Abstract
The objective of this study was to evaluate the clinical value of combination of diagnostic computed tomography (CT) and somatostatin receptor imaging with 99mTc-octreotide acetate SPECT/CT in differentiation of benign pulmonary nodules from cancers.
Methods
This was a retrospective study, 29 patients with suspected pulmonary neoplasm underwent diagnostic CT and 99mTc-octreotide SPECT/CT scans, and the tumor-to-normal tissue tracer value (T/N) for 99mTc-octreotide was measured. Diagnosis was confirmed by histological analysis.
Results
Eighteen of the 29 patients included in this study had lung cancer: 2 with small cell lung cancer and 16 with nonsmall cell lung cancer. The other 11 patients had benign lung lesions: 5 with tuberculosis, 4 with nontuberculosis infection, 1 with hematoma, and 1 with fibroma. 99mTc-octreotide uptake (expressed as mean T/N±SD) was significantly higher in lung cancers (2.58±0.91) than benign lesions (1.38±0.79) (p=0.002). Specificity for pulmonary malignant nodule diagnosis was 63.6% for diagnostic CT, 72.7% for somatostatin receptor SPECT/CT imaging, and 81.8% for the combined use of diagnostic CT and somatostatin receptor SPECT/CT imaging.
Conclusion
Somatostatin receptor imaging with 99mTc-octreotide SPECT/CT is useful for the differentiation of benign pulmonary nodules from lung cancers, the combination of diagnostic CT and 99mTc-octreotide SPECT/CT further increases the specificity of malignant pulmonary nodule detection.
Key words: computed tomography, lung cancer, pulmonary nodules, somatostatin receptor, SPECT/CT
Introduction
Lung cancer is the leading cause of cancer death worldwide. It is estimated that 226,160 patients will be diagnosed with, and 160,340 patients will die of cancer of the lung and bronchus in US in 2012, the overall 5-year relative survival was 15.9%.1 A solitary pulmonary nodule is defined as “a round opacity, at least moderately well-marginated and no greater than 3 cm in maximum diameter.”2 The 5-year survival rate of patients after surgical removal of earlier stage primary lung cancer, without regional lymph nodes spreading and distal metastases, was reported more than 50%, however, more than 50% resected pulmonary nodules were benign.3,4 Therefore, early detection and differentiation of malignant solitary pulmonary nodule is important for lung cancer therapy, which may avoid unnecessary surgical procedure for patients with benign nodules.
Noninvasive diagnostic modalities currently used in evaluating lung tumors such as chest X-ray, computed tomography (CT), and sputum cytology, have a high percentage of indeterminate diagnosis.5,6 Although CT plays a critical role on the differentiating diagnosis of pulmonary nodules, it provides only morphologic information and reflects little biology of pulmonary nodules. 18F-FDG PET/CT have been widely used in the detection of lung cancers for diagnosis and anticancer management after staging,7–12 however, infection and inflammation lung diseases have been reported 18F-FDG uptake, 18F-FDG PET/CT may also result indeterminate diagnosis of lung nodules.13,14
The affinity of receptor analogs such as somatostatin has been demonstrated overexpressing in some malignant lung tumors.15–20 It has been reported that somatostatin receptor imaging has a high value in differentiating the property of pulmonary nodules with equal sensitivity and specificity to 18F-FDG PET.19–21 Somatostatin receptor imaging is beneficial to both small cell lung cancer and nonsmall cell lung cancer; this is a specific receptor imaging and has considerable diagnostic values in the group of patients with suspected lung cancers by diagnostic CT.22 The objective of this study was to test the hypothesis that, the combination of somatostatin receptor imaging with 99mTc-octreotide acetate and diagnostic CT is able to further improve lung cancer diagnosis noninvasively.
Materials and Methods
Patients
The Institutional Review Board of the Nanjing Medical University and the local ethics committee approved this investigation. Written consent was obtained from all patients with indeterminate pulmonary nodules. This is a retrospective study, 29 consecutive cases of pulmonary nodules (19 men, 10 women; 63±10 years [mean±SD]) were enrolled. Diagnostic CT and somatostatin receptor imaging with 99mTc-octreotide acetate performed within a week in patients with solitary nodules were included. The final diagnosis was confirmed by surgically removed tissue or transthoracic needle biopsy histopathology.
Diagnostic CT study
A Siemens sensation 16-slice CT scanner (Germany) was used with fixed scanning protocol (collimation 16×0.75 mm, 120 kV, 180 mA). Omnipaque was used for enhanced imaging; the contrast agent (1.5 mL/kg body weight) was injected at 3 mL/s through the elbow vein.
