Abstract
A crude hydromethanolic extract from Pinus brutia bark and its fractions (diethyl ether, ethyl acetate, n-butanol, and aqueous fractions) were studied with regard to their phenolic content and antioxidant activities. The total phenolics and proanthocyanidins in each extract were quantified by spectrophotometric methods; the polyphenolic profile was analyzed by RP-HPLC-DAD-ESI-MS. All extracts were tested with regard to their ability to scavenge free radicals (ABTS radical cation, superoxide and hydroxyl radicals), reduce ferric ions, and inhibit 15-lipoxygenase. P. brutia bark extracts had high phenolic contents (303.79±7.34–448.90±1.39 mg/g). Except diethyl ether extract, all other extracts contained proanthocyanidins ranging from 225.79±3.94 to 250.40±1.44 mg/g. Several polyphenols were identified by RP-HPLC-DAD-ESI-MS: taxifolin in diethyl ether extract, a taxifolin-O-hexoside, catechin, procyanidin dimers, and trimers in ethyl acetate extract. Except diethyl ether extract, all other extracts were effective scavengers of superoxide and hydroxyl radicals (EC50=33.5±1.1–54.93±2.85 μg/mL and 0.47±0.06–0.6±0.0 mg/mL, respectively). All extracts had noticeable 15-lipoxygenase inhibitory effects (EC50=22.47±0.75–34.43±2.25 μg/mL). We conclude that P. brutia bark is very promising for the dietary supplements industry due to its high free radical scavenging and 15-lipoxygenase inhibitory effects.
Key Words: Calabrian pine; hydroxyl radical; 15-lipoxygenase,; proanthocyanidins; RP-HPLC-DAD-ESI-MS; superoxide anion radical; taxifolin
Introduction
Barks of different pine species have been used as food and medicine for more than 2000 years. In ancient times, pine bark was used to treat inflammatory conditions. European herbals of the fifteenth and sixteenth century mention the efficacy of pine bark in skin disorders, mainly wounds and ulcers. In North America, indigenous peoples used pine bark to prevent and treat scurvy. It is worthy to note that the older bark was often used for therapeutic purposes, while the younger bark was used as food. In addition, in northern Scandinavia, Sami people used pine inner bark as food.1–4
Nowadays, bark extracts of different Pinus species have been intensively investigated with regard to their chemical constituents and biological effects. The most studied one is Pycnogenol®—a standardized extract prepared from the bark of French maritime pine (Pinus pinaster Aiton, subspecies Atlantica des Villar syn. P. maritima). Pycnogenol contains procyanidins (65–75%), mainly oligomers of catechin and epicatechin, monomeric catechin and epicatechin, taxifolin, phenolic acids, and their glycosides.1,3 It is widely used as a nutritional supplement and provides numerous health benefits due to its antioxidant, antiinflammatory, and enzyme-modulation effects.1,4–6 Human studies have proved that Pycnogenol has beneficial effects in cardiovascular disorders, type 2 diabetes mellitus and its complications, asthma, osteoarthritis, muscular pain, cognitive decline in Alzheimer's disease, UV-induced skin inflammation, and sexual disorders.3,4 Bark extracts from other Pinus species have been reported to possess potent antioxidant activity.7–10 Extracts of Pinus sylvestris, Pinus pinea, and Pinus massoniana barks showed cytotoxic activity against human cancer cell lines.8,9 In addition, P. sylvestris and P. massoniana bark extracts reduced the production of several inflammatory mediators (nitric oxide, prostaglandin E2, and intercellular adhesion molecule-1).8,11 P. pinea and Pinus densiflora bark extracts have been found very promising for diabetes treatment due to their inhibitory effects on glucose absorption and carbohydrate-hydrolysing enzymes, respectively.12,13 Taken together, all these data show that bark extracts from different pine species have remarkable biological effects, thus making pine bark a valuable raw material for the food and pharmaceutical industries.
Pinus brutia Ten. (Pinaceae, Calabrian pine, Turkish pine) is naturally spread in the eastern Mediterranean region being one of the most important timber trees. The bark is the main waste from the industrial processing of the wood of mature trees that is still useless and discarded.14–16 There are recent reports on the chemical composition and biological activities of the bark collected from pines growing in western Turkey (Izmir-Deliomer). Several low-molecular-weight compounds (taxifolin isomers, catechins, and procyanidins) have been identified in bark extracts. Bark extracts showed moderate cytotoxic effects against certain human cancer cell lines and antiinflammatory effects.9,17 To the best of our knowledge, the only published data on the antioxidant activity of P. brutia bark extracts are those reporting radical scavenging effects assessed by the chemiluminescence and diphenylpicrylhydrazyl (DPPH) assays.9,18
Although P. brutia is the dominant tree species in Cyprus,19 no investigations concerning the valorization of its bark, the main waste from the timber industry, have been carried out. As a part of an extensive study on the possible use of bark waste as raw material in the dietary supplements industry, the present work evaluated the antioxidant activity of P. brutia bark by several in vitro assays; in addition, the polyphenolic content and profile of bark extracts were studied.
