Abstract
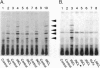
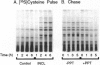
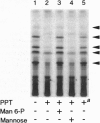
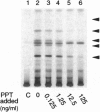
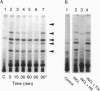
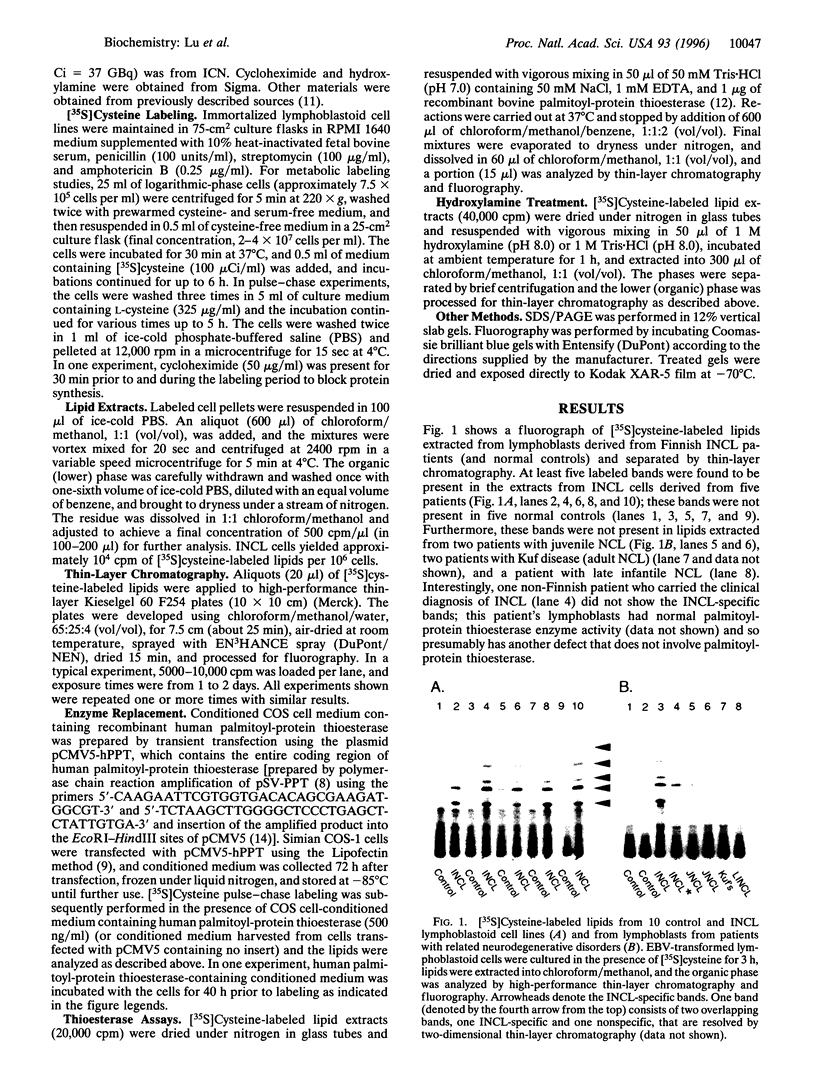
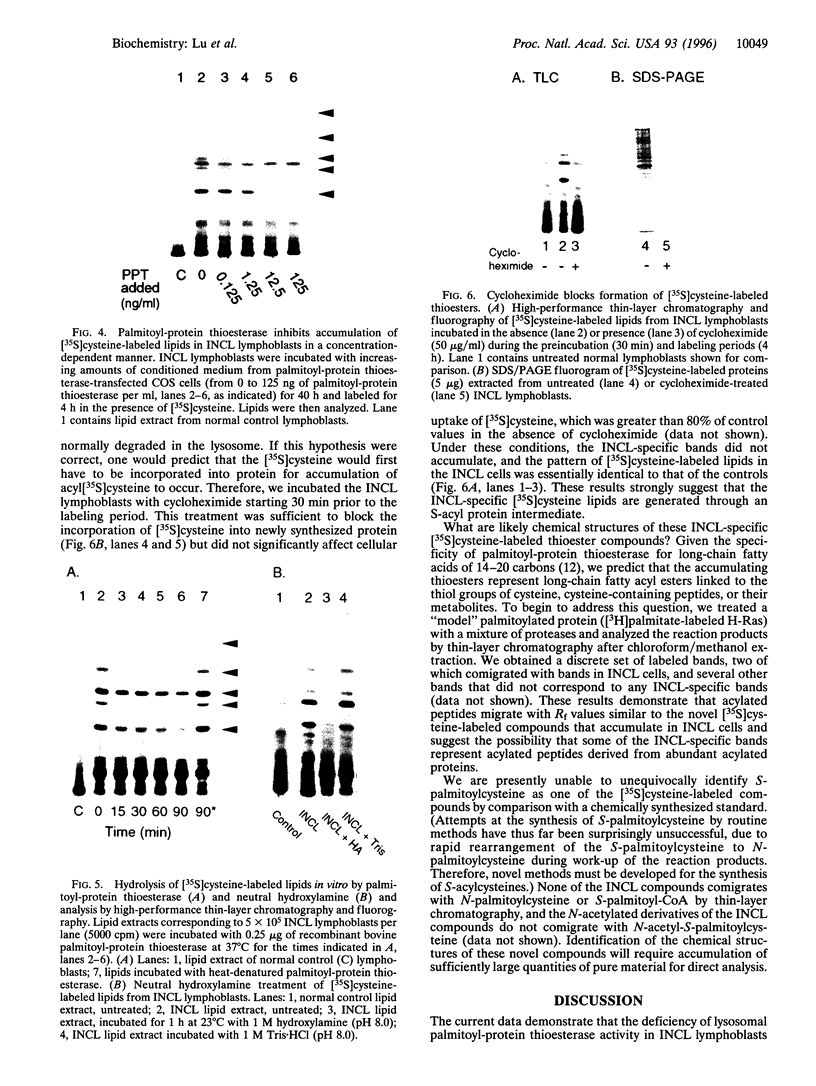
Palmitoyl-protein thioesterase is a lysosomal long-chain fatty acyl hydrolase that removes fatty acyl groups from modified cysteine residues in proteins. Mutations in palmitoyl-protein thioesterase were recently found to cause the neurodegenerative disorder infantile neuronal ceroid lipofuscinosis, a disease characterized by accumulation of amorphous granular deposits in cortical neurons, leading to blindness, seizures, and brain death by the age of three. In the current study, we demonstrate that [35S]cysteine-labeled lipid thioesters accumulate in immortalized lymphoblasts of patients with infantile neuronal ceroid lipofuscinosis. The accumulation in cultured cells is reversed by the addition of recombinant palmitoyl-protein thioesterase that is competent for lysosomal uptake through the mannose-6-phosphate receptor. The [35S]cysteine-labeled lipids are substrates for palmitoyl-protein thioesterase in vitro, and their formation requires prior protein synthesis. These data support a role for palmitoyl-protein thioesterase in the lysosomal degradation of S-acylated proteins and define a major new pathway for the catabolism of acylated proteins in the lysosome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Bolanowski M. A., Earles B. J., Lennarz W. J. Fatty acylation of proteins during development of sea urchin embryos. J Biol Chem. 1984 Apr 25;259(8):4934–4940. [PubMed] [Google Scholar]