99mTc-octreotide SPECT/CT study
Octreotide (10 mg, sandostatin; Novartis Pharma Schweiz AG) was labeled with 99mTc-pertechnetate. Radiochemical purity was great than 95% for each study. As described previously,20 99mTc-octreotide [1110 MBq (30mCi)] was injected intravenously to each patient without fasting. The imaging apparatus was a dual-head detector SPECT/CT (Millennium VG, Hawkeye; GE Healthcare) equipped with low-energy and high-resolution collimation, the peak energy is 140 keV, window width was set as 20%. Planar images were collected at 1 and 4 hours, SPECT/CT images of the chest were obtained followed 4 hours planar scan. SPECT data were acquired in a 128×128 matrix through a 360° rotation with 64 projections. The acquisition time for each projection was 40 seconds. Transverse slices were reconstructed with ordered subset expectation maximization iterative algorithm and formatted as a 128×128 matrix. SPECT image attenuation correction was performed. Regions of interest were drawn over lung lesions and the contralateral normal lung tissue based on CT imaging of SPECT/CT; tumor uptake of 99mTc-octreotide activity was expressed as tumor-to-normal tissue activity ratios (T/N).
Imaging analysis
Diagnostic CT images were read by 2 radiologists who were blinded to pathologic diagnosis. The diagnosis was made according as internal and margin characteristics of lesions. Somatostatin receptor 99mTc-octreotide SPECT/CT imaging was evaluated by two nuclear medicine physicians blinded to pathologic findings. In case of disagreement, a third radiologist/nuclear medicine physician made a diagnostic conclusion.
Statistical analysis
Data were presented as the mean±SD. The sensitivity, specificity, positive predictive value, and negative predictive value were estimated and compared between these two modalities using SPSS software (version 15.0). The Student's t-test was used to assess statistical difference. A p-value less than 0.05 was considered statistically significant.
Results
The patients' information is summarized in Table 1, of 29 lung nodules, 18 were identified as lung cancers by histological findings: nonsmall cell lung cancer in 16 and small cell lung cancer in 2. Eleven lung nodules were benign: 5 tuberculosis, 4 inflammatory pseudotumors, 1 hematoma, and 1 fibroma.
Table 1.
Clinical and Histological Information of Patients Included in the Study
| Patient no. | Sex | Age (years) | Location | Size in diameter (cm) | Histology |
|---|---|---|---|---|---|
