Abstract
Purpose
This study was aimed to investigate whether the tumor necrosis induced by radiofrequency ablation (RFA) can improve the ratio of tumor-to-normal tissue (T/NT) after intratumoral injection of 131I-chTNT.
Materials and Method
Eighteen New Zealand rabbits bearing VX2 tumor on the thigh were randomly divided into two treatment groups (control group: intratumoral injection of 131I-chTNT alone; RFA group: RFA + intratumoral injection of 131I-chTNT 3 days after RFA) and each group was further divided into three subgroups I, II, and III (1–2 cm, 2–3 cm, and 3–4 cm in maximum diameter, respectively), by the tumor size. SPECT was performed to evaluate the T/NT on days 1, 8, and 15 after 131I-chTNT injection.
Results
After treatment, all rabbits underwent the SPECT whole-body scan and the T/NT was analyzed. The results showed that T/NT in the RFA group (55.45±41.83) was significantly higher compared with the control group (7.23±5.61) (F=18.89, p=0.001). Meanwhile, a linear ascending trend was found for T/NT in the RFA group along with the follow-up time (r=0.47, p=0.01). The tumor size or the dose of 131I-TNT injection had no significant effect on the variation of T/NT in both groups (p>0.05).
Conclusion
RFA before intratumoral injection of 131I-chTNT can dramatically improve T/NT, demonstrating the potential application of this combination therapy.
Key words: radioimmunotherapy, radiofrequency ablation, ratio of tumor to normal tissue, tumor necrosis therapy
Introduction
Radioimmunotherapy (RIT), using the radiolabeled monoclonal antibody (MAB) targeted against tumor-associated antigens, delivers cytotoxic decay radiation to kill the tumor. RIT has a long history beginning from the end of 19th century, but until recent decades, a remarkable progress of tumor RIT has been made with the development of biologic and immunologic techniques.1,2 Nowadays, the promising results have been obtained in RIT for the treatment of B-cell non-Hodgkin's lymphoma (NHL) by using two RIT agents: yttrium-90 (90Y)-ibritumomabtiuxetan (Zevalin) and iodine-131 (131I)-tositumomab (Bexxar) (20%–40% complete response rates, 60%–80% overall response rates, and mild toxicity), which have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of NHL.1–3 However, for treating solid cancers, the results of both preclinical studies and clinical trials with RIT have still been modest.4–8
The unsatisfactory therapeutic response of solid tumor RIT mainly attributes to the limited penetration or poor targeting capability, undesirable tumor radiosensitivity, and inadequate dose for consideration of excessive hematopoietic toxicity.2,4,8 Several strategies have been explored with an aim to improve the therapeutic efficacy of RIT for solid tumors, for example, application of the pretargeting technique, novel isotope, and combination with chemotherapy, and radiofrequency ablation (RFA).5,7–13 Fortunately, combination therapies by coupling or sequencing RIT with surgical excision, conventional cytotoxic chemotherapy, and RFA are likely to reflect potential clinical perspective for solid tumors.2,6,14 In a word, no matter which strategy is used, the application of RIT aims to achieve a higher ratio of tumor-to-normal tissue (T/NT) of RIT agents, which might result in an anticipatory therapeutic response.
Conventionally, RIT uses MABs to bind cell-surface tumor-associated antigens. In contrast, the tumor necrosis therapy (TNT) antibody targets intracellular nuclear antigens that exist throughout tumors at sites of degenerating and necrotic cells. Some previous studies have already demonstrated its practical applicability experimentally and clinically.15–19 RFA has been demonstrated to be an effective method for some small or early-stage solid tumors, the thermal damage of which can result in peripheral degeneration and central necrosis of solid tumors.20–23 On the basis of these theories, we assumed that an artificial necrosis induced by RFA inside solid tumors might be an ideal targeting site for the TNT antibody, which might improve its T/NT accordingly.
The aim of the present study was to test the hypothesis whether intratumoral 131I-chTNT injection after RFA can improve the T/NT. For this purpose, we designed the following study on rabbits bearing VX2 tumors.
