Abstract
Objective
This research was to investigate the role of goserelin in combination with endocrine therapy for the treatment of advanced breast cancer in premenopausal women positive for hormone receptors.
Methods
We retrospectively analyzed 40 patients as the treatment group with advanced breast cancer who, were positive for hormone receptors, received goserelin in combination with endocrine therapy and 40 patients as the control group received endocrine therapy alone, matched for age, gender, receptor status, and tumor stage.
Results
The median period of follow-up was 38.9 months. The response status at 6 months, the overall clinical benefit rate was 87.5% and 70.0% in the treatment group and control group, respectively. The mean progression-free survival (PFS) in the treatment group and control group was 27.9 and 16.9 months, respectively. The 1-, 2-, and 3-year PFS rates were 87.5%, 66.2%, and 49.7%, respectively, in the treatment group and 59.2%, 38.8%, and 35.3%, respectively, in the control group (p=0.076). The 1-, 2-, and 3-year overall survival (OS) rates were 100%, 87.2%, and 76.6%, respectively, in the treatment group and 90.0%, 74.2%, and 55.8%, respectively, in the control group (p=0.048). For the treatment group with age <40 years, PFS (p=0.036) and OS (p=0.014) were significantly longer than the control group, but it was no effect on the prognosis with the patients aged ≥40 years. Continued use of goserelin after disease progress again in the median survival time was significantly longer than nonusers (28.2 months vs. 7.0 months), and there is the potential benefit of OS (p=0.070).
Conclusions
For premenopausal hormone receptor-positive advanced breast cancer, goserelin-combined endocrine therapy can be used for those <40 years, the standard endocrine treatment for patients, we recommend continued use of goserelin for patients with disease progress again.
Key words: breast cancer, endocrine therapy, goserelin, premenopausal
Introduction
Breast cancer is the most common malignancy in women.1 With recent advancements in the comprehensive treatment of breast cancer, the mortality from breast cancer has declined.2 However, a fraction of patients still develop locoregional recurrence or distant metastasis after comprehensive therapy due to the biological behavior of breast cancer. Once recurrence or distant metastases occur, the quality of life of these patients declines significantly.3
Compared to Western countries, breast cancer largely affects younger women in China, and most of these patients are premenopausal.4 Thus, it is imperative to develop therapeutic strategies applicable to Chinese patients with breast cancer, especially younger women. The goal of advanced breast cancer treatment is to improve symptoms, delay disease progression, increase the quality of life, and prolong survival.5 Ovarian function suppression is achieved by suppressing the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone by the pituitary and inhibiting the production of estrogen. Boccardo et al. found that, for patients with metastatic breast cancer who were positive for the estrogen receptor (ER), luteinizing hormone releasing hormone (LHRH) agonists (e.g., goserelin) in combination with tamoxifen (TAM) could achieve similar outcomes to those following surgical oophorectomy or ovarian irradiation, and the objective response (OR) rate was between 45% and 46%.6 In addition, the suppression of ovarian function was reversible, and thus, patients were more likely to undergo this treatment. Related research in Chinese women is limited. In the present study, advanced breast cancer in premenopausal Chinese women positive for the hormone receptor were recruited and retrospectively analyzed to explore the therapeutic efficacy of goserelin in combination with endocrine therapy.
Materials and Methods
Patients
The study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Sun Yat-sen University Cancer Center. Written consent was given by the patients for their information to be stored in the hospital database and used for research. Premenopausal patients with advanced breast cancer who developed locoregional recurrence or distant metastasis were recruited from the Sun Yat-sen University Cancer Center from April 2002 to March 2009.
Inclusion criteria were as follows: (1) women who had unilateral breast cancer, received (modified) radical mastectomy, had a complete postoperative medical record, and underwent at least 4 courses of adjuvant chemotherapy; (2) pathological examination demonstrated locoregional recurrence, or more than 2 imaging examinations revealed distant metastasis; (3) patients were positive for ER and/or progesterone receptor; (4) detection levels of estradiol (E2), FSH, and luteinizing hormone (LH) confirmed that these patients were premenopausal; and (5) patients received regular goserelin treatment for ≥6 months. Exclusion criteria involved (1) breast cancer patients receiving initial treatment who developed distant metastasis; (2) goserelin was administered before disease progression; (3) patients had second primary cancer; and (4) patients had incomplete medical information. A total of 40 patients were enrolled as the treatment group (patients receiving goserelin and endocrine therapy). For this frequency-matched case–control study, another 40 premenopausal patients, who developed locoregional recurrence or distance metastasis, received endocrine therapy alone and were matched in age, gender, receptor status, and stage (as determined by 2002 6th edition of UICC/AJCC staging system) were selected for the control group.