| 1 | F | 74 | Right upper | 3.0 | Squamous cell carcinoma |
| 2 | M | 72 | Left lower | 3.9 | Squamous cell carcinoma |
| 3 | M | 47 | Left upper | 3.5 | Squamous cell carcinoma |
| 4 | M | 70 | Left lower | 3.4 | Squamous cell carcinoma |
| 5 | F | 60 | Right upper | 2.7 | Squamous cell carcinoma |
| 6 | M | 71 | Left upper | 2.9 | Squamous cell carcinoma |
| 7 | F | 70 | Left upper | 4.0 | Squamous cell carcinoma |
| 8 | M | 42 | Right upper | 2.3 | Squamous cell carcinoma |
| 9 | M | 63 | Right upper | 0.8 | Squamous cell carcinoma |
| 10 | F | 63 | Left upper | 2.0 | Adenocarcinoma |
| 11 | M | 76 | Left upper | 3.2 | Adenocarcinoma |
| 12 | M | 73 | Left lower | 3.1 | Adenocarcinoma |
| 13 | F | 71 | Right lower | 3.6 | Adenocarcinoma |
| 14 | F | 64 | Right upper | 1.7 | Adenocarcinoma |
| 15 | M | 51 | Right lower | 2.0 | Adenocarcinoma |
| 16 | M | 68 | Right lower | 1.9 | Small cell lung carcinoma |
| 17 | F | 79 | Right upper | 3.8 | Small cell lung carcinoma |
| 18 | F | 35 | Right lower | 4.0 | Adenocarcinoma |
| 19 | M | 68 | Right lower | 3.2 | Tuberculosis |
| 20 | M | 47 | Left upper | 4.0 | Tuberculosis |
| 21 | M | 55 | Right upper | 2.1 | Tuberculosis |
| 22 | M | 70 | Right upper | 2.6 | Tuberculosis |
| 23 | F | 62 | Left lower | 2.2 | Tuberculosis |
| 24 | M | 71 | Right upper | 2.6 | Granuloma |
| 25 | M | 62 | Left upper | 1.0 | Granuloma |
| 26 | M | 65 | Right lower | 2.2 | Granuloma |
| 27 | F | 69 | Right lower | 2.8 | Granuloma |
| 28 | M | 62 | Left upper | 1.6 | Hamartoma |
| 29 | M | 70 | Right upper | 2.1 | Fibroma |
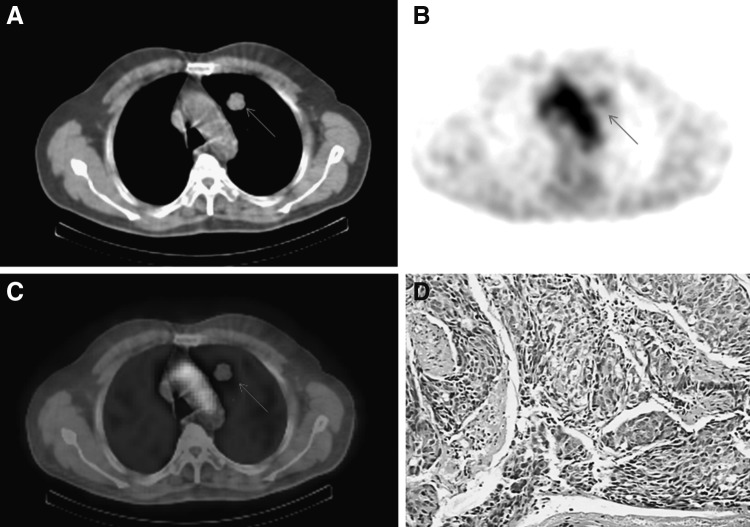
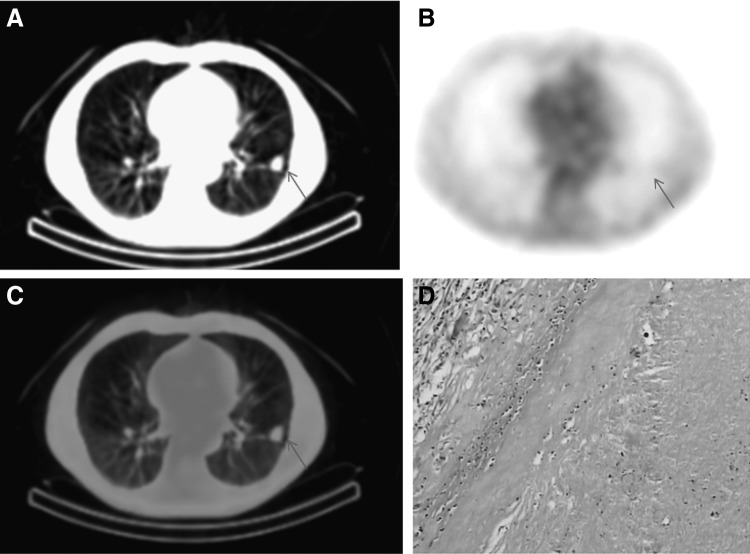
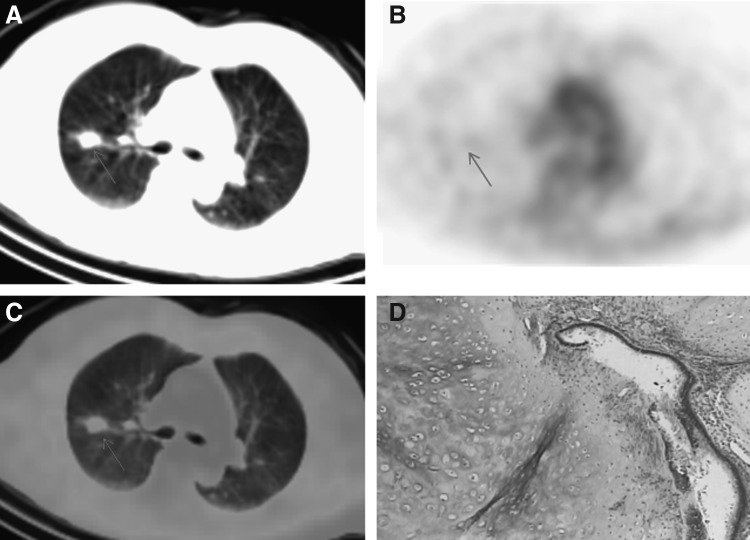
Four hours after injection of 99mTc-octreotide, the maximal radioactivity accumulation in lung nodules was observed; SPECT/CT scans were performed at 4-hour time point. Lung cancer had a higher 99mTc-octreotide accumulation, a representative SPECT/CT imaging showed intense 99mTc-octreotide uptake in lung cancer of a 76-year-old male (Fig. 1). Lung nodules with pathological confirmations of infection or inflammation generally had low 99mTc-octreotide accumulation, Figure 2 showed low 99mTc-octreotide uptake in a tuberculosis lesion. Benign tumors included in this study had very low 99mTc-octreotide accumulation, 99mTc-octreotide SPECT/CT imaging of a patient with hamartoma is presented in Figure 3. 99mTc-octreotide uptake was significantly higher in malignant lung nodules (2.58±0.91) than benign lesions (1.38±0.79) (p=0.002) (Table 2).