Materials and Methods
Animal model
All experiments had institutional animal care and obtained the approval from the university animal research committee. A total of 18 New Zealand white rabbits, each weighing 2.0–2.7 (2.23±0.42) kg, were purchased from the Laboratory Animal Center and housed in the Laboratory Animal Center of the institution. Food and water were given ad libitum. A VX2 tumor tissue (courtesy of the Laboratory Animal Center of Sun Yat-Sen University) was cut into pieces less than 1 mm3 in size under sterile conditions. The fragments of tumor tissue were kept in 4°C in a Hanks solution. All the recipient rabbits were anesthetized by injection with 3% of the pentobarbital solution (1 mL/kg) through the ear vein, and the right thigh of rabbits were shaved and prepared with povidone–iodine, 0.5 mL VX2 tumor tissue suspension (containing 3–5 fragments) was injected into the right thigh muscle of each rabbit using a 16-gauge trocar.
After the VX2 tumor was implanted successfully, all the rabbits were randomly allocated to two groups on the basis of the random number generation method; those were, the control group (intratumoral injection of 131I-chTNT alone) and the RFA group (RFA + intratumoral injection of 131I-chTNT 3 days after RFA). Nine rabbits were allocated in each group. When the tumors developed to the expected size, the rabbits in the two groups were further randomly and equally assigned to three subgroups I, II, and III (1–2 cm, 2–3 cm, and 3–4 cm in maximum diameter, respectively), by the tumor size and underwent treatment thereafter. On days 1, 8, and 15 after 131I-chTNT injection, all rabbits underwent SPECT scanning for investigating the whole-body distribution of 131I-chTNT.
Treatment
All rabbits received a solution of potassium iodine orally, beginning 3 days before treatment and continuing until 15 days after 131I-chTNT injection, to block the uptake of 131I by the thyroid. The rabbits were fixed on the bed in left lateral decubitus and anesthetized with intravenous injection of 3% pentobarbital sodium (1 mL/kg), then 131I-chTNT injection or RFA under the guidance of ultrasound (US) was performed.
Ultrasound
The US system was a Sonosite US unit (M-Turbo Ultrasound system; Sonosite, Inc., Bothell, WA). US equipped with a 13-6 MHz transducer was applied in the experiment for monitoring tumor growth, puncture guidance, and measuring the tumor volume according to the following formula: π·xyz/6 (x, y, z were the three orthogonal greatest dimensions of the VX2 tumors, respectively).
Radiofrequency ablation
RFA procedures were performed using a Cool-tip system (Valleylab, Boulder, CO), which consists of a radiofrequency generator with a maximum power of 200 W, a 20-cm-long 17-gauge internally cooled electrode with a 3-cm active tip, and two dispersive grounding pads. The electrode contains two lumina, which enables the circulation of cooled saline solution in the tip of the shaft. A steady flow pump (Valleylab) is used to push the chilled saline solution circulating within the lumina of the electrode shaft at 30 mL/min, and the radiofrequency electrode temperature is maintained at less than 21°C.
A radiofrequency electrode was inserted into the tumor percutaneously under US guidance. Grounding pads were attached on the depilated back of each rabbit for RFA. Single application of energy was manually adjusted at 50 W for 3 minutes.
131I-chTNT injection
131I-chTNT (Vivatuxin, Shanghai Medipharm Biotech Co. Ltd, Shanghai, China) is a radiolabeled recombinant human–mouse chimeric TNT (chTNT) MAB. The purified chTNT antibody with purity of at least 98% is radiolabeled with Na131I, which has an average radioactive range of 2 mm in tissue and a half-life of 8 days. The purity of 131I-chTNT is over 95% with a specific radioactivity of about 10 mCi/mL (370 MBq/mL).17,24
131I-chTNT was injected into the VX2 tumor by using a 22-gauge fine needle at a dose of 1.4 mCi/cm3 VX2 tumor. This dose was calculated according to the reported formula for dose translation based on the body surface area by Reagan-Shaw et al.25 The US guidance made sure of the accurate placement of the needle along the periphery of the tumor at multiple sites (0, 3, 6, 9 o'clock direction), monitored the 131I-chTNT injection, and observed its distribution in the VX2 tumor simultaneously. After injection of 131I-chTNT, a following 0.5 mL of normal saline was applied to flush the needle path, and the puncture site was gently compressed using alcoholic cotton gauze for 2 minutes to avoid leakage of 131I-chTNT or bleeding after withdrawal of the needle.
SPECT imaging
On days 1, 8, and 15 after 131I-chTNT injection, all rabbits were fixed on the examination bed in a prone position and underwent a whole-body scanning on SPECT/CT (Symbia T2 SPECT/CT system, Siemens Munich, Germany). The acquisition parameters for whole-body scanning were used as follows: high-energy parallel-hole collimators, 20% energy window at 364 keV photon peak, 10 cm/min for whole-body scanning speed. The whole-body distribution of radionuclide imaged on SPECT (anterior and posterior images) was analyzed by an experienced radiologist. Regions of interest (ROIs) of tumor, thyroid, and normal tissue (the homogenous abdominal area) were drawn on the anterior image, then the mean K count of ROIs was used to calculate T/NT and the ratio of thyroid to normal tissue (THY/NT).
