Abstract
Bark infusion of Tabebuia avellanedae Lorentz ex Griseb is consumed in tropical America folk medicine for the treatment of several diseases, including depressive disorders. It was recently demonstrated that the extract from this plant has antidepressant properties. The present study was aimed at investigating the contribution of N-methyl-D-aspartate (NMDA) receptors and the L-arginine–nitric oxide (NO)–cyclic guanosine 3′5′-monophosphate (cGMP) pathway to the antidepressant-like action of the ethanolic extract from T. avellanedae (EET) in the tail suspension test (TST). The anti-immobility effect of the extract (30 mg/kg, orally [p.o.]) was prevented by pretreatment of mice with NMDA (0.1 pmol/site, intracerebroventicular [i.c.v.]), L-arginine (750 mg/kg, intraperitoneally [i.p.]), and sildenafil (5 mg/kg, i.p.). Additionally, the combination of MK-801 (0.01 mg/kg, p.o.), 7-nitroindazole (25 mg/kg, i.p.), and 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) (30 pmol/site, i.c.v.) with a subeffective dose of EET (1 mg/kg, p.o.) produced a synergistic antidepressant-like effect in the TST, without causing significant alterations in the locomotor activity. Moreover, the administration of an effective dose of EET (30 mg/kg, p.o.) produced a reduction in NOx levels in the cerebral cortex. Conversely, a subeffective dose of EET (1 mg/kg, p.o.) caused no changes in the cortical NOx levels. Results suggest that the antidepressant-like effect of EET in the TST is dependent on a blockade of NMDA receptor activation and inhibition of NO-cGMP synthesis, significantly extending literature data about the antidepressant-like action of this plant and reinforcing the notion that this plant may be useful in the management of depressive disorders.
Key Words: depression, L-arginine–nitric oxide–cGMP pathway, NMDA receptors, Tabebuia avellanedae, tail suspension test
Introduction
Depression is a common mental disorder associated with high rates of suicide, severe functional impairment, high rates of comorbid mental disorders, intensive use of treatment, and high costs to society.1,2 According to projections from the World Health Organization, depression will be the second leading cause of disability in the developed world by 2020.3
Despite the advances in the treatment of depression with selective serotonin reuptake inhibitors and serotonin and noradrenaline reuptake inhibitors, the major drawbacks of the conventional antidepressant therapy are related to its relatively low efficacy and side effects.4 Therefore, considerable efforts are invested in the search for better drugs and even combined treatments approaches for the management of depression.
Tabebuia avellanedae Lorentz ex Griseb (Bignoniaceae) is a tree native to tropical rain forests in the northeast of Brazil commonly known as “paud'arco” or “ipê-roxo.” This species has been traditionally used for various ethnopharmacological purposes. Colombians drink the bark infusion as stimulant of central nervous system;5 Bahamians commonly use the bark decoction to prepare an energizing tonic for strength,5 and Brazilians drink the bark infusion of this plant to treat malaria, cancer, fever, stomach disorders, bacterial and fungal infections, and to the relief of a variety of mental and emotional states such as anxiety, poor memory, irritability, and depression.6,7 Moreover, it was demonstrated that T. avellanedae ethanolic extract (EET) has a protective action against gastric lesions in rats,8,9 and a recent study by Freitas et al.10 demonstrated that the administration of EET exerts an antidepressant-like effect in the tail suspension test (TST), a behavioral test used to assess the efficacy of antidepressant compounds.11 The antidepressant-like action of this extract was reported to be mediated by an activation of the monoaminergic systems. Further, EET produced a synergistic antidepressant-like effect when combined with conventional antidepressants.10 Additionally, a recent study performed by our group demonstrated that the administration of EET during 14 days is able to abolish the increased immobility time in the TST in bulbectomized mice.12
Besides the monoaminergic systems, several other targets have been implicated in the pathogenesis of depressive disorders. Special emphasis has been given to the N-methyl-D-aspartate (NMDA) receptors and the L-arginine–nitric oxide (NO)–cyclic guanosine 3′5′-monophosphate (cGMP) pathway.13,14 Several biochemical,15 electrophysiological,16 and behavioral17 studies have shown that increased monoaminergic neurotransmission by treatment with antidepressants is associated with a reduction of either NMDA receptor function or NO synthesis.18,19
Glutamatergic function is altered in mood disorders and evidence from literature has shown that antidepressants may exert its action by blocking NMDA receptors.13,20 In addition, glutamatergic modulators, including NMDA receptor antagonists, exhibit antidepressant-like actions in several behavioral paradigms, such as inescapable stress,21 chronic mild stress,22 and forced swimming test (FST).23
Calcium influx, via NMDA receptors, induces the activation of the enzyme nitric oxide (NO) synthase (NOS). The activated NOS then converts L-arginine to NO and L-citrulline.24 NO, a messenger molecule in the brain has been implicated in pathophysiology of depression.14,25 In neurons, the best characterized target for NO is soluble guanylate cyclase (sGC), which is activated by NO and produces the intracellular messenger cGMP.24 Several studies have demonstrated that NOS inhibitors produce antidepressant-like actions in a variety of animal paradigms.14,25
Considering that (1) NMDA receptors and the NO–cGMP pathway are involved in the pathogenesis of depression;13,14 (2) the importance of these molecular targets for the efficacy of antidepressants;26,27 (3) the relationship between the monoaminergic systems and the blockade of the NMDA receptors and the modulation of NO synthesis;13,19 and (4) the antidepressant-like effect of EET in the TST was reported to be dependent on the modulation of the monoaminergic systems,10 but its mechanisms of action still remain to be fully established; the aim of the present study was to investigate the participation of NMDA receptors and L-arginine–NO–cGMP pathway in the antidepressant-like effect of EET in the TST.
