fatigue, two episodes of grade 3 palmar-plantar erythema (PPE), three episodes of pneumonitis, one episode of neutropenic infection and two episodes of grade 3 neuropathy.
The subject treated at 35 mg m–2 developed grade 3 fatigue during cycle 3. No dose reductions were required although there were three subsequent treatment delays totalling 1 week, and ultimately the subject terminated treatment following five cycles of therapy for non-medical reasons.
The DLTs observed at 45 mg m–2 were both pneumonitis. One subject experienced G3 dyspnoea at cycle 4 day 1; bleomycin toxicity was confirmed radiologically and this drug omitted from subsequent cycles. However, symptoms deteriorated at cycle 4 day 15 and the subject was admitted to hospital for 5 days and treated with steroids and intravenous antibiotics for possible superimposed infection (subsequently disproved). Cycle 5 was delayed by 2 weeks. The other DLT encountered at 45 mg m–2 was an episode of G4 dyspnoea in cycle 4, which was treated as pneumocystis jiroveci pneumonia, but as bleomycin toxicity could not be discounted, the subject was withdrawn from the study and completed frontline therapy with alternative agents.
Multiple episodes of DLT were observed at 55 mg m–2. One patient developed grade 3 PPE at cycle 2, which was treated symptomatically and resolved by cycle 3, but necessitated a subsequent dose reduction to 45 mg m–2. Another patient developed grade 3 PPE and neuropathy during cycle 2; this required a dose delay of 1 week in cycle 3 by which time the neuropathy had completely resolved. The PPE was treated with topical steroids and resolved to grade 1 by cycle 3 day 15. Following cycle 3, the same patient was admitted with grade 3 neutropenic infection (source unknown; nadir neutrophil count 0.8 × 109 l–1). This was treated with fluids and antibiotics, required a hospital stay of 5 days and resulted in a 1-week delay to cycle 4.
The third subject who experienced DLT at 55 mg m–2 complained of intermittent G1 sensory neuropathy affecting the legs from cycle 1, this escalated to G3 by cycle 3, necessitating the subsequent omission of vinblastine. He also developed dyspnoea in cycle 4, with radiological evidence of pneumonitis. He was therefore removed from the study and completed frontline therapy with alternative agents.
Response to therapy
Eighteen patients had a complete response to therapy, five patients had a partial remission and one patient developed progressive disease.
At the time of analysis (February 2012) with a median follow-up of 792 days (range 407–1104), 23 of 24 subjects are alive and disease free. However, two patients who had initial partial remissions after ABVD subsequently progressed and received salvage chemotherapy followed by stem-cell transplantation (one allogeneic and one autologous). Both remain in remission. There has been one death in the subject with progressive disease at the completion of ABVD. This individual received salvage chemotherapy followed by autologous stem-cell transplantation and subsequent treatment for a second recurrence with brentuximab vedotin (Younes et al, 2012).
Non-DLT toxicity
Episodes/grade of infection
There were 11 episodes of infection, including the dose-limiting neutropenic event. Four infections were associated with peripherally inserted central catheters inserted to allow administration of therapy and not considered directly protocol related. All other toxicities were in keeping with the spectrum commonly observed with ABVD (Table 3) and manageable with standard measures.
Table 3. Toxicity (maximum grade per subject) by cohort in the phase 1 study of dose-escalated doxorubicin in cycles 1–3 ABVD.