After the whole-body scanning on day 1, a supplemental SPECT/CT fusion image acquisition was performed to investigate whether 131I-chTNT was injected accurately into the tumor and to confirm the anatomic location of the jugular high radioactive spot detected on the SPECT whole-body scanning. The acquisition parameters for SPECT/CT fusion image were used as follows: zoom 1.5, a 128×128 matrix with a pixel size of 4.8 mm, 6°/step for 180° rotating for 30 second/step acquisition, the fusion CT acquisition used full-circle rotation, 130 kV, 35 mAs, and 5-mm slices. SPECT/CT reconstruction was performed under the integrated multimodality image fusion system.
Statistical analysis
Continuous data are expressed as mean±standard deviation. Repeated measures analysis of variance (ANOVA) and Pearson's correlation analysis were used to perform the data analysis. Two-tailed p<0.05 was considered to indicate statistical significance. SPSS software (version 16.0; SPSS, Inc., Chicago, IL) was used to perform the statistical analysis.
Results
Both of the two groups received intratumoral 131I-chTNT injection at a dose of 1.4 mCi/cm3 tumor. The US guidance guaranteed the accuracy of 131I-chTNT injection. In the RFA group, 131I-chTNT injection was given on day 3 after RFA. Unlike the control group, no obvious tumor progression in the RFA group was found until day 6–12 after RFA. Until the end of the follow-up (day 15 after 131I-chTNT injection), no rabbit death or severe complications were encountered.
After 131I-chTNT injection, the SPECT whole-body scanning (Fig. 1) and the fusion images were acquired successfully from all rabbits. On day 1, the typical outline of the rabbit with the two high radioactive spots was displayed on the whole-body SPECT images. The fused SPECT/CT images demonstrated that the jugular high radioactive spot was the thyroid (Fig. 2) and the intratumoral injected 131I-chTNT was displayed as another high radioactive spot on the right thigh (Fig. 3). There was no macroscopic difference between the two groups on day 1. On day 8, the whole-body SPECT images showed that the two high radioactive spots were still conspicuous, whereas the outline of the rabbit was blurred. On day 15, there were some obvious macroscopic variations on the whole-body SPECT images, and the outline of the rabbit was hardly discernible, especially for the rabbits in subgroup I of the two groups. The radioactivity of tumor in the control group dramatically decayed in comparison with that in the RFA group; even in a rabbit in subgroup I of the control group, the radioactivity of tumors could not be detected (the default T/NT was 1). However, the thyroids of the rabbits in the two groups showed remarkable uptake of radioactive iodine.
FIG. 1.
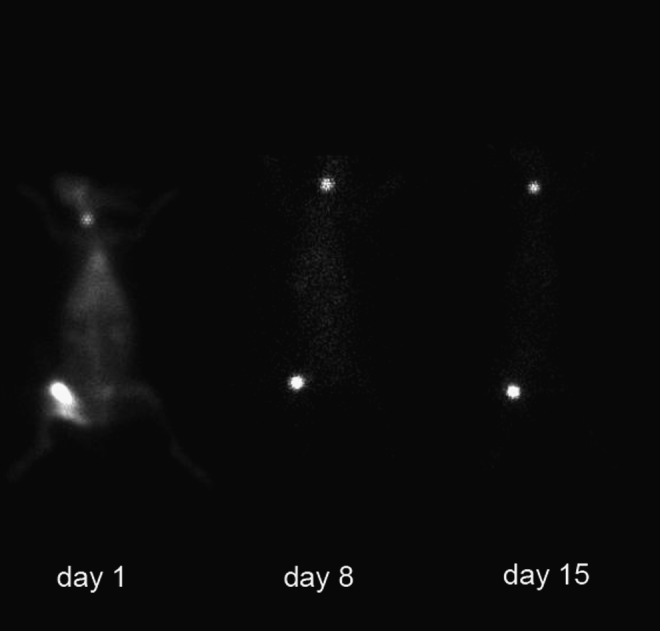
A typical outline of a rabbit in the radiofrequency ablation group with two spots displayed on whole-body SPECT images on days 1, 8, and 15.
FIG. 2.
