Abstract
A major hurdle in permanently eliminating HIV from the body is the persistence of viral reservoirs, including those of the brain. One potential strategy towards eradicating HIV reservoirs of the brain is to block efflux transporters, such as P-glycoprotein (P-gp), that contribute to the limited penetration of antiviral agents across the blood-brain barrier (BBB). Herein, we described a series of dimeric inhibitors of P-gp based on the nucleoside reverse transcriptase inhibitor and P-gp substrate, abacavir. Varying tether lengths were used to generate abacavir dimers to probe tether requirements for inhibitory potency. These dimeric agents were evaluated in two cell lines that express P-gp at varying levels: a P-gp over-expressing CD4+ T-lymphocyte cell line (12D7-MDR) and a human brain capillary endothelial cell line as an in vitro model of the BBB (hCMEC/D3) that expresses endogenous levels of P-gp. All dimeric abacavir analogs were inhibitors of P-gp efflux in the two cell lines with potencies that varied with tether length; the most potent agents displayed low micromolar inhibition. P-gp inhibition in a highly P-gp over-expressing cell line (MCF-7/DX1) was also observed with a range of therapeutic substrates. Competition studies with the photoaffinity substrate [125I]iodoarylazidoprazosin demonstrated that abacavir dimers act by competing for the substrate binding sites of P-gp. These data demonstrate that the tether length of dimeric abacavir derivatives has a significant effect on inhibition of P-gp drug efflux, with up to a 35-fold increase in potency observed with longer tether linkages.
Introduction
Significant progress has been made in developing therapeutic approaches for treatment of HIV using combination antiretroviral therapy (ART). Treatment with ART has successfully reduced viral plasma loads to undetectable levels in HIV infected individuals. Nevertheless, complete eradication of HIV by ART currently remains unattainable, as the virus persists in cellular and anatomical reservoirs, such as resting memory CD4+ cells, macrophages and the central nervous system (CNS).1 Sanctuaries of HIV, such as those in the brain, are believed to occur, in part, due to limited penetration of ART into these sites.2
The multidrug resistance transporter, P-glycoprotein (P-gp), is the most highly expressed member of the ATP-binding cassette (ABC) family of transporters at the blood brain barrier (BBB).3 P-gp is localized to the apical membrane of the brain capillary endothelial cells where it actively transports a wide number of therapies, thereby limiting accumulation of these agents in the brain.4 In vitro experiments have identified many substrates for P-gp, including a number of antiretrovirals, such as the protease inhibitors saquinavir, darunavir, amprenavir, nelfinavir, ritonavir, and indinavir, and the reverse transcriptase inhibitor (RTI) prodrug abacavir.2,5 Additionally maraviroc, the HIV entry inhibitor that blocks the chemokine receptor CCR5, and the HIV integrase inhibitor raltegravir have been shown to be P-gp substrates.6 The effect of P-gp on in vivo accumulation of antiretrovirals has also been investigated. For instance, experiments with P-gp-null mice have found a 20-fold increase in brain levels of the RTI abacavir versus wild-type mice.7
The role of P-gp in limiting the penetration of ART across membranes such as those found in the endothelial cells of the BBB, points to the need for developing potent inhibitors of P-gp. In one example, the P-gp inhibitor ritonavir is often co-administered to promote the brain penetration of antiviral therapies. However, ritonavir has a number of off-target interactions and extensive undesirable drug-drug interactions. Therefore, there is a great need for the development of new modulators of P-gp for co-administration with antiviral agents. Herein, we probe the role of tether length in a class of dimeric agents based on the antiviral agent abacavir. These agents effectively inhibit P-gp efflux, by targeting the membrane-bound drug binding region of P-gp, in a tether length-dependent fashion
