Abstract
Background
As antiretroviral treatment (ART) for HIV/AIDS is scaled-up globally, information on per-person costs is critical to improve efficiency in service delivery and maximize coverage and health impact.
Objective
To review studies on delivery unit costs for adult and pediatric ART provision per-patient-year, and prevention of mother-to-child transmission (PMTCT) interventions per mother-infant pair screened or treated, in low- and middle-income countries.
Methods
Systematic review of English, French and Spanish publications from 2001 to 2009, reporting empirical costing that accounted for at least antiretroviral (ARV) medicines, laboratory testing and personnel. Expenditures were analyzed by country income level and cost component. All costs were standardized to 2009 US dollars.
Results
Analyses covered 29 eligible, comprehensive costing studies. In the base case, in low-income countries (LIC), median, ART cost per patient-year was $792 (mean: $839, range: $682-$1089); for lower-middle-income countries (LMIC), the median was $932 (mean: $1246, range: $156-$3904); and for upper-middle-income countries (UMIC) the median was $1454 (mean: $2783, range: $1230-$5667). ARV drugs were largest component of overall ART cost in all settings (62%, 50% and 47% in LIC, LMIC and UMIC respectively). Out of 26 ART studies, 14 report which drug regimes were used, and only one study explicitly reported second line treatment costs. The second cost driver was laboratory cost in LIC and LMIC (14% and 19.5%) whereas it was personnel costs in UMIC (26%). Two studies specified the types of laboratory tests costed, and three studies specifically included above-facility-level personnel costs. Three studies reported detailed PMTCT costs, and two studies reported on pediatric ART.
Conclusions
There is a paucity of data on the full ART and PMTCT delivery unit costs, in particular for low-and middle-income countries. Heterogeneity in activities costed and insufficient detail regarding components included in the costing hampers standardization of unit cost measures. Evaluation of program-level unit costs would benefit from international guidance on standardized costing methods, and expenditure categories and definitions. Future work should help elucidate the sources for the large variations in delivery unit costs across settings with similar income and epidemiological characteristics.
Keywords: HIV treatment costs, ART, PMTCT, developing countries, systematic review, meta-analysis
1. Introduction
Cost evaluations support program planning and budgeting, can help to ensure program sustainability, and are a pre-requisite in identifying opportunities for efficiency gains. [1, 2] As global health institutions strive to ensure “value for money,” they are committed to supporting countries to measure per-person delivery costs of key services.[3–8] Since 2008, many national HIV/AIDS programs have made an important advance in complying with the bi-annual routine reporting of nationally aggregated expenditures as stated in the 2001 Declaration of Commitment on HIV/AIDS (UNGASS) to UNAIDS.[9] However, UNGASS reporting does not express program expenditures as per-person unit costs, nor does it request a comprehensive break-down of the cost components included in each service area. As of 2010, most HIV/AIDS programs do not routinely assess their cost per person or per unit of service delivery, nor do they have expenditure breakdowns by cost components using standardized methods.
For antiretroviral treatment (ART), scale-up of treatment access in low- and middle-income studies started in 2004–5, when funding increased substantially with new donor funding from the Global Fund to Fight AIDS, Tuberculosis and Malaria, and the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), among others. As of the end of 2009, an estimated 5.2 million HIV-infected people were receiving ART globally; with the WHO’s revised 2010 guidelines on HIV treatment in resource-limited settings, the total immediate need is now estimated at 15 million eligible people worldwide.[10] The ongoing scale-up and progress toward Universal Access will depend on both total available funding and the delivery cost per patient-year in high-HIV countries.
Previous reviews of ART – facility-level or per-patient – costs found only a very small number of studies from low- and middle-income countries [11, 12] and estimated the cost of antiretroviral (ARV) medicines from manufacturers’ procurement price lists, as an average per country income-group.[12] For Prevention of Mother-to-Child Transmission of HIV (PMTCT), (unit) delivery costs have not been reviewed since 2000.[13] The most recent ART costs review [14] included more studies from low- and middle-income countries and presented a scoring system to rate studies’ methodological quality – without attempting a quantitative meta-analysis of cost results.
To complement these overviews, we conducted a systematic literature review and a meta-analysis of per-patient unit expenditures on adult and pediatric ART as well as ARV prophylaxis used for PMTCT, using explicit guidelines for systematic reviews, and standardizing data from eligible studies into common service delivery units and cost component categories. Per-patient costs are presented as median, arithmetic average, and ranges across eligible study sites, studies, countries and country-income-level groupings, separately for the most important standardized cost components. We discuss results in the light of information requirements anticipated from National AIDS Programs from major global financiers of HIV/AIDS program scale-up.
2. Methods
2.1. Literature Search
The literature search followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[15] Original articles published in English, French and Spanish from January 2001 to October 2009 were searched using PubMed, EconLit and Cochrane Library, and gray literature through Google Scholar and POPLINE. In addition, websites of international donor organizations such as UNAIDS, WHO, World Bank, PEPFAR, UNICEF were searched. We also screened abstracts from the International AIDS Conferences 2001–2008, International Health Economics Association 2006–2008, American Society for Health Economists 2006–08, International AIDS Economics Network 2008, and PEPFAR Implementer’s meetings 2008–09 for contextual information to the published data. References identified as relevant served to track additional studies of interest. Annex A (all annexes available in Supplemental Digital Contents only) details the search terms used on electronic databases. Annex B shows the search commands and number of studies found.
2.1A. Inclusion criteria
Country-specific, empirical measurement of actual expenditures, from micro-costing or ingredients approach. (If the study used solely a step-down approach it was excluded);
Study (or study data point) included at least 25 patients;
Low- and middle-income countries, according to the July 2009 World Bank list of economies[16] with both publication and data collection between January 2001 and October 2009;
Costing included at least antiretroviral drugs (ARVs), laboratory expenses and personnel, as three individually reported expenditure components, or as explicitly named components of a comprehensive but unbundled overall expenditure;
Economic costs from the health care provider perspective; financial costs included if economic costs were not available. (Economic costs accruing to patients such as waiting time, transportation time, and productivity losses were not considered because of the inherent difficulties associated with assigning opportunity costs in the absence of accurate data on wages and time costs). (A list of excluded studies is available in Annex D).