Materials and Methods
Plant material
P. brutia bark was generously supplied by the Cyprus Department of Forests of the Ministry of Agriculture, Natural Resources, and Environment in March 2008. The bark was dried in the dark at room temperature, milled, and then sieved to select particles smaller than 4 mm. The plant material was stored at +4°C until use. A voucher specimen has been deposited in the Department of Pharmacognosy, the University of Medicine and Pharmacy “Gr. T. Popa” Iasi, Romania.
Extraction
An amount of 150 g of bark powder was extracted thrice (each time for 3 h) with 1.5 L of 80% aqueous methanol (v/v) at ambient temperature under constant stirring. The supernatants were filtered, combined, evaporated under reduced pressure at 40°C, and lyophilized, yielding 20.07 g of crude extract. A quantity of 18.36 g of the crude extract was suspended in ultrapure water (1:10) and successively partitioned with diethyl ether (7×190 mL), ethyl acetate (9×190 mL), and n-butanol (10×190 mL). The organic solvents were removed by low-pressure evaporation at 40°C. The remaining aqueous phase was concentrated under reduced pressure at 40°C and then lyophilized. All extracts were stored at −20°C for further studies.
Total phenolic content
The total phenolic content was quantified spectrophotometrically using the Folin–Ciocalteu method as previously described.20–22 The results were expressed as gallic acid equivalents (mg/g extract).
Proanthocyanidin content
The proanthocyanidin content was determined by the vanillin-hydrochloric acid method as previously described.23,24 The results were expressed as (+)-catechin equivalents (mg/g extract).
RP-HPLC-DAD-ESI-MS analysis
Reversed-phase high-performance liquid chromatography coupled with diode array detection and electrospray ionization mass spectrometry (RP-HPLC-DAD-ESI-MS) analysis was performed in the negative ion mode.25 The equipment consisted of a Perkin–Elmer HPLC-ESI-MS system (SCIEX API 365 LC/MS/MS mass spectrometer connected to a Series 200 HPLC system with UV/VIS detector and Analyst Software 1.1 data system). A Merck Superspher 100 RP-18 column (75 mm×4 mm×4 μm) was used. The mobile phase consisted of solvent A (acetonitrile) and solvent B (water and formic acid; 99:1, v/v). The elution profile was as follows: 0 min, 100% B; 3 min, 100% B; 30 min, 30% A in B; 33 min, 70% A in B; 42 min, 70% A in B; and 45 min, 100% B. All gradients were linear. The flow rate was set to 1 mL/min. Phenolic compounds were detected at 280 nm. Extracts were dissolved in ethanol–water mixtures at a concentration of 20 mg/mL and filtered through 0.45 μm PTFE filters; the injection volume was 20 μL. The spray needle voltage, dry temperature, and nebulizer gas were set to −4200 V, 320°C, and 10, respectively; a split ratio of 7:3 was used.25
Trolox equivalent antioxidant capacity assay
The Trolox equivalent antioxidant capacity (TEAC) assay is based on the ability of antioxidants to scavenge the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation.26–28 The assay was performed as described by Re et al.26 using quercetin as a positive control; quercetin was the positive control in all antioxidant assays. To calculate the TEAC values, a (R)-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) curve was plotted. TEAC values (mM Trolox equivalent to 1 mg/mL extract or quercetin) were calculated as follows:
TEAC=gradient of the plot for test samples (extracts, quercetin)/gradient of the plot for Trolox.26
Reducing power assay
The assay was performed to evaluate the electron-donating ability of P. brutia bark extracts.29,30 Within this assay, the absorbance at 700 nm is an indicator of the reducing power.
Superoxide anion radical scavenging assay
The assay was performed according to previously described procedures.31,32 Superoxide anion radical, chemically generated in a beta-nicotinamide adenine dinucleotide-reduced (NADH)-phenazine methosulfate system, was detected by its ability to reduce nitroblue tetrazolium to a formazan dye.32 The percent of superoxide anion radical scavenging activity was calculated as 100×(C–S)/(C), where C is the absorbance of the control and S is the absorbance in the presence of the sample (extracts, quercetin).
Hydroxyl radical scavenging assay
The salicylate hydroxylation method was used to measure hydroxyl radicals generated via Fenton reaction.33,34 The percent of hydroxyl radical scavenging activity was calculated using the following formula: 100×(C−S)/(C), where C is the absorbance of the control and S is the absorbance in the presence of extracts or quercetin.