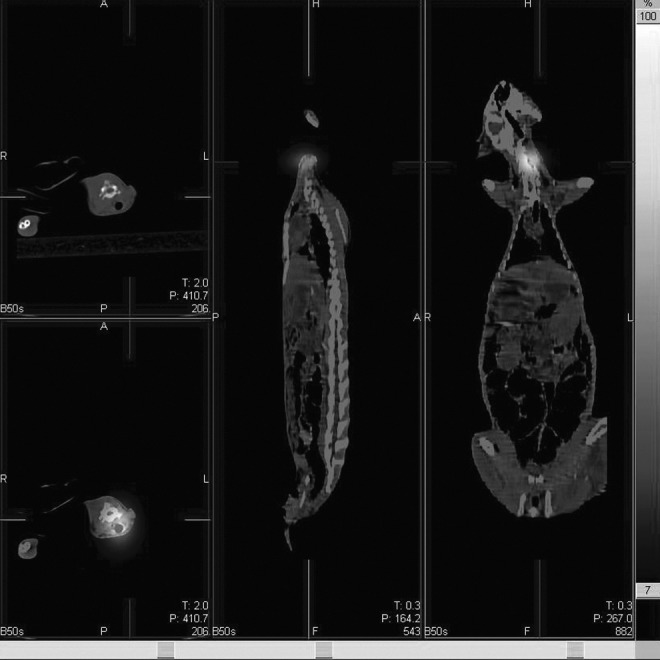
The fusion images illustrate that the jugular high radioactive spot is the high concentration of 131I-chTNT absorbed by thyroid.
FIG. 3.
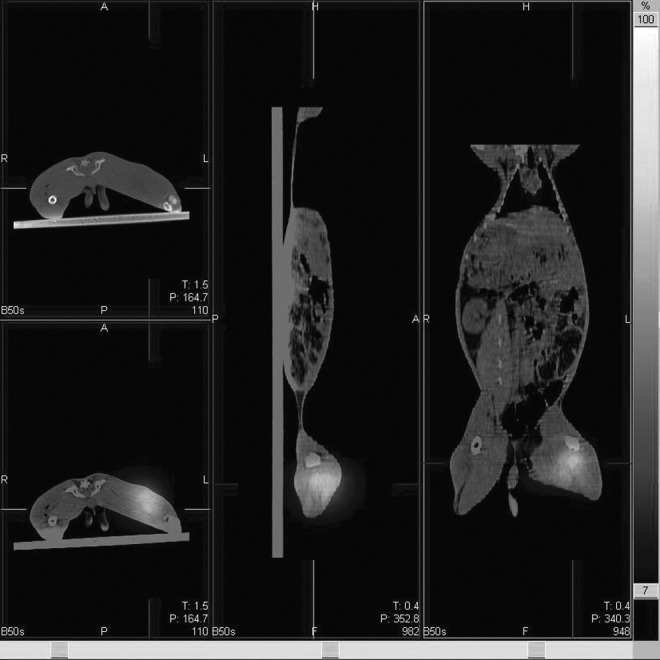
The fusion images show the high radioactive spot on the rabbit's right thigh precisely overlaps with the tumor.
On the basis of the mean K count of ROIs on the whole-body images (Fig. 4), T/NT and THY/NT were calculated and listed in Table 1 and Table 2, respectively.
FIG. 4.
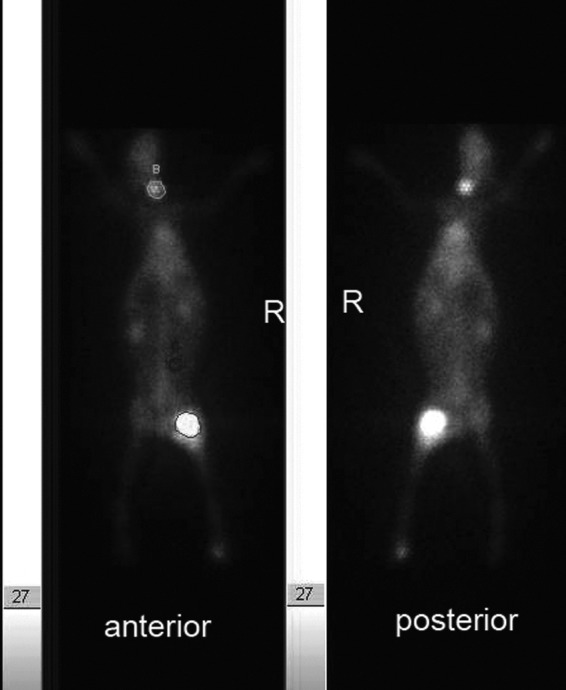
The regions of interest (ROIs) are draw on the anterior image for calculating the ratio of tumor to normal tissue (T/NT) and the ratio of thyroid to normal tissue (THY/NT).