|
35 mg m2 |
45 mg m2 |
55 mg m2 |
|
||||
|---|---|---|---|---|---|---|---|
| Grades 1-2 (%) | Grades 3-4 (%) | Grades 1-2 (%) | Grades 3-4 (%) | Grades 1-2 (%) | Grades 3-4 (%) | Published grades 3–5 toxicity rates with standard ABVD | |
| Anaemia |
2 (33) |
0 (0) |
9 (75) |
1 (8) |
4 (66) |
0 (0) |
5%2 |
| Neutropaenia |
2 (33) |
0 (0) |
5 (42) |
5 (42) |
1 (17) |
3 (50) |
34%2 |
| Thrombocytopaenia |
1 (17) |
0 (0) |
1 (8) |
0 (0) |
1 (17) |
0 (0) |
3%2 |
| Nausea |
4 (67) |
1 (17) |
9 (75) |
2 (17) |
6 (100) |
0 (0) |
13%2 |
| Vomiting |
4 (67) |
1(17) |
8 (67) |
1 (8) |
5 (83) |
0 (0) |
13%2 |
| Mucositis |
5 (83) |
0 (0) |
7 (58) |
0 (0) |
2 (33) |
0 (0) |
1–3%2,4 |
| Diarrhoea |
4 (67) |
0 (0) |
7 (58) |
0 (0) |
2 (17) |
0 (0) |
— |
| Constipation |
5 (83) |
0 (0) |
11 (92) |
0 (0) |
5 (83) |
0 (0) |
2%2 |
| Neuropathy |
4 (63) |
(0) |
9 (75) |
0 (0) |
4 (67) |
2 (33) |
0–3%2,4 |
| Palmar Plantar Erythema (PPE) |
0 (0) |
0 (0) |
1 (8) |
0 (0) |
1 (17) |
2 (33) |
— |
| Pulmonary |
0 (0) |
0 (0) |
0 (0) |
2 (17) |
0 (0) |
1 (17) |
18–24.5%1,3 |
| Infection |
3 (50) |
0 (0) |
7 (58) |
1 (8) |
3 (50) |
1 (17) |
2% |
| Fatigue |
3 (50) |
1 (17 ) |
8 (75) |
0 (0) |
6 (100) |
0 (0) |
7%1 |
| Other* | 1(17) | 0 (0) | 2(17) | 0(0) | 2 (33) | 0 (0) | — |
Abbreviation: ABVD=doxorubicin, bleomycin, vinblastine, dacarbazine.
Other includes back pain, tumour pain, dyspnea, hearing impairment and palpitations.
(1) Duggan et al, 2003; (2) Federico et al, 2009 (3) Martin et al, 2005 (4) Johnson et al, 2005.
Changes in cardiac function
No clinically significant fall in LVEF was seen with an average change of −3% (range −12 to +6%). Only one subject had a LVEF below the lower limit of normal at study completion (49% compared with 57% at baseline) and this was asymptomatic. There have been no reported cardiac events.
Circulating biomarkers
Nucleosomal DNA
Published data suggest that decreasing nDNA may be used as a pharmacodynamic biomarker to monitor therapeutic response. As we hypothesised that escalating doxorubicin in the first three cycles could be associated with improved efficacy, we assessed whether there was any evidence of greater decrease in nDNA levels at higher doses of doxorubicin both during the first cycle and throughout the whole six cycles of therapy (using the AUC for nDNA; Holdenrieder et al, 2008; Greystoke et al, 2011).
The median change in nDNA AUC over cycle 1 was 13 (range −21 to 22) for the cohort receiving doxorubicin 35 mg m–2, −1 (range −42 to 51) for the cohort receiving 45 mg m–2 and −3 (range −45 to 19) for the cohort receiving 55 mg m–2 (P=0.25).
Levels of nDNA in the cohort receiving 55 mg m–2 doxorubicin were significantly lower throughout therapy than in the 35 mg m–2 cohort (median nDNA AUC 37 (range 11 to 68) vs 69 (range 62 to 117) P=0.03).
Lactate dehydrogenase (LDH)
As elevated LDH is associated with poor prognosis in HL and falls following therapy, we evaluated how changes in nDNA corresponded to changes in LDH. There were no significant differences observed between the changes in LDH levels following therapy within different cohorts. The median change during cycle 1 was −33 (range −867 to +161) U l–1 in the 35 mg m–2 cohort, −23.5 (range −158 to +141) U l–1 in the 45 mg m–2 cohort and −42.5 (range −319 to +206) U l–1 in the 55 mg m–2 cohort (P=0.34).
Cytokeratin 18
We evaluated whether larger changes following therapy were seen in the putative epithelial toxicity biomarker CK18 at higher doses of doxorubicin. However, there was no significant difference in CK18 changes during the first three cycles of therapy between the different dose cohorts; the median change in CK18 at day 3 was 18% (range −31 to 40%) in the 35 mg m–2 cohort, 20% (range −29 to 56%) in the 45 mg m–2 cohort and 17% (range −23 to 108%) in the 55 mg m–2 cohort (P=0.45 for trend).