Results and Discussion
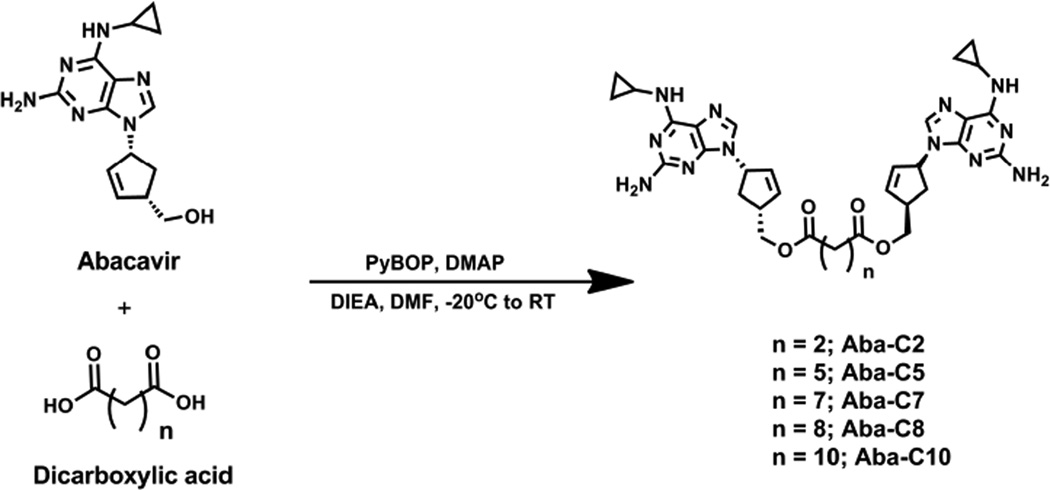
Design and Synthesis of Library of Abacavir Dimers
A number of biochemical studies have demonstrated that there are multiple substrate binding sites within the transporter region of P-gp.8 Recent crystal structures of P-gp have also shown that the substrate binding region of P-gp is large enough to accommodate multiple substrate molecules, such as two cyclic peptides.9 With these data in mind, we have developed a general strategy to convert therapeutic substrates of P-gp into P-gp inhibitors through dimerization.10 In this way a monomeric P-gp substrate can be very quickly converted into an inhibitor through dimerization with the appropriate crosslinking agent. Using this strategy, we recently developed prodrug dimeric inhibitors of P-gp based on the RTI abacavir with invariant tether lengths.10 However, we envisioned that by varying the length of the linker in the abacavir dimers we could optimize the inhibitory potency toward P-gp. With this in mind, we investigated five homodimeric compounds consisting of abacavir linked to flexible alkyl-based tethers via intervening ester bonds (Aba-C2 – Aba-C10) (Scheme 1). The dimers were synthesized by treating abacavir with the corresponding PyBOP-activated bis-carboxylic acids in the presence of DIEA and DMAP (Scheme 1). The resulting abacavir dimers were purified to homogeneity by reversed phase HPLC.
Scheme 1.
Synthesis of library of Abacavir dimers.
Inhibition of P-gp by Abacavir Dimers is Dependent on Tether Length
CD4+ T-lymphocyte cells are a well-defined HIV reservoir that is established during early infection of HIV.11 We employed the P-gp over-expressing CD4+ T-lymphocyte cell line, 12D7-MDR, as a model to evaluate the activity of the abacavir-based dimers.12 Inhibition of P-gp efflux was determined by monitoring the cellular accumulation of the fluorescent P-gp substrates calcein-AM and NBD-Aba in 12D7-MDR cells in the presence and absence of the dimers. In these assays, the P-gp over-expressing cells were incubated with the fluorescent substrates and cellular fluorescence was quantified by flow cytometry. The known P-gp inhibitor GF120918 was used as a positive control and DMSO (1%) was used as the negative control. An increase in cellular fluorescence is indicative of inhibition of fluorescent substrate efflux by P-gp from the cells.
All of the abacavir dimers were found to inhibit P-gp mediated efflux of both calcein-AM and NBD-Aba in a concentration dependent manner (Table 1). The abacavir monomer, however, demonstrated no inhibition of efflux with either substrate up to a concentration of 500 µM. The efficacy of P-gp inhibition was dependent on the length of the tether; Aba-C2 was the least potent dimer in the library with an IC50 value of 50 µM for inhibition of calcein-AM efflux. As the tether length of the abacavir dimers increased, a concomitant increase in potency was observed. For instance, Aba-C7 to Aba-C10 were found to be 20- to 35-fold more potent than the C2-analog with calcein-AM in 12D7-MDR cells.
Table 1.
Inhibition of P-gp-mediated efflux of calcein-AM and NBD-Aba in 12D7 MDR and hCMEC/D3 cellsa
| Compound | 12D7-MDR Cells Calcein-AM (IC50 in µM) |
12D7-MDR Cells NBD-Aba (IC50 in µM) |
hCMEC/D3 Cells NBD-Aba (IC50 in µM) |
|---|---|---|---|
| abacavir | >500 | >500 | >500 |
| Aba-C2 | 50 ± 11.7 | ND | ND |
| Aba-C5 | 7.7 ± 1.3 | 10.5 ± 2.3 | 4.1 ± 0.3 |
| Aba-C7 | 2.6 ± 0.1 | 2.6 ± 0.4 | 3.8 ± 0.3 |
| Aba-C8 | 2.3 ± 0.2 | 2.1 ± 0.2 | 3.6 ± 0.9 |
| Aba-C10 | 1.4 ± 0.1 | 1.8 ± 0.3 | 1.1 ± 0.02 |
Cells were treated with calcein-AM (0.25 µM) or NBD-Aba (5 µM) and various concentrations of compounds for 30 min at 37 °C. Cells were analyzed for fluorescence by flow cytometery.