15-Lipoxygenase inhibition assay
The assay is based on the property of 15-lipoxygenase (15-LO) to catalyze the peroxidation of polyunsaturated fatty acids (linoleic acid) to hydroperoxide derivatives that strongly absorb at 234 nm.21 The assay was carried out using soybean 15-LO according to a procedure described in literature21 except that the enzyme and the inhibitor were incubated for 10 min at 25°C. The percent inhibition of 15-LO activity was calculated using the formula: 100×[(C90−C30)−(S90−S30)/(C90−C30)], where C30, C90 and S30, S90 are the values of absorbance at 234 nm after 30 and 90 s reaction time for the control (mixtures without extracts or quercetin) and sample (mixtures with extracts or quercetin), respectively.
Statistics
All assays were carried out in triplicate, and the results were expressed as mean±SD. The EC50 values were calculated by linear interpolation between values above and below 50% activity except the reducing power assay; in this assay, the EC50 values were the effective concentrations at which the absorbance was 0.5.30 Statistical analyses were performed using the one-way analysis of variance and Duncan's multiple-range tests (SPSS version 17.0).
Results
The extraction of P. brutia bark with 80% (v/v) methanol followed by fractionation of the crude extract (PbE; 18.36 g) by successive liquid–liquid partition led to four extractive fractions: diethyl ether (PbE1; 2.18 g), ethyl acetate (PbE2; 1.84 g), n-butanol (PbE3; 8.59 g), and aqueous (PbE4; 5.37 g) fractions. The results of the quantitative analyses showed that the extracts had high phenolic contents ranging from 303.79±7.34 in PbE3 to 448.90±1.39 mg/g in PbE2. Except PbE1, all other extracts contained proanthocyanidins in the range of 225.79±3.94 to 250.40±1.44 mg/g (Table 1).
Table 1.
Total Phenolic and Proanthocyanidin Content in Pinus brutia Bark Extracts
| Total phenolic content (mg gallic acid/g extract) | Proanthocyanidin content (mg (+)-catechin/g extract) | |
|---|---|---|
| PbE | 412.42 ± 7.56††††,‡‡‡‡,####,$ | 225.79 ± 3.94††††,$$$$ |
| PbE1 | 366.71 ± 5.63****,‡‡‡‡,####,$$$ | 10.05 ± 0.22****,‡‡‡‡,####,$$$$ |
| PbE2 | 448.90 ± 1.39****,††††,####,$$$$ | 231.41 ± 1.24††††,$$$$ |
| PbE3 | 303.79 ± 7.34****,††††,‡‡‡‡,$$$$ | 229.10 ± 1.13††††,$$$$ |
| PbE4 | 393.49 ± 5.39*,†††,‡‡‡‡,#### | 250.40 ± 1.44****,††††,‡‡‡‡,#### |
Significant differences between extracts are indicated: *P < .05, ****P < .001 vs. PbE; †††P < .002, ††††P < .001 vs. PbE1; ‡‡‡‡P < .001 vs. PbE2; ####P < .001 vs. PbE3; $P < .05, $$$P < .002, $$$$P < .001 vs PbE4.
PbE, crude extract of P. brutia bark with 80% (v/v) methanol (18.36 g); PbE1, PbE fractionated with diethyl ether (2.18 g); PbE2, PbE fractionated with ethyl acetate (1.84 g); PbE3, PbE fractionated with n-butanol (8.59 g); PbE4, remaining aqueous phase (5.37 g).
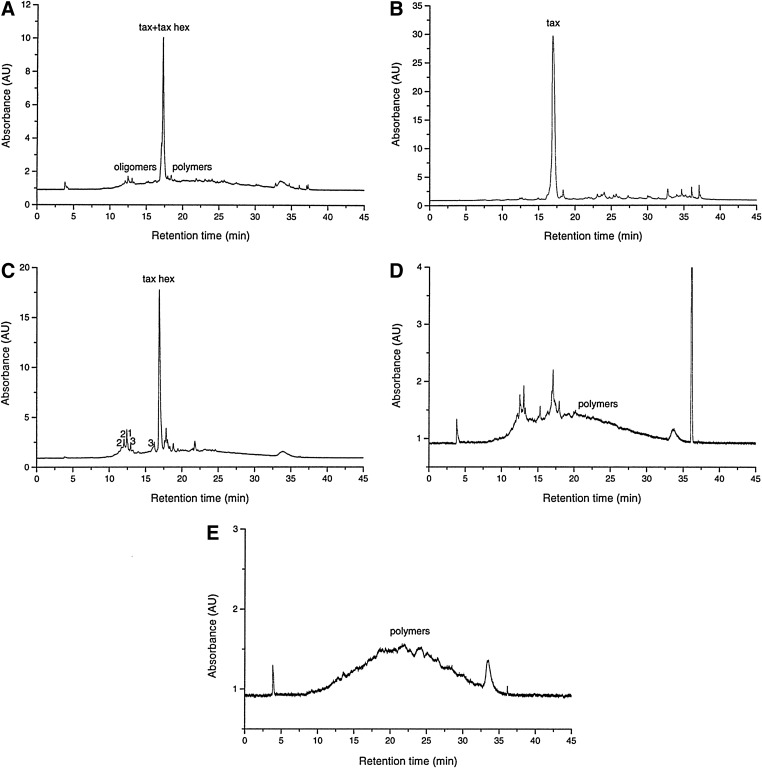
