Abstract
Background:
Although regulatory T cells (Treg) are highly enriched in human tumours compared with peripheral blood, expression of the immune-checkpoint receptors, immunosuppressive molecules and function of Treg in these two sites remains undefined.
Methods:
Tumour-infiltrating lymphocytes and peripheral blood lymphocytes were isolated from a cohort of head and neck squamous cell carcinoma (HNSCC) patients. The immunosuppressive phenotypes and function of intratumoral Treg were compared with those of peripheral blood Treg.
Results:
The frequency of immune-checkpoint receptor-positive cells was higher on intratumoral FOXP3+CD25hi Treg compared with circulating Treg (CTLA-4, P=0.002; TIM-3, P=0.002 and PD-1, P=0.002). Immunosuppressive effector molecules, LAP and ectonucleotidase CD39 were also upregulated on intratumoral FOXP3+ Treg (P=0.002 and P=0.004, respectively). CTLA-4 and CD39 were co-expressed on the majority of intratumoral FOXP3+CD4+ Treg, suggesting that these molecules have a key role in regulatory functions of these cells in situ. Notably, intratumoral Treg exhibited more potently immunosuppressive activity than circulating Treg.
Conclusion:
These results indicate that intratumoral Treg are more immunosuppressive than circulating Treg and CTLA-4 and CD39 expressed can be potential target molecules to inhibit suppressive activities of intratumoral Treg in situ.
Keywords: regulatory T cells, checkpoint receptors, tumour-infiltrating lymphocytes, head and neck cancer
The frequency and function of regulatory T cells (Treg) are elevated in the peripheral blood and at the tumour sites of patients with cancers including head and neck squamous cell carcinoma (HNSCC; Ichihara et al, 2003; Schaefer et al, 2005; Hiraoka et al, 2006). This enrichment of Treg in tumour-infiltrating lymphocytes (TIL) might be responsible for suppressing anti-tumour immunity in the tumour microenvironment (TME). Mechanisms underlying Treg accumulation at tumour sites are not clear. It has been suggested that enrichment of Treg in tumours could be induced by the conversion of FOXP3− T cells into FOXP3+ T cells in the presence of TGF-β1 and retinoic acid (Coombes et al, 2007; Sun et al, 2007). Alternatively, peripheral Treg might be recruited into tumour sites and expanded as a result of specific self-antigen recognition by memory Treg (Darrasse-Jeze et al, 2009). This alternative implies that Treg activation and expansion takes place in the TME. Although these studies demonstrate that TCR triggering is a central event to Treg induction and expansion in the TME, molecules that contribute to the expansion and suppressive function of Treg remain largely unidentified.
Immune-checkpoint receptors (ICRs) including cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death-1 (PD-1), T-cell immunoglobulin and mucin protein-3 (TIM-3) and lymphocyte activation gene-3 (LAG-3) have been proposed as key molecules involved in generation and/or suppressive functions of Treg (Fife and Bluestone, 2008; Francisco et al, 2010; Joller et al, 2012; Pardoll, 2012). In both mice and human, blockade of CTLA-4 abrogated immunosuppressive activity of Treg and enhanced effector T-cell functions (Tang et al, 2004; Zheng et al, 2008). A recent study using PD-1-deficient mice suggested that PD-1 is involved in the development of inducible Treg but not in their immunosuppressive functions (Qiao et al, 2012). In addition to the ICRs, Treg also express ectonucleotidases, CD39 and CD73, which have been proposed as immunosuppression-inducing molecules on Treg (Mandapathil et al, 2010). CD39 converts ATP and ADP into cAMP, which is further converted into adenosine, a potent immunosuppressive agent, by CD73 (Bastid et al, 2013). In addition to blocking lymphocyte functions, adenosine exerts immunosuppressive activity on macrophages and DCs and may promote tumour development by inhibiting innate immune responses, including phagocytosis and antigen presentation, and by secreting VEGF, TGF-β and IL-6 (Bastid et al, 2013). Despite the potential importance of ICRs and CD39/CD73 molecules in the development and immunosuppressive functions of Treg, a comparative analysis of these molecules on Treg in the blood and tumour sites has not been performed.
In this study, we investigated the expression levels of ICRs and other immunosuppressive molecules on Treg isolated from tumour sites and peripheral circulation of head and neck cancer (HNC) patients. Using paired autologous TIL and peripheral blood lymphocyte (PBL) specimens, we observed that intratumoral Treg were more immunosuppressive than circulating Treg and suggest that elevated expression of ICRs on TIL may contribute to their expansion and/or suppressive activity in the TME.