2.1B. Data extraction
Eligible articles were reviewed for inclusion by at least two independent reviewers (OG and VW or AML), using a pre-determined data extraction form (see Annex C). When the decision was “in doubt”, a third or fourth reviewer (YST or AFL) analyzed the article; inclusion was solved by consensus. If the information contained in the full-text article was incomplete, its authors were contacted to obtain missing data.
2.1C. Standardization of data
ART delivery unit cost data were standardized as estimates per patient-year, separately for first-line and second-line ART, or weighed by their local mix of patients. Pediatric ART was distinguished from adult ART using an age cut-off of 15 years. Components of total costs were categorized as follows:
Antiretroviral medicines (ARVs); including, if available, transport of drugs to point of care, and storage;
Personnel involved in delivering ART, including education and training, as well as supra-facility level human resources, if specified (such as personnel in distribution centers or district/provincial/national program management, or monitoring and evaluation);
Laboratory tests, including CD4 cell counts and viral load, as well as other tests, if specifically mentioned.
Overhead costs including administration and utilities (electricity, water, phone, rent, maintenance costs, cleaning, security);
Other components considered and reported as part of delivery unit costs, such as: concomitant medications (for opportunistic infections or prophylaxis such as Septran), other supplies (e.g., cotton, syringes, gloves), staff transport for home visits (i.e., trained field officers visit patients’ homes periodically to deliver drugs, clinically monitor participants with a checklist on signs and symptoms of drug toxicity or disease progression, and provide adherence support), depreciated capital costs (medical and non-medical equipment), as well as other recurrent costs, when those breakdowns were available.
For PMTCT, reported costs were either per HIV-infected pregnant woman receiving ARV prophylaxis, or per pregnant woman initially tested and counseled for HIV. In each case, costs covered the woman-neonate pair throughout the pregnancy/birth period. Cost components were categorized as follows:
ARVs given before, during and/or shortly after birth to mother and/or child to prevent transmission (including transport cost of ARVs to point of care and storage);
Personnel involved in delivering PMTCT, including education and training, as well as supra-facility level personnel, if specified;
Laboratory tests (including HIV testing, CD4 cell counts and viral load);
Overhead costs including administration and utilities (electricity, water, phone, rent, maintenance costs, cleaning, security);
Other components, as listed by specific studies.
We report all costs in October 2009 US dollars (USD), after foreign currency conversion using average annual exchange rates provided by original study authors or from the International Monetary Fund[17] (except, in the case of a study of ART in Lesotho[18], directly from a Central Bank[19] source for lack of the relevant exchange rate from the IMF). Once converted into USD, costs were adjusted for inflation using the U.S. Consumer Price Index (CPI).[20]
2.2. Data aggregation and sensitivity analysis
The analysis focused on economic costs from the perspective of the provider. Specified economic costs to the patient were included only if a monetary transaction took place so that the service would occur (e.g., a co-payment or recuperation fee paid for by the patient); in which case they were added to the overall costs.
Unit costs and unit cost components were summarized as mean, median and range across countries studied (after aggregation across multiple sites, or data points within each country), within country income level groups: low-income countries (LIC), lower-middle-income countries (LMIC), and upper-middle-income countries (UMIC), according to the World Bank’s 2009 country classification.[16] This method of aggregation prevented countries with relatively large numbers of data points to skew the results. In sensitivity analyses, we explored the robustness of results against alternative ways of aggregating results across sites, countries and country-income groupings, and of different study inclusion criteria.
3. Results
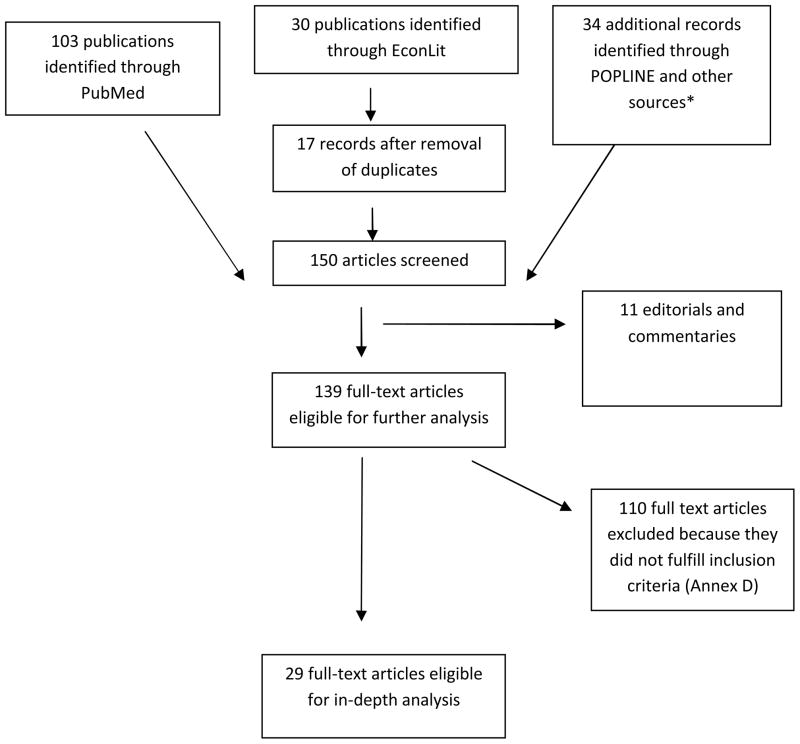
Out of 574 abstracts retrieved, 150 full-text studies were assessed for eligibility; of those, 29 were included in analysis: 26 on ART (23 on adult ART, two on adult and pediatric ART[21, 22], and one on pediatric ART only) [23] (tables I, II and III). Table IV provides results for the base case and the sensitivity analysis for ART unit costs, and fig. 2 summarizes the most relevant findings. Three studies for PMTCT are detailed in table V. (Expanded versions of tables with additional information and annexes are available as Supplemental Digital Content).
Table I.