- Camp L. A., Hofmann S. L. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993 Oct 25;268(30):22566–22574. [PubMed] [Google Scholar]
- Camp L. A., Verkruyse L. A., Afendis S. J., Slaughter C. A., Hofmann S. L. Molecular cloning and expression of palmitoyl-protein thioesterase. J Biol Chem. 1994 Sep 16;269(37):23212–23219. [PubMed] [Google Scholar]
- DeArmond S. J., Prusiner S. B. Prion protein transgenes and the neuropathology in prion diseases. Brain Pathol. 1995 Jan;5(1):77–89. doi: 10.1111/j.1750-3639.1995.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Haas C., Hung A. Y., Citron M., Teplow D. B., Selkoe D. J. beta-Amyloid, protein processing and Alzheimer's disease. Arzneimittelforschung. 1995 Mar;45(3A):398–402. [PubMed] [Google Scholar]
- Hellsten E., Vesa J., Speer M. C., Mäkelä T. P., Järvelä I., Alitalo K., Ott J., Peltonen L. Refined assignment of the infantile neuronal ceroid lipofuscinosis (INCL, CLN1) locus at 1p32: incorporation of linkage disequilibrium in multipoint analysis. Genomics. 1993 Jun;16(3):720–725. doi: 10.1006/geno.1993.1253. [DOI] [PubMed] [Google Scholar]
- Ivy G. O. Protease inhibitors as a model for NCL disease, with special emphasis on the infantile and adult forms. Am J Med Genet. 1992 Feb 15;42(4):555–560. doi: 10.1002/ajmg.1320420427. [DOI] [PubMed] [Google Scholar]
- Price D. L., Borchelt D. R., Sisodia S. S. Alzheimer disease and the prion disorders amyloid beta-protein and prion protein amyloidoses. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6381–6384. doi: 10.1073/pnas.90.14.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider J. A., Dawson G., Siakotos A. N. Perspective of biochemical research in the neuronal ceroid-lipofuscinosis. Am J Med Genet. 1992 Feb 15;42(4):519–524. doi: 10.1002/ajmg.1320420419. [DOI] [PubMed] [Google Scholar]