Materials and methods
Patients and specimens
Peripheral venous blood samples and tumours were obtained from 27 patients with HNSCC as a baseline. All patients were seen in the Department of Otolaryngology at the University of Pittsburgh Medical Center. All subjects signed written informed consent approved by the Institutional Review Board of the University of Pittsburgh (IRB no. 99-06). The patient cohort included 10 females and 15 males with a mean age of 64.7±9.9 years (range: 40–83 years) and the tumours were isolated from different sites as described in Table 1.
Table 1. Demographics of the HNC patients in this study.
| No. of patients | Tumour site | Previous treatment | Mean age | Male | Female | ||
|---|---|---|---|---|---|---|---|
| 25 |
OC |
15 |
None |
16 |
64.7 |
15 |
10 |
| |
OP |
9 |
S |
2 |
|
|
|
| |
Other |
1 |
RC |
1 |
|
|
|
| |
|
|
SR |
2 |
|
|
|
| |
|
|
SC |
1 |
|
|
|
| SCR | 3 |
Abbreviations: C=chemotherapy; HNC=head and neck cancer; OC=oral cavity; OP=oropharynx; R=radiation; S=surgery.
Collection of PBMC and TIL
Blood samples from cancer patients and healthy donors (30–40 ml) were drawn into heparinized tubes and centrifuged on Ficoll–Hypaque gradients (GE Healthcare Bioscience, Piscataway, NJ, USA). Peripheral blood mononuclear cells (PBMC) were recovered, washed in RPMI-1640 or AIM-V medium (Invitrogen, Carlsbad, CA, USA) and immediately used for experiments. For TIL isolation, freshly isolated tumours from HNC patients were minced into small pieces, which then were transferred to a cell strainer (70 μm Nylon) and mechanically separated by using a syringe plunge. The cells passing through the cell strainer were collected and subjected to Ficoll–Hypaque gradient centrifugation. After centrifugation, mononuclear cells were recovered and stored at −80 °C until flow cytometry analysis.
Antibodies and reagents
The following anti-human monoclonal antibodies (mAb) were used for staining: CD39-FITC/PC7, FOXP3-FITC (clone PCH101), LAP-PE, PD-1-APC (all eBioscience, San Diego, CA, USA), CD73-PE, CTLA-4-PE, TIM-3-Brillian violet 421, CD25-PE-Cy7, Ganzyme B-FITC, Perforin-APC, CD39-APC, CD86-PE (all Biolegend, San Diego, CA, USA), LAG-3-ATTO647N conjugate (Enzo Life Sciences, Farmingdale, NY, USA), CD4-PE-Texas Red, CD3-Alexa Fluor 405 conjugate (Invitrogen) and CD4-AF700, CD80-FITC, HLA-DR-APC (all BD Biosciences, San Jose, CA, USA) including their respective isotypes, which served as negative controls for surface as well as intracellular staining. All Abs were pre-titrated using activated as well as non-activated PBMC to determine optimal staining dilutions.
Flow cytometry
For cell surface staining, PBMCs and TIL were washed twice in staining buffer (2% w/v fetal bovine serum) and stained for cell surface markers as described (Lopez-Albaitero et al, 2009). Briefly, cells were incubated with relevant Abs for 20 min at room temperature (RT) in the dark, washed twice and re-suspended in staining buffer. Intracellular staining for FOXP3 was performed according to the manufacturer's protocol (eBioscience). Briefly, PBMCs or TIL were stained with mAb for surface markers, washed and subsequently fixed and permeabilized by using Fix/Perm buffer. After washing, cells were stained for their intracellular FOXP3. Flow cytometry was performed using a CyAn flow cytometer (Dako, Ft. Collins, CO, USA), or Fortesa cytometry (Becton Dickinson, Franklin Lakes, NJ, USA) and data analysed using Summit V4.3 software or flowJo software (TreeStar, Inc., Ashland, OR, USA). The acquisition and analysis gates were restricted to the lymphocyte gate based on characteristic properties of the cells in the forward and side scatter. At least 1 × 105 events were acquired for analysis and, where applicable, gates were restricted to the CD3+CD4+ subset for Treg analysis.