Delivery Unit Costs of Antiretroviral Treatment, per patient-year, low-income countries
| Country | Reference | City/Setting | Scope | Provider type | ART unit costs, in 2009 USD | Costs components, in 2009 USD (% of total cost) | ART Regimen | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Facilities | Patients | ARV drugs | Personnel | Laboratory tests | Overheads | ||||||
| Benin | Hounton S, et al. 2008a[24] | Cotonou/U | 1 | 122 Outpatients |
Nat/NGO/D | 681.9 | 500.5 (73.4%) | 51.4 (7.5%) | 99.5 (14.6%) | 21.1 (3.1%) | NA |
| Ethiopia | Bikilla A, et al. 2009b[25] | Arba Minch/U | 1 | 209 Outpatients |
Nat | 262.8 | 205 (78%) | 37.8 (14.4%) | 15.7 (6%) | Included but not unbundled | (d4T/3TC + NVP) or (AZT +
3TC + NVP) or (d4T/3TC + EFV) or (AZT + 3TC
+ EFV) All regimens are first-line treatments |
| Kombe G, et al. 2004* [26] | Nazareth/U; Addis/U; Bahir Dar/U | 6 | NA | Nat | 804.1 | 548.3 (68.2%) | 14.2
(1.8%) Doctor, nurse, counselor, pharmacist, lab technician |
241.5 (30%) Full blood count, blood chemistry, blood sugar, CD4 count, viral load |
Not included | AZT+ d4T +
NVP First-line treatment |
|
| The PEPFAR ART Costing Project Team, 2009c,w[22] | Urban | 9 | NA Outpatients |
Nat | 741.7 New adult patients |
428.3 (57.7%) | 21.8 (2.9%) | 191.1 (25.8%) | 59.9 (8%) | NA | |
| 610.8 Established-adult patients |
428.3 (70.1%) | 12 (2%) | 80.8 (13.2%) | 33.7 (5.5%) | |||||||
| 960.8 New pediatric patients |
606.7 63.1%) | 21.8 (2.2%) | 191.1 (19.9%) | 62.7 (6.5%) | |||||||
| 933.4 Established pediatric patients |
606.7 (65%) | 12.3} (1.3%) | 94.5 (10.1%) | 39.4 (4.2%) | |||||||
| Haiti | Koenig S, et al. 2008d [27] | Port-au-Prince/U | 1 | 218 Inpatients-Outpatients |
NGO | 1,088.7 | 402.6 (37%) | Included but not unbundled | 147.4 (13.5%) | 129.6 (11.9%) | NA |
| Uganda | Jaffar S, et al. 2009e [28] | Jinja/R | 1 | NA Outpatients |
NGO | 789 | 416.6 (52.8%) | 181.5 (23%) | Included but not unbundled | Included but not unbundled | NA |
| 833.8 | 417.7 (50.1%) | 242.6 (29.1%) | |||||||||
General: The tables are ordered alphabetically by country name and then by author. Dollar amounts and proportions of overall costs may not add to 100% due to rounding and because some of costs do not fit into the classifications used. For example, transportation costs to visit patients’ homes, non-ARV medicines, OI medicines, and recurrent costs such as cotton, syringes, gloves, etc. are in the “Other” category, which has been excluded from the table (but could be estimated subtracting the current total from 100%). ARV medicine costs include only the costs of antiretrovirals, mostly first-line unless otherwise noted in Regimen type. Personnel costs include human resources expenses incurred in services delivery, including health workers doing patient care and in some studies also administrative personnel. Laboratory costs may include HIV testing, CD4 cell count and viral load measurement. Overheads may include capital costs (medical equipment, computers) and/or recurrent costs (utilities: electricity, water, phone, rent, maintenance costs, storage, cleaning, or security). If the study reviewed provided details about what was included in each category, those details have been incorporated in the tables. Costs were converted into 2009 USD using average exchange rates during the study period (see sources in the Methods section).
City/setting: Refers to the city or area name and the type of location where the study took place, including U= Urban and R= Rural
Facilities: Refers to the number of facilities in which the costs were collected
Patients: Refers to the number of patients for whom cost data were collected for ART delivery
Provider type: Refers to the type of provider delivering ART: Nat=National; D=Donor; NGO= Non-government organization ; FBO= Faith-based organization
Regimen: Refers to the ARV line costed by the study. The acronyms for ARV drugs were taken from the World Health Organization classification: Cotrimoxazole = CTX; Didanosine = ddl; Efavirenz = EFV; Indinavir = IDV; Lamivudine = 3TC; Lopinavir = LPV; Nevirapine = NVP; Ritonavir =RTV; Saquinavir = SQV; Stavudine= d4T; Zidovudine = AZT
NA=Not available in the study reviewed.
The study includes programmatic cost
The costs collected are financial costs.
Specific:
International dollars convert into USD using the purchase power parity conversion factor provided by the U.N. Statistical Office.[61]
Outpatients only. It includes patients who are 15 years old and above.
The study reports the median costs.
Costs calculated using patients retained “at the end of the study” as denominator.
The study reports median costs.
Costs taken were for “first-line, annually thereafter” (i.e., first-line patients only, from their 6th month after ART initiation onwards).
Including patients who started visiting the hospital in 1997, as it was not possible to single-out those patients who initiated ART after 2001.
Public sector only, with patients paying a fixed portion of ART (included in the costs listed).
Annual patient cost derived by multiplying cost per visit with an average number of visits per year. Because the hospital charged an extra 15%, we multiplied reported results obtain the actual cost of ART delivery.
Study does not specify what “other” specialists or what “other” laboratory tests were costed out.
Patients who, every month throughout the study period, received ART. Costs presented cover patients through 2006, with only 41% having started after the year 2000.
Costs include patients who initiated ART in 2000, as it was not possible to single-out those patients who started after 2001.
Costs concern ART given for the treatment occurring between 6–12 months for first-line ART (patient specific) and separately for second-line treatment.
Average between first year and second year of follow-up.
Average between first year and second year of treatment.
Includes patients who are 15 years old or above.
Includes children: patients who are younger than 15 years of age.
For further details see the Supplementary Digital Content version of the tables.
Table II.