Table 1.
The Ratio of Tumor-to-Normal Tissue in the Control and the Radiofrequency Ablation Groups
| |
Control group |
RFA group |
||||
|---|---|---|---|---|---|---|
| Day 1 | Day 8 | Day 15 | Day 1 | Day 8 | Day 15 | |
| Subgroups | ||||||
| I | 3.21±1.18 | 10.76±4.04 | 7.59±6.35 | 34.23±26.08 | 62.47±32.98 | 59.02±26.50 |
| II | 5.68±3.88 | 4.89±3.72 | 3.99±3.27 | 23.57±10.28 | 49.76±34.15 | 58.22±23.80 |
| III | 13.98±8.98 | 5.04±1.97 | 7.66±7.10 | 23.54±20.74 | 83.47±74.73 | 104.75±61.00 |
| Total | 7.23±5.61 | 55.45±41.83 | ||||
Control group: 131I-chTNT alone; RFA group: RFA+131I-chTNT combination.
Table 2.
The Ratio of Thyroid-to-Normal Tissue in the Control and the Radiofrequency Ablation Groups
| |
Control group |
RFA group |
||||
|---|---|---|---|---|---|---|
| Day 1 | Day 8 | Day 15 | Day 1 | Day 8 | Day 15 | |
| Subgroups | ||||||
| I | 1.67±0.34 | 4.73±2.75 | 8.24±9.13 | 1.96±0.58 | 14.78±14.73 | 14.20±14.65 |
| II | 1.20±0.32 | 3.76±0.62 | 2.64±0.97 | 2.39±1.38 | 8.56±6.29 | 11.15±5.79 |
| III | 1.40±0.55 | 4.42±1.70 | 6.97±5.71 | 1.83±0.66 | 14.55±13.39 | 23.95±21.76 |
| Total | 3.89±3.94 | 10.38±11.87 | ||||
Control group: 131I-chTNT alone; RFA group: RFA+131I-chTNT combination.
The analysis results with repeated measures ANOVA showed that the T/NT in the RFA group (55.45±41.83) was significantly higher than that in the control group (7.23±5.61) (F=18.89, p=0.001); the variation of T/NT among different subgroups (different size tumors or at different doses of 131I-TNT injection) was not significantly different (F=0.66, p=0.54); the variation of T/NT at follow-up time points (days 1, 8, and 15) was significantly different (F=7.61, p=0.003); meanwhile, the variation trend of T/NT between the two groups during follow-up was significantly different (F=8.37, p=0.002), T/NT in the RFA group ascended along with the follow-up time (r=0.47, p=0.01), while no linear trend was found for T/NT in the control group along with the follow-up time (r=−0.03, p=0.87); the variation trend of T/NT among the subgroups during follow-up was not significant (F=0.75, p=0.57); there was no group (treatment)×subgroup (the dose of 131I-TNT injection) interaction (F=0.37, p=0.70).
At the same time, the analysis results with repeated measures ANOVA showed that the variations of THY/NT between the groups or among the subgroups were not significantly different (F=4.25, p=0.06; F=0.53, p=0.60, respectively); while the variation of THY/NT at follow-up time points (days 1, 8, and 15) was significantly different (F=7.61, p=0.003); the group×time interaction, the subgroup×time interaction, and the group×subgroup interaction, all were not significantly different (F=2.97, p=0.07; F=0.77, p=0.55; F=0.19, p=0.83, respectively). The same ascending linear trend was found for the THY/NT along with the follow-up time (r=0.42, p=0.002).