The polyphenolic profile of P. brutia extracts was studied by RP-HPLC-DAD-ESI-MS. The RP-HPLC-UV traces (280 nm) are shown in Figure 1. Taxifolin and a taxifolin-O-hexoside were, by far, the dominant polyphenols in PbE1 and PbE2, respectively; in addition, PbE2 contained catechin and shorter procyanidin oligomers (dimers, trimers; Fig. 1B, C). Taxifolin and catechin were identified by comparing their UV spectra and MS fragmentation patterns with those of standard compounds analyzed in the same experimental conditions. On the basis of UV and MS data, the major polyphenol in PbE2 was tentatively identified as a O-hexoside of taxifolin. Its UV spectral characteristics (λmax= 226, 290, 340 sh nm) suggested a taxifolin-type structure.35,36 The negative ion ESI-MS fragmentation pattern of taxifolin-O-hexoside showed the deprotonated molecules [M−H]− at m/z 465.1 and [2M−H]− at m/z 931.4 along with two fragment ions corresponding to the aglycone: [A]− at m/z 303.2, generated by the loss of a hexose moiety (162 amu) from the deprotonated molecule [M−H]− and [A−H2O]− at m/z 284.6 (Table 2). These spectral data are in agreement with those reported in literature for taxifolin-O-glucosides.11,37 Procyanidin oligomers (two dimers, two trimers) were tentatively identified on the basis of their MS spectral data (Table 2).9,11,25 A broad, unresolved hump corresponding to high oligomeric and polymeric procyanidins is visible in PbE3 and PbE4 chromatograms (Fig. 1D, E).
FIG. 1.
RP-HPLC-UV traces (280 nm) of Pinus brutia extracts runs with a Merck Superspher 100 RP-18 column (75 mm×4 mm×4 μm) and a gradient consisting of solvent A (acetonitrile) and solvent B (water and formic acid; 99:1, v/v): 0 min, 100% B; 3 min, 100% B; 30 min, 30% A in B; 33 min, 70% A in B; 42 min, 70% A in B; 45 min, 100% B; flow rate: 1 mL/min. (A) PbE, (B) PbE1, (C) PbE2, (D) PbE3, (E) PbE4. Tax, taxifolin; tax hex, taxifolin-O-hexoside; 1, catechin; 2, dimer; 3, trimer.
Table 2.
Main Polyphenolic Constituents in Pinus Brutia Bark Extracts
| Polyphenolic constituent | Extractive fraction | Retention time (min) | MS data | Identification |
|---|---|---|---|---|
| Dimer 1 | PbE2 | 11.78 | [M−H]− at m/z 577.3 | Lit.e |
| [2M−H]− at m/z 1155.7 | ||||
| fragment ions at m/z 286.7a, m/z 289.2a, m/z 407.6b, m/z 425.3c, m/z 451.2d | ||||
| Dimer 2 | PbE2 | 12.10 | [M−H]− at m/z 577.3 | Lit.e |
| [2M−H]− at m/z 1155.4 | ||||
| fragment ions at m/z 286.8a, m/z 289.2a, m/z 407.2b, m/z 425.2c, m/z 451.2d | ||||
| Catechinf | PbE2 | 12.53 | [M−H]− at m/z 289.0 | St.g |
| [2M−H]− at m/z 579.1 | ||||
| fragment ion at m/z 244.7h | ||||
| Trimer 1 | PbE2 | 12.95 | [M−H]− at m/z 865.5 | Lit.e |
| Trimer 2 | PbE2 | 15.89 | [M−H]− at m/z 865.4 | Lit.e |
| Taxifolin-O-hexoside | PbE2 | 16.83 | [M−H]− at m/z 465.1 | Lit.e |
| [2M−H]− at m/z 931.4 | ||||
| [A]− at m/z 303.2 | ||||
| [A−H2O]− at m/z 284.6 | ||||
| Taxifolin | PbE1 | 16.92 | [M−H]− at m/z 302.9 | St.g |
| [2M−H]− at m/z 607.3 | ||||
| [M−H2O]− at m/z 284.8 |
Fragment ions formed through aquinone methide cleavage of the interflavonoid bond; bloss of water from the ion at m/z 425; cretro–Diels–Alder fragmentation; dheterocyclic ring fission; hloss of CH2=CH-OH from [M−H]− at m/z 289.
Co-elution with an unidentified compound.
Comparison of UV and MS spectral data with those of the standard.