Delivery Unit Costs of Antiretroviral Treatment, per patient-year, lower-middle-income countries
| Country | Reference | City/Setting | Scope | Provider type | ART unit costs, in 2009 USD | Costs components, in 2009 USD (% of total cost) | ART Regimen | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Facilities | Patients | ARV drugs | Personnel | Laboratory tests | Overheads | ||||||
| India | Gupta I, et al. 2009f * β[29] | Chennai/U | 1 | 2,606 | Nat | 96.7 | 46.2 (47.8%) | 7.1 (7.3%) | 18.9 (19.5%) | Not included | d4T + 3TC +
NVP First-line treatment |
| Imphal/U | 1 | 226 | 377.2 | 130.3 (34.5%) | 33.3 (8.8%) | 97.8 (25.9%) | |||||
| Ahmedabad/U | 1 | 1,210 | 133.2 | 60.7 (45.5%) | 8.5 (6.4%) | 42.4 (31.8%) | |||||
| New Delhi/U | 2 | 1,728 | 120 | 53.4 (44.5%) | 11.4 (9.5%) | 20.3 (16.9%) | |||||
| Trivandrum/U | 1 | 498 | 217.9 | 118.3 (54.2%) | 12.8 (5.9%) | 60.3 (27.6%) | |||||
| Thrissur/U | 1 | 308 | 155.5 | 101.4 (65.1%) | 8.7 (5.6%) | 0 (0%) | |||||
| John K, et al. 2006g β[30] | Bangalore/U | 1 | 25 Inpatients-Outpatients |
NGO | 461.8 | 257.7 (55.8%) | Included but not
unbundled Project coordinator, medical officer, nurses, laboratory technician, counselors |
17.4 (3.8%) | Included but not unbundled | AZT/d4T + 3TC +
NVP First-line treatment |
|
| Lesotho | Cleary S, et al. 2007h[18] | Maseru/U | 1 | 271 Inpatients |
Nat/NGO | 161.7 | 127.4 (78.8%) | Included but not unbundled | 15.3 (9.5%) | Included but not unbundled | 3TC + d4T +
NVP First-line treatment |
| Morocco | Loubiere S, et al. 2008i[31] | Casa Blanca/U | 1 | 167 Inpatients-Outpatients |
Nat | 1,076.9 | Included but not unbundled | Included but not unbundled | Included but not unbundled | Included but not unbundled | NA |
| Nigeria | Kombe G, et al. 2004 j [32] | Lagos/U; Abuja/U | 5 | NA | Nat | 841 | 420.5 (50%) | 185 (22%) | 193.4 (23%) | 33.6 (4%) | d4T + 3TC +
NVP First-line treatment |
| PHR, 2004k * β[33] | Anambra/U; Bauchi/R; Edo/U; Federal Capital Territory/U; Kano/U; Lagos/U; Nassarawa/U; Rivers/U | 66 | NA Inpatients-Outpatients |
Nat/FBO/NGO | 1,023.3 | 362.1 (35.4%) | 391.2 (38.2%) | 237.5 (23.2%) | 18.6 (1.8%) | 3TC + d4T +
NVP First-line treatment |
|
| Thailand | Kitajima T, et al. 2003l[34] | Khon Kaen/R | 2 | 106 Outpatients |
Nat | 3,903.8 | 3,415.1 (87.5%) | Included but not unbundled | 462 (11.8%) | 26.7 (0.7%) | NA |
General: The tables are ordered alphabetically by country name and then by author. Dollar amounts and proportions of overall costs may not add to 100% due to rounding and because some of costs do not fit into the classifications used. For example, transportation costs to visit patients’ homes, non-ARV medicines, OI medicines, and recurrent costs such as cotton, syringes, gloves, etc. are in the “Other” category, which has been excluded from the table (but could be estimated subtracting the current total from 100%). ARV medicine costs include only the costs of antiretrovirals, mostly first-line unless otherwise noted in Regimen type. Personnel costs include human resources expenses incurred in services delivery, including health workers doing patient care and in some studies also administrative personnel. Laboratory costs may include HIV testing, CD4 cell count and viral load measurement. Overheads may include capital costs (medical equipment, computers) and/or recurrent costs (utilities: electricity, water, phone, rent, maintenance costs, storage, cleaning, or security). If the study reviewed provided details about what was included in each category, those details have been incorporated in the tables. Costs were converted into 2009 USD using average exchange rates during the study period (see sources in the Methods section).
City/setting: Refers to the city or area name and the type of location where the study took place, including U= Urban and R= Rural
Facilities: Refers to the number of facilities in which the costs were collected
Patients: Refers to the number of patients for whom cost data were collected for ART delivery
Provider type: Refers to the type of provider delivering ART: Nat=National; D=Donor; NGO= Non-government organization ; FBO= Faith-based organization
Regimen: Refers to the ARV line costed by the study. The acronyms for ARV drugs were taken from the World Health Organization classification: Cotrimoxazole = CTX; Didanosine = ddl; Efavirenz = EFV; Indinavir = IDV; Lamivudine = 3TC; Lopinavir = LPV; Nevirapine = NVP; Ritonavir =RTV; Saquinavir = SQV; Stavudine= d4T; Zidovudine = AZT
NA=Not available in the study reviewed.
The study includes programmatic cost
The costs collected are financial costs.
Specific:
International dollars convert into USD using the purchase power parity conversion factor provided by the U.N. Statistical Office.[61]
Outpatients only. It includes patients who are 15 years old and above.
The study reports the median costs.
Costs calculated using patients retained “at the end of the study” as denominator.
The study reports median costs.
Costs taken were for “first-line, annually thereafter” (i.e., first-line patients only, from their 6th month after ART initiation onwards).
Including patients who started visiting the hospital in 1997, as it was not possible to single-out those patients who initiated ART after 2001.
Public sector only, with patients paying a fixed portion of ART (included in the costs listed).
Annual patient cost derived by multiplying cost per visit with an average number of visits per year. Because the hospital charged an extra 15%, we multiplied reported results obtain the actual cost of ART delivery.
Study does not specify what “other” specialists or what “other” laboratory tests were costed out.
Patients who, every month throughout the study period, received ART. Costs presented cover patients through 2006, with only 41% having started after the year 2000.
Costs include patients who initiated ART in 2000, as it was not possible to single-out those patients who started after 2001.
Costs concern ART given for the treatment occurring between 6–12 months for first-line ART (patient specific) and separately for second-line treatment.
Average between first year and second year of follow-up.
Average between first year and second year of treatment.
Includes patients who are 15 years old or above.
Includes children: patients who are younger than 15 years of age.
For further details see the Supplementary Digital Content version of the tables.
Table III.