ND – not determined
Given that our goal is to inhibit P-gp at the BBB as a means to increase brain penetration of ART, we also utilized an immortalized human brain capillary endothelial cell line as an in vitro model of the BBB (hCMEC/D3 cells). Importantly, this cell line expresses endogenous levels of P-gp13 and also retains many of the key characteristics of primary brain endothelial cells without the need to co-culture with glial cells.14 We performed substrate accumulation assays with NBD-Aba in the presence and absence of the abacavir dimer series. As above, DMSO (1%) alone was used as the negative control and GF10918 (1 µM) was the positive control. The abacavir dimers all inhibited P-gp efflux of NBD-Aba in the human brain capillary cells to varying degrees (Table 1). The most potent dimer, Aba-C10, was greater than 500-fold more potent than monomeric abacavir, with an IC50 of 1.1 µM. These data indicate that abacavir dimers were effective inhibitors of P-gp expressed at endogenous levels in capillary endothelial cells of the BBB.
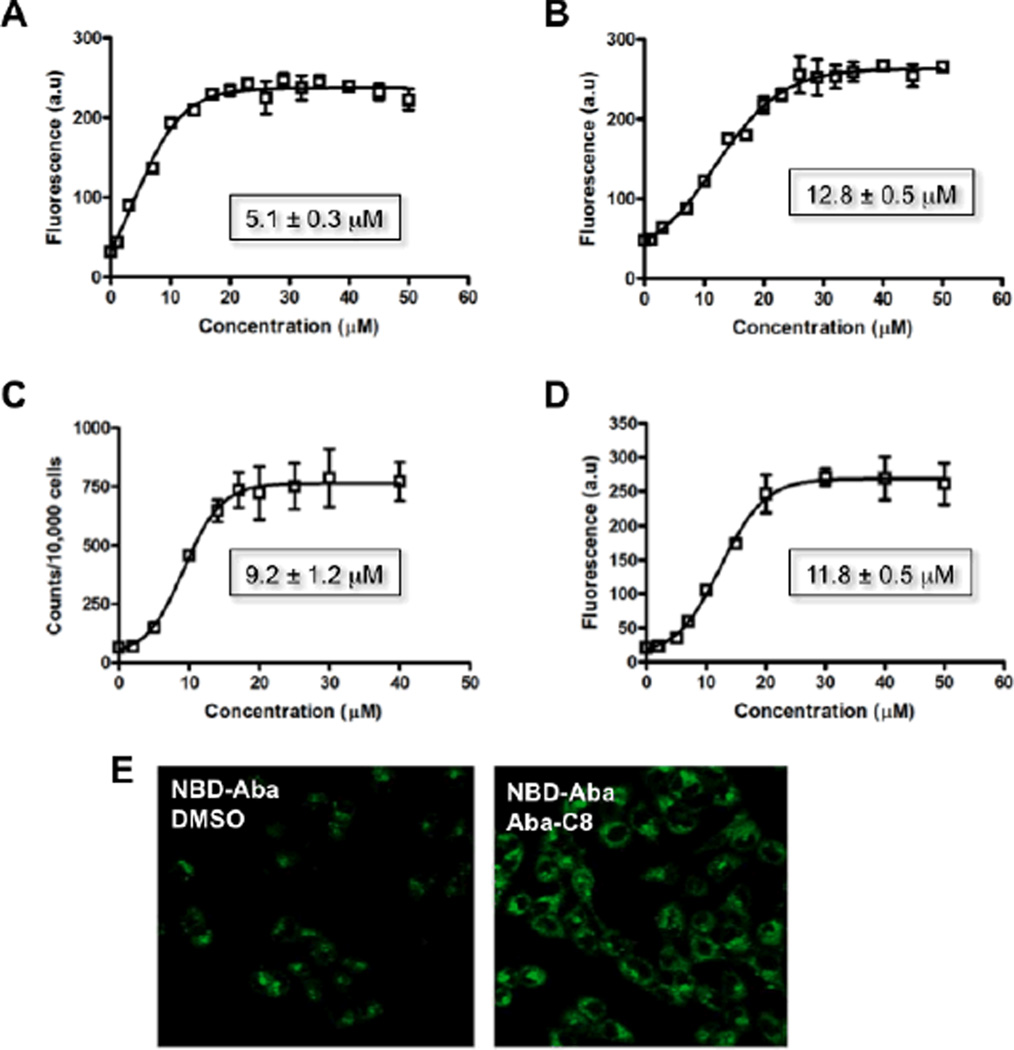
A major concern for long-term administration of ART is the potential for some agents, like the protease inhibitors atazanavir and ritonavir, to induce the expression of efflux transporters such as P-gp. This up-regulation of P-gp expression can serve to further limit the penetration P-gp sensitive ART into cells.15 To test how well the abacavir dimers function in a P-gp over-expressing environment, we used the human breast carcinoma cell line MCF-7/DX1 that expresses high levels of P-gp (10-fold greater levels than 12D7 MDR cells16). Substrate accumulation assays were performed to determine the efficacy of Aba-C8 with four different P-gp substrates, BODIPY-prazosin, doxorubicin, [3H]-daunomycin and NBD-Aba; Aba-C8 was used due to more favorable solubility in aqueous buffer as compared to Aba-C10. Cellular accumulation of the fluorescent substrates in the presence of increasing concentrations of the dimers was quantified by flow cytometry, and the accumulation of [3H]-daunomycin was quantified by scintillation counting. In the absence of Aba-C8, very low levels of cellular fluorescence or radioactivity was observed with the four different P-gp substrates as they are effectively effluxed from the cells by P-gp (Figure 1A–D). As the concentration of Aba-C8 was increased, we observed an increased level of the fluorescent or radioactive substrates within cells due to inhibition of P-gp. Aba-C8 was found to effectively inhibit efflux for each of the substrates (Figure 1), whereas monomeric abacavir had no effect on transport up to a concentration of 100 µM (data not shown). These data demonstrate that one of the more potent abacavir dimers of this series was capable of inhibiting the efflux of therapeutics in cells that express high levels of P-gp. The potency of Aba-C8 in MCF-7/DX1 cells, however, was decreased about 3- to 6-fold using NBD-Aba as a substrate in hCMEC/D3 and 12D7-MDR, respectively.