All P. brutia extracts scavenged ABTS•+ in a concentration-dependent manner; at 2.5 mg/mL, all extracts were very effective in eliminating the radical, showing more than 94% scavenging activity (data not shown). All tested extracts (TEAC=0.89±0.01−1.54±0.00) were less active than the positive control, quercetin (TEAC=5.62±0.04). On the basis of the TEAC values, the ABTS•+-scavenging effects of P. brutia extracts and quercetin can be ranked in the following order: quercetin > PbE4 > PbE > PbE2 > PbE1 > PbE3 (Table 3).
Table 3.
Antioxidant Activities of Pinus brutia Bark Extracts
| Trolox equivalent antioxidant capacity assay TEAC | Reducing power assay EC50 (μg/mL) | Superoxide anion radical scavenging assay EC50 (μg/mL) | Hydroxyl radical scavenging assay EC50 (mg/mL)a | 15-LO inhibition assay EC50 (μg/mL) | |
|---|---|---|---|---|---|
| PbE | 1.47 ± 0.02††††,‡,####,$$$$,¶¶¶¶ | 9.17 ± 0.13††††,‡,####,$,¶¶¶¶ | 39.37 ± 0.85††††,‡‡‡,####,$$$,¶¶¶¶ | 0.5 ± 0.0†††† | 22.47 ± 0.75††††,‡,##,¶¶ |
| PbE1 | 1.08 ± 0.04****,‡‡‡‡,####,$$$$,¶¶¶¶ | 10.27 ± 0.11****,‡‡‡‡,###,$$,¶¶¶¶ | 70.30 ± 2.65****,‡‡‡‡,###,$$$$,¶¶¶¶ | 1.07 ± 0.06****,‡‡‡‡,####,$$$$,¶¶¶¶ | 34.43 ± 2.25****,‡‡‡,###,$$$$,¶¶¶¶ |
| PbE2 | 1.43 ± 0.01*,††††,####,$$$$,¶¶¶¶ | 9.49 ± 0.01*,††††,####,¶¶¶¶ | 51.10 ± 2.66***,††††,$$$$,¶¶¶¶ | 0.53 ± 0.06††††,¶¶¶¶ | 24.3 ± 0.4*,†††,$,¶¶¶¶ |
| PbE3 | 0.89 ± 0.01****,††††,‡‡‡‡,$$$$,¶¶¶¶ | 11.38 ± 0.24****,†††,‡‡‡‡,$$$$,¶¶¶¶ | 54.93 ± 2.85****,†††,$$$$,¶¶¶¶ | 0.6 ± 0.0††††,$ | 24.9 ± 0.5**,†††,$$$$,¶¶¶¶ |
| PbE4 | 1.54 ± 0.00****,††††,‡‡‡‡,####,¶¶¶¶ | 9.54 ± 0.18*,††,####,¶¶¶¶ | 33.5 ± 1.1***,††††,‡‡‡‡,####,¶¶¶¶ | 0.47 ± 0.06††††,#,¶¶¶¶ | 22.93 ± 0.55††††,‡,##,¶¶¶ |
| Quercetin | 5.62 ± 0.04****,††††,‡‡‡‡,####,$$$$ | 3.4 ± 0.2****,††††,‡‡‡‡,####,$$$$ | 26.63 ± 0.75****,††††,‡‡‡‡,####,$$$$ | 0.2 ± 0.0††††,‡‡‡‡,$$$$ | 18.70 ± 0.85**,††††,‡‡‡‡,####,$$$ |
For the hydroxyl radical scavenging assay, statistical comparisons for PbE vs. PbE3, PbE vs, quercetin, and PbE3 vs. quercetin were not determined.
Significant differences between samples (extracts, quercetin) are indicated: *P < .05, **P < .01, ***P < .002, ****P < .001 vs. PbE; ††P < .01, †††P < .002, ††††P < .001 vs. PbE1; ‡P < .05, ‡‡‡P < .002, ‡‡‡‡P < .001 vs. PbE2; #P < .05, ##P < .01, ###P < .002, ####P < .001 vs. PbE3; $P < .05, $$P < .01, $$$P < .002, $$$$P < .001 vs. PbE4; ¶¶P < .01, ¶¶¶P < .002, ¶¶¶¶P < .001 vs. quercetin.
The reducing power assay showed no important differences among P. brutia extracts; the EC50 values ranged from 9.17±0.13 to 11.38±0.24 μg/mL. Quercetin (EC50=3.40±0.20 μg/mL) was more active than P. brutia extracts. In terms of the EC50 values, the reducing power decreased in the following order: quercetin > PbE > PbE2 > PbE4 >PbE1 > PbE3 (Table 3).
The results of the superoxide scavenging assay showed significant differences in activity. According to the EC50 values, PbE4 (33.5±1.1 μg/mL) and PbE (39.37±0.85 μg/mL) were very efficient in scavenging the superoxide anion radical; their activity was slightly lower than that of quercetin (26.63±0.75 μg/mL). The lowest scavenging activity was found for PbE1 (70.30±2.65 μg/mL). With regard to the EC50 values, the superoxide scavenging effects might be ranked as follows: quercetin > PbE4 > PbE >PbE2 > PbE3 > PbE1 (Table 3).