Delivery Unit Cost of Antiretroviral Treatment, per patient-year, upper-middle-income countries
| Country | Reference | City/Setting | Scope | Provider type | ART unit costs, in 2009 USD | Costs components, in 2009 USD (% of total cost) | ART Regimen | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Facilities | Patients | ARV drugs | Personnel | Laboratory tests | Overheads | ||||||
| Brazil | Acurcio A, et al. 2006m * [35] | Belo Horizonte/U | 2 | 157 adherents patients |
Nat | 1,454 | 1,290.3 (88.7%) | 6.2
(0.4%) Infectious diseases specialist and other specialists |
45 (3%) Lymphocyte CD4/CD8, viral load test, other tests |
Not included | NA |
| 40 non-adherents patients |
1,340.1 | 1,290.3 (96.3%) | 8.7
(0.6%) Infectious diseases specialist and other specialists |
39.9
(2.9%) Lymphocyte CD4/CD8, viral load test, other tests |
|||||||
| Marques H, et al. 2007 w[23] | Sao Paulo/U | 1 | 140 Outpatient children |
Nat | 2,038.8 | Included but not unbundled | Included but not unbundled | Included but not unbundled | Included but not unbundled | RTV+NFV+3CT+ZDV+ddI+IDV | |
| Mexico | Aracena-Genao B, et al. 2008n[36] | Mexico City/U | 1 | 80 | Nat | 7,688 | Included but not unbundled | Included but not unbundled | Included but not unbundled | Included but not unbundled | NA |
| Bautista-Arredondo S, et al. 2003; Bautista-Arredondo S, et al. 2008o[37, 38] | Mexico City/U; Cuernavaca/U; Guadalajara/U | 11 | 902 | Nat | 6,651.3 First year after ART treatment |
6,024.4 (90.6%) | Included but not unbundled | 223.4 (3.4%) | Included but not unbundled | NA | |
| 4,256.8 Second year after ART treatment |
3, 809.4 (89.5%) | 165.8 (3.9%) | |||||||||
| 4,682.9 Third year after ART treatment |
3,960.7 (84.6%) | 254.6 (5.4%) | |||||||||
| South Africa | Cleary S, et al. 2006p[39] | Cape Town/U | 3 | 1,729 | Nat | 987.3 | 338.8 (34.3%) | Included but not unbundled | 96 (9.7%) | Included but not unbundled | AZT +
3TC+NVP/EFV First-line Treatment |
| 1,774.3 | 1,108.3 (62.5%) | 112 (6.3%) | AZT + ddl +
LPV/RTV Second-line treatment |
||||||||
| Deghaye N, et al. 2006q [40] | Durban/U | 2 | 41 | Nat | 1,022.3 | 411 (40.2%) | 330 (32.3%) | 218.8 (21.4%) | 16.4 (1.6%) | d4T + 3TC+
EFV First-line treatment |
|
| Harling G, Bekker J, et al 2007r[41] | Cape Town/U | 1 | NT | Nat/D | 521.6 | Included but not unbundled | Included but not
unbundled Doctor, nurse, counselor, pharmacist |
Included but not unbundled | Included but not unbundled | NT | |
| Harling G, Wood R. 2007s[42] | Cape Town/U | 1 | 172 outpatients |
Nat/NGO | 1,296.5 | 535.9 (41.3%) | 203.3 (15.7%) | 341 (26.3%) | 25.2 (1.9%) | d4T + 3TC +
EFV First-line treatment |
|
| 129 inpatients |
1,834.3 | 535.9 (29.2%) | 545.5 (29.7%) | 442.1 (24.1%) | 212.7 (11.6%) | ||||||
| Kevany S, et al. 2009t[43] | Cape Town/U | 1 | 48 outpatients |
Nat | 1,795.8 | 103.9 (5.8%) | 988.6
(55.1%) Consultant, medical officer, nursing sister, medical ward staff |
478.1 (26.6%) | Not included | 3TC + d4T +
NVP First-line Treatment |
|
| 25 inpatients |
6,579.5 | 271.7 (4.1%) | 2,097.8
(31.9%) Consultant, medical officer, nursing sister, medical ward staff |
847.6 (12.9%) | |||||||
| Martinson N, et al. 2009u [44] | Soweto/U | 1 | 591 | D | 2,853.6 Newly ART patients |
958 (33.6%) | 435.5
(15.3%) Primary health care nurse, pharmacist, medical officer |
1,174.4 (41.2%) | 190.5 (6.7%) | d4T + 3TC +
NVP First-line treatment |
|
| 1,770.4 Established-ART patients |
958 (54.1%) | 435.5
(24.6%) Primary health care nurse, pharmacist, medical officer |
114.5 (6.5%) | 190.5 (10.8%) | |||||||
| Rosen S, et al. 2008v [45] | Gauteng/U | 2 | 200 | Nat/NGO | 1,122.5 Patient responding |
Included but not unbundled | Included but not unbundled | Included but not unbundled | Included but not unbundled | 3TC + d4T +
EFV First-line treatment |
|
| 1,210.3 Patient not responding | |||||||||||
| Eastern Cape/U | 1 | 100 | NGO | 1,241.1 Patient responding |
|||||||
| 1,177.3 Patient not responding | |||||||||||
| Mpumalanga/R | 1 | 100 | 1,229.4 Patient responding |
||||||||
| 1,182.7 Patient not responding | |||||||||||
| Vella V, et al. [21] 2008w | KwaZulu-Natal/U | 32 | 2,835 | Nat | 1,068.1 | 500 (46.8%) | 300 (28.1%) | 236 (22.1%) | 16.6 (1.5%) | NA | |
General: The tables are ordered alphabetically by country name and then by author. Dollar amounts and proportions of overall costs may not add to 100% due to rounding and because some of costs do not fit into the classifications used. For example, transportation costs to visit patients’ homes, non-ARV medicines, OI medicines, and recurrent costs such as cotton, syringes, gloves, etc. are in the “Other” category, which has been excluded from the table (but could be estimated subtracting the current total from 100%). ARV medicine costs include only the costs of antiretrovirals, mostly first-line unless otherwise noted in Regimen type. Personnel costs include human resources expenses incurred in services delivery, including health workers doing patient care and in some studies also administrative personnel. Laboratory costs may include HIV testing, CD4 cell count and viral load measurement. Overheads may include capital costs (medical equipment, computers) and/or recurrent costs (utilities: electricity, water, phone, rent, maintenance costs, storage, cleaning, or security). If the study reviewed provided details about what was included in each category, those details have been incorporated in the tables. Costs were converted into 2009 USD using average exchange rates during the study period (see sources in the Methods section).