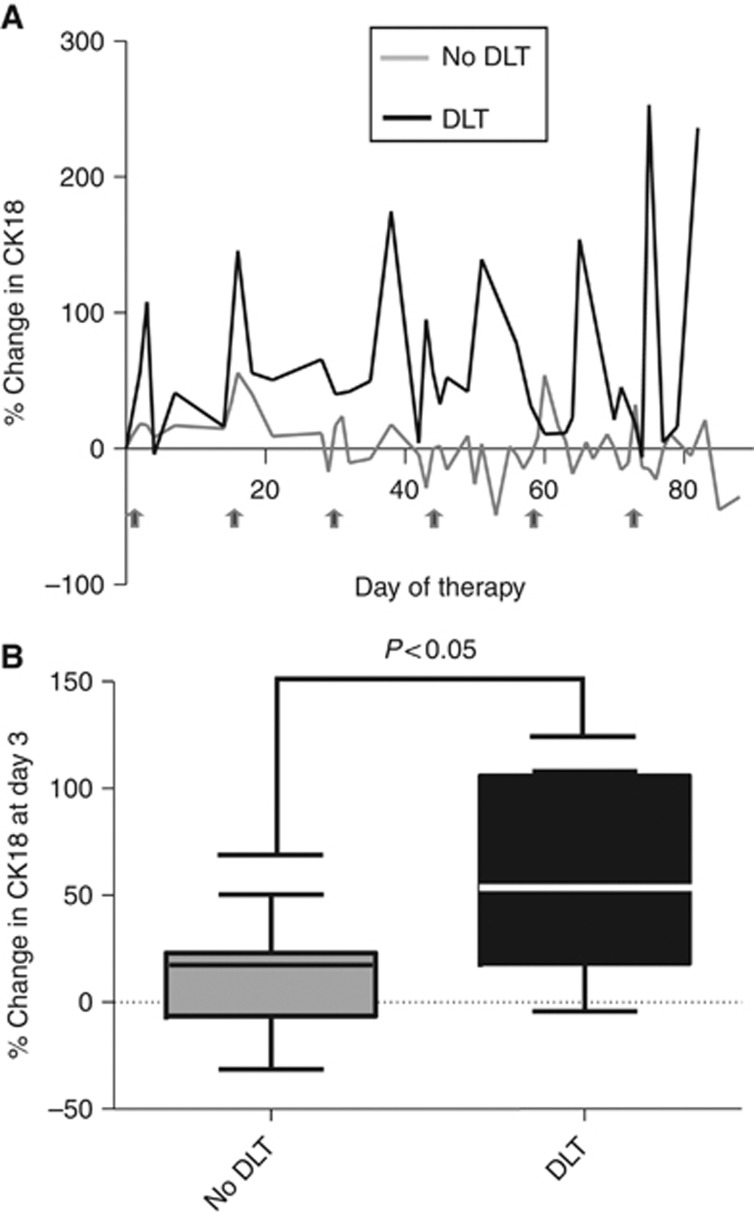
However, in patients who subsequently experienced DLT, larger increases in CK18 following therapy could be seen by day 3 of cycle 1 and these were maintained throughout therapy (Figure 1). The median change in patients without DLT was 17% (range −31 to 50%) compared with 56% in patients who experienced DLT (range −4 to 108% P=0.03).
Figure 1.
(A) Median changes from baseline in CK18 during therapy according to whether the subject developed subsequent DLT (n=6) or not (n=18). (B) Change in CK18 at day 3 of cycle 1 of therapy according to whether the subject developed subsequent DLT.
Discussion
This study has shown that using pegfilgrastim support it is both feasible and safe to deliver 45 mg m–2 doxorubicin during cycles 1–3 ABVD. The dose and schedule used produces a dose intensity of doxorubicin relative to standard doses of 180% in cycles 1–3 while maintaining cumulative doses over six cycles of 110% (Table 1).
High rates of low-grade nausea and vomiting occurred but were easily controlled with standard anti-emetic therapy. Other toxicities (Table 3) were observed at frequencies comparable to the literature (Martin et al, 2005; Younes et al, 2006). Most infections were either minor or related to vascular devices inserted for patient convenience and not mandated by the protocol. Two cases of grade 3 neuropathy were seen. Low rates of severe neuropathy can be seen with standard ABVD (Johnson et al, 2005) and while doxorubicin is not generally considered to be neurotoxic, neuropathy can be seen in both animal models (Eddy, 1983) and rarely following the administration of pegylated doxorubicin (Julius et al, 2013). As this severity of toxicity was only seen in two patients, it is uncertain whether escalated ABVD is associated with an increased risk of neurotoxicity.