In the hydroxyl radical scavenging assay, the activity decreased in the following order: quercetin > PbE4 > PbE >PbE2 > PbE3 > PbE1. According to the EC50 values, PbE4 (0.47±0.06 mg/mL) was the most potent hydroxyl radical scavenger, while PbE1 (1.07±0.06 mg/mL) showed the lowest scavenging activity. All extracts were less active than quercetin (0.2±0.0 mg/mL; Table 3).
All P. brutia bark extracts exerted noticeable inhibitory effects on 15-LO. In terms of the EC50 values, the effects of PbE, PbE2, PbE3, and PbE4 (22.47±0.75–24.9±0.5 μg/mL) were slightly lower than that of quercetin (18.70±0.85 μg/mL). The 15-LO inhibitory effects might be ranked as follows: quercetin > PbE > PbE4 > PbE2 > PbE3 > PbE1 (Table 3).
Discussion
This study showed that a polyphenol-rich extract from P. brutia bark and its fractions possess strong free radical scavenging and 15-LO inhibitory effects. Since 15-LO and reactive oxygen species such as superoxide and hydroxyl radicals promote pathological conditions leading to the development of many diseases, these extracts are, undoubtedly, interesting for therapeutics.
Despite a low reactivity, superoxide anion radical is highly toxic due to its conversion into more aggressive reactive species (hydroxyl radical, peroxynitrite anion, and singlet oxygen).38,39 There is scientific evidence for the involvement of superoxide anion in atherosclerosis, ischemia-reperfusion injury, high microvascular permeability, neurodegeneration, inflammation, and gout.40,41 Quercetin, the positive control in our study, is an efficient scavenger of superoxide anion radical. In different enzymatic and nonenzymatic superoxide-generating systems, quercetin exerted stronger scavenging effects than other natural or synthetic antioxidants (quercitrin, cyanidol, rutin, myricetin, Ginkgo biloba extract, and butylated hydroxyanisole).31,42,43 The EC50 values suggest high abilities to neutralize the superoxide anion radical for all P. brutia bark extracts, but to a lesser extent, for PbE1.
Hydroxyl radical is one of the most harmful reactive oxygen species damaging molecules (DNA, polyunsaturated fatty acids, proteins, and sugars) in or close to the generation site. Hydroxyl radical-induced oxidative damage is known to be involved in cancer, inflammation, neurodegeneration, and also in aging.34,39 Since quercetin is a highly effective hydroxyl radical scavenger,42,43 it is obvious that, except PbE1, all other P. brutia bark extracts, having EC50 values about 2.3–3 times higher than that of quercetin, have good hydroxyl radical scavenging effects.
Mammalian 15-LO is involved in many processes that play important roles in the development of early atherosclerotic lesions such as the oxidation of low-density lipoproteins, the recruitment of circulating monocytes to the vessel wall, and the proliferation of vascular smooth muscle cells. On the other hand, 15-hydroperoxy eicosatetraenoic acid (15-HPETE), a 15-LO arachidonic acid metabolite, has been reported to have not only pro-thrombotic but also beneficial effects as a precursor of vasodilator and anti-inflammatory lipoxins.44 Although the role of 15-LO in atherosclerosis remains controversial, there is emerging evidence for its involvement in diabetes, hypertension, renal disease, Alzheimer's disease, and Parkinson's disease.45 Within this assay, P. brutia extracts were slightly less active than quercetin, a well-known 15-LO inhibitor.46
According to the TEAC and EC50 values, the crude extract (PbE) and its ethyl acetate (PbE2) and aqueous (PbE4) fractions were the most potent in all antioxidant assays. The fractionation of bark crude extracts by immiscible solvent–solvent partitioning has been reported to separate antioxidant-rich fractions.7,47 On the contrary, in our study, the fractionation of the crude extract did not afford fractions with a considerably higher activity, supporting the idea that the phytocomplex in the crude extract can be as effective or even more effective than the fractions containing a part of its constituents. It is noteworthy that the use of the crude extract might have economic benefits, as it saves the fractionation costs.
Literature abounds in reports on the polyphenol content and antioxidant effects of plant extracts. In this regard, it needs to be mentioned that the antioxidant activity revealed for P. brutia bark extracts has both therapeutic and economic significance. The extracts showed strong antioxidant effects when compared with quercetin, a very potent antioxidant agent; these strong antioxidant effects represent a promising result for the valorization of a by-product of the wood industry. A literature survey showed no information regarding toxic constituents in P. brutia bark. Besides, extracts from P. pinaster and P. radiata barks were reported to have no toxic effects in human studies being considered safe.4,48 P. brutia bark is, undoubtedly, a valuable source of antioxidants and can be used as raw material in the development of antioxidant dietary supplements. Further in vivo studies are needed to assess the efficacy and toxicity of P. brutia bark extracts.