City/setting: Refers to the city or area name and the type of location where the study took place, including U= Urban and R= Rural
Facilities: Refers to the number of facilities in which the costs were collected
Patients: Refers to the number of patients for whom cost data were collected for ART delivery
Provider type: Refers to the type of provider delivering ART: Nat=National; D=Donor; NGO= Non-government organization ; FBO= Faith-based organization
Regimen: Refers to the ARV line costed by the study. The acronyms for ARV drugs were taken from the World Health Organization classification: Cotrimoxazole = CTX; Didanosine = ddl; Efavirenz = EFV; Indinavir = IDV; Lamivudine = 3TC; Lopinavir = LPV; Nevirapine = NVP; Ritonavir =RTV; Saquinavir = SQV; Stavudine= d4T; Zidovudine = AZT
NA=Not available in the study reviewed.
The study includes programmatic cost
The costs collected are financial costs.
Specific:
International dollars convert into USD using the purchase power parity conversion factor provided by the U.N. Statistical Office.[61]
Outpatients only. It includes patients who are 15 years old and above.
The study reports the median costs.
Costs calculated using patients retained “at the end of the study” as denominator.
The study reports median costs.
Costs taken were for “first-line, annually thereafter” (i.e., first-line patients only, from their 6th month after ART initiation onwards).
Including patients who started visiting the hospital in 1997, as it was not possible to single-out those patients who initiated ART after 2001.
Public sector only, with patients paying a fixed portion of ART (included in the costs listed).
Annual patient cost derived by multiplying cost per visit with an average number of visits per year. Because the hospital charged an extra 15%, we multiplied reported results obtain the actual cost of ART delivery.
Study does not specify what “other” specialists or what “other” laboratory tests were costed out.
Patients who, every month throughout the study period, received ART. Costs presented cover patients through 2006, with only 41% having started after the year 2000.
Costs include patients who initiated ART in 2000, as it was not possible to single-out those patients who started after 2001.
Costs concern ART given for the treatment occurring between 6–12 months for first-line ART (patient specific) and separately for second-line treatment.
Average between first year and second year of follow-up.
Average between first year and second year of treatment.
Includes patients who are 15 years old or above.
Includes children: patients who are younger than 15 years of age.
For further details see the Supplementary Digital Content version of the tables.
Table IV.
Base Case and Sensitivity Analysis: Delivery Unit Cost (USD 2009) of ART per patient per year, by country income level
| Low income | Lower middle income | Upper middle income | All | ||
|---|---|---|---|---|---|
|
|
|||||
| Base case | Mean | 839 | 1,246 | 2,783 | 1,494 |
| Median | 792 | 932 | 1,454 | 1,005 | |
| SD | 175 | 1,546 | 2,500 | 1,628 | |
| Min | 682 | 156 | 1,230 | 156 | |
| Max | 1,089 | 3,904 | 5,667 | 5,667 | |
| Countries | 4 | 5 | 3 | 12 | |
| Studies (N) | 6 | 7 | 13 | 26 | |
| Data points/sites (n) | 10 | 12 | 24 | 45 | |
|
|
|||||
| 1. Weighing all study sites, instead of all countries equally | Mean | 770 | 714 | 2,366 | 1,600 |
| Median | 797 | 298 | 1,397 | 1,077 | |
| SD | 226 | 1,067 | 2 | 1,772 | |
| Min | 263 | 97 | 522 | 97 | |
| Max | 1,089 | 3,904 | 7,688 | 7,688 | |
| Countries | 4 | 5 | 3 | 12 | |
| Studies (N) | 6 | 7 | 13 | 26 | |
| Data points/sites (n) | 10 | 12 | 24 | 46 | |
|
|
|||||
| 2. Adding studies reporting total unit cost without ARV/lab/HR break-down | Mean | 713 | 1,314 | Unchanged from base-case | 1,422 |
| Median | 773 | 932 | 932 | ||
| SD | 319 | 1,637 | 1,639 | ||
| Min | 211 | 162 | 162 | ||
| Max | 1,089 | 4,212 | 5,667 | ||
| Countries | 5 | 5 | 13 | ||
| Studies (N) | 7 | 9 | 29 | ||
| Data points/sites (n) | 11 | 14 | 48 | ||
|
|
|||||
| 3. Country income level according to costing year | Mean | 565 | 1,683 | 3,068 | 1,496 |
| Median | 646 | 1,454 | 1,241 | 1,073 | |
| SD | 346 | 886 | 2,537 | 1,559 | |
| Min | 97 | 522 | 1,068 | 156 | |
| Max | 1,089 | 3,904 | 7,688 | 5,667 | |
| Countries | 7 | 4 | 2 | 12 | |
| Studies (N) | 11 | 9 | 6 | 26 | |
| Data points/sites (n) | 20 | 13 | 13 | 45 | |
Notes: The base case aggregates total ART unit costs per person per year (in 2009 USD) by first taking the median within each country. Sensitivity analyses are as follows: 1. All study sites weighted equally, regardless of country or number of sites per country. 2) Expanded dataset to include studies which did not include a break-down of cost components, but had data on total ART costs. 3) Use World Bank GNI per capita data at the time of the study costing, instead of 2009 GNI, to classify countries according to income level. Baseline estimates based on more than 14,765 patients from 26 studies, plus additional studies that did not disaggregate costs by cost component. Twelve studies specifically reported costs for first-line treatment; one study [39] specifically included second-line treatment but those specific costs were excluded. Total data points aggregate to 45 because two studies from Mexico [37, 38] were considered as only one data point as they referred to the same study population, but we needed both sources to obtain all the necessary information. See tables I, II, and III, text and annexes for further details.