Treg suppression assays
The CD4+CD25+ T cells were enriched from TIL or PBL using human CD4+CD25+ Treg isolation kit (STEMCELL Technologies, Vancouver, BC, Canada) and tested for their immunosuppressive activity in co-culture with autologous CD4+CD25− responder cells (RC) in the presence of CD14+ antigen-presenting cells (APCs), which is slightly modified from the previously described method (Strauss et al, 2007). CFSE-labelled autologous CD4+CD25− cells (4 × 104 cells/well) were incubated in wells of round-bottom 96-well plates at RC to Treg ratio of 1 : 1, 1 : 0.5, 1 : 0.25 and 1 : 0. To induce proliferation, RC were stimulated with soluble OKT3 (anti-human CD3, 10 μg/ml) in the presence of autologous CD14+ APC for 4 days. All CFSE data were collected by a Fortesa cytometry (Becton Dickinson) and analysed using the flowJo software (TreeStar, Inc.). The percentages of suppression were calculated based on the proliferation index (PI) of RCs alone compared with the PI of cultures containing responders and Treg. The program determines the per cent of cells within each peak, and the sum of all peaks in the control culture is taken as 100% of proliferation and 0% of suppression.
Statistical analysis
Averages were calculated as means. For non-parametric distribution of samples, P-values were calculated by Wilcoxon-matched pair test or Mann–Whitney test using GraphPad Prism. P-values<0.05 were considered to be significant.
Results
Increased frequency of FOXP3+ cells and immune-checkpoint receptors on Treg at tumour sites of HNSCC patients
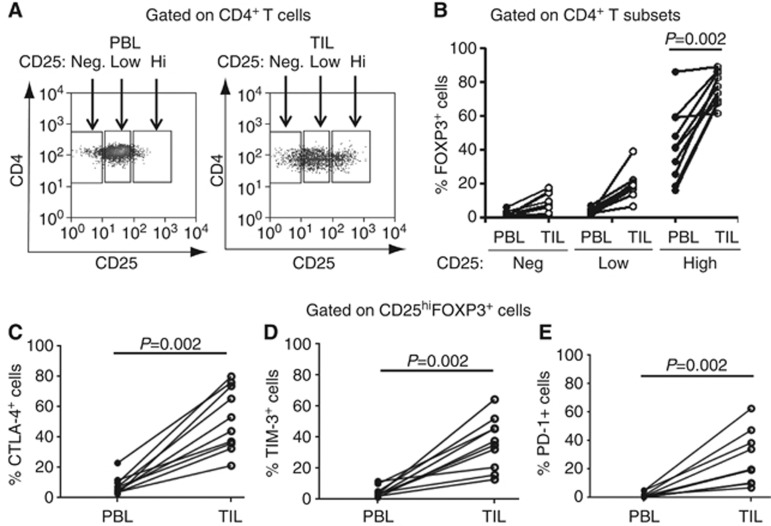
To compare the Treg frequency in tumour sites with that in the circulation, CD4+CD25neg, CD4+CD25low and CD4+CD25hi T cells from paired PBL and TIL were evaluated for percentages of FOXP3+ cells (Figures 1A and B). FOXP3+ cells were consistently observed in the CD4+CD25hi subset of PBL and TIL. However, the proportion of FOXP3+ cells was significantly higher in CD4+CD25hi cells in TIL compared with PBL (Figures 1B; P=0.002). We also found that percentages of CTLA-4+, TIM-3+ and PD-1+ cells in CD25hiFOXP3+ Treg were significantly higher in TIL compared with those in paired PBL (Figures 1C∼E and Supplementary Figure 1A, CTLA-4, P=0.002; TIM-3, P=0.002 and PD-1, P=0.002). CTLA-4 and TIM-3 were preferentially expressed by FOXP3+CD25hiCD4+ TIL and PBL, defining a distinct subset of Treg. Although PD-1 was predominantly expressed by the CD4+CD25hi T subset compared with CD4+CD25med and CD4+CD25neg T-cell subsets, it was also expressed by some FOXP3-negative cells regardless of CD25 expression (Supplementary Figure 1A). Therefore, PD-1 was not a specific biomarker for intratumoral CD25hiFOXP3+ Treg. For LAG-3, no significant expression was found among CD4+ TIL subsets, but LAG-3 was predominantly expressed by CD4+CD25hi PBL subsets (Supplementary Figure 1B), consistent with previous report that LAG-3 predominates on CD4+CD25hiFOXP3+ Treg in PBL (Camisaschi et al, 2010). Taken together, these results indicate that the frequency of cells expressing immune-checkpoint receptors, including CTLA-4, TIM-3 and PD-1, is significantly increased on intratumoral FOXP3+CD25hi Treg compared with circulating Treg.