Pneumonitis was seen in 3 out of 24 (12.5%) subjects as a DLT, and was presumed to be secondary to bleomycin, although an interaction with the higher doses of doxorubicin has to be considered. This is also comparable to published rates with standard ABVD (Martin et al, 2005; Younes et al, 2006). Some investigators have hypothesised that G-CSF administration is a risk factor for pneumonitis (Matthews, 1993), but this has not been borne out in this small study. However, higher-than-expected frequencies of PPE were observed. Although this is a common side-effect with liposomal doxorubicin, where it occurs in up to 50% of patients, it is seen in only 1–2% of patients receiving standard doxorubicin (Lorusso et al, 2007; Tanyi et al, 2009). More frequent dosing schedules and higher doses of standard doxorubicin are associated with a greater incidence of PPE (Bronchud et al, 1989; Ranson et al, 2001), but the other agents in ABVD (especially bleomycin) may have contributed to the skin toxicity.
Phase I studies do not adequately capture the incidence of late, chronic or rare toxicities (Edgerley and Fojo, 2008; Greystoke and Ranson, 2012). These toxicities are particularly important in HL given the good prognosis and young age of many of the patients. Although the patients enrolled in this study are continuing to be observed for late toxicities, future studies evaluating this regimen will need to include long-term follow-up of larger cohorts.
This study demonstrates the potential of using validated pharmacodynamic circulating cell death biomarkers to monitor efficacy and toxicity in patients with HL. The pharmacodynamic potential of nDNA in lymphoma has been previously reported (Greystoke et al, 2011). Higher doses of doxorubicin led to more pronounced reduction of nDNA levels throughout therapy but no direct association with outcome was seen. However, the study was primarily aimed at assessing feasibility and toxicity, and not powered for efficacy end points.
Changes in nDNA level alone may not occur sufficiently early or with suitable discrimination to change therapy in the case of inadequate response to ABVD. FDG-PET after two cycles of ABVD has improved prognostic value over the IPS in predicting FFTF (Gallamini et al, 2007), and this imaging biomarker emerges as the single most important tool for planning risk-adapted treatment protocols in advanced HL. With increasing interest being shown in both escalation and de-escalation of subsequent therapy based on the results of interim FDG-PET (UK NCRI RATHL Study, GHSG BEACOPP studies GHSG HD9, 12, 18, and EORTC No. 20012), dose escalation of doxorubicin to 45 mg m–2 in early cycles ABVD may hold promise in terms of increasing the PET negativity rate after two cycles of therapy, potentially improving long-term outcomes. The combined predictive power of early FDG PET negativity and nDNA change in cycle 1 is worthy of evaluation in therapy optimisation strategies
The CK18 data are intriguing as it suggests that it is possible to identify as early as day 3 of cycle 1 ABVD, patients who are at higher risk of experiencing toxicity in any subsequent escalated cycle. This confirms data previously observed in a mixed cohort of patients with lymphoma receiving standard chemotherapy (Greystoke et al, 2011). We believe therefore that CK18 could be used within future trials to give an objective measure of impending epithelial toxicity, one of the main factors limiting dose escalation of chemotherapy. In addition, the integration of toxicity biomarkers such as CK18 and FLT3 ligand (a circulating biomarker of myelosuppression; Molyneux et al, 2008) may facilitate the identification of subjects for whom dose escalation is inappropriate because of excessive risk of toxicity.
In terms of next steps a randomised trial addressing the hypothesis that a strategy of escalated ABVD with early biomarker-driven adjustments of dose is more effective than standard ABVD is currently under consideration by the UK NCRI Lymphoma Clinical Studies Group. End points would include FDG-PET negative rates after two cycles, progression-free survival and toxicity.
Acknowledgments
We thank Cancer Research UK Clinical Trials Awards and Advisory Committee who provided feasibility grant funding for this study (award number CRUK/08/013), clinical staff at all the Centres involved and Amgen (Thousand Oaks, CA, USA) for supplying pegfilgrastim (Neulasta) free of charge.