Treatment
Fifty-one patients received surgical excision of the locoregional recurrence lesion, including 30 patients with isolated locoregional recurrence, and 21 patients with locoregional recurrence plus distant metastasis. The other 29 patients with distant metastasis did not receive surgery. All patients (n=80) received 4–8 courses of palliative chemotherapy, the chemotherapy regimen included mainly with capecitabine, taxol, and anthracycline. None of the Her-2-positive patients were treated with trastuzumab. Thirty patients with isolated locoregional recurrence without distant metastasis, received palliative radiotherapy to the ipsilateral chest wall and supraclavicular region, the total radiation dose was 50–60 Gy with 2 Gy delivered over 25–30 times. Patients with bone metastasis received palliative radiotherapy at metastatic sites with a total dose of 30–40 Gy.
Following chemotherapy and radiotherapy, all patients received endocrine therapy. In the treatment group, patients received a subcutaneous abdominal injection of goserelin (3.6 mg, once per 28–30 days) and TAM. The hormone level was measured monthly. When the hormone level was stable and menopause was clinically demonstrated, all patients were administered aromatase inhibitors (AI), the treatment has been recommended by the National Comprehensive Cancer Network (NCCN) breast cancer guidelines, and hormone levels were measured once every 3–6 months. In the control group, 40 patients were treated with TAM.
Follow-up
All patients received clinic visits or telephone follow-up, with the endpoints defined as disease progression (newly developed recurrence or metastatic at 3 months after treatment for initial recurrence or metastasis) and overall survival (OS). Follow-up was performed once every 1–3 months, and the response to goserelin treatment, disease progression, survival time, and current survival status were recorded. The progression-free survival (PFS) and OS were calculated since the initial diagnosis of recurrence or metastasis.
Assessment of therapeutic responses
For all patients, response status was defined as previously described for complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), OR=CR+PR, and clinical benefit (CB)=CR+PR+SD for ≥6 months.7,8 Duration of CB was calculated as the duration of the respective treatment in patients who achieved CB.
Statistical analysis
Statistical analysis was performed using SPSS version 16.0. Univariable survival analysis was done using the Kaplan–Meier method and comparisons of difference were performed with the log-rank test. A value of p<0.05 was considered statistically significant. The remaining comparisons were done using the chi square test.
Results
Patients and tumor characteristics
General demographic features and disease recurrence patterns of all patients are summarized in Table 1. The median age was 39 years (range, 27–50 years) in the treatment group and 39 years (range, 27–48 years) in the control group. In addition, 30 patients were diagnosed with isolated locoregional recurrence (n=15 per group) and 50 with distant metastasis with and without locoregional recurrence (n=25 per group).
Table 1.
The Clinical Characteristic of 80 Patients with Progressive Disease
| Characteristic | Treatment group (n) | Control group (n) | p |
|---|---|---|---|
| Age (y) | |||
| <40 | 20 | 20 | – |
| ≥40 | 20 | 20 | |
| Pathology type | |||
| Invasive ductal carcinoma | 39 | 37 | 0.304 |
| Mucinous adenocarcinoma | 1 | 3 | |
| ER/PR status | |||
| ER(+) and PR(+) | 37 | 35 | 0.456 |
| ER(+) and/or PR(+) | 3 | 5 | |
| Her-2 | |||
| Positive | 10 | 12 | 0.617 |
| Negative | 30 | 28 | |
| Site of disease progression | |||
| Chest wall | 6 | 5 | – |
| Regional lymph nodes | 8 | 9 | |
| Chest wall and regional lymph nodes | 1 | 1 | |
| Bone | 10 | 9 | |
| Liver | 2 | 1 | |
| Lung | 3 | 4 | |
| Chest wall and bone | 3 | 4 | |
| Regional lymph nodes and bone | 6 | 6 | |
| Chest wall, regional lymph nodes, and bone | 1 | 1 | |
ER, estrogen receptor; PR, progesterone receptor; Her-2, human epidermal growth factor receptor 2.