Figure 1.
The frequency of FOXP3+, CTLA-4+, TIM-3+ and PD-1+ cells are higher on intratumoral CD4+CD25hi Treg compared with circulating Treg. (A) Flow cytometry gating strategy for analysing CD4+ T-cell subsets in PBL and TIL isolated from 10 HNSCC patients. Using paired PBL and TIL CD4+ T cells isolated from these patients, gates were set to include CD25 negative (CD25neg), CD25 low (CD25lo) and CD25 high (CD25hi) CD4+ T cells. Then, the frequency of FOXP3+ cells (B) in CD25hi CD4+ T-cell subset and CTLA-4+ (C), TIM-3+ (D) and PD-1+ (E) cells in CD25hi FOXP3+ Treg was compared between paired PBL and TIL subsets of the same HNSCC patients (n=10).
CD39 and LAP are predominantly upregulated by intratumoral CD4+FOXP3+ Treg
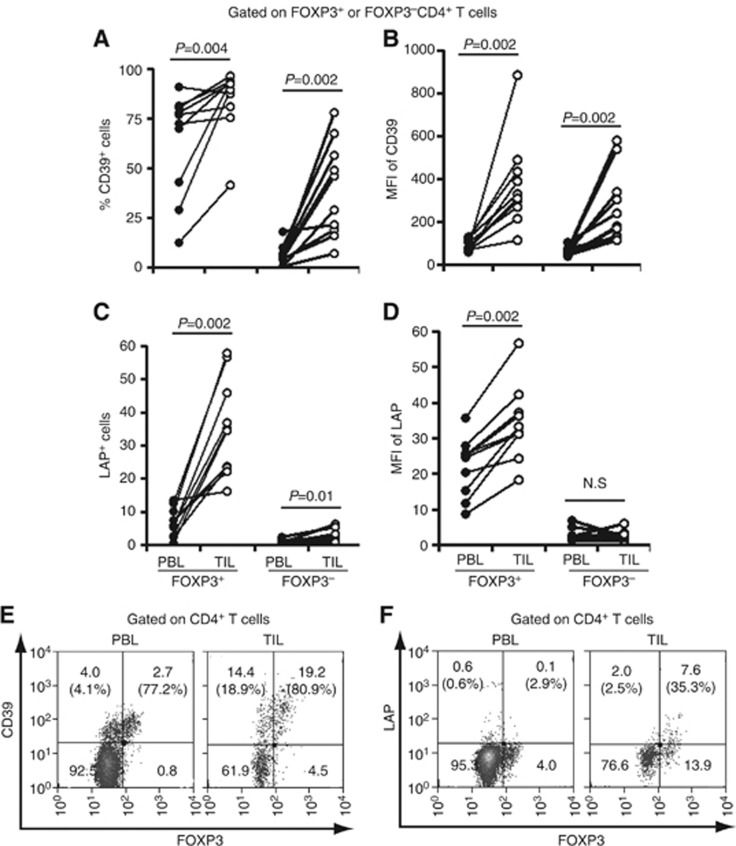
As concordant expression of the adenosine pathway ectoenzymes CD39 and CD73, has been strongly associated with human inducible FOXP3+ Treg (Mandapathil et al, 2010, Schuler et al, 2012), we investigated whether there is differential expression of these molecules between intratumoral FOXP3+ Treg and circulating Treg. To do this, CD4+ T cells in PBL and TIL were divided into FOXP3+ and FOXP3− cells to examine the correlation between CD39/CD73 and FOXP3 expression. Interestingly, the frequency of CD39+ cells was higher among intratumoral FOXP3+CD4+ T cells compared with circulating FOXP3+CD4+ T cells (Figures 2A, P=0.004). In addition, CD39 expression levels (mean fluorescent intensity, MFI) were also significantly higher in FOXP3+CD4+ TIL than in circulating FOXP3+CD4+ PBL (Figures 2B, P=0.002). However, no significant difference in the frequency of CD73+ cells was detected between FOXP3+ and FOXP3− CD4+ T cells in both TIL and PBL (Supplementary Figure 2). The frequency of CD73+ cells in some intratumoral FOXP3+CD4+ T cells was higher than that in circulating FOXP3−CD4+ T cells. Although CD39 expression was found on both FOXP3+ and FOXP3− CD4+ T cells, the fraction of CD39+ cells was disproportionately higher on FOXP3+CD4+ T cells in TIL and PBL than FOXP3−CD4+ T cells (Figure 2E). Analysis of LAP (membrane-bound active form of TGF-β) expression on CD4+ T cells showed that both frequency and expression of LAP+ cells were significantly higher on FOXP3+CD4+ TIL than circulating FOXP3+CD4+ PBL (Figures 2C and D, P=0.002). In addition, the fraction of LAP+ cells was significantly higher on FOXP3+CD4+ T cells than FOXP3−CD4+ T cells in both TIL and PBL (Figure 2F). Together, these observations suggest that both immunosuppressive molecules, CD39 and LAP, are more robustly expressed by intratumoral FOXP3+ Treg than by circulating FOXP3+ Treg.