Discussion
The abilities to kill the tumor cells selectively and avoid damage to normal tissue are the essential characteristics of RIT, which mainly depends on the specificity of MAB.2,8 At present, a number of MABs have been developed for RIT, and several isotopes have been successfully tested and applied to antitumor therapy.2,8,12,26,27
In this study, the radioactive iodine (131I) labeled MAB (-chTNT), as RIT agents, was applied to treat the solid tumor (VX2 tumor). 131I is a mixed β-γ emitter with a half-life of 8 days. The short-range β ray is the major cytotoxic radiation and can induce the tumor necrosis; the long-range γ ray can be imaged on SPECT for observing the biodistribution of 131I.2,26,28 The TNT antibody can bind specifically to the nuclear antigens of the degenerated cells, regardless of the origin of cells. Once binding with the necrotic tissue, 131I-chTNT will be cytotoxic to adjacent viable tumor cells.16,17,24
At present, RIT for solid tumors still has not produced satisfactory responses. One of the main reasons is the relatively low target concentration of RIT agents.2,8 For consideration of the excessive hematopoietic toxicity and other side effects, the administration dose was strictly limited. The improvement of T/NT indicates a better efficacy of RIT for solid tumor. Conventionally, most of the RIT agents are administrated systemically through oral or intravenous injection, then are diluted by the circulation, and distributed into the whole body, which results in undesirable low T/NT (low target concentration). The locoregional route is preferable for the short-lived radionuclides when targeted by antibodies because using the intravenous route results in significant radionuclide decay before the antibody reaches its target.12 Therefore, the RIT agents were tentatively administrated locally through the tumor supply blood vessel and some clinical studies using RIT (131I-Metuximab administrated through hepatic artery intubation) for hepatocellular carcinoma showed promising efficacy.29–32 However, the T/NT was observed to be only 0.7–2.7.30,31 If the T/NT can be improved, the tumor response will be more encouraging. In addition, in the treatment of hepatic metastases with RFA before 131I-chTNT-1/B infusion, Anderson et al. reported that an average target to the background ratio of 2.9 (ranged from 1.3 to 6.0) was observed at the 3th day after 131I-chTNT-1/B infusion.33 It is critical to alleviate or avoid the effect of the circulation dilution and clearance and directly delivering the RIT agents to the target tumor can solve this problem. In a phase II clinical trial in China, 43 patients with advanced lung cancer were treated with administration of 131I-chTNT through intravenous and intratumoral injection, the intratumoral injection group (T/NT=14.6, n=15) showed a higher T/NT and higher efficacy than the intravenous injection group (T/NT=1.36, n=19).24 Although there is no comparability between these previous studies, the contribution of intratumoral injection to the improvement of T/NT is significant.
Similarly, in this study, the mean T/NT in the control group was not high enough (just 7.23), because the VX2 tumor has rich blood supply, the washing of blood still plays a role locally as the effect of the circulation dilution and clearance; furthermore, because of the absence of necrosis, most of the VX2 tumor in the control group has no ideal target site for 131I-chTNT to dock and lock; thus, on SPECT images, the radioactivity of tumors in the control group faded quickly, especially close to the late period of follow-up. By comparison, T/NT (55.45) in the RFA group improved dramatically and contributed to the effect of RFA significantly. This result not only embodied the expectant characteristics of RIT, but also presented a potential application in clinic. The tumor size and the administration dose of 131I-chTNT had no significant effect on the improvement of T/NT. Moreover, the T/NT in the RFA group, unlike in the control group, showed an ascending trend during the follow-up. It can be inferred that intratumoral injection after RFA can not only increase the target concentration of 131I-chTNT, but also prolong its intratumoral retention time. These results were mainly caused by two factors induced by RFA: the first is that RFA induces tumor necrosis, which provides the target site for 131I-chTNT; and the second is that RFA destroys the tumor blood vessel so that it decreases the effect of the circulation dilution and clearance.
In addition, although previous blockade by using potassium iodine orally in this study, radioactive iodine uptake in the thyroid, unlike that seen in patients, was unavoidable in rabbits. The results showed that the administration dose of 131I-chTNT and treatment both had no impact on the variation of TYH/NT, which in both groups also showed the same ascending trend during the follow-up. These phenomena like T/NT in the RFA group, we thought, were attributed to the decrease of radioactive iodine in the normal tissue; the macroscopic SPECT images also illustrated these phenomena, which mainly resulted from circulating dehalogenated iodine absorbed by thyroid and circulating 131I-chTNT reabsorbed by necrotic tumor tissue, except for the physical decay and metabolic clearance.26 As far as the influence of iodine absorbed by the thyroid is concerned, if it can be avoided, the results will be more compelling. Based on such advantages, we believe that 90Y might be a better alternative choice for solid tumor RIT in future studies.26
Although the results of T/NT were encouraging, several limitations in this study should be mentioned: first, the biodistribution of 131I-chTNT only expressed by using T/NT, the precise dosimetric investigations of 131I-chTNT biodistribution in tumor and organs were not available due to the technical limitation; second, the inadequate blockade of thyroid may lead to bias of the results of T/NT; third, the detailed contribution to the improvement of T/NT is not clear; thus, in future studies, the therapeutic effects of 131I-chTNT and its retention in tumor tissue need to be addressed to evaluate the potential application of this combination therapy, by comparing the tumor's volume and adding the RFA alone group as control. Finally, the detailed mechanism of RFA in improving the T/NT ratio of 131I-chTNT should be further explored by injecting a nonbinding radiolabeled antibody or free iodine-131.