Therapeutic efficacy
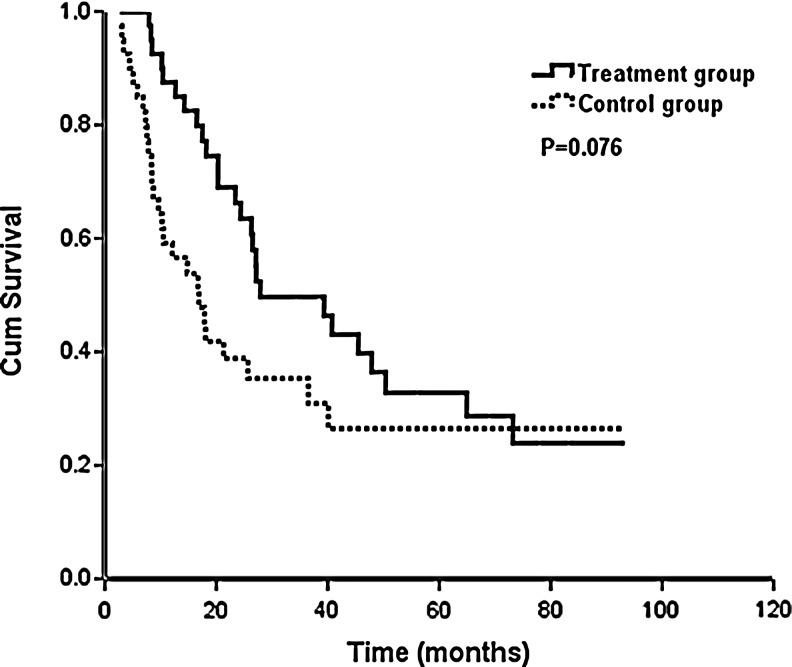
The median period of follow-up was 38.9 months (range, 6.7–105.4 months) among all patients. In the treatment group, the response status at 6 months of the 40 patients, 9 patients (22.5%) achieved CR, 13 patients (32.5%) experienced a PR, for an OR rate of 55.0%. An additional 13 patients (32.5%) experienced SD 6 months or longer, giving an overall clinical benefit rate of (87.5%) (Table 2). Twenty-six patients (65.0%) developed disease progression in the follow-up time, and the 1-year, 2-year, and 3-year PFS rates were 87.5%, 66.2%, and 49.7%, respectively. The median PFS was 27.9 months. In the control group, the response status at 6 months of the 40 patients, 5 patients (12.5%) achieved CR, 11 patients (27.5%) experienced a PR, for an OR rate of 40.0%. An additional 12 patients (30.0%) experienced SD 6 months or longer, giving an overall CB rate of (70.0%) (Table 2). Twenty-six patients (65.0%) showed disease progression in the follow-up time, and the 1-year, 2-year, and 3-year PFS rates were 59.2%, 38.8%, and 35.3%, respectively (p=0.076), and the median PFS was 16.9 months (Fig. 1).
Table 2.
Response Status at 6 Months on Treatment Group and Control Group
| Parameter | Treatment group (%) | Control group (%) |
|---|---|---|
| CR | 9(22.5) | 5(12.5) |
| PR | 13(32.5) | 11(27.5) |
| SD | 13(32.5) | 12(30.0) |
| CB(CR+PR+SD) | 35(87.5) | 28(70.0) |
| PD | 5(12.5) | 12(30.0) |
CR, complete response; PR, partial response, SD, stable disease; CB, clinical benefit; PD, progressive disease.
FIG. 1.
Impact of goserelin (n=40 with the treatment group and n=40 with the control group) on progression-free survival.