Materials and Methods
Plant material and preparation of EET
T. avellanedae barks were provided by Chamel Indústria e Comércio de Produtos Naturais Ltda (Campo Largo, Brazil), lot 4753. The identification was performed by the botanist Elide Pereira dos Santos and a voucher specimen has been deposited at the Herbarium of the Department of Botany at the Universidade Federal do Paraná (UFPR), Brazil. Dried and powdered barks (5 kg) were extracted three times by maceration with 95% ethanol for 7 days at room temperature. The combined ethanolic extract was filtered, the solvent evaporated under reduced pressure (40–50°C), and lyophilized to give a red-brown solid (919.2 g; 18.4% yield), as described previously.8
Phytochemical analyses of EET
The analyses of EET was performed in a capillary electrophoresis system (CE; HP3DCE, Agilent Technologies, Palo Alto, CA, USA) equipped with a diode array detector set a 200 nm. The measurements were conducted at 25°C in an uncoated fused-silica capillary (48.5 cm×50 μm inner diameter×375 μm outer diameter) obtained from Polymicro (Phoenix, AZ, USA). In the first conditioning, the capillary was washed for 30 min with sodium hydroxide 1.0 M followed by deionized water for 30 min. Between runs the capillary was rinsed for 5 min with running electrolyte (sodium tetraborate 20 mmol L-1 and methanol 10%, pH 9.0). Standard solutions and samples were introduced from the inlet capillary extremity and hydrodynamically injected at 50 mbar (50 mbar=4996.2 Pa) for 6 s. The applied separation voltage was 30 kV, positive polarity in the injection side. Caffeic acid (100 mg/L) was utilized as internal standard and detection was done at 330 nm. Data acquisition and analysis were performed with HP Chemstation software. Sample preparation: 0.5299 g of EET were solubilized into 10 mL of methanol:water 50% (v/v). As reported previously8 the following compounds were identified by the electropherogram of a sample of EET: p-hydroxybenzoic acid, anisic acid, veratric acid, and caffeic acid.
Animals
Adult female Swiss mice (30–40 g) were maintained at constant room temperature (20–22°C) with free access to water and food, under a 12:12 h light:dark cycle (lights on at 7:00 a.m.). The cages were placed in the experimental room 24 h before the test for acclimatization. All manipulations were carried out between 9:00 a.m. and 5:00 p.m., with each animal used only once (n=8–9 animals per group). The procedures in this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the local Ethics Committee. All efforts were made to minimize animal suffering and the number of animals used in the experiments.
Drugs and treatment
The following drugs were used: L-arginine, NMDA (N-methyl-D-aspartate), 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ), 7-nitroindazole (Sigma Chemical Co., St. Louis, MO, USA), MK-801 (RBI, Boston, MA, USA), and sildenafil (Pfizer, São Paulo, SP, Brazil). All drugs were dissolved in saline, except ODQ, which was dissolved in saline with 1% DMSO and 7-nitroindazole that was dissolved in 1%Tween 80. All drugs were administered by intraperitoneal (i.p.) route in a constant volume of 10 ml/kg body weight, except NMDA and ODQ, which were administered by intracerebroventricular (i.c.v.) route. Appropriate vehicle-treated groups were also assessed simultaneously.
The i.c.v. injections were performed under ether anesthesia according to the procedure described previously.28 Briefly, a 0.4 mm external diameter hypodermic needle attached to a cannula, which was linked to a 25 μL Hamilton syringe, was inserted perpendicularly through the skull and no more than 2 mm into the brain of the mice. A volume of 5 μL was then administered into the left lateral ventricle. The injection was given over 30 s, and the needle remained in place for another 30 s to avoid the reflux of the substances injected. The injection site was 1 mm to the left from the mid-point on a line drawn through to the anterior base of the ears. To ascertain that the drugs were administered exactly into the cerebral ventricle, the brains were dissected and macroscopically examined after the test. Results from mice presenting misplacement of the injection site or any signs of cerebral hemorrhage (<5%) were discarded from statistical analysis. Worth of note is the previous data from our laboratory that do not show significant differences from immobility time in the TST and locomotor activity in the open-field test in control mice that received an i.c.v. injection (previously submitted to ether anesthesia),when compared to animals that received i.p. or oral (p.o.) administration of drugs (without ether anesthesia).29,30
EET (1–30 mg/kg) was dissolved in distilled water with 5% Tween 80 and was administered acutely p.o. by gavage 60 min before the TST or the open-field test. The dissolution of the extract was freshly done from the lyophilized power immediately before its administration. A control group received distilled water with 5% Tween 80 as vehicle. All the animals were fasted for 120 min before the oral treatment.
In the experiments designed to study whether the antidepressant-like effect of the extract in the TST is mediated through the inhibition of NMDA receptors, mice were pretreated with vehicle or extract (30 mg/kg, p.o.) and 30 min later they received saline or NMDA (0.1 pmol/site, i.c.v.). Sixty minutes later, the TST or the open-field test were carried out.
To investigate a possible synergistic effect of the extract with a NMDA receptor antagonist, animals received by p.o. route a subeffective of MK-801 (0.01 mg/kg, p.o., a noncompetitive NMDA-receptor antagonist) or saline and immediately after, a subeffective dose of the extract (1 mg/kg, p.o.) or vehicle were administered. Sixty minutes later, the TST or the open-field test were carried out.
In a separate series of experiments, the involvement of the L-arginine–NO pathway in the anti-immobility actions of the extract in the TST was verified. To this end, mice were pretreated with L-arginine, a precursor of NO (750 mg/kg, i.p., a dose that produces no effect in the TST31) or saline and after 30 min they received extract (30 mg/kg, p.o.) or vehicle injection before being tested in the TST after 60 min.
To study the effect of the combined administration of subeffective doses of the extract (1 mg/kg, p.o.) with subeffective doses of 7-nitroindazole (25 mg/kg, i.p., a specific neuronal NO synthase inhibitor) or ODQ (30 pmol/site i.c.v., a specific sGC inhibitor), extract or vehicle was administered 30 min before of the drugs and 30 min later the animals were tested in the TST.
To verify the role of cyclic GMP (cGMP) in the antidepressant action of the extract mice received an injection of sildenafil (5 mg/kg, i.p., a phosphodiesterase (PDE) inhibitor), or saline 30 min before the extract (30 mg/kg, p.o.), and 60 min later, the TST or the open-field test were carried out.
We also assessed the effect of the extract on NO levels by measuring the production of nitrite formed from the metabolism of NO. Animals received distilled water or extract (1 or 30 mg/kg, p.o.). After 60 min, animals were killed by decapitation and cerebral cortices were rapidly removed. Briefly, cerebral cortices were homogenized with 25% trichoroacetic acid and centrifuged at 1800 g for 10 min. The supernatant was immediately neutralized with 2 M potassium bicarbonate. Nitrate (NO3−) was reduced to nitrite (NO2−) by nitrate reductase. The total NO2− in the incubation was measured by a colorimetric assay read at 540 nm, based on the Griess reaction. A standard curve was performed using sodium nitrate (0–80 μM).32
The doses of the drugs used were chosen on the basis of literature and are previously reported not to increase locomotor activity.23,25,28,31 The dose–response curve of EET in the TST was established in a work previously performed by our group.10 The doses of the extract used in the present study were selected on the basis of these experiments.