Figure 2.
The frequency and expression of CD39 and LAP are significantly increased by intratumoral FOXP3+ Treg. (A, B) The fraction of CD39+ cells in FOXP3+ TIL-Treg and their CD39 expression levels (MFI) were compared with those in FOXP3+ PBL-Treg isolated from 10 HNC patients. (C, D) Similarly, the frequency and expression levels of LAP+ cells in FOXP3+ TIL-Treg was compared with those in FOXP3+ PBL-Treg isolated from HNSCC patients (n=10). (E, F) Representative flow cytometry analysis for CD39 (E) and LAP (F) expression in the FOXP3+ or FOXP3− CD4+ TIL and PBL isolated from one HNSCC patient. The numbers represent the percentage of CD39+ and LAP+ cells in CD4+ T cells for each quadrant, and the percentage values represent the fraction of CD39+ or LAP+ cells in FOXP3+CD4+ T (top right) or in FOXP3−CD4+ T cells (top left).
CTLA-4 and CD39 are co-expressed on the majority of intratumoral FOXP3+ Treg
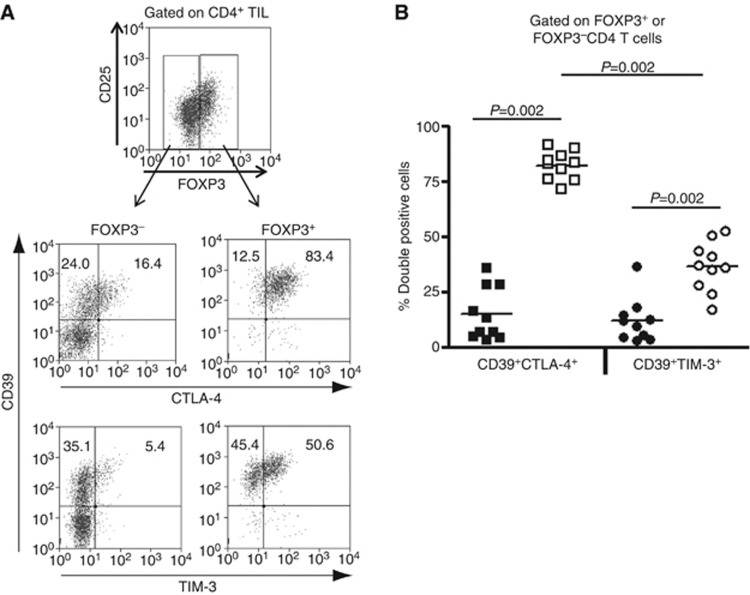
As FOXP3 expression was strongly associated with expression of CTLA-4, TIM-3 and CD39, we compared expression of these receptors on FOXP3+CD4+ TIL with those of FOXP3−CD4+ TIL to identify molecules that best characterise intratumoral FOXP3+ Treg. We found that the majority of intratumoral CD4+FOXP3+ Treg (>80%) concomitantly expressed CTLA-4 and CD39, and only about half co-expressed CD39 and TIM-3 (Figure 3). However, these highly co-expressed CTLA-4 and CD39 molecules were not found in FOXP3−CD4+ TIL. In addition, FOXP3−CD4+ TIL expressed relatively high level of CD39 on their surface, compared with relatively poorly expressed CTLA-4 and TIM-3 molecules (Figure 3A).
Figure 3.
CTLA-4 and CD39 are co-expressed on the majority of intratumoral FOXP3+ Treg. (A) CTLA-4, TIM-3 and CD39 expression on intratumoral FOXP3+ Treg was analysed by flow cytometry. FOXP3+ and FOXP3− cells were, respectively, evaluated for their frequency of CTLA-4+, CD39+ and TIM-3+ cells. The numbers represent fraction of CTLA-4+, TIM-3+ and CD39+ cells in FOXP3+ or FOXP3− CD4+ T cells. (B) Percentages of double-positive CD39+CTLA-4+ or CD39+TIM-3+ cells in FOXP3+CD4+ T cells (open squares and circles) are compared with those in FOXP3−CD4+ T cells (filled squares and circles, n=9). Then, the percentage of double-positive CD39+CTLA-4+ cells was compared with that of CD39+TIM-3+ cells in FOXP3+ Treg.