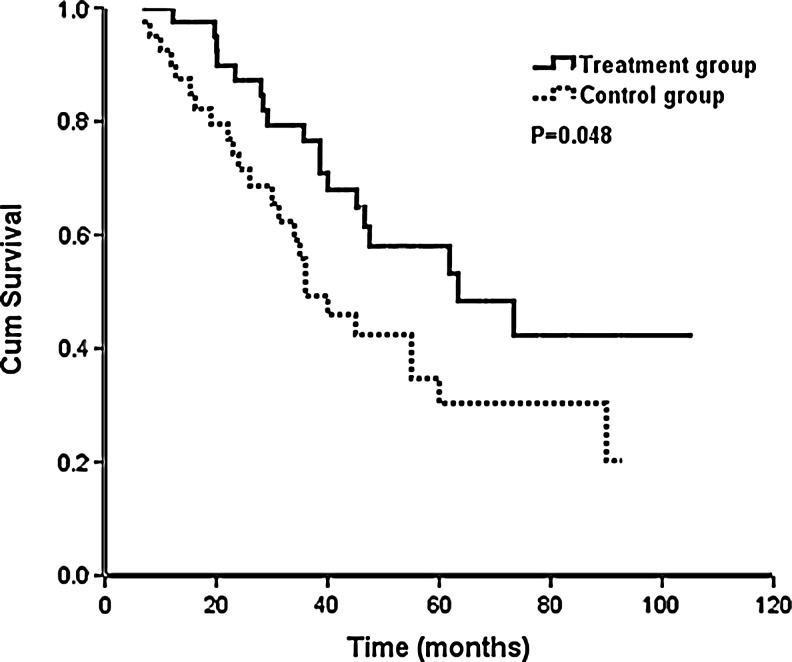
In the treatment group, 18 patients died, and the 1-year, 2-year, and 3-year OS was 100%, 87.2%, and 76.6%, respectively. The median survival time was 64.0 months. In the control group, 24 patients died, and the 1-year, 2-year, and 3-year OS were 90.0%, 74.2%, and 55.8%, respectively (p=0.048). The median survival time was 36.0 months (Fig. 2).
FIG. 2.
Impact of goserelin (n=40 with the treatment group and n=40 with the control group) on overall survival.
Correlation between age and prognosis
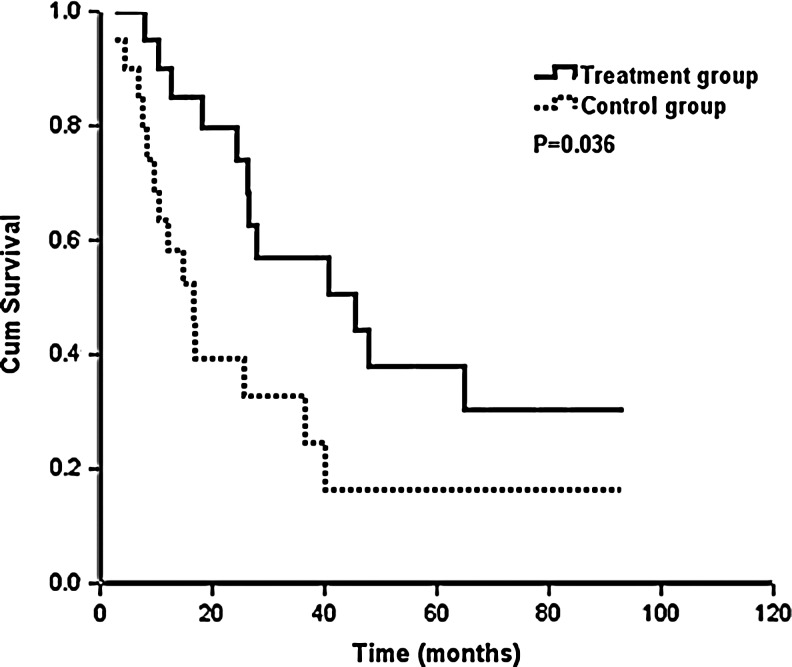
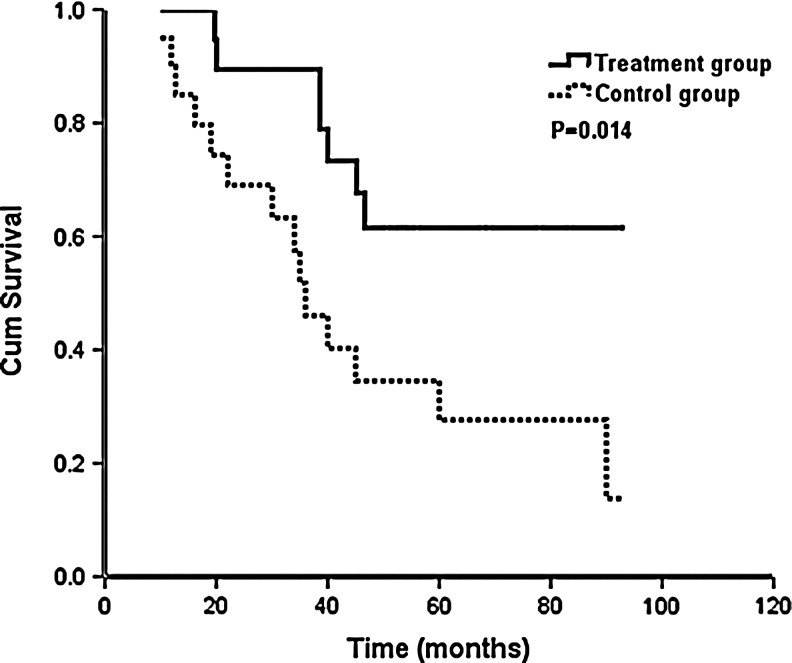
For patients aged <40 years, PFS in the treatment group was statistically significantly longer than that in the control group (median PFS, 45.5 vs. 16.7 months, p=0.036) (Fig. 3). OS was also statistically significantly increased (median survival time was not achieved in the treatment group and median survival time was 36.0 months in the control group, p=0.014) (Fig. 4). For patients aged ≥40, there were no significant differences in PFS and OS in the treatment group versus control group, but PFS and OS in the treatment group were longer compared to those in the control group (median PFS: 27.1 vs. 17.9 months, p=0.635; median OS: 61.9 vs. 55.0 months, p=0.702) (Table 3).