Acknowledgments
E.C. gratefully acknowledges the financial support provided by POSDRU/88/1.5/S/58965. The support from the Cyprus Research Promotion Foundation (CRPF grant KY-POY/0407/03) and the National Authority for Scientific Research in Romania (Bilateral Cooperation 311/2009) is greatly appreciated.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Packer L. Rimbach G. Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, Pycnogenol. Free Radic Biol Med. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 2.Östlund L. Ahlberg L. Zackrisson O. Bergman I. Arno S. Bark-peeling, food stress and tree spirits-the use of pine inner bark for food in Scandinavia and North America. J Ethnobiol. 2009;29:94–112. [Google Scholar]
- 3.D'Andrea G. Pycnogenol: a blend of procyanidins with multifaceted therapeutic applications? Fitoterapia. 2010;81:724–736. doi: 10.1016/j.fitote.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Maimoona A. Naeem I. Saddiqe Z. Jameel K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J Ethnopharmacol. 2011;133:261–277. doi: 10.1016/j.jep.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Bito T. Roy S. Sen CK. Packer L. Pine bark extract Pycnogenol downregulates IFN-γ-induced adhesion of T cells to human keratinocytes by inhibiting inducible ICAM-1 expression. Free Radic Biol Med. 2000;28:219–227. doi: 10.1016/s0891-5849(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 6.Grimm T. Schäfer A. Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (Pycnogenol) Free Radic Biol Med. 2004;36:811–822. doi: 10.1016/j.freeradbiomed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Ku CS. Mun SP. Antioxidant properties of monomeric, oligomeric and polymeric fractions in hot water extract from Pinus radiata bark. Wood Sci Technol. 2008;42:47–60. [Google Scholar]
- 8.Yu L. Zhao M. Wang JS, et al. Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb) bark. Innov Food Sci Emerg Technol. 2008;9:122–128. [Google Scholar]
- 9.Yesil-Celiktas O. Ganzera M. Akgun I. Sevimli C. Korkmaz KS. Bedir E. Determination of polyphenolic constituents and biological activities of bark extracts from different Pinus species. J Sci Food Agric. 2009;89:1339–1345. [Google Scholar]
- 10.Apetrei CL. Tuchilus C. Aprotosoaie AC. Oprea A. Malterud KE. Miron A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules. 2011;16:7773–7788. doi: 10.3390/molecules16097773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karonen M. Hämäläinen M. Nieminen R, et al. Phenolic extractives from the bark of Pinus sylvestris L. and their effects on inflammatory mediators nitric oxide and prostaglandin E2. J Agric Food Chem. 2004;52:7532–7540. doi: 10.1021/jf048948q. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y-M. Wang M-H. Rhee H-I. A novel α-glucosidase inhibitor from pine bark. Carbohydr Res. 2004;339:715–717. doi: 10.1016/j.carres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 13.El-Zein O. Kreydiyyeh SI. Pine bark extract inhibits glucose transport in enterocytes via mitogen-activated kinase and phosphoinositol 3-kinase. Nutrition. 2011;27:707–712. doi: 10.1016/j.nut.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Roussis V. Petrakis PV. Ortiz A. Mazomenos BE. Volatile constituents of needles of five Pinus species grown in Greece. Phytochemistry. 1995;39:357–361. [Google Scholar]
- 15.Lardos A. The botanical materia medica of the Iatrosophikon—a collection of prescriptions from a monastery in Cyprus. J Ethnopharmacol. 2006;104:387–406. doi: 10.1016/j.jep.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Ustun O. Senol FS. Kurkcuoglu M. Orhan IE. Kartal M. Baser KHC. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind Crops Prod. 2012;38:115–123. [Google Scholar]
- 17.Ince I. Yesil-Celiktas O. Karabay-Yavasoglu NU. Elgin G. Effects of Pinus brutia bark extract and Pycnogenol® in a rat model of carrageenan induced inflammation. Phytomedicine. 2009;16:1101–1104. doi: 10.1016/j.phymed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Guri A. Kefalas P. Roussis V. Antioxidant potential of six pine species. Phytother Res. 2006;20:263–266. doi: 10.1002/ptr.1848. [DOI] [PubMed] [Google Scholar]
- 19.Ciesla WM. Forests and forest protection in Cyprus. The Forestry Chronicle. 2004;80:107–113. [Google Scholar]
- 20.Singleton VL. Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- 21.Wangensteen H. Samuelsen AB. Malterud KE. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–297. [Google Scholar]
- 22.Ozcan B. Yilmaz M. Caliskan M. Antimicrobial and antioxidant activities of various extracts of verbascum antiochium Boiss. (Scrophulariaceae) J Med Food. 2010;13:1147–1152. doi: 10.1089/jmf.2009.0213. [DOI] [PubMed] [Google Scholar]