The intratumoral FOXP3+ Treg are more suppressive than circulating FOXP3+ Treg
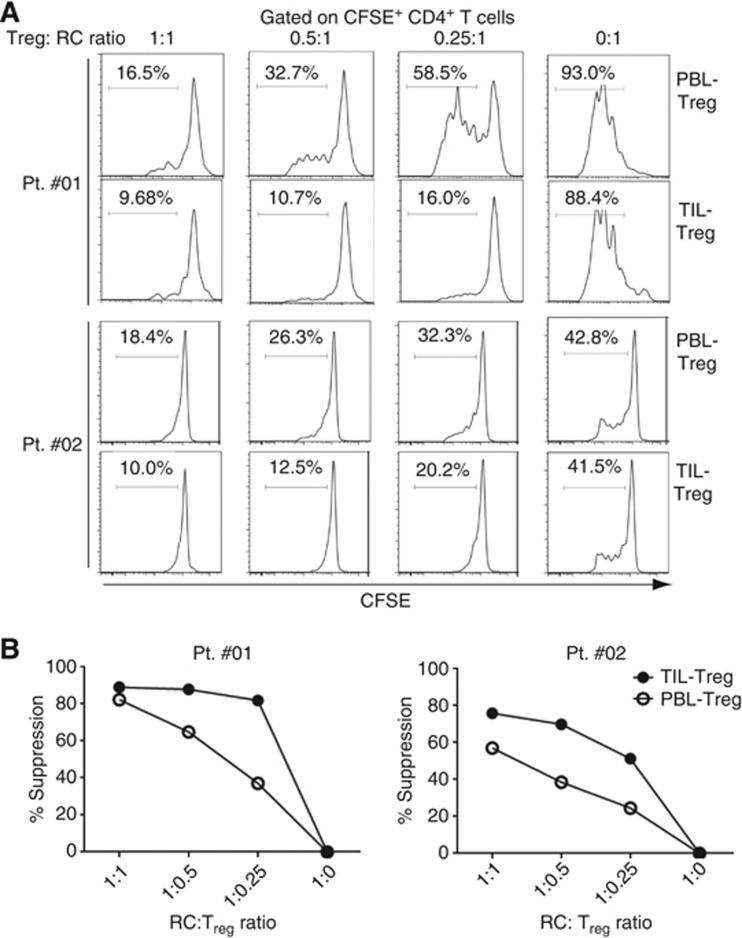
As intratumoral Treg exhibited more immunosuppressive phenotypes than circulating Treg, we determined whether they are functionally more suppressive than the peripheral blood Treg. To do this, we enriched CD4+CD25+ T cells from TIL and PBL isolated from two patients and performed Treg suppressive assay to compare their suppressive activity. We designated these cells as TIL-Treg and PBL-Treg, respectively. As shown in Figure 4, TIL-Treg were more potently suppressive in regulating T-cell proliferation than PBL-Treg in both patients. In patient no. 01, TIL-Treg potently suppressed autologous CD4+ T-cell proliferation (CFSElow cells) even at 1 : 0.25, the ratio of Treg to RCs. Although PBL-Treg was as suppressive as TIL-Treg at 1 : 1 ratio of Treg to RC, they gradually lost their suppressive activity as the ratio decreased (1 : 0.5 and 1 : 0.25). In the case of patient no. 02, suppressive activity of TIL-Treg was more potent than that of PBL-Treg at all the ratio of Treg to RC. This result strongly suggests that intratumoral Treg are functionally more suppressive than circulating Treg, which also indicates that immunosuppressive phenotype of TIL-Treg is positively correlated with their suppressive function.
Figure 4.
The intratumoral FOXP3+ Treg are more suppressive than circulating FOXP3+ Treg. (A) Suppression of autogolous RC proliferation by CD25+CD4+ T cells isolated from TIL or PBL (from two patients). CFSE-labelled CD4+CD25− T cells were co-cultured with unlabelled autologous CD4+CD25+ T cells isolated from TIL or PBL as indicated. After 4 days of stimulation with anti-CD3 antibody, CFSE+ CD4+ T cells were analysed for their proliferation by CFSE dilution. (B) Percentage of suppression of TIL-Treg was compared with that of PBL-Treg from two patients, respectively.