Tail suspension test
A previous study performed by our group showed that EET is able to produce an antidepressant-like effect in the TST (10–100 mg/kg, p.o.), and in the FST (100 mg/kg, p.o.).10 In the present study, we choose TST, because in this test EET is more effective, that is, produces a significant effect at lower doses compared with the FST. The total duration of immobility induced by tail suspension was measured according to the method described by Steru et al.11 Mice both acoustically and visually isolated were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was registered during a 6-min period.10,33–35
Open-field test
To assess the effects of EET on locomotor activity, mice were evaluated in the open-field paradigm as previously described.35 Animals were individually placed in a wooden box (40 cm×60 cm×50 cm) with the floor divided into 12 rectangles. The number of squares crossed with all paws (crossing) was counted in a 6 min session. The apparatus was cleaned with a solution of 10% ethanol between tests to hide animal clues.
Statistical analysis
For behavioral tests, comparisons between experimental and control groups were performed by two-way ANOVA followed by Tukey's HSD test when appropriate. For NOx assay, unpaired Student's t-test was used. A value of P<.05 was considered significant.
Results
Involvement of NMDA receptors in the antidepressant-like effect of EET in the TST
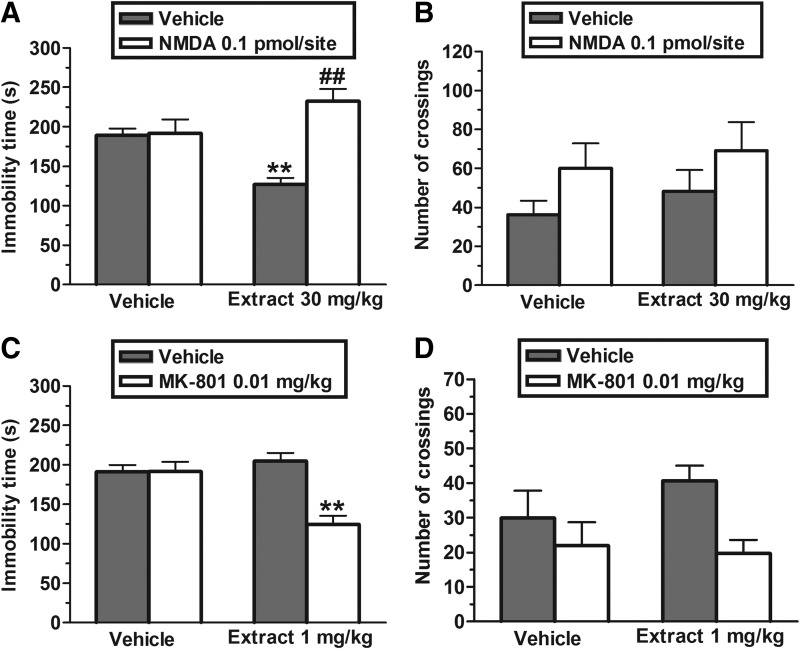
The results depicted in Figure 1A show that the pretreatment of mice with NMDA (0.1 pmol/site, i.c.v.) prevented the antidepressant-like effect of EET (30 mg/kg, p.o.) in the TST. The two-way ANOVA revealed significant differences of NMDA pretreatment [F(1,30)=18.44, P<.01] and NMDA pretreatment×EET treatment interaction [F(1,30)=16.46, P<.01], but not of EET treatment [F(1,30)=0.72, P=.40]. Post hoc analyses indicated that the pretreatment with NMDA blocked the decrease in immobility time produced by EET in the TST (P<.01). The administration of NMDA alone or in combination with EET did not affect the ambulation in the open-field (Fig. 1B). The two-way ANOVA revealed no differences for NMDA pretreatment [F(1,29)=3.60, P=.07], EET treatment [F(1,29)=0.81, P=.38], and NMDA pretreatment×EET treatment interaction [F(1,29)=0.02, P=.90].
FIG. 1.
Effect of the pretreatment of mice with NMDA (0.1 pmol/site, i.c.v.) on the anti-immobility effect of EET (30 mg/kg, p.o.) in the TST (A) and on locomotor activity in the open-field test (B). Effect of the combined treatment of mice with EET (1 mg/kg, p.o) and MK-801 (0.01 mg/kg, p.o.) in the TST (C) and on the number of crossings in the open-field test (D). Each column represents the mean±SEM of 8–9 animals. **P<.01 compared with the vehicle-treated control. ##P<.01 as compared with the extract alone. NMDA, N-methyl-D-aspartate; EET, ethanolic extract from Tabebuia avellanedae; TST, tail suspension test; i.c.v., intracerebrovascular route; p.o., oral route; SEM, standard error of the mean.
Figure 1C illustrates the effect of the administration of subeffective doses of MK-801 (0.01 mg/kg, p.o, a noncompetitive NMDA-receptor antagonist) and EET (1 mg/kg, p.o.) in the TST. The two-way ANOVA revealed significant differences of MK-801 treatment [F(1,30)=14.20, P<.01], EET treatment [F(1,30)=6.23, P<.01], and MK-801×EET treatment interaction [F(1,30)=14.31, P<.01]. Post hoc analyses indicated that the pretreatment with a subeffective dose of EET (1 mg/kg, p.o.) produced a synergistic effect with MK-801 (P<.01). However, Figure 1D shows that the co-administration of MK-801 and EET did not change the ambulation in the open-field test. A two-way ANOVA revealed no differences for EET treatment [F(1,29)=0.48, P=.49] and MK-801×EET treatment interaction [F(1,29)=1.12, P=.30], but showed a significant main effect for MK-801 treatment [F(1,29)=5.59, P<.05].