Fig. 1.
(A–D) Inhibition of P-gp efflux with varying concentrations of inhibitor Aba-C8 using constant concentrations of the following substrates (A) BODIPY-prazosin, (B) doxorubicin, (C) [3H]-daunomycin and (D) NBD-Aba in MCF-7/DX1 cells using flow cytometry (A, B and D) and a scintillation counter (C). (E) Confocal microscopy of MCF-7/DX1 cells treated with NBD-Aba (5 µM) in the absence (left) and presence (right) of Aba-C8 (20 µM).
Abacavir Dimers Inhibit P-gp by Competing for Substrate Binding Sites
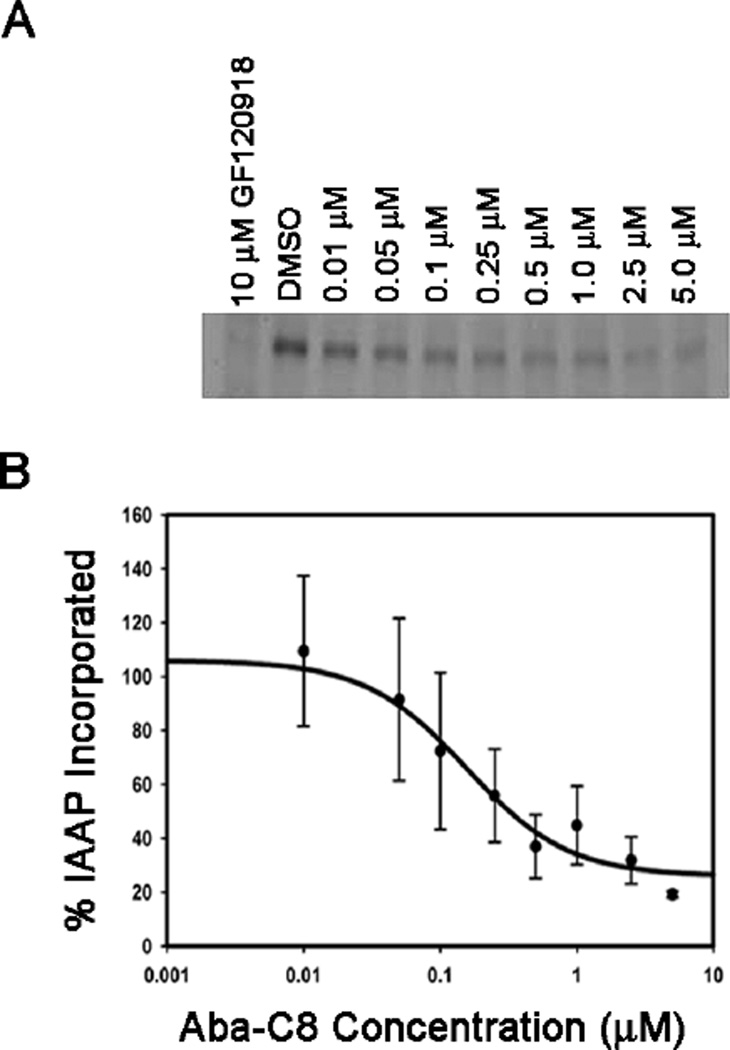
We designed the abacavir dimers to interact with substrate binding sites located within the transmembrane region of P-gp. Therefore, the dimers should compete with P-gp substrates for these binding sites. To test this hypothesis, we probed the ability of the dimers to compete for the binding sites with a photoactive analog of the P-gp substrate prazosin, [125I]-iodoarylazidoprazosin ([125I]-IAAP).17 Crude Sf9 membranes expressing P-gp were treated with [125I]-IAAP with and without the abacavir dimer, Aba-C8, followed by photo-crosslinking. Covalent modification of P-gp by [125I]-IAAP was detected using autoradiography of the resulting SDS-PAGE gels as a function of increasing concentrations of the dimer (Figure 2A). The observed band in the DMSO lane indicates that [125I]-IAAP was successfully binding and crosslinked to P-gp. However, as the concentration of Aba-C8 was increased, the band intensity of the [125I]-IAAP/P-gp conjugate decreased, indicating that the prazosin analog is no longer binding and is being effectively displaced by Aba-C8. Aba-C8 was found to effectively compete for the [125I]-IAAP binding sites on P-gp in a concentration dependent manner, with an IC50 value for inhibition of binding of approximately 240 nM (Figure 2B). These data support the hypothesis that Aba-C8 inhibits P-gp by interacting with the substrate binding sites.