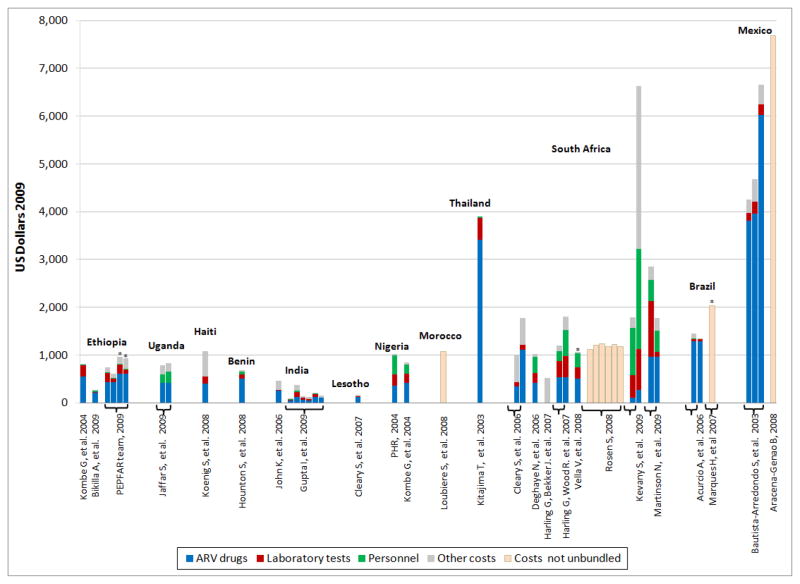
Fig. 2. Delivery unit costs of ART, per patient-year, by cost component, country and site.
Countries ranked from left to right according to increasing per capita gross national income (GNI) and then by year of study publication. Studies including (or limited to) pediatric ART are indicated with an asterisk (*) over the bar. ‘Other costs’ refer to components of ART other than ARV medicines, laboratory and personnel costs; ‘other costs’ are not comparable across studies because they do not contain the same elements. Unbundled costs refer to the overall cost of ART in case the study did not report the separate cost components – but these still included at least the three main components under review. Each bar represents a separate data point within each country or study (e.g., different region, health facility/site, patient type, delivery modality, temporality, etc.). GNI per capita (2009): Ethiopia: $280, Uganda: $420, Haiti: $660, Benin: $690, India: $1,070, Lesotho: $1,080, Nigeria: $1,160, Thailand: $2,840, South Africa: $5,820, Brazil: $7,350, Mexico: $9,980. See tables I, II and III for additional details.
Table V.
Delivery Unit Cost of Prevention of Mother to Child Transmission of HIV
| Country | Reference | City/Setting Costing year | Scope | Provider type | PMTCT unit cost (2009USD) | Cost in 2009 USD (% of total cost) | Regimen | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Facilities | Patients | ARV drugs | Personnel | Laboratory tests | Overheads | ||||||
| India | Dandona L, et al. 2008 [46] | Andhra Pradesh/U2005–06 | 16 | 1,212ϒ | Nat | 2.4 Post test counseling |
Included but not unbundled | 1.1 (47.3%) | Included but not unbundled | 0.35 (14.7%) | NVP |
|
251.1# Mother neonate pair receiving nevirapine |
118.7 (47.3%) | 36.9 (14.7%) | |||||||||
| South Africa | Desmond C, et al. 2004 [47] | Durban/U; Paarl/U; Siloam/R Frankfort/R; 2002 | 9 | 709 | Nat/D | 42.4 Pretest counseling |
Included but not unbundled | Included but not unbundled | Included but not unbundled | Not included | NVP + CTX |
| 570 | 32.5 Testing |
||||||||||
| 532 | 23.8 Post test counseling |
||||||||||
| 117ϒ |
208.8¥ Mother neonate pair receiving nevirapine |
||||||||||
| 63 | 327.2‡ Mother neonate pair follow up |
||||||||||
| Rwanda | McMennamin T, Fellow A. 2007 [48] | Gicumbi/R 2005 | 6 | NA | Nat/FBO | 6.42 Specific intervention |
Included but not unbundled | Included but not unbundled | Included but not unbundled | Included but not unbundled | Not reported |
NA: not available in the study
Costs have been converted into USD$ of 2009 using average exchange rates during the study period
City/setting: Refers to the city and the type of location: U= Urban; R= Rural
Costing year: Refers to the year for which the study reported the costs
Facilities: Refers to the number of facilities in which the costs were collected
Patients: Refers to the number of patients studied to cost the ART delivery:
mother-neonate pairs
Provider type: Refers to the type of ART provider: Nat=National; D=Donor; NGO= Non-government organization ; FBO= Faith based organization
Regimen: ARV line costed by the study using acronyms for ARV drugs taken from the World Health Organization classification: Cotrimoxazole = CTX; Nevirapine = NVP
The mother was given one tablet of 200 mg at the onset of labor and the neonate was given 2 mg/kg body weight within 72 hours of birth.
This cost includes re-issue of nevirapine to mothers, and provision of nevirapine suspension to the child and any formula milk for the duration of the hospital stay.
PMTCT activities delivered after the mother and infant are discharged from hospital after delivery, including cotrimoxazole and the provision of formula feeding. It does not include infant HIV testing.
3.1. Antiretroviral Treatment
Six studies from four low-income countries (LIC) were included (Benin, Ethiopia, Haiti and Uganda); the median cost per patient-year of ART was $792 (mean $839, range $682 to $1,089) (tables I & IV).[22, 24–28] Studies for lower-middle-income countries (LMIC) were found for India[29, 30], Lesotho[18], Morocco[31], Nigeria[32, 33], and Thailand [34]; the median cost of ART was $932 (mean $1,246, range $156 to $3,904) (tables II & IV). In upper-middle-income countries (UMIC), Brazil [23, 35], Mexico [36–38] and South Africa [21, 39–45] the median ART cost was $1,454 (mean $2,783; range $1,230 to $5,667) (tables III & IV).
ART unit costs and its components varied considerably both between countries and studies,– such as South Africa[21, 39–45] – and between sites within one study (e.g., Gupta et al, 2009[29]) (fig. 2). While variations between studies may in part reflect differences in costing methods and definitions, variations within studies point to the existence of real differences in actual cost between sites and/or patient populations.
3.1A. ARV medicines
In all three income groups, ARV medicines were the largest cost component. In low-income countries, they represented 64% of the overall costs, at a median $428 per patient-year (mean: $456 (62%), range: $205–$607 (37% – 78%)) (table I). In lower-middle income countries, ARV medicines represented 50% of overall cost at a median $127 (mean: $463 (54%), range: $46–$3415 (35%–88%)) (table II). In upper-middle income countries, ARV medicines covered 47% of cost, at a median $958 per patient-year (mean: $1473 (53%), range: $104 – $6,024 (4%–96%)) (table III).