Discussion
In this study, we demonstrated that the frequency of not only immune-checkpoint receptors, including CTLA-4+, TIM-3+ and PD-1+ cells, but also immune suppressive molecules, such as CD39+ and TGF-β1+ cells, were significantly increased on intratumoral Treg compared with circulating Treg. Particularly, we found that CTLA-4 and CD39 were co-expressed on the majority of intratumoral Treg, which suggests that the blockade of CTLA-4 or CD39 enzymatic activity might enhance anti-tumour immunity in the TME by inhibiting immunosuppressive activities of intratumoral Treg. We also observed that intratumoral Treg exhibited more suppressive activity than peripheral blood Treg. Taken together, this study provides insight into mechanisms and markers of intratumoral Treg, which are potently immunosuppressive cells. These CTLA-4+/CD39+ Treg are major targets to inhibit their suppressive function, thus promoting anti-tumour immunity in the TME.
Many studies define CTLA-4 as an effector receptor in mediating Treg immunosuppressive functions (Walker and Sansom, 2011). For example, the FOXP3+ Treg-specific loss of CTLA-4 is sufficient to induce fatal lymphoproliferative disease, which is similar to that observed in CTLA-4-deficient mice (Wing et al, 2008). It has also been reported that Treg can induce indoleamine 2,3-dioxygenase (IDO) activity in APCs through CTLA-4 and CD80/CD86 interaction. Indoleamine 2,3-dioxygenase catabolizes tryptophan, depleting its local supply to immune cells and subsequently inhibiting effector T- and NK-cell functions (Pietra et al, 2012). Similarly, it has been suggested that antibody-triggering of CTLA-4 can induce the release of TGFβ1 from Treg (Oida et al, 2006). However, the link between CTLA-4 and IDO/TGFβ1 currently remains controversial, albeit their critical roles in Treg-mediated immune suppressive activities have been recognised (Walker and Sansom, 2011). Ectonucleotidases, CD39 and CD73, have been proposed as important contributors to Treg-mediated immunosuppressive functions, thereby promoting tumour progression (Bastid et al, 2013). One recent study demonstrated that anti-CD39 specific blocking mAb attenuated immunosuppressive functions of Treg and restored CD4+ and CD8+ T cells (Nikolova et al, 2011), suggesting a potential role for CD39 in regulating T-cell-mediated antitumour immunity. Although it has been well known that intratumoral Treg are strongly immunosuppressive, the molecules mediating immunosuppressive activities have not been thoroughly investigated in paired intratumoral Treg and circulating Treg. We observed that CTLA-4, CD39 and TGFβ1 molecules are predominantly expressed by intratumoral FOXP3+ Treg compared with circulating Treg (Figures 1 and 2), suggesting that these molecules may be involved in enhancing immune suppressive activities of Treg in TME. Particularly, we observed that CD39 and CTLA-4 were co-expressed on the majority of intratumoral Treg, suggesting that these two molecules may be key modulators of functional FOXP3+ Treg in the TME. Therefore, these results indicate that the blockade of CTLA-4, TGFβ1 or CD39 on Treg may facilitate anti-tumour immunity by enhancing functional activation of immune cells such as DC, T and NK cells in the TME.
Although several studies of human cancer indicate that intratumoral FOXP3+ Treg may be associated with poor prognosis (deLeeuw et al, 2008), little information is available regarding the mechanisms underlying the enrichment of Treg in the TME. One study showed that FOXP3+Treg are rapidly recruited into tumour sites and expanded as a consequence of triggering through self-antigen reactive TCR (Darrasse-Jeze et al, 2009). A recent report analysing the T-cell receptor (TCR) repertoire of intratumoral Treg suggested that a few dominant Treg clones containing the so-called public TCR sequences were markedly expanded through TCR responses in the TME (Sainz-Perez et al, 2012). However, the mechanism underlying the expansion of Treg at the tumour sites has not been thoroughly investigated in these studies. Further, it has been reported that CTLA-4 interacts with CD80 with affinity, which is more than 20 times stronger than that for CD86 (Walker and Sansom, 2011). Also, it has been proposed that the preferential engagement of CTLA-4 by CD80 during antigen presentation is one of the critical events allowing for the contact between DC and Treg, thus leading to expansion of Treg (Perez et al, 2008). In this respect, during antigen presentation in the TME, Treg preferentially interact with intratumoral APCs through strong CTLA-4–CD80 interaction, which may affect not only expansion of Treg but also their potently immunosuppressive functions at tumour sites. Given that the interaction between Treg and APCs is one of the critical events to determine the expansion and suppressive functions of Treg, it is important area to investigate the molecules that mediate this cellular interaction to understand the mechanism whereby relatively more enriched and immunosuppressive Treg are induced in the TME. For example, interaction between PD-1 on T cells and PD-L1 on APCs regulates the development, maintenance and function of induced Treg (Francisco et al, 2009). Therefore, it would be interesting to identify the molecules mediating this interaction between Treg and APCs to gain better understanding the mechanism by which enhanced frequency and functions of Treg are consistently induced, thus maintaining potent immunosuppressive microenvironment at cancer patients' tumour sites.