Fig. 2.
The ability of Aba-C8 to compete with [125I] IAAP for P-gp substrate binding sites with GF120918 (10 µM) used as a positive control. (A) Autoradiograph and (B) densitometric quantitation of the autoradiograph.
Experimental Procedures
General Synthesis of Abacavir Dimers
To a solution of bis-carboxylic acid (0.06 mmol) in dry dimethylformamide (DMF) (2 mL) at −20°C was added benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) (0.18 mmol), 4-dimethylaminopyridine (DMAP) (0.03 mmol), and diisopropylamine DIEA (0.6 mmol). After 20 min at −20°C, abacavir (0.18 mmol) was added. The mixture was stirred for 8 hours at −20°C, allowed to warm to room temperature and stirred for an additional 8 hours. The solvent was removed in vacuo, the resulting material was dissolved in dimethylsulfoxide (DMSO) and purified by reverse phase HPLC using a C5 semi-preparative column (Phenomenex, USA) with an eluent consisting of solvent A (methanol and 0.1% trifluoroacetic acid (TFA)) and solvent B (water and 0.1% TFA) with a 60 min gradient of 20–95% solvent A, a flow rate of 20.0 mL/min and UV detection at 214 nm and 285 nm. Fractions consisting of the desired dimers were collected and lyophilized to obtain Aba-C2 (22% yield), Aba-C5 (26% yield), Aba-C7 (68% yield), Aba-C8 (70% yield) and Aba-C10 (58% yield).
Aba-C8
1H NMR (300 MHz, DMSO-d6) δ 9.90 (broad singlet, 2H), 7.96 (s, 2H), 7.70 (broad singlet, 4H), 6.13 – 6.12 (m, 2H), 5.97 – 5.96 (m, 2H), 5.40 (m, 2H), 4.05 (d, J = 5.7 Hz, 4H), 3.10 (m, 2H), 2.85 (m, 2H), 2.70 (2H), 2.25 – 2.21 (m, 4H), 1.60 (m, 2H), 1.43 (m, 4H), 1.19 – 1.16 (m, 8H), 0.90 – 0.88 (m, 4H), 0.76 (m, 4H). 13C NMR (300 MHz, DMSO-d6) δ172.9, 158.9, 158.4, 152.5, 138.4, 137.5, 129.8, 111.6, 65.9, 59.3, 45.8, 45.1, 44.0, 34.1, 33.7, 33.3, 28.7, 28.5, 28.3, 25.6, 24.4, 23.9, 6.9. MS (MALDI-TOF) calculated for C38H50N12O4 739.41, found 740 (M+H)+. HRMS (ESI) calculated 739.4156 (M+H)+, found 739.4165 (M+H)+. Purity (~96%) was characterized by analytical HPLC, retention time 21.9 min using a C5 analytical column (Phenomenex, USA) with an eluent consisting of solvent A (methanol and 0.1% trifluoroacetic acid (TFA)) and solvent B (water and 0.1% TFA) with a 30 min gradient of 20–95% solvent A, and a flow rate of 1.2 mL/min.
Aba-C10
MS (MALDI-TOF): calculated for C40H54N12O4 766.4, found 768.3 (M+H)+. Purity (~95%) was characterized by analytical HPLC, retention time 21.1 min using a C5 analytical column (Phenomenex, USA) with an eluent consisting of solvent A (acetonitrile and 0.1% TFA) and solvent B (water and 0.1% TFA) with a 30 min gradient of 2–70% solvent A, and a flow rate of 1.2 mL/min.
Aba-C7
MS (MALDI-TOF): calculated for C37H48N12O4 724.4, found 725.3 (M+H)+. Purity (~96%) was characterized by analytical HPLC, retention time 18.5 min using a C5 analytical column (Phenomenex, USA) with an eluent consisting of solvent A (acetonitrile and 0.1% TFA) and solvent B (water and 0.1% TFA) with a 30 min gradient of 2–70% solvent A, and a flow rate of 1.2 mL/min.