FIG. 1.
A 76-year-old male patient with adenocarcinoma lung cancer in left upper lobe. (A) CT scans showed abnormality in left upper lobe; (B) 99mTc-octreotide images showed intense uptake in the lung lesion (arrow); (C) the overlay of (A) and (B); (D) adenocarcinoma was verified on histology. CT, computed tomography.
FIG. 2.
A 63-year-old male patient with tuberculosis, (A) CT scans showed abnormality in the right lower lobe. (B) 99mTc-octreotide images were negative in the lung lesion (arrow). (C) Overlay of (A) and (B); (D) tuberculosis was verified on histology.
FIG. 3.
A 62-year-old female patient with hamartoma. (A) CT scans showed neoplasm of the upper lobe of the right lung. (B) 99mTc-octreotide images had little uptake in the lesion. (C) Overlay of (A) and (B). (D) Hamartoma was verified on histology.
Table 2.
99mTc-octreotide Uptake in Benign and Malignant Pulmonary Nodules
| Parameter | Malignant | Benign | p-Value |
|---|---|---|---|
| Age (years) | 63.83±12.37 | 63.72±7.38 | 0.98 |
| Diameter (cm) | 2.88±0.92 | 2.40±0.79 | 0.17 |
| T/N | 2.58±0.91 | 1.38±0.79 | 0.0002 |
T/N, tumor-to-normal tissue tracer value.
The specificity for pulmonary malignant nodule diagnosis was 63.6% for diagnostic CT, 72.7% for somatostatin receptor imaging, and 81.8% for combined use of diagnostic CT and somatostatin receptor imaging.
Discussion
Overexpressing somatostatin receptor in many malignant tumors is the fundamental of somatostatin receptor nuclear imaging for differentiating malignant tissues from benign diseases.23 Studies have confirmed the presence of somatostatin receptors in nonsmall cell lung cancer and their cell lines.16,24 It has demonstrated the usefulness of somatostatin analog scintigraphy on detection of solitary pulmonary nodules (discussed in ref. Wang et al.20). In this study, overexpression of somatostatin receptor, we have observed that 99mTc-octreotide uptake in malignancy is significantly higher than benign tumors and infectious diseases (Figs. 1–3 and Table 2); therefore, somatostatin receptor imaging has advantages than diagnostic CT on pulmonary solitary nodule characterization.
Although 18F-FDG PET/CT has been widely used for lung cancer diagnosis, staging and following up of cancer management, high 18F-FDG uptake in infection and inflammation lung diseases have been reported, 18F-FDG PET/CT may also result in indeterminate diagnosis of lung nodules.13,14 On the other hand, low18F-FDG uptake in malignancy has also reported.20 We have recently found in animal models that 18F-FDG uptake in lung cancer as well as colon cancer is hypoxia dependent, oxic cancer cells associate with low18F-FDG accumulation,25–28 accordingly, we reasonably assume that oxic cancer lesions may result in 18F-FDG PET/CT negative imaging. Higher 99mTc-octreotide uptake in malignancy than benign neoplasm and infection lesions could be the advantage of somatostatin receptor imaging than 18F-FDG PET/CT for lung malignancy characterization. Considering the pilot nature of this study, we had not compared 99mTc-octreotide SPECT/CT with 18F-FDG PET/CT in the same patients in this study.
In this study, we used 99mTc-octreotide instead of 111In-Octreotide; 99mTc is much convenient for daily use, 99mTc has a much shorter half-life than 111In, which may decrease irradiation to patients and public population, and Octreotide reaches maximal accumulation 4 hours after injection, prolonging scan with a longer half-life 111In-Octreotide seems unnecessary.