Conclusion
RFA before intratumoral injection of 131I-chTNT can dramatically improve the T/NT, demonstrating the potential application of this combination therapy.
Acknowledgments
This work was supported, in part, by grant 30970837 from the National Scientific Foundation Committee of China and grant NCET-06-0723 from the Chinese Ministry of Education.
The authors would like to thank Feng-Ying Chen and Wen-Ge Huang from the Laboratory Animal Center of Sun Yat-Sen University, for their assistance in the preparation of the animal model. We are grateful for the help in the SPECT scan by Chang Yi from the Department of Nuclear Medicine of the First Affiliated Hospital, Sun Yat-Sen University.
Disclosure Statement
No financial conflict of interests exist.
References
- 1.Tomblyn M. Radioimmunotherapy for B-cell non-hodgkin lymphomas. Cancer Control. 2012;19:196. doi: 10.1177/107327481201900304. [DOI] [PubMed] [Google Scholar]
- 2.Pohlman B. Sweetenham J. Macklis RM. Review of clinical radioimmunotherapy. Expert Rev Anticancer Ther. 2006;6:445. doi: 10.1586/14737140.6.3.445. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan A. Mukherji SK. Tositumomab and iodine I131 tositumomab (Bexaar) AJNR Am J Neuroradiol. 2011;32:637. doi: 10.3174/ajnr.A2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CY. Pourgholami MH. Allen BJ. Optimizing radioimmunoconjugate delivery in the treatment of solid tumor. Cancer Treat Rev. 2012;38:854. doi: 10.1016/j.ctrv.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Chatal JF. Davodeau F. Cherel M, et al. Different ways to improve the clinical effectiveness of radioimmunotherapy in solid tumors. J Cancer Res Ther. 2009;5(Suppl 1):S36. doi: 10.4103/0973-1482.55139. [DOI] [PubMed] [Google Scholar]
- 6.de Jong GM. Hendriks T. Eek A, et al. Adjuvant radioimmunotherapy improves survival of rats after resection of colorectal liver metastases. Ann Surg. 2011;253:336. doi: 10.1097/SLA.0b013e3181ff313a. [DOI] [PubMed] [Google Scholar]
- 7.de Jong G. Hendriks T. Franssen G, et al. Adjuvant radioimmunotherapy after radiofrequency ablation of colorectal liver metastases in an experimental model. Eur J Surg Oncol. 2011;37:258. doi: 10.1016/j.ejso.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Koppe MJ. Postema EJ. Aarts F, et al. Antibody-guided radiation therapy of cancer. Cancer Metast Rev. 2005;24:539. doi: 10.1007/s10555-005-6195-z. [DOI] [PubMed] [Google Scholar]
- 9.Lindegren S. Frost SH. Pretargeted radioimmunotherapy with alpha-particle emitting radionuclides. Curr Radiopharm. 2011;4:248. doi: 10.2174/1874471011104030248. [DOI] [PubMed] [Google Scholar]
- 10.Schoffelen R. van der Graaf WT. Franssen G, et al. Pretargeted 177Lu radioimmunotherapy of carcinoembryonic antigen-expressing human colonic tumors in mice. J Nucl Med. 2010;51:1780. doi: 10.2967/jnumed.110.079376. [DOI] [PubMed] [Google Scholar]
- 11.Salaun PY. Bodet-Milin C. Frampas E, et al. Toxicity and efficacy of combined radioimmunotherapy and bevacizumab in a mouse model of medullary thyroid carcinoma. Cancer. 2010;116:1053. doi: 10.1002/cncr.24792. [DOI] [PubMed] [Google Scholar]
- 12.Barbet J. Chatal JF. The best radionuclide for radioimmunotherapy of small tumors: Beta- or alpha-emitter? Eur J Nucl Med Mol Imaging. 2011;38:271. doi: 10.1007/s00259-010-1707-7. [DOI] [PubMed] [Google Scholar]
- 13.Sharkey RM. Rossi EA. McBride WJ, et al. Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy. Semin Nucl Med. 2010;40:190. doi: 10.1053/j.semnuclmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharkey RM. Karacay H. Govindan SV, et al. Combination radioimmunotherapy and chemoimmunotherapy involving different or the same targets improves therapy of human pancreatic carcinoma xenograft models. Mol Cancer Ther. 2011;10:1072. doi: 10.1158/1535-7163.MCT-11-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Street HH. Goris ML. Fisher GA, et al. Phase I study of 131I-chimeric(ch) TNT-1/B monoclonal antibody for the treatment of advanced colon cancer. Cancer Biother Radiopharm. 2006;21:243. doi: 10.1089/cbr.2006.21.243. [DOI] [PubMed] [Google Scholar]