Aba-C5
MS (MALDI-TOF): calculated for C35H44N12O4 696.4, found 697.1 (M+H)+. Purity (~95%) was characterized by analytical HPLC, retention time 18.2 min using a C5 analytical column (Phenomenex, USA) with an eluent consisting of solvent A (acetonitrile and 0.1% TFA) and solvent B (water and 0.1% TFA) with a 30 min gradient of 2–70% solvent A, and a flow rate of 1.2 mL/min.
Aba-C2
MS (MALDI-TOF): calculated for C32H38N12O4 654.3, found 655.2 (M+H)+. Purity (~97%) was characterized by analytical HPLC, retention time 15.8 min using a C5 analytical column (Phenomenex, USA) with an eluent consisting of solvent A (acetonitrile and 0.1% TFA) and solvent B (water and 0.1% TFA) with a 30 min gradient of 2–70% solvent A, and a flow rate of 1.2 mL/min.
Cell Culture
12D7-MDR cells (CD4+ human T-lymphocytic cell line) expressing P-gp were cultured in RPMI 1640 supplemented with 10% Fetal Bovine Serum (Cambrex Bioscience, WalkersVille, Inc.), 5 mM L-glutamine, 50 units/mL penicillin and 50 µg/mL streptomycin (Cellgro Mediatech). Cells were incubated at 37 °C with 5% carbon dioxide. Sf9 cells were cultured at 27 °C in Sf-900 II SFM medium supplemented with 0.5× antibiotic-antimycotic (Invitrogen). The hCMEC/D3 cell line was cultured as described previously with slight modifications.15 The cells were maintained in endothelial growth medium-2 (EGM-2) from Lonza (Walkersville, MD), supplemented with 5% fetal bovine serum, 1% penicillin-streptomycin, 0.5% human basic fibroblast growth factor (hbFGF), 0.05% hydrocortisone, 0.5% ascorbic acid, 1% HEPES and 1% lipid concentrate at 37 °C and 5% CO2. Cells were passaged into collagenated culture flasks every 3–4 days at approximately 85%-95% confluence. MCF-7/DX1 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), 2 mM L-glutamine (Mediatech, Herndon, VA), 50 units/mL penicillin, 50 µg/mL streptomycin (Mediatech), and 1 µM doxorubicin.
Expression of P-gp in Insect Cells
Sf9 cells in 150-cm2 flasks (1.86 × 107 cells/flask) were infected with BV-MDR1 that expresses human P-gp as previously described.18 After 2 hours of incubation at 27 °C, the cells were treated with 15 mL of culture medium and incubated at 27 °C for another 72 hours.
Preparation of Crude Insect Cell Membranes
BV-MDR1 infected Sf9 cells were collected and crude membrane extracts were prepared as described previously.18 P-gp expression was verified by immunoblot analysis with C219 primary antibody (1:8000) and HRP-conjugated anti-mouse secondary antibody (1:8000). Probe bands were visualized using enhanced chemiluminescence (ECL) (Pierce).
Flow Cytometry Assays
Flow cytometry assays were performed as previously described, with a few modifications.19 For assays with 12D7-MDR cells or hCMEC/D3 cells, an aliquot of the substrate fluorophore (calcein-AM or NBD–Aba) dissolved in DMSO was added to pre-warmed basal medium eagle (BME) media to give a final concentration of 0.25 µM for calcein-AM or 5 µM for NBD-Aba. The fluorophore treated BME media (1 mL) was incubated with 125,000 cells (in suspension) in the presence of increasing concentrations of the dimeric abacavir agents at 37 °C for 30 min, keeping the DMSO concentration at a constant 1%. 1 µM GF120918 was used as a positive control. Cells were collected by centrifugation and re-suspended in ice-cold phosphate buffered saline (PBS, pH 7.4). The cells were analyzed for fluorescent substrate accumulation using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) equipped with a 488 nm argon laser and a 530 nm band pass filter (FL1). Ten thousand cells were counted for each data point and the mean fluorescence was used to determine the IC50 values using Sigma plot.
For assays with MCF-7/DX1 cells, 125,000 cells suspended in BME media (1 mL) that were previously treated with either doxorubicin (final concentration of 3 µM), BODIPY-prazosin (final concentration of 0.5 µM) or NBD-Aba (final concentration 5 µM) were incubated alone or with increasing concentrations of abacavir dimers for 30 min at 37 °C, keeping the concentration of DMSO at a constant 1%. 1µM GF120918 was used as a positive control. For doxorubicin, cells were harvested by centrifugation followed by re-suspension in pre-warmed BME media with 1 µM GF120918 or varying concentrations of abacavir dimers. The cells were incubated for an additional 30 min at 37 °C. These cells, and those treated with BODIPY-prazosin and NBD-Aba were harvested by centrifugation, the media was removed and the cells were resuspended in 350 µL of ice cold PBS, pH 7.4. The cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) equipped with a 488 nm argon laser and a 530 band pass filter (FL1) for NBD-Aba and BODIPY-prazosin, and a 585/42 band pass filter (FL2) for doxorubicin. IC50 values were obtained as described above.