Acknowledgments
The authors thank Michael Meyer and Bratislav Janjic from the flow cytometry facility, University of Pittsburgh Cancer Institute for assisting with the flow cytometry.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32:1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, Parmiani G, Belli F, Rivoltini L, Castelli C. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumour sites. J Immunol. 2010;184:6545–6551. doi: 10.4049/jimmunol.0903879. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse-Jeze G, Bergot AS, Durgeau A, Billiard F, Salomon BL, Cohen JL, Bellier B, Podsypanina K, Klatzmann D. Tumour emergence is sensed by self-specific CD44hi memory Tregs that create a dominant tolerogenic environment for tumours in mice. J Clin Invest. 2009;119:2648–2662. doi: 10.1172/JCI36628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumour-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2008;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumour-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- Joller N, Peters A, Anderson AC, Kuchroo VK. Immune checkpoints in central nervous system autoimmunity. Immunol Rev. 2012;248:122–139. doi: 10.1111/j.1600-065X.2012.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Albaitero A, Mailliard R, Hackman T, Andrade Filho PA, Wang X, Gooding W, Ferrone S, Kalinski P, Ferris RL. Maturation pathways of dendritic cells determine TAP1 and TAP2 levels and cross-presenting function. J Immunother. 2009;32:465–473. doi: 10.1097/CJI.0b013e3181a1c24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova M, Carriere M, Jenabian MA, Limou S, Younas M, Kok A, Hue S, Seddiki N, Hulin A, Delaneau O, Schuitemaker H, Herbeck JT, Mullins JI, Muhtarova M, Bensussan A, Zagury JF, Lelievre JD, Levy Y. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog. 2011;7:e1002110. doi: 10.1371/journal.ppat.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oida T, Xu L, Weiner HL, Kitani A, Strober W. TGF-beta-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J Immunol. 2006;177:2331–2339. doi: 10.4049/jimmunol.177.4.2331. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez N, Karumuthil-Melethil S, Li R, Prabhakar BS, Holterman MJ, Vasu C. Preferential costimulation by CD80 results in IL-10-dependent TGF-beta1(+) -adaptive regulatory T cell generation. J Immunol. 2008;180:6566–6576. doi: 10.4049/jimmunol.180.10.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S, Solari N, Gualco M, Queirolo P, Moretta L, Mingari MC. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012;72:1407–1415. doi: 10.1158/0008-5472.CAN-11-2544. [DOI] [PubMed] [Google Scholar]
- Qiao G, Yang L, Li Z, Ying H, Hassen Y, Yin F, Zhang J. Program death-1 regulates peripheral T cell tolerance via an anergy-independent mechanism. Clin Immunol. 2012;143:128–133. doi: 10.1016/j.clim.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz-Perez A, Lim A, Lemercier B, Leclerc C. The T-cell receptor repertoire of tumour-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res. 2012;72:3557–3569. doi: 10.1158/0008-5472.CAN-12-0277. [DOI] [PubMed] [Google Scholar]
- Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913–920. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler PJ, Schilling B, Harasymczuk M, Hoffmann TK, Johnson J, Lang S, Whiteside TL. Phenotypic and functional characteristics of CD4+ CD39+ FOXP3+ and CD4+ CD39+ FOXP3neg T-cell subsets in cancer patients. Eur J Immunol. 2012;42:1876–1885. doi: 10.1002/eji.201142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Manzotti CN, Burke F, Dussably L, Qureshi O, Walker LS, Sansom DM. Acquisition of suppressive function by activated human CD4+ CD25- T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol. 2008;181:1683–1691. doi: 10.4049/jimmunol.181.3.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.