- 16.Chen FM. Taylor CR. Epstein AL. Tumor necrosis treatment of ME-180 human cervical carcinoma model with 131I-labeled TNT-1 monoclonal antibody. Cancer Res. 1989;49:4578. [PubMed] [Google Scholar]
- 17.Chen S. Yu L. Jiang C, et al. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J Clin Oncol. 2005;23:1538. doi: 10.1200/JCO.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 18.Epstein AL. Chen D. Ansari A, et al. Radioimmunodetection of necrotic lesions in human tumors using I-131 labeled TNT-1 F (ab') 2 monoclonal antibody. Antibody Immunoconj Radiopharm. 1991;4:151. [Google Scholar]
- 19.Chen FM. Liu CZ. Epstein AL. Effects of 131I-labeled TNT-1 radioimmunotherapy on HT-29 human colon adenocarcinoma spheroids. Cancer Immunol Immunother. 1991;33:158. doi: 10.1007/BF01756136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruix J. Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirza A. Fornage B. Sneige N, et al. Radiofrequency ablation of solid tumors. Cancer J. 2001;7:95. [PubMed] [Google Scholar]
- 22.Forner A. Llovet JM. Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 23.Xu HX. Xie XY. Lu MD, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol. 2004;59:53. doi: 10.1016/j.crad.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Yu L. Ju DW. Chen W, et al. 131I-chTNT radioimmunotherapy of 43 patients with advanced lung cancer. Cancer Biother Radiopharm. 2006;21:5. doi: 10.1089/cbr.2006.21.5. [DOI] [PubMed] [Google Scholar]
- 25.Reagan-Shaw S. Nihal M. Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 26.Govindan SV. Goldenberg DM. New antibody conjugates in cancer therapy. Sci World J. 2010;10:2070. doi: 10.1100/tsw.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldenberg DM. Sharkey RM. Novel radiolabeled antibody conjugates. Oncogene. 2007;26:3734. doi: 10.1038/sj.onc.1210373. [DOI] [PubMed] [Google Scholar]
- 28.Dewaraja YK. Wilderman SJ. Koral KF, et al. Use of integrated SPECT/CT imaging for tumor dosimetry in I-131 radioimmunotherapy: A pilot patient study. Cancer Biother Radiopharm. 2009;24:417. doi: 10.1089/cbr.2008.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frampas E. Maurel C. Thedrez P, et al. The intraportal injection model for liver metastasis: Advantages of associated bioluminescence to assess tumor growth and influences on tumor uptake of radiolabeled anti-carcinoembryonic antigen antibody. Nucl Med Commun. 2011;32:147. doi: 10.1097/MNM.0b013e328341b268. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z. Bian H. Feng Q, et al. Biodistribution and localization of iodine-131-labeled metuximab in patients with hepatocellular carcinoma. Cancer Biol Ther. 2006;5:318. doi: 10.4161/cbt.5.3.2431. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZN. Mi L. Xu J, et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: Clinical phase I/II trials. Int J Radiat Oncol Biol Phys. 2006;65:435. doi: 10.1016/j.ijrobp.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Wu L. Yang YF. Ge NJ, et al. Hepatic arterial iodine-131-labeled metuximab injection combined with chemoembolization for unresectable hepatocellular carcinoma: Interim safety and survival data from 110 patients. Cancer Biother Radiopharm. 2010;25:657. doi: 10.1089/cbr.2010.0801. [DOI] [PubMed] [Google Scholar]
- 33.Anderson PM. Wiseman GA. Lewis BD, et al. A phase I safety and imaging study using radiofrequency ablation (RFA) followed by 131I-chTNT-1/B radioimmunotherapy adjuvant treatment of hepatic metastases. Cancer Ther. 2003;1:297. [Google Scholar]