Radioactive Substrate Accumulation Assays
Radioactive assays were performed as previously described with minor modifications.19 Six-well plates were seeded with 500,000 MCF-7/DX1 cells per well in RPMI 1640 media a day before the assay. The next day, the media in the wells was removed and the cells were washed twice with ice cold PBS. The cells were incubated with BME media supplemented with 5% calf serum and 0.25 µCi/mL [3H]-daunomycin alone or with varying concentrations of abacavir dimers or GF120918 (1 µM) for 40 min at 37 °C. The media was removed and the cells were washed twice with 1 mL ice cold PBS. Pre-warmed trypsin-EDTA (1 mL) was added and the cells were incubated for 1 h at 37 °C. The contents of the wells were transferred into scintillation vials containing 18 mL of EcoLite(+) scintillation fluid (MR Research Products, Irvine, CA). Each well was washed twice with 500 µL ice cold PBS and the contents added to the scintillation vials. Each vial was counted using a 1600 CA tri-CARB liquid scintillation analyzer (Packard Instrument Company, Downers Grove, IL). The data obtained was normalized to counts per 10,000 cells. The IC50 values were acquired from the concentration-dependent data that was fitted using GraphPad Prism 4.
IAAP Photoaffinity Labeling
Photoaffinity labeling was performed as described previously with some modifications.19 Crude Sf9 membranes expressing P-gp (25 µg) were incubated for 10 min at room temperature in the dark in assay buffer (Tris-HCl (50 mM), pH 7.5, 1% aprotinin, 1 mM DTT, and 2 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride) with either 2.5% DMSO or GF120918 (a final concentration of 10 µM) or increasing concentrations of the abacavir dimers and [125I]-IAAP (1 µL, specific activity of ~2200 Ci/mmol). The samples were illuminated with UV light (365 nm) for 20 min on ice. Samples were separated by SDS-PAGE using a 7.5% tris gel. The gel was fixed and allowed to dry overnight. Following exposure to x-ray film at −80 °C, the gel band corresponding to P-gp was analyzed using ImageJ (NIH) to determine the amount of [125I]-IAAP photocrosslinked to P-gp. Values are represented as a percent of the DMSO control sample, and the IC50 values were determined by GraphPad Prism 4.
Conclusion
A significant obstacle to delivering ART at therapeutic levels to viral reservoirs, such as those in the brain, is the efflux activity of P-gp. With the ultimate goal of increasing the brain penetration of ART, we developed a series of dimeric inhibitors of P-gp based on the P-gp substrate and RTI abacavir. These dimers were designed with varying tether lengths, and were found to inhibit P-gp mediated efflux of substrates such as calcein-AM and NBD-Aba in several P-gp expressing mammalian cell lines. Importantly, the dimers inhibited P-gp both in human brain capillary cells that express endogenous levels of the transporter and in CD4+ T-cells that overexpress P-gp. In both cell lines, inhibitory potency increased with tether length, with Aba-C7 to Aba-C10, being the most potent agents tested. Furthermore, the representative dimeric agent Aba-C8 was shown to inhibit P-gp efflux of four therapeutic agents in a cell line that highly over-expresses P-gp, MCF-7/DX1 cells. Aba-C8 was also shown to inhibit P-gp by competing for substrate binding sites. Ultimately, agents such as the designed abacavir dimers could be co-administered with monomeric abacavir to improve brain penetration in antiviral treatment.
Acknowledgements
We acknowledge support from the National Institutes of Health (R21NS084913). The hCMEC/D3 cell line was kindly donated by Dr. P. Couraud from the Institut Cochin, Universite´ Rene´ Descartes, Paris, France.