Author contributions
JR and PJ developed the study concept. JR wrote the protocol with advice from MR, obtained funding and ethics committee approval and oversaw study management. JR, AGi, KL, TI, ES, AP and AL assessed toxicity in individual patients taking part in the study. AGr, GH and CD did the biomarker analysis and SN supervised all blood-borne biomarker collections. AGi, AGr, MR, CD and JR analysed the data. AGi and AGr wrote the initial draft of the manuscript and all the authors contributed to subsequent revisions and final approval.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bains OS, Grigliatti TA, Reid RE, Riggs KW. Naturally occurring variants of human aldo-keto reductases with reduced in vitro metabolism of daunorubicin and doxorubicin. J Pharmacol Exp Ther. 2010;335:533–545. doi: 10.1124/jpet.110.173179. [DOI] [PubMed] [Google Scholar]
- Bauer K, Skoetz N, Monsef I, Engert A, Brillant C.2011Comparison of chemotherapy including escalated BEACOPP vs chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma Cochrane Database Syst Rev(8): CD007941. [DOI] [PubMed]
- Bronchud MH, Howell A, Crowther D, Hopwood P, Souza L, Dexter TM. The use of granulocyte colony-stimulating factor to increase the intensity of treatment with doxorubicin in patients with advanced breast and ovarian cancer. Br J Cancer. 1989;60:121–125. doi: 10.1038/bjc.1989.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD. Is BEACOPP better than ABVD. Curr Hematol Malig Rep. 2007;2:161–166. doi: 10.1007/s11899-007-0022-2. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on Lymphoma Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Duggan DB, Petroni GR, Johnson JL, Glick JH, Fisher RI, Connors JM, Canellos GP, Peterson BA. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: report of an intergroup trial. J Clin Oncol. 2003;21 (4:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- Eddy EL. Neuronal loss from cervical dorsal root ganglia in adriamycin induced peripheral neuropathy—a quantitative study. Anat Anz. 1983;153 (1:83–90. [PubMed] [Google Scholar]
- Edgerly M, Fojo T. Is there room for improvement in adverse event reporting in the era of targeted therapies. J Natl Cancer Inst. 2008;100 (4:240–242. doi: 10.1093/jnci/djm324. [DOI] [PubMed] [Google Scholar]
- Elis A, Lishner M, Walker S, Atias D, Korenberg A, Koren G. Doxorubicin in lymphoma: association between pharmacokinetic variability and clinical response. Ther Drug Monit. 2010;32:50–52. doi: 10.1097/FTD.0b013e3181c3a16d. [DOI] [PubMed] [Google Scholar]
- Engert A, Diehl V, Franklin J, Lohri A, Dorken B, Ludwig WD, Koch P, Hanel M, Pfreundschuh M, Wilhelm M, Trumper L, Aulitzky WE, Bentz M, Rummel M, Sezer O, Muller-Hermelink HK, Hasenclever D, Loffler M. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- Federico M, Luminari S, Iannitto E, Polimeno G, Marcheselli L, Montanini A, La Sala A, Merli F, Stelitano C, Pozzi S, Scalone R, Di Renzo N, Musto P, Baldini L, Cervetti G, Angrilli F, Mazza P, Brugiatelli M, Gobbi PG. HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27 (5:805–811. doi: 10.1200/JCO.2008.17.0910. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, Patti C, Loft A, Di Raimondo F, D'Amore F, Biggi A, Vitolo U, Stelitano C, Sancetta R, Trentin L, Luminari S, Iannitto E, Viviani S, Pierri I, Levis A. Early interim 2-(18F)fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to International Prognostic Score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- Greystoke A, Cummings J, Ward T, Simpson K, Renehan A, Butt F, Moore D, Gietema J, Blackhall F, Ranson M, Hughes A, Dive C. Optimisation of circulating biomarkers of cell death for routine clinical use. Ann Oncol. 2008;19 (5:990–995. doi: 10.1093/annonc/mdn014. [DOI] [PubMed] [Google Scholar]
- Greystoke A, O'Connor JP, Linton K, Taylor MB, Cummings J, Ward T, Maders F, Hughes A, Ranson M, Illidge TM, Radford J, Dive C. Assessment of circulating biomarkers for potential pharmacodynamic utility in patients with lymphoma. Br J Cancer. 2011;104:719–725. doi: 10.1038/sj.bjc.6606082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greystoke A, Ranson M. Learning from toxicity patterns in phase I trials during the era of mechanism targeted agents. Ann Oncol. 2012;23 (8:1934–1936. doi: 10.1093/annonc/mds116. [DOI] [PubMed] [Google Scholar]