Involvement of L-arginine–NO–cGMP pathway in the anti-immobility effect of EET in the TST
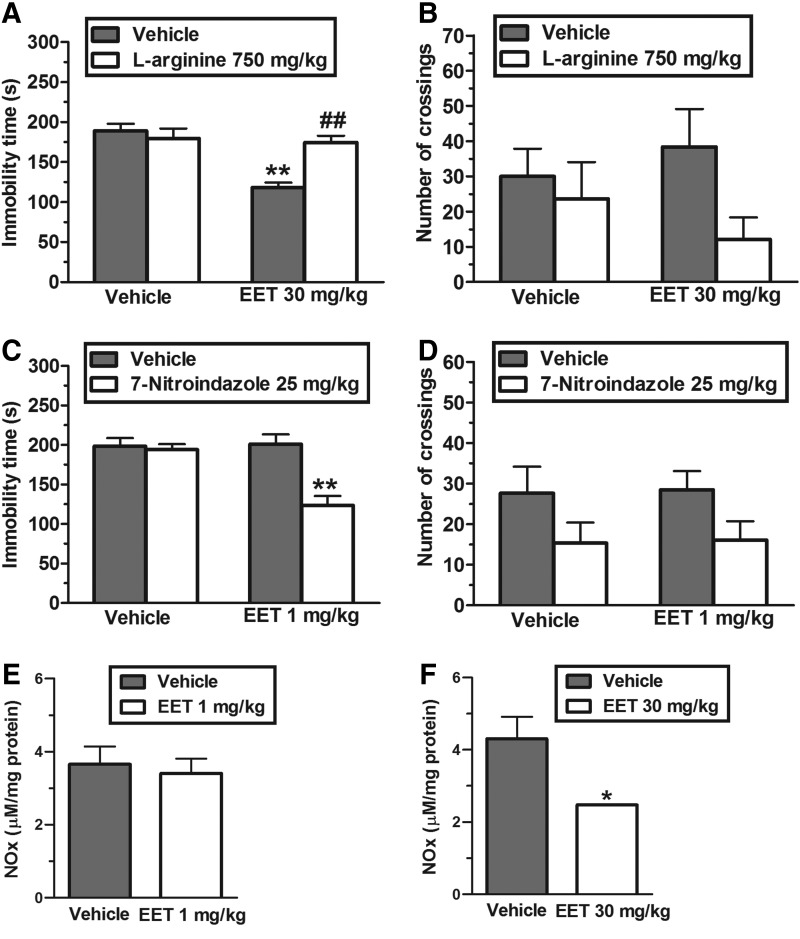
The results illustrated in Figure 2A show that the pretreatment of mice with L-arginine (750 mg/kg, i.p., an NO precursor) prevented the antidepressant-like effect of the EET (30 mg/kg, p.o.) in the TST. The two-way ANOVA revealed significant differences of L-arginine pretreatment [F(1,30)=6.51, P<.01], EET treatment [F(1,30)=17.16, P<.01], and L-arginine pretreatment×EET treatment interaction [F(1,30)=12.91, P<.01]. Post hoc analyses indicated that the anti-immobility effect of EET was completely prevented by pretreatment of animals with L-arginine (P<.01). Figure 2B shows that the administration of L-arginine and EET did not influence the locomotor activity of mice. The two-way ANOVA revealed no differences for L-arginine pretreatment [F(1,30)=3.20, P=.08], EET treatment [F(1,30)=0.03, P=.86] and L-arginine pretreatment×EET treatment interaction [F(1,30)=1.18, P=.30].
FIG. 2.
Effect of the pretreatment of mice with L-arginine (750 mg/kg, intraperitoneally [i.p.]) on the anti-immobility effect of EET (30 mg/kg, p.o.) in the TST (A) and on locomotor activity in the open-field test (B). Effect of the administration of a subeffective dose of EET (1 mg/kg, p.o.) with subeffective dose of 7-nitroindazole (25 mg/kg, i.p.) in the TST (C) and in the open-field test (D). The effect of EET administration (1 or 30 mg/kg, p.o., 1 h before decapitation) in the NOx levels in the cerebral cortex is shown in (E) and (F), respectively. Each column represents the mean±SEM of 8–9 animals. *P<.05 and **P<.01 compared with the vehicle-treated control. ##P<.01 as compared with the extract alone.
The results depicted in Figure 2C show that the combination of a subeffective dose of EET (1 mg/kg, p.o.) and a subeffective dose of 7-nitroindazole (25 mg/kg, i.p., a neuronal NOS inhibitor), reduced the immobility time of mice submitted to the TST. The two-way ANOVA revealed significant differences of 7-nitroindazole treatment [F(1,30)=14.65, P<.01], EET treatment [F(1,30)=10.14, P<.01], and 7-nitroindazole×EET treatment interaction [F(1,30)=11.65, P<.01]. Post hoc analyses revealed a synergistic antidepressant-like effect induced by 7-nitroindazole when co-administered with EET in the TST (P<.01). Figure 2D illustrates that the administration of 7-nitroindazole in combination with EET did not affect animal ambulation. A two-way ANOVA revealed no differences for EET treatment [F(1,29)=0.02, P=.88] and 7-nitroindazole×EET treatment interaction [F(1,29)=0.01, P=.99], but showed a significant main effect for 7-nitroindazole treatment [F(1,29)=5.27, P<.05].
Corroborating the results presented above, which suggest that a putative reduction in NO levels are implicated in the antidepressant-like effect of the extract, Figure 2D shows that a subeffective dose of EET (1 mg/kg, p.o.) caused no changes in the cortical NOx levels. However, an effective dose of EET in the TST (30 mg/kg, p.o.) significantly reduced the NOx levels in the cerebral cortex (P<.05), as compared with the control group (Fig. 2E).