The objective of this study was to evaluate the clinical value of the combination of diagnostic CT and somatostatin receptor 99mTc-octreotide acetate SPECT/CT imaging in differentiation of benign pulmonary nodules from cancers. We found that the specificity for pulmonary malignant nodule diagnosis was 63.6% for diagnostic CT, 72.7% for somatostatin receptor imaging, and 81.8% for the combined use of diagnostic CT and somatostatin receptor imaging. Therefore, the combination of diagnostic CT and somatostatin receptor 99mTc-octreotide acetate SPECT/CT in characterization of malignancy from benign pulmonary nodules is superior to 99mTc-octreotide acetate SPECT/CT and, much superior than diagnostic CT.
Conclusions
Somatostatin receptor imaging with 99mTc-octreotide SPECT/CT is useful for the differentiation of benign pulmonary nodules from lung cancers, the combination of diagnostic CT and 99mTc-octreotide SPECT/CT further increases the specificity of malignant pulmonary nodule detection.
Acknowledgments
We thank Professor Zizheng Wang for the invaluable comments and thoughtful suggestions. This study was supported by the Jiangsu Province Nature Foundation Grants (BK2006010 and BK2011104), a funding from the Nanjing health personnel training plan 2011, and the Jiangsu Provincial Government Overseas Scholarship (2009). The authors have no conflicts of interest relevant to this article.
Disclosure Statement
There are no existing financial conflicts.
References
- 1.Howlader N. Noone AM. Krapcho M, et al. based on November 2011 SEER data submission, posted to the SEER web site. National Cancer Institute; Bethesda, MD: 2012. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) [Google Scholar]
- 2.Ost D. Fein A. Management strategies for the solitary pulmonary nodule. Curr Opin Pulm Med. 2004;10:272. doi: 10.1097/01.mcp.0000130322.11513.c8. [DOI] [PubMed] [Google Scholar]
- 3.Bernard A. Resection of pulmonary nodules using video-assisted thoracic surgery. Ann Thorac Surg. 1996;61:202. doi: 10.1016/0003-4975(95)01014-9. [DOI] [PubMed] [Google Scholar]
- 4.van Rens MT. de la Riviere AB. Elbers HR, et al. Prognostic assessment of 2361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II and IIIA. Chest. 2000;117:374. doi: 10.1378/chest.117.2.374. [DOI] [PubMed] [Google Scholar]
- 5.Deslauriers J. Gregoire J. Clinical and surgical staging of non-small cell lung cancer. Chest. 2000;117:96S. doi: 10.1378/chest.117.4_suppl_1.96s. [DOI] [PubMed] [Google Scholar]
- 6.Webb WR. Gatsouris S. Zerhouni EA, et al. CT and MR imaging in staging nonsmall cell bronchogenic carcinoma: Report of the Radiology Diagnostic Oncology Group. Radiology. 1992;182:319. doi: 10.1148/radiology.178.3.1847239. [DOI] [PubMed] [Google Scholar]
- 7.Gupta NC. Maloof J. Gunel E. Probability of malignancy in solitary pulmonary nodules using fluorine-18-FDG and PET. J Nucl Med. 1996;37:943. [PubMed] [Google Scholar]
- 8.Dewan NA. Shehan CJ. Reeb SD, et al. Likelihood of malignancy in a solitary pulmonary nodule. Chest. 1997;112:416. doi: 10.1378/chest.112.2.416. [DOI] [PubMed] [Google Scholar]
- 9.Herder GJ. Golding RP. Gobar L, et al. The performance of 18-F-fluorodeoxyglucose positron emission tomography in small solitary pulmonary nodules. Eur J Nucl Med Mol Imaging. 2004;31:1231. doi: 10.1007/s00259-004-1552-7. [DOI] [PubMed] [Google Scholar]
- 10.Van Tinteren H. Hoekstra OS. Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small cell lung cancer: The PLUS multicentre randomised trial. Lancet. 2002;359:1388. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- 11.Pieterman RM. van Putten JWG. Meuzelaar JJ, et al. Preoperative staging of non-small cell lung cancer with positron emission tomography. N Engl J Med. 2000;343:254. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 12.Port JL. Kent MS. Korst RJ, et al. Positron emission tomography scanning poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. Ann Thorac Surg. 2004;77:254. doi: 10.1016/s0003-4975(03)01457-7. [DOI] [PubMed] [Google Scholar]