FIG. 3.
Impact of goserelin (n=20 with the treatment group and n=20 with the control group) on progression-free survival with age <40 years.
FIG. 4.
Impact of goserelin (n=20 with the treatment group and n=20 with the control group) on overall survival with age <40 years.
Table 3.
Impact of Age on the Effect of Goserelin
| |
|
PFS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | 1-year (%) | 2-year (%) | 3-year (%) | p | 1-year (%) | 2-year (%) | 3-year (%) | p |
| <40 years | |||||||||
| Treatment group | 20 | 90.0 | 79.9 | 56.9 | 0.036 | 100 | 94.7 | 89.5 | 0.014 |
| Control group | 20 | 63.5 | 39.3 | 32.8 | 90.0 | 69.1 | 51.8 | ||
| ≥40 years | |||||||||
| Treatment group | 20 | 85.0 | 53.3 | 42.7 | 0.635 | 100 | 85.0 | 68.7 | 0.702 |
| Control group | 20 | 55.0 | 38.5 | 38.5 | 90.0 | 74.1 | 60.6 | ||
PFS, progression-free survival; OS, overall survival.
Efficacy of further goserelin treatment
In the treatment group, of the 26 patients with disease recurrence, 5 patients with locoregional recurrence developed distant metastasis (1 patient received continuous goserelin treatment) and 21 patients again developed distant metastasis (6 patients received continuous goserelin treatment). For patients continuously receiving goserelin treatment after disease progression, there was no significant difference in OS between groups, but patients potentially benefited from continuous therapy (p=0.070) and the median survival time was also prolonged (28.2 vs. 7.0 months).
Post-treatment menstrual cycle
In the treatment group, goserelin was administered for 6–86 months and the median duration was 31 months. At the end of follow-up, there were 20 patients still receiving goserelin treatment. The majority of patients (n=37, 92.5%) achieved menopause at 1–2 months after goserelin treatment, and 2 patients developed menopause at 3–4 months after goserelin treatment. The major adverse effects included fever (n=25, 62.5%), bone and joint discomfort (n=27, 67.5%), anxiety and irritability (n=9, 22.5%), and insomnia (n=2, 5.0%).
During the follow-up period, 20 patients in the treatment group discontinued treatment due to disease progression or completion of the treatment course. Sixteen of these 20 patients recovered their menstrual cycle (80.0%) and the remaining 4 developed amenorrhea (20.0%). Among patients aged <40 years in the control group (n=20), 15 patients (75.0%) recovered their menstrual cycle and 5 developed amenorrhea (25.0%). In the control group, among the patients aged ≥40 years (n=20), 3 (15.0%) recovered their menstrual cycle and 17 patients developed amenorrhea (85.0%).
Discussion
Within the limitations of the present study, not being a randomized study and with small number of subjects, goserelin in combination with endocrine therapy appears to be an effective therapy for premenopausal Chinese women with hormone receptor-positive advanced breast cancer.