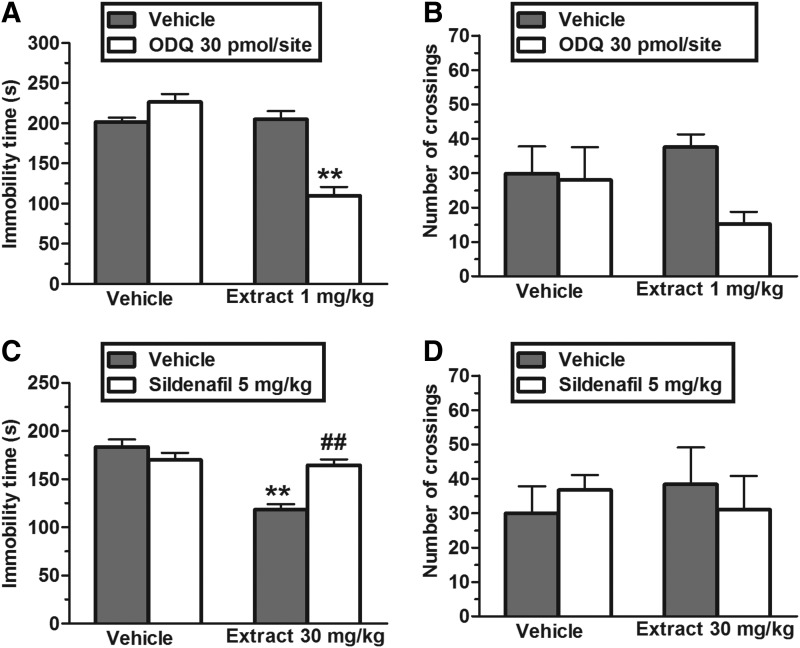
Figure 3A shows that the administration of a subeffective dose of ODQ (30 pmol/site, i.c.v., a sGC inhibitor) exhibited an antidepressant-like effect when combined with a subeffective dose of EET (1 mg/kg, p.o.). The two-way ANOVA revealed significant differences of ODQ treatment [F(1,30)=13.35, P<.01], EET treatment [F(1,30)=35.17, P<.01], and ODQ×EET treatment interaction [F(1,30)=39.84, P<.01]. Post hoc analyses indicated that the pretreatment with a subeffective dose of EET (1 mg/kg, p.o.) produced a synergistic effect with ODQ (P<.01). In addition, the administration of ODQ (30 pmol/site, i.c.v.) alone or in combination with EET did not significantly affect the ambulation in the open-field (Fig. 3B). A two-way ANOVA revealed no differences for ODQ treatment [F(1,30)=3.28, P=.08], EET treatment [F(1,30)=0.14, P=.71], and ODQ×EET treatment interaction [F(1,30)=2.34, P=.14].
FIG. 3.
Effect of the administration of a subeffective dose of EET (1 mg/kg, p.o.) with subeffective dose of ODQ (30 pmol/site i.c.v.) in the TST (A) and in the open-field test (B). Effect of the pretreatment of mice with sildenafil (5 mg/kg, i.p.) on the anti-immobility effect of EET (30 mg/kg, p.o.) in the TST (C) and on the locomotor activity in the open-field test (D). Each column represents the mean±SEM of 8–9 animals. **P<.01 compared with the vehicle-treated control. ##P<.01 as compared with the extract alone.
Figure 3C shows that the anti-immobility effect of EET (30 mg/kg, p.o.) was completely prevented by pretreatment of animals with sildenafil (5 mg/kg, i.p., a PDE5 inhibitor). The two-way ANOVA revealed significant differences of sildenafil pretreatment [F(1,29)=5.66, P<.05], EET treatment [F(1,29)=26.57, P<.01], and sildenafil pretreatment×EET treatment interaction [F(1,29)=18.93, P<.01]. Post hoc analyses indicated that the pretreatment of mice with sildenafil prevented the decrease in immobility time in the TST produced by the administration of EET (P<.01). The administration of sildenafil (5 mg/kg, i.p.) alone or in combination with EET did not affect the ambulation of mice (Fig. 3D). A two-way ANOVA revealed no differences for sildenafil pretreatment [F(1,30)=0.01, P=.98], EET treatment [F(1,30)=0.02, P=.88], and sildenafil pretreatment×EET treatment interaction [F(1,30)=0.65, P=.43].
Discussion
The preset work further contributes to the understanding of the mechanisms underlying the antidepressant-like action of EET in the TST, which was recently demonstrated, by our group, to be dependent on an interaction with the monoaminergic systems.10 EET given systemically (by p.o. route) is able to produce an anti-immobility effect through the modulation of either NMDA receptors or the L-arginine–NO–cGMP signaling pathway. In addition, this study extends literature by reinforcing the pivotal role of inhibition of NMDA receptors and NO-cGMP synthesis in the mechanism underlying the effects of antidepressant agents.
In the present study, the phytochemical analyses of EET identified the following compounds: p-hydroxybenzoic acid, anisic acid, veratric acid, and caffeic acid, as reported previously.8 Data from literature have shown that p-hydroxybenzoic acid has antimicrobial properties,36 anisic acid is an ant-inflammatory compound,37 veratric acid is antihypertensive and antioxidant,38 and caffeic acid is an antioxidant compound39 that has antidepressant effects.40–43 Thus, it is likely that caffeic acid alone or in combination with the other compounds identified in EET contributes to the antidepressant action of T. avellanedae.
The TST is a behavioral tool widely used for screening antidepressant activity of different classes of drugs.11,44 This test is based on the observation that animals, after initial escape-oriented movements, develop an immobile posture when placed in an inescapable stressful situation. When antidepressant treatments are given prior to the tests, the subjects will actively persist engaging in escape-directed behavior for longer periods of time than after vehicle treatment.45 In this study, a subeffective (1 mg/kg) and an effective (30 mg/kg) dose of EET were chosen on the basis of a previous study performed by our group.10 Noteworthy, these doses did not change the ambulatory activity of mice. It is relevant to mention that this study was performed in female mice, since several studies have shown that the prevalence of depression is about two fold higher in women than in men.46
The glutamatergic system plays a significant role in depressive disorders.13,47 The implication of NMDA inhibition in the mechanism of action of conventional antidepressants and putative antidepressant agents has been suggested by several studies, which show that the administration of NMDA is able to reverse the antidepressant-like effects of these compounds.15,19,26 In the present study, the pretreatment of mice with NMDA blocked the antidepressant-like effect of EET in the TST, also suggesting that the antidepressant-like effect of EET may be dependent on the inhibition of NMDA receptor activation. Further reinforcing this notion, the co-administration of mice with subeffective doses of EET and MK-801, a noncompetitive NMDA receptor antagonist, produced a synergistic antidepressant-like effect in the mouse TST. The reduction of locomotor activity observed in the group of mice that received MK-801 and EET did not account for the antidepressant-like effect induced by the combined administration of them. Noteworthy, MK-801, and other competitive and uncompetitive NMDA antagonists, exhibit antidepressant-like actions in the FST13,21 and TST.31 Our results are consistent with the fact that compounds that reduce transmission at NMDA receptors exert antidepressant-like actions.23,28,31 However, our results do not allow us to conclude about the mechanism by which the EET interacts with the NMDA receptor. Preclinical and clinical observations have shown that monoaminergic-based antidepressants reduce NMDA binding, expression and function.13,48 Additionally, several highly selective GluN2B antagonists have been reported to improve depressive symptoms in patients resistant to biogenic-amine-based agents.49
This study also evaluated the involvement of the L-arginine–NO–cGMP signaling pathway in the antidepressant-like action of EET. It has become generally accepted that NO plays a significant neuromodulatory role in the nervous system and the pharmacological manipulation of the NO–cGMP signaling pathway may constitute a novel therapeutic approach for the management of depression.14,25
In the present study we demonstrate that pretreatment with the NOS substrate, L-arginine, significantly prevented the anti-immobility effect of EET. Similarly, in another study, the antidepressant effects of imipramine were also blocked by pretreatment with L-arginine and contrary to this, NOS inhibitor, NG-nitro-Larginine augmented the behavioral effect of imipramine or fluoxetine in the FST.50 Thus, our result indicated that the inhibition of NO synthesis may underlie the reduction of the immobility time in the TST elicited by EET.