- 13.Chun EJ. Lee HJ. Kang WJ, et al. Differentiation between malignancy and inflammation in pulmonary ground-glass nodules: The feasibility of integrated (18)F-FDG PET/CT. Lung Cancer. 2009;65:180. doi: 10.1016/j.lungcan.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Brandman S. Ko JP. Pulmonary nodule detection, characterization, and management with multidetector computed tomography. J Thorac Imaging. 2011;26:90. doi: 10.1097/RTI.0b013e31821639a9. [DOI] [PubMed] [Google Scholar]
- 15.Kwekkeboom DJ. Kho GS. Lamberts SW, et al. The value of octreotide scintigraphy in patients with lung cancer. Eur J Nucl Med. 1994;221:1106. doi: 10.1007/BF00181066. [DOI] [PubMed] [Google Scholar]
- 16.O'Byrne KJ. Carney D. Somatostatin and the lung. Lung Cancer. 1993;10:151. doi: 10.1016/0169-5002(93)90177-y. [DOI] [PubMed] [Google Scholar]
- 17.Blum J. Handmaker H. Rinne N, et al. The utility of a somatostatin-type receptor binding peptide radiopharmaceutical (P829) in the evaluation of solitary pulmonary nodules. Chest. 1999;115:224. doi: 10.1378/chest.115.1.224. [DOI] [PubMed] [Google Scholar]
- 18.Blum J. Handmaker H. Lister-James J, et al. A multicenter trial with a somatostatin analog 99mTc depreotide in the evaluation of solitary pulmonary nodules. Chest. 2000;117:1232. doi: 10.1378/chest.117.5.1232. [DOI] [PubMed] [Google Scholar]
- 19.Shih WJ. Samayoa L. Tc-99m depreotide detecting malignant pulmonary nodules: Histopathologic correlation with semiquantitative tumor-to-normal lung ratio. Clin Nucl Med. 2004;29:171. doi: 10.1097/01.rlu.0000113855.93504.03. [DOI] [PubMed] [Google Scholar]
- 20.Wang F. Wang Z. Yao W, et al. Role of 99mTc-octreotide acetate scintigraphy in suspected lung cancer compared with 18F-FDG dual-head coincidence imaging. J Nucl Med. 2007;48:1442. doi: 10.2967/jnumed.107.040824. [DOI] [PubMed] [Google Scholar]
- 21.Halley A. Hugentobler A. Icard P, et al. Efficiency of 18F-FDG and 99mTc-depreotide SPECT in the diagnosis of malignancy of solitary pulmonary nodules. Eur J Nucl Med Mol Imaging. 2005;32:1026. doi: 10.1007/s00259-005-1812-1. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen K. Madsen HH. Rasmussen F, et al. The value of HRCT and Tc-depreotide in the evaluation of pulmonary lesions. J Thorac Oncol. 2006;1:296. [PubMed] [Google Scholar]
- 23.Reubi JC. Laissue J. Krenning EP, et al. Somatostatin receptors in human cancer: Incidence, characteristics, functional correlates and clinical application. J Steroid Biochem Mol Biol. 1992;43:27. doi: 10.1016/0960-0760(92)90184-k. [DOI] [PubMed] [Google Scholar]
- 24.Virgolini I. Leimer M. Handmaker H, et al. Somatostatin receptor subtype specificity and in vitro binding of a novel tumour tracer, 99mTc-P829. Cancer Res. 1998;58:1850. [PubMed] [Google Scholar]
- 25.Huang T. Civelek AC. Li J, et al. Tumor microenvironment–dependent 18F-FDG, 18F-fluorothymidine, and 18F-misonidazole uptake: A pilot study in mouse models of human non–small cell lung cancer. J Nucl Med. 2012;53:1262. doi: 10.2967/jnumed.111.098087. [DOI] [PubMed] [Google Scholar]
- 26.Li XF. Ma Y. Sun X, et al. High 18F-FDG uptake in microscopic peritoneal tumors requires physiological hypoxia. J Nucl Med. 2010;51:632. doi: 10.2967/jnumed.109.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XF. Ma Y. Jiang H. Understanding hypoxia microenvironment of micro-metastases. J Solid Tumors. 2012;2:28. [Google Scholar]
- 28.Li XF. Jiang H. Ma Y, et al. A model system for validation of PET radiopharmaceuticals: Focusing on tumor microenvironment. Int J Med Phys Clin Eng Radiat Oncol. 2013;2:19. [Google Scholar]