- Rider J. A., Rider D. L. Batten disease: past, present, and future. Am J Med Genet Suppl. 1988;5:21–26. doi: 10.1002/ajmg.1320310606. [DOI] [PubMed] [Google Scholar]
- Santavuori P., Haltia M., Rapola J. Infantile type of so-called neuronal ceroid-lipofuscinosis. Dev Med Child Neurol. 1974 Oct;16(5):644–653. doi: 10.1111/j.1469-8749.1974.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Sleat D. E., Sohar I., Lackland H., Majercak J., Lobel P. Rat brain contains high levels of mannose-6-phosphorylated glycoproteins including lysosomal enzymes and palmitoyl-protein thioesterase, an enzyme implicated in infantile neuronal lipofuscinosis. J Biol Chem. 1996 Aug 9;271(32):19191–19198. doi: 10.1074/jbc.271.32.19191. [DOI] [PubMed] [Google Scholar]
- Tayama M., O'Brien J. S., Kishimoto Y. Distribution of saposins (sphingolipid activator proteins) in tissues of lysosomal storage disease patients. J Mol Neurosci. 1992;3(4):171–175. doi: 10.1007/BF03380135. [DOI] [PubMed] [Google Scholar]
- Tyynelä J., Palmer D. N., Baumann M., Haltia M. Storage of saposins A and D in infantile neuronal ceroid-lipofuscinosis. FEBS Lett. 1993 Sep 6;330(1):8–12. doi: 10.1016/0014-5793(93)80908-d. [DOI] [PubMed] [Google Scholar]
- Verkruyse L. A., Hofmann S. L. Lysosomal targeting of palmitoyl-protein thioesterase. J Biol Chem. 1996 Jun 28;271(26):15831–15836. doi: 10.1074/jbc.271.26.15831. [DOI] [PubMed] [Google Scholar]
- Vesa J., Hellsten E., Verkruyse L. A., Camp L. A., Rapola J., Santavuori P., Hofmann S. L., Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995 Aug 17;376(6541):584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]