Supporting the notion that the effect of EET in the TST is, at least in part, due to an inhibition of NO synthesis, in our study we also demonstrated that the co-treatment of 7-nitroindazole (a specific neuronal NO synthase inhibitor) or ODQ (an inhibitor of sGC) with a subeffective dose of EET produced a synergistic antidepressant-like effect in the TST. Considering that NO activates guanylate cyclase that generates cGMP, which mediates many of the effects of NO,24 our results also suggest that the antidepressant-like effect of EET may be mediated through the reduction of cGMP levels, probably as a consequence of the reduction of NO synthesis. Corroborating these results, the administration of an effective, but not a subeffective, dose of EET was able to decrease NOx levels in the cerebral cortex of mice. The role of NOx levels in depressive patients is controversial. Clinical evidence has shown that plasma NOx levels and platelet NOS activity are both decreased in depression51 and that the treatment with antidepressants significantly increase plasma NOx levels.45 In contrast, NOS expression is increased in brain obtained post mortem from patients suffering from depression.52 The reason for this contradiction could be explained by the distinct roles of NO in the brain and in the periphery. It is well known that in human plasma, NO is involved in cardiovascular functions.53 In the brain, however, NO is a messenger molecule that has been implicated in pathophysiology of depression.14,25 Similar to our result, an acute administration of an effective dose of the antidepressant duloxetine is able to decrease NOx levels in the cerebral cortex of mice.54
Taken together, these results are in accordance with the finding that NOS inhibitors, depending on their concentrations, exert antidepressant-like effects in a variety of animal models of depression,14,25,55 and they are able to enhance the activity of antidepressants that act through serotonergic mechanisms.50 In addition, a study by Krass and co-workers18 showed that the treatment with imipramine and venlafaxine decreased the nitrite plus nitrate content in brain, and this mechanism appears to be important for the antidepressant action of these drugs.
Another piece of evidence that EET exerts its effect in the TST by decreasing cGMP levels is given by the reversal of the anti-immobility effect of EET by the PDE5 inhibitor sildenafil. PDE5 catalyses the hydrolysis of the second messenger cGMP and is particularly expressed in the cerebellum and hippocampus.56 Therefore, the results are in agreement with the notion that the inhibition of cGMP synthesis may be an important target to promote antidepressant effects.
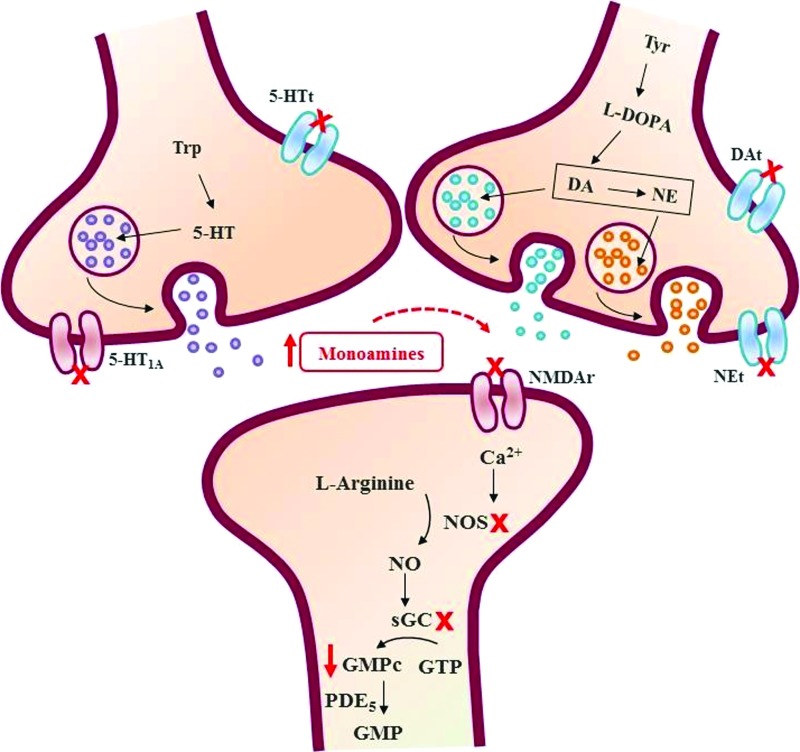
In conclusion, the present study indicates, to our knowledge for the first time, that T. avellanedae produces an antidepressant-like action in the TST by modulation of NMDA receptors and L-arginine-NO-cGMP signaling cascade. Taken together, our results support evidence that these pathways could be relevant to the antidepressant effect of this plant, reinforcing the notion that this plant may be useful for the treatment of depressive disorders, and validating the consumption of this plant as folk medicine (Fig. 4).
FIG. 4.