For premenopausal patients with hormone receptor-positive advanced breast cancer, the estradiol level may increase significantly when endocrine therapy is performed alone, but the hormone level may be maintained at the menopausal level when endocrine therapy is given in combination with ovarian function suppression.9 Thus, theoretically, we can postulate that endocrine therapy and pharmacological castration of the ovary may exert a synergistic effect. Klijn et al. proposed that, for premenopausal patients with advanced breast cancer and positive ER status, the clinical response rate was as high as 33% following LHRH agonists, but that rate could be increased to 42% after LHRH agonists and TAM treatment.10 Meta-analysis showed that, for premenopausal patients with advanced breast cancer, the overall effective rate (39% vs. 30%), median PFS (8.7 vs. 5.4 months), and median OS (2.9 vs. 2.5 years) after treatment with goserelin and TAM were superior to those treated with goserelin alone.11 In the present study, systemic chemotherapy comprising goserelin in combination with endocrine therapy was found to increase PFS (borderline significant, p=0.076) and OS (p=0.048).
Young age is an important factor in the progression of breast cancer.12,13 Based on the hormone-dependent characteristics of breast cancer, the optimum method to block hormone secretion is to promote an artificial menopausal status in patients. For patients younger than 40 years, ovarian function is still active after chemotherapy in 50% patients, which may lead to cancer growth in the presence of estrogen stimulation resulting in recurrence or distant metastasis, 80% developed menopause after chemotherapy for patients ages ≥40 years.14,15 Therefore, inhibition of ovarian function may have limited benefit for ages ≥40 years. Two large randomized studies have confirmed that patients aged <40 years could benefit from ovarian function suppression to reduce the incidence of disease recurrence when compared with those aged ≥40 years.16,17 To date, the studies investigating the role of age in the therapeutic efficacy of ovarian function suppression in premenopausal patients with advanced breast cancer are limited.
Our findings suggest that, in premenopausal patients with breast cancer, age is still an important factor affecting the therapeutic efficacy of ovarian function suppression. NCCN guidelines for breast cancer have proposed that ovarian function suppression in combination with endocrine therapy is recommended for patients with advanced breast cancer and positive hormone receptor status, but the optimum age of these patients was not addressed. On the basis of our findings, the optimum age for premenopausal patients with advanced breast cancer and positive hormone receptor status may be under 40 years of age, but randomized, controlled studies are required to confirm our findings.
LHRH agonists can change patients to a menopausal status, allowing for the application of AI. The median time to achieve benefit (12.1 vs. 8.3 months, p=0.0506) and median survival time (18.9 vs. 14.3 months, p=0.0001) following treatment with goserelin and anastrozole were superior to those following treatment with goserelin and TAM.18 Yao et al. found that the CB rate was 68.8% following treatment with goserelin and anastrozole.19 Carlson et al. also showed that the CB rate was as high as 71.9% after treatment with goserelin and anastrozole.20 These findings are consistent with our results, which may be attributed to the fact that anastrozole further decreases estrogen levels and reverses the reduction of FSH following treatment with goserelin+TAM.21
In the present study, the main side effects were acceptable. Severe side effects were not observed. This indicates that goserelin, in combination with AI, is safe. Our results also showed that, for patients developing disease progression following goserelin in combination with endocrine therapy, continuous treatment with goserelin can potentially benefit patients although there was no significant difference in the OS. Few studies have reported continuous treatment following treatment failure with goserelin and AI. Forward et al. proposed that the CB rate of goserelin+anastrozole treatment was as high as 65% after patients failed to respond to goserelin+TAM treatment, and the median time to response was 17 months, but the benefit was less than that found with initial treatment.21 The above findings together with our results indicate that patients may benefit from continuous inhibition of ovarian function after disease progression.
In summary, we show that the hormone combination of goserelin and endocrine therapy is effective in premenopausal Chinese women with hormone receptor-positive advanced breast cancer in patients <40 years, we recommend continued use of goserelin for patients with disease progression. Further study and expanded use of this combination in current practice are warranted.
Acknowledgments
This study was supported by a grant from the Sci-Tech Office of Guangdong Province (No. 2008B060 600019) and the Youth Foundation of the First Affiliated Hospital of Xiamen University (No. XYY2012005).
Disclosure Statement
No actual or potential conflicts of interest existed.