- 23.Sun JS. Tsuang YW. Chen IJ. Huang WC. Hang YS. Lu FJ. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns. 1998;24:225–231. doi: 10.1016/s0305-4179(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 24.Adedapo AA. Jimoh FO. Afolayan AJ. Masika PJ. Antioxidant properties of the methanol extracts of the leaves and stems of Celtis africana. Rec Nat Prod. 2009;3:23–31. [Google Scholar]
- 25.Karonen M. Loponen J. Ossipov V. Pihlaja K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal Chim Acta. 2004;522:105–112. [Google Scholar]
- 26.Re R. Pellegrini N. Proteggente A. Pannala A. Yang M. Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 27.Cai Y-Z. Sun M. Xing J. Luo Q. Corke H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Bouayed J. Hoffmann L. Bohn T. Antioxidative mechanisms of whole-apple antioxidants employing different varieties from Luxembourg. J Med Food. 2011;14:1631–1637. doi: 10.1089/jmf.2010.0260. [DOI] [PubMed] [Google Scholar]
- 29.Berker KI. Güçlü K. Tor İ. Apak R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP) and ferricyanide reagents. Talanta. 2007;72:1157–1165. doi: 10.1016/j.talanta.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira ICFR. Baptista P. Vilas-Boas M. Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem. 2007;100:1511–1516. [Google Scholar]
- 31.Robak J. Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z. Luo D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr Polym. 2007;68:54–58. [Google Scholar]
- 33.Jeong JB. Hong SC. Jeong HJ. 3,4-Dihydroxybenzaldehyde purified from the barley seeds (Hordeum vulgare) inhibits oxidative DNA damage and apoptosis via its antioxidant activity. Phytomedicine. 2009;16:85–94. doi: 10.1016/j.phymed.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Guo T. Wei L. Sun J. Hou C-L. Fan L. Antioxidant activities of extract and fractions from Tuber indicum Cooke & Massee. Food Chem. 2011;127:1634–1640. [Google Scholar]
- 35.Tsimogiannis D. Samiotaki M. Panayotou G. Oreopoulou V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules. 2007;12:593–606. doi: 10.3390/12030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gođevac D. Tešević V. Veličković M. Vujisić L. Vajs V. Milosavljević S. Polyphenolic compounds in seeds from some grape cultivars grown in Serbia. J Serb Chem Soc. 2010;75:1641–1652. [Google Scholar]
- 37.Saleem A. Kivelä H. Pihlaja K. Antioxidant activity of pine bark constituents. Z Naturforsch C. 2003;58:351–354. doi: 10.1515/znc-2003-5-611. [DOI] [PubMed] [Google Scholar]
- 38.Afonso V. Champy R. Mitrovic D. Collin P. Lomri A. Reactive oxygen species and superoxide dismutases: role in joint disease. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Goetz ME. Luch A. Reactive species: A cell damaging rout assisting to chemical carcinogens. Cancer Lett. 2008;266:73–83. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 40.White CR. Brock TA. Chang LY, et al. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci USA. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mancuso C. Siciliano R. Barone E. Preziosi P. Natural substances and Alzheimer's disease: from preclinical studies to evidence based medicine. Biochim Biophys Acta. 2012;1822:616–624. doi: 10.1016/j.bbadis.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Wang L. Tu Y-C. Lian T-W. Hung J-T. Yen J-H. Wu M-J. Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem. 2006;54:9798–9804. doi: 10.1021/jf0620719. [DOI] [PubMed] [Google Scholar]
- 43.Zhu L. Tan J. Wang B. He R. Liu Y. Zheng C. Antioxidant activities of aqueous extract from Agrimonia pilosa Lebed and its fractions. Chem Biodivers. 2009;6:1716–1726. doi: 10.1002/cbdv.200800248. [DOI] [PubMed] [Google Scholar]
- 44.Wittwer J. Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Dobrian AD. Lieb DC. Cole BK. Taylor-Fishwick DA. Chakrabarti SK. Nadler JL. Functional and pathological roles of 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wangensteen H. Miron A. Alamgir M. Rajia S. Samuelsen AB. Malterud KE. Antioxidant and 15-lipoxygenase inhibitory activity of rotenoids, isoflavones and phenolic glycosides from Sarcolobus globosus. Fitoterapia. 2006;77:290–295. doi: 10.1016/j.fitote.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Diouf PN. Stevanovic T. Cloutier A. Antioxidant properties and polyphenol contents of trembling aspen bark extracts. Wood Sci Technol. 2009;43:457–470. [Google Scholar]
- 48.Frevel MAE. Pipingas A. Grigsby WJ. Frampton CM. Gilchrist NL. Production, composition and toxicology studies of Enzogenol® Pinus radiata bark extract. Food Chem Toxicol. 2012;50:4316–4324. doi: 10.1016/j.fct.2012.08.051. [DOI] [PubMed] [Google Scholar]