Schematic illustration for the different targets or pathways involved in the antidepressant-like effect of T. avellanedae taking into account present results and previous literature findings. As described previously by Freitas et al.,10 T. avellanedae can exert an antidepressant-like effect through the increase monoamines availability. The present study provides evidence that the antidepressant-like effect of T. avellanedae is dependent, at least in part, on the inhibition of NMDA receptor (NMDAR) activation; inhibition of NO synthesis, and reduction of cGMP levels. 5-HT, serotonin; 5-HTt, serotonin transporter; Ca2+, calcium; cGMP, cyclic guanosine 3′5′-monophosphate; DA, dopamine; DAt, dopamine transporter; GMP, guanosine 3′5′-monophosphate; GTP, guanosine-triphosphate; L-DOPA, L-3,4-dihydroxyphenylalanine; NE, noradrenaline; NEt, noradrenaline transporter; NMDAR, N-methyl-D-aspartate receptor; NO, nitric oxide; NOS, nitric oxide synthase; PDE5, phosphodiesterase 5; sGC, soluble guanylate cyclase; Trp, tryptofan; Tyr, tyrosine. Color images available online at www.liebertpub.com/jmf
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), Fundação de Apoio a Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC), and “Rede Instituto Brasileiro de Neurociência IBNNet/CNPq and NENASC project (PRONEX-CNPq/FAPESC), Brazil. ALSR and MGP are recipients of CNPq fellowship.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nestler EJ. Barrot M. DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 2.Holtzheimer PE. Numeroff CB. Advances in the treatment of depression. NeuroRx. 2006;3:42–56. doi: 10.1016/j.nurx.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ. Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Morilak DA. Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2007;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 5.Jones K. Pau D'Arco: Immune Power from the Rain Forest. Healing Arts Press; Rochester: 1995. [Google Scholar]
- 6.Gómez Castellanos JR. Prieto JM. Heinrich M. Red Lapacho (Tabebuia impetiginosa)-a global ethnopharmacological commodity? J Ethnopharmacol. 2009;121:1–13. doi: 10.1016/j.jep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Lüebeck W. The Healing Power of Pau d'Arco. Lotus Press; United States: 1999. [Google Scholar]
- 8.Pereira IT. Burci LM. da Silva LM, et al. Antiulcer effect of bark extract of Tabebuia avellanedae: activation of cell proliferation in gastric mucosa during the healing process. Phytother Res. 2013;27:1067–1073. doi: 10.1002/ptr.4835. [DOI] [PubMed] [Google Scholar]
- 9.Twardowschy A. Freitas CS. Baggio CH, et al. Antiulcerogenic activity of bark extract of Tabebuia avellanedae, Lorentz ex Griseb. J Ethnopharmacol. 2008;118:455–459. doi: 10.1016/j.jep.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Freitas AE. Budni J. Lobato KR, et al. Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: evidence for the involvement of the monoaminergic system. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:335–343. doi: 10.1016/j.pnpbp.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Steru L. Chermat R. Thierry B, et al. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 12.Freitas AE. Machado DG. Budni J, et al. Antidepressant-like action of the bark ethanolic extract from Tabebuia avellanedae in the olfactory bulbectomized mice. J Ethnopharmacol. 2013;145:737–45. doi: 10.1016/j.jep.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 13.Skolnick P. Antidepressants for the new millennium. Eur J Pharmacol. 1999;375:31–40. doi: 10.1016/s0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- 14.Harkin AJ. Bruce KH. Craft B, et al. Nitric oxide synthase inhibitors have antidepressant-like properties in mice. 1. Acute treatments are active in the forced swim test. Eur J Pharmacol. 1999;372:207–313. doi: 10.1016/s0014-2999(99)00191-0. [DOI] [PubMed] [Google Scholar]
- 15.Nowak G. Trullas R. Layer RT, et al. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther. 1993;265:1380–1386. [PubMed] [Google Scholar]
- 16.Bobula B. Tokarski K. Hess G. Repeated administration of antidepressants decreases field potentials in rat frontal cortex. Neuroscience. 2003;120:765–769. doi: 10.1016/s0306-4522(03)00380-4. [DOI] [PubMed] [Google Scholar]
- 17.Popik P. Wróbel M. Nowak G. Chronic treatment with antidepressants affects glycine/NMDA receptor function: Behavioral evidence. Neuropharmacology. 2000;39:2278–2287. doi: 10.1016/s0028-3908(00)00090-3. [DOI] [PubMed] [Google Scholar]
- 18.Krass M. Wegener G. Vasar E, et al. The antidepressant action of imipramine and venlafaxine involves suppression of nitric oxide synthesis. Behav Brain Res. 2011;218:57–63. doi: 10.1016/j.bbr.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Zomkowski AD. Engel D. Gabilan NH, et al. Involvement of NMDA receptors and L-arginine–nitric oxide–cyclic guanosine monophosphate pathway in the antidepressant-like effects of escitalopram in the forced swimming test. Eur Neuropsychopharmacol. 2010;20:793–801. doi: 10.1016/j.euroneuro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Szasz BK. Mike A. Karoly R, et al. Direct inhibitory effect of fluoxetine on N-methyl-D-aspartate receptors in the central nervous system. Biol Psychiatry. 2007;62:1303–1309. doi: 10.1016/j.biopsych.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Trullas R. Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 22.Papp M. Moryl E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol. 1994;263:1–7. doi: 10.1016/0014-2999(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 23.Rosa AO. Lin J. Calixto JB, et al. Involvement of NMDA receptors and L-arginine–nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav Brain Res. 2003;144:87–93. doi: 10.1016/s0166-4328(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S. Palmer RM. Higgs EA. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989;38:1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- 25.Da Silva GD. Matteussi AS. dos Santos AR, et al. Evidence for dual effects of nitric oxide in the forced swimming test and in the tail suspension test in mice. Neuroreport. 2000;11:3699–3702. doi: 10.1097/00001756-200011270-00022. [DOI] [PubMed] [Google Scholar]
- 26.Rogóz Z. Skuza G. Maj J, et al. Synergistic effect of uncompetitive NMDA receptor antagonists and antidepressant drugs in the forced swimming test in rats. Neuropharmacology. 2002;42:1024–1030. doi: 10.1016/s0028-3908(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 27.Pruus K. Rudissaar R. Allikmets L, et al. The effect of the NMDA receptor antagonist dizocilpine on behavioral manifestations of serotonin and adrenergic antidepressants in rats. Methods Find Exp Clin Pharmacol. 2010;32:123–128. doi: 10.1358/mf.2010.32.2.1481727. [DOI] [PubMed] [Google Scholar]