References
- 1.DeSantis C. Siegel R. Bandi P, et al. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A. Siegel R. Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Oh S. Heflin L. Meyerowitz BE, et al. Quality of life of breast cancer survivors after a recurrence: a follow-up study. Breast Cancer Res Treat. 2004;87:45. doi: 10.1023/B:BREA.0000041580.55817.5a. [DOI] [PubMed] [Google Scholar]
- 4.Li J. Zhang BN. Fan JH, et al. A nation-wide multicenter 10-year (1999–2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011;11:364. doi: 10.1186/1471-2407-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleator SJ. Ahamed E. Coombes RC, et al. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2009;9(suppl1):S6–S17. doi: 10.3816/CBC.2009.s.001. [DOI] [PubMed] [Google Scholar]
- 6.Boccardo F. Rubagotti A. Perrotta A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-/perimenopausal patients with advanced breast cancer: Results of a multicentric Italian study. Ann Oncol. 1994;5:337. doi: 10.1093/oxfordjournals.annonc.a058837. [DOI] [PubMed] [Google Scholar]
- 7.Hayward J. Carbone P. Heuson J, et al. Assessment of response to therapy in advanced breast cancer. Cancer. 1977;39:1289. doi: 10.1002/1097-0142(197703)39:3<1289::aid-cncr2820390340>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.British Breast Group. Assessment of response to treatment in advanced breast cancer. Lancet. 1974;2:38. [PubMed] [Google Scholar]
- 9.Klijn JG. Beex LV. Mauriac L, et al. Combined treatment with buserelin and tamoxifen in premenopausal metastatic breast cancer: A randomized study. J Natl Cancer Inst. 2000;92:903. doi: 10.1093/jnci/92.11.903. [DOI] [PubMed] [Google Scholar]
- 10.Klijn JG. Blamey RW. Boccardo F, et al. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: A meta-analysis of four randomized trials. J Clin Oncol. 2001;19:343. doi: 10.1200/JCO.2001.19.2.343. [DOI] [PubMed] [Google Scholar]
- 11.Robertson JF. Blamey RW. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur J Cancer. 2003;39:861. doi: 10.1016/s0959-8049(02)00810-9. [DOI] [PubMed] [Google Scholar]
- 12.El Saghir NS. Seoud M. Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foo CS. Su D. Chong CK, et al. Breast cancer in young Asian women: Study on survival. ANZ J Surg. 2005;75:566. doi: 10.1111/j.1445-2197.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- 14.Goldhirsch A. Gelber RD. Castiglione M. The magnitude of endocrine effect of adjuvant chemotherapy for premenopausal breast cancer patients. Ann Oncol. 1990;1:183. doi: 10.1093/oxfordjournals.annonc.a057718. [DOI] [PubMed] [Google Scholar]
- 15.Tancini G. Valagussa P. Bajetta E, et al. Preliminary 3-year results of 12 versus 6 cycles of surgical adjuvant CMF in premenopausal breast cancer. Cancer Clin Trials. 1979;2:285. [PubMed] [Google Scholar]
- 16.Bernhard J. Zahrieh D. Castiglione-Gertsch M, et al. Adjuvant chemotherapy followed by goserelin compared with either modality alone: The impact on amenorrhea, hot flashes, and quality of life in premenopausal patients-the International Breast Cancer Study Group Trial VIII. J Clin Oncol. 2007;25:263. doi: 10.1200/JCO.2005.04.5393. [DOI] [PubMed] [Google Scholar]
- 17.The Adjuvant Breast Cancer Trials Collaborative Group. Ovarian ablation or suppression in premenopausal early breast cancer: Results from the international adjuvant breast cancer ovarian ablation or suppression randomized trial. J Natl Cancer Inst. 2007;99:516. doi: 10.1093/jnci/djk109. [DOI] [PubMed] [Google Scholar]
- 18.Milla-Santos A. Milla L. Portella J, et al. A randomized trial of goserelin+tamoxifenversus goserelin+anastrozole in pre/perimenopausal patients with hormone dependent advanced breast cancer. Breast Cancer Res Treat. 2002;76(suppl1):S32. [Google Scholar]
- 19.Yao SY. Xu BH. Li Q, et al. Goserelin plus anastrozole in the treatment of premenopausal patients with metastatic breast cancer. Zhonghua Yi Xue Za Zhi. 2010;90:526. [PubMed] [Google Scholar]
- 20.Carlson RW. Theriault R. Schurman CM, et al. Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor-positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol. 2010;28:3917. doi: 10.1200/JCO.2009.24.9565. [DOI] [PubMed] [Google Scholar]
- 21.Forward DP. Cheung KL. Jackson L, et al. Clinical and endocrine data for goserelin plus anastrozole as second-line endocrine therapy for premenopausal advanced breast cancer. Br J Cancer. 2004;90:590. doi: 10.1038/sj.bjc.6601557. [DOI] [PMC free article] [PubMed] [Google Scholar]