Notes and references
- 1.Dahl V, Josefsson L, Palmer S. Antiviral Res. 2010;85:286. doi: 10.1016/j.antiviral.2009.09.016. [DOI] [PubMed] [Google Scholar]; Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. Science. 2009;323:1304. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]; Trono D, Van Lint C, Rouzioux C, Verdin E, Barre-Sinoussi F, Chun TW, Chomont N. Science. 2010;329:174. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]; Pomerantz RJ. Clin Infect Dis. 2002;34:91. doi: 10.1086/338256. [DOI] [PubMed] [Google Scholar]
- 2.Varatharajan L, Thomas SA. Antiviral Res. 2009;82:A99. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Arch Neurol. 2008;65:65. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Proc Natl Acad Sci U S A. 1989;86:695. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schinkel AH. Adv Drug Deliv Rev. 1999;36:179. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 4.Loscher W, Potschka, H H. Nat Rev Neurosci. 2005;6:591. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet L, Roucairol C, Hembury A, Nevers MC, Creminon C, Farinotti R, Mabondzo A. AIDS Res Hum Retroviruses. 2008;24:1147. doi: 10.1089/aid.2007.0022. [DOI] [PubMed] [Google Scholar]; de Souza J, Benet LZ, Huang Y, Storpirtis S. J Pharm Sci. 2009;98:4413. doi: 10.1002/jps.21744. [DOI] [PubMed] [Google Scholar]; Fujimoto H, Higuchi M, Watanabe H, Koh Y, Ghosh AK, Mitsuya H, Tanoue N, Hamada A, Saito H. Biol Pharm Bull. 2009;32:1588. doi: 10.1248/bpb.32.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pal D, Kwatra D, Minocha M, Paturi DK, Budda B, Mitra AK. Life Sci. 2011;88:959. doi: 10.1016/j.lfs.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker DK, Bowers SJ, Mitchell RJ, Potchoiba MJ, Schroeder CM, Small HF. Xenobiotica. 2008;38:1330. doi: 10.1080/00498250802447409. [DOI] [PubMed] [Google Scholar]; Moss D, Kwan W, Liptrott N, Siccardi M, Anstee D, Khoo S, Back D, Owen A. 17th CROI: Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: 2010. [Google Scholar]
- 7.Shaik N, Giri N, Pan G, Elmquist WF. Drug Metab Dispos. 2007;35:2076. doi: 10.1124/dmd.107.017723. [DOI] [PubMed] [Google Scholar]
- 8.Dey S, Ramachandra M, Pastan I, Gottesman MM, Ambudkar SV. Proc Natl Acad Sci U S A. 1997;94:10594. doi: 10.1073/pnas.94.20.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shapiro AB, Ling V. Eur J Biochem. 1997;250:130. doi: 10.1111/j.1432-1033.1997.00130.x. [DOI] [PubMed] [Google Scholar]; Loo TW, Bartlett MC, Clarke DM. J Biol Chem. 2003;278:50136. doi: 10.1074/jbc.M310448200. [DOI] [PubMed] [Google Scholar]; Loo TW, Bartlett MC, Clarke DM. J Biol Chem. 2003;278:39706. doi: 10.1074/jbc.M308559200. [DOI] [PubMed] [Google Scholar]
- 9.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrel PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Science. 2009;323:1718. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jin MS, Oldham ML, Zhang Q, Chen J. Nature. 2012;490:566. doi: 10.1038/nature11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namanja HA, Emmert D, Davis DA, Campos C, Miller DS, Hrycyna CA, Chmielewski J. J Am Chem Soc. 2012;134:2976. doi: 10.1021/ja206867t. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sauna ZE, Andrus MB, Turner TM, Ambudkar SV. Biochemistry. 2004;43:2262. doi: 10.1021/bi035965k. [DOI] [PubMed] [Google Scholar]; Chan KF, Zhao Y, Burkett BA, Wong IL, Chow LM, Chan TH. J. Med. Chem. 2006;49:6742. doi: 10.1021/jm060593+. [DOI] [PubMed] [Google Scholar]; Pires MM, Hrycyna CA, Chmielewski J. Biochemistry. 2006;45:11695. doi: 10.1021/bi0608109. [DOI] [PubMed] [Google Scholar]
- 11.Alexaki A, Liu Y, Wigdahl B. Curr HIV Res. 2008;6:388. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CG, Pastan I, Gottesman MM. Methods Enzymol. 1998;292:557. doi: 10.1016/s0076-6879(98)92044-4. [DOI] [PubMed] [Google Scholar]
- 13.Poller B, Gutmann H, Krahenbuhl S, Weksler B, Romero I, Couraud PO, Tuffin G, Drewe J, Huwyler J. J Neurochem. 2008;107:1358. doi: 10.1111/j.1471-4159.2008.05730.x. [DOI] [PubMed] [Google Scholar]
- 14.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. FASEB J. 2005;19:1872. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 15.Chandler B, Almond L, Ford J, Owen A, Hoggard P, Khoo S, Back DJ. Acquir Immune Defic Syndr. 2003;33:551. doi: 10.1097/00126334-200308150-00001. [DOI] [PubMed] [Google Scholar]; Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, Weksler B, Bendayan M, Bendayan R. J Neurosci Res. 2009;87:1023. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]
- 16.Emmert DM. Ph.D. Thesis. Purdue University; 2011. [Google Scholar]
- 17.Shapiro AB, Fox K, Lam P, Ling V. Eur J Biochem. 1999;259:841. doi: 10.1046/j.1432-1327.1999.00098.x. [DOI] [PubMed] [Google Scholar]
- 18.Germann UA, Willingham MC, Pastan I, Gottesman MM. Biochemistry. 1990;29:2295. doi: 10.1021/bi00461a013. [DOI] [PubMed] [Google Scholar]
- 19.Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Methods Enzymol. 1998;292:456. doi: 10.1016/s0076-6879(98)92035-3. [DOI] [PubMed] [Google Scholar]