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International prognostic factors project on advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Nagel D, Schalhorn A, Heinemann V, Wilkowski R, von Pawel J, Raith H, Feldmann K, Kremer AE, Muller S, Geiger S, Hamann GF, Seidel D, Stieber P. Clinical relevance of circulating nucleosomes in cancer. Ann N Y Acad Sci. 2008;1137:180–189. doi: 10.1196/annals.1448.012. [DOI] [PubMed] [Google Scholar]
- Joerger M, Huitema AD, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, Vermorken JB, Strocchi E, Martoni A, Sorio R, Sleeboom HP, Izquierdo MA, Jodrell DI, Fety R, de Bruijn E, Hempel G, Karlsson M, Tranchand B, Schrijvers AH, Twelves C, Beijnen JH, Schellens JH, EORTC-PAMM-NDDG Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients: a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet. 2007;46:1051–1068. doi: 10.2165/00003088-200746120-00005. [DOI] [PubMed] [Google Scholar]
- Johnson PW, Radford JA, Cullen MH, Sydes MR, Walewski J, Jack AS, MacLennan KA, Stenning SP, Clawson S, Smith P, Ryder D, Hancock BW, United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519) Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin's lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519) J Clin Oncol. 2005;23:9208–9218. doi: 10.1200/JCO.2005.03.2151. [DOI] [PubMed] [Google Scholar]
- Julius JM, Tanyi JL, Nogueras-Gonzalez GM, Watkins JL, Coleman RL, Wolf JK, Smith JA. Evaluation of pegylated liposomal doxorubicin dose on the adverse drug event profile and outcomes in treatment of recurrent endometrial cancer. Int J Gynecol Cancer. 2013;23 (2:348–354. doi: 10.1097/IGC.0b013e31827c18f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimm B, Reineke T, Haverkamp H, Behringer K, Eich HT, Josting A, Pfistner B, Diehl V, Engert A, German Hodgkin Study Group Role of hematotoxicity and sex in patients with Hodgkin's lymphoma: an analysis from the German Hodgkin Study Group. J Clin Oncol. 2005;23:8003–8011. doi: 10.1200/JCO.2005.205.60. [DOI] [PubMed] [Google Scholar]
- Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot' syndrome) Ann Oncol. 2007;18:1159–1164. doi: 10.1093/annonc/mdl477. [DOI] [PubMed] [Google Scholar]
- Martin WG, Ristow KM, Habermann TM, Colgan JP, Witzig TE, Ansell SM. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin's lymphoma. J Clin Oncol. 2005;23:7614–7620. doi: 10.1200/JCO.2005.02.7243. [DOI] [PubMed] [Google Scholar]
- Matthews JH. Pulmonary toxicity of ABVD chemotherapy and G-CSF in Hodgkin's disease: possible synergy. Lancet. 1993;342:988. doi: 10.1016/0140-6736(93)92033-p. [DOI] [PubMed] [Google Scholar]
- Molyneux G, Gibson FM, Whayman M, Turton JA. Serum FLT-3 ligand in a busulphan-induced model of chronic bone marrow hypoplasia in the female CD-1 mouse. Int J Exp Pathol. 2008;89:159–170. doi: 10.1111/j.1365-2613.2008.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson MR, Cheeseman S, White S, Margison J. Caelyx (stealth liposomal doxorubicin) in the treatment of advanced breast cancer. Crit Rev Oncol Hematol. 2001;37:115–120. doi: 10.1016/s1040-8428(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Tanyi JL, Smith JA, Ramos L, Parker CL, Munsell MF, Wolf JK. Predisposing risk factors for palmar-plantar erythrodysesthesia when using liposomal doxorubicin to treat recurrent ovarian cancer. Gynecol Oncol. 2009;114:219–224. doi: 10.1016/j.ygyno.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Younes A, Fayad L, Romaguera J, Pro B, Goy A, Wang M. Safety and efficacy of once-per-cycle pegfilgrastim in support of ABVD chemotherapy in patients with Hodgkin lymphoma. Eur J Cancer. 2006;42:2976–2981. doi: 10.1016/j.ejca.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, de Vos S, Forero-Torres A, Moskowitz CH, Connors JM, Engert A, Larsen EK, Kennedy DA, Sievers EL, Chen R. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]