- 28.Brocardo PdeS. Budni J. Lobato KR, et al. Antidepressant-like effect of folic acid: involvement of NMDA receptors and L-arginine–nitric oxide–cyclic guanosine monophosphate pathway. Eur J Pharmacol. 2008;598:37–42. doi: 10.1016/j.ejphar.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Bettio LE. Cunha MP. Budni J, et al. Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide–cGMP and PI3K/mTOR pathways. Behav Brain Res. 2012;234:137–148. doi: 10.1016/j.bbr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Moretti M. Budni J. Ribeiro CM, et al. Involvement of different types of potassium channels in the antidepressant-like effect of ascorbic acid in the mouse tail suspension test. Eur J Pharmacol. 2012;687:21–27. doi: 10.1016/j.ejphar.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani M. Pértile R. Calixto JB, et al. Melatonin exerts an antidepressant-like effect in the tail suspension test in mice: evidence for involvement of N-methyl-D-aspartate receptors and the L-arginine–nitric oxide pathway. Neurosci Lett. 2003;343:1–4. doi: 10.1016/s0304-3940(03)00306-9. [DOI] [PubMed] [Google Scholar]
- 32.Hevel JM. Marletta MA. Nitric-oxide synthase assays. Methods Enzymol. 1994;233:250–258. doi: 10.1016/s0076-6879(94)33028-x. [DOI] [PubMed] [Google Scholar]
- 33.Binfaré RW. Rosa AO. Lobato KR, et al. Ascorbic acid administration produces an antidepressant-like effect: evidence for the involvement of monoaminergic neurotransmission. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:530–540. doi: 10.1016/j.pnpbp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Machado DG. Bettio LE. Cunha MP, et al. Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: involvement of the monoaminergic system monoaminergic system. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:642–650. doi: 10.1016/j.pnpbp.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues AL. Rocha JB. Mello CF, et al. Effect of perinatal lead exposure on rat behavior in open-field and two-way avoidance tasks. Pharmacol Toxicol. 1996;79:150–156. doi: 10.1111/j.1600-0773.1996.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez-Maldonado AF. Schieber A. Gänzle MG. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J Appl Microbiol. 2011;111:1176–1184. doi: 10.1111/j.1365-2672.2011.05141.x. [DOI] [PubMed] [Google Scholar]
- 37.Singh N. Jabeen T. Pal A, et al. Crystal structures of the complexes of a group IIA phospholipase A2 with two natural anti-inflammatory agents, anisic acid, and atropine reveal a similar mode of binding. Proteins. 2006;64:89–100. doi: 10.1002/prot.20970. [DOI] [PubMed] [Google Scholar]
- 38.Saravanakumar M. Raja B. Veratric acid, a phenolic acid attenuates blood pressure and oxidative stress in L-NAME induced hypertensive rats. Eur J Pharmacol. 2011;671:87–94. doi: 10.1016/j.ejphar.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 39.Simić A. Manojlović D. Segan D, et al. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules. 2007;12:2327–2340. doi: 10.3390/12102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda H. Tsuji M. Inazu M, et al. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur J Pharmacol. 2002;449:261–267. doi: 10.1016/s0014-2999(02)02037-x. [DOI] [PubMed] [Google Scholar]
- 41.Takeda H. Tsuji M. Miyamoto J, et al. Caffeic acid produces antidepressive- and/or anxiolytic-like effects through indirect modulation of the alpha 1A-adrenoceptor system in mice. Neuroreport. 2003;14:1067–1070. doi: 10.1097/01.wnr.0000073427.02536.b0. [DOI] [PubMed] [Google Scholar]
- 42.Takeda H. Tsuji M. Yamada T, et al. Caffeic acid attenuates the decrease in cortical BDNF mRNA expression induced by exposure to forced swimming stress in mice. Eur J Pharmacol. 2006;534:115–121. doi: 10.1016/j.ejphar.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Dzitoyeva S. Imbesi M. Uz T, et al. Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J Neural Transm. 2008;115:823–827. doi: 10.1007/s00702-008-0034-7. [DOI] [PubMed] [Google Scholar]
- 44.Cryan JF. Mombereau C. Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Lara N. Archer SL. Baker GB, et al. Paroxetine-induced increase in metabolic end products of nitric oxide. J Clin Psychopharmacol. 2003;23:641–645. doi: 10.1097/01.jcp.0000085416.08426.1d. [DOI] [PubMed] [Google Scholar]
- 46.Wong ML. Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 47.Sanacora G. Zarate CA. Krystal JH, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul IA. Nowak G. Layer RT, et al. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J Pharmacol Exp Ther. 1994;269:95–102. [PubMed] [Google Scholar]
- 49.Skolnick P. Popik P. Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Harkin A. Connor TJ. Burns MP, et al. Nitric oxide synthase inhibitors augment the effects of serotonin reuptake inhibitors in the forced swimming test. Eur Neuropsychopharmacol. 2004;14:274–281. doi: 10.1016/j.euroneuro.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Chrapko WE. Jurasz P. Radomski MW, et al. Decreased platelet nitric oxide synthase activity and plasma nitric metabolites in major depressive disorder. Biol Psychiatry. 2004;56:129–134. doi: 10.1016/j.biopsych.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Oliveira RM. Guimarães FS. Deakin JF. Expression of neuronal nitric oxide synthase in the hippocampal formation in affective disorders. Braz J Med Biol Res. 2008;41:333–341. doi: 10.1590/s0100-879x2008000400012. [DOI] [PubMed] [Google Scholar]
- 53.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Zomkowski AD. Engel D. Cunha MP, et al. The role of the NMDA receptors and L-arginine–nitric oxide–cyclic guanosine monophosphate pathway in the antidepressant-like effect of duloxetine in the forced swimming test. Pharmacol Biochem Behav. 2012;103:408–417. doi: 10.1016/j.pbb.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Jesse CR. Wilhelm EA. Bortolatto CF, et al. Involvement of L-arginine–nitric oxide–cyclic guanosine monophosphate pathway in the antidepressant-like effect of bis selenide in the mouse tail suspension test. Eur J Pharmacol. 2010;635:135–141. doi: 10.1016/j.ejphar.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Bender AT. Beavo JA. Specific localized expression of cGMP PDEs in Purkinje neurons and macrophages. Neurochem Int. 2004;45:853–857. doi: 10.1016/j.neuint.2004.03.015. [DOI] [PubMed] [Google Scholar]