3.1B. Laboratory
In LIC and LMIC, laboratory costs were the second most important cost component. In LIC they accounted for 14% of overall costs (median $123; mean: $133 (17%), range: $16 – $242 (6–30%)) (table I). In LMIC laboratory costs corresponded to 20% of overall costs (median cost of $42; mean: $106 (18%), range: <$1 – $462 (<1% – 32%)) (table II). In UMIC, laboratory costs accounted for 10% (median of $223; mean: $319 (14%), range: $40 – $1174 (3% – 41%) of ART cost (table III), being the third largest cost component after personnel.
3.1C. Personnel
In LIC and LMIC, personnel costs were the third most important cost component, corresponding to 3% in LIC [median $22; mean: $66 (9%), range: $12 – $243 (1%–29%)], (table I), and 8% in LMIC [median $12; mean: $82 (13%), range: $7–$391 (6%–38%)], (table II) of overall costs, respectively. In UMIC, in contrast, personnel costs were the second most important cost component at 26% of overall cost [median cost of $383; mean: $535 (24%), range: $6 – $2098 (0.4% – 55%)] (table III).
Most studies for ART (23 out of 26) included the cost of personnel only at the facility level. While types of services costed varied, only seven studies reported details of the types of personnel included [26, 30, 35, 39, 41, 43, 44]. Of these, three costing studies included above-facility level personnel such as human resources at distribution centers or district/provincial/national program management, or monitoring and evaluation in the personnel cost component.[29, 30, 33] (See section 3.1D below).
3.1D. Program-level costs
Three studies included program-level costs associated with ART delivery.[29, 30, 33] Across two urban sites in India [29] program-level costs on medical and administrative personnel other than those directly involved in ART delivery to patients were reported to be about 16% of per patient-year recurrent costs during the first year of the program, falling to about 7% in the second year. Another study in India[30] included a project coordinator working across different health facilities under personnel cost. In urban Nigeria[33], reported above-facility expenses included transport of drugs from federal central facilities to the ARV centers.
3.2. Pediatric ART
We identified two studies reporting costs of ART for patients below 15 years of age. In UMIC Brazil, costs of ART in a university hospital were $2,039 per year for outpatient children [23]; weighed across all children (14% of whom were inpatient), the average yearly cost was $2,826. In LIC Ethiopia [22] a total cost per child-year was found of $961 for new patients (within first 6 month of treatment) and $933 for established patients, with ARVs covering $607 (over 60% of total cost), and laboratory tests $191 (20%) in new or $94 (10%) in established patients.
3.3. PMTCT
Three eligible PMTCT costing studies were analyzed. The cost per mother-neonate pair receiving nevirapine around child delivery was $251 in India[46], $ 209 in South Africa [47] (both middle-income countries), but only $6.4 in Rwanda[48], a LIC (table V). (The large difference in cost in the latter study suggests that not all components were comparable).
The South Africa study[47] presented a thorough costing in already existing facilities, focusing on the added financial costs utilized specifically by the PMTCT program. Across four facilities, the average unit cost per HIV-infected mother-neonate pair receiving prophylactic nevirapine services was $209. This cost included nevirapine to mothers at delivery, as well as provision of nevirapine suspension to the infant and formula milk for the duration of the hospital stay. Additional costs estimated in the study were: $42.4 for the pre-test counseling, and $32.5 for testing costs. The study reported a substantially higher cost for post-discharge follow-up care at $327 per HIV-infected mother/infant pair covering cotrimoxazole and provision of infant formula after birth for the duration of the hospital stay (table V). The unit costs varied according to the denominator unit used (pregnant women screened, women tested, HIV-infected women and neonate pair, number followed) as well as between the four locations according to their economic and epidemiological differences: e.g., a high HIV prevalence, resource-poor setting (Frankfort, Free State) compared to a low-HIV-prevalence, better resourced setting (Paarl, Western Cape).
In Andhra Pradesh, India[46] across 16 PMTCT centers 1,212 HIV-infected pregnant women received PMTCT, out of 125,073 pregnant women counseled and tested. The average economic cost per HIV-infected woman receiving nevirapine around child delivery was $251. Expressed per pregnant women counseled and tested irrespective of HIV status, average unit cost was $2.40. Given the low price of nevirapine, personnel was the major cost component, at $119 or 47% of overall cost per HIV-infected woman, followed by $37 (15%) on overhead.
In Rwanda[49], the PMTCT unit cost was much lower, at $6.4 for a specific intervention. This marked contrast is in part explained by Rwanda’s much lower per-capita income level and health worker salaries. Equally important, this unit cost was achieved in a large-scale program in a high-HIV prevalence setting, with the six health centers covering a catchment population of 148,151 persons with a high PMTCT uptake rate of 71.6% that likely achieved significant economies of scale. However, the study does not specify number of pregnant women screened for PMTCT, the number of HIV-infected women, or the number of mother-infant pairs given NVP.
3.4. Sensitivity Analysis
A first sensitivity analysis assessed the effect of aggregating unit cost within country income groups by weighing all data points and sites across studies and countries equally, instead of the base-case model (presented above) that weighed all countries equally irrespective of their number of data points. In this variant model, median unit costs of ART were nearly unchanged for LIC and UMIC: in LIC the median was $797 (compared to $792 in base-case model) and for UMIC 1,397 (compared to $1,454 in base-case model, table IV). For LMIC, however, this alternative aggregation method resulted in a three-fold reduced median cost estimate per patient-year, of $298 compared to $932 in the base-case. This difference reflects an overrepresentation of study sites and data points from India among LMIC, with exceptionally low unit costs reflecting India’s uniquely low ARV prices related to the country’s important generic ARV industry and resulting strong pharmaceutical negotiation capacity.
A second sensitivity analysis, to further check on representativeness, expanded the data set to include a number of studies which did not include a break-down of cost components, but had data on total ART costs. Adding one study from Kenya[50], the LIC median cost per patient per year became $773 (mean $713, range $211 to $1,089), similar to the original estimate of $ 792. Among LMIC, adding data points from Thailand and India [51–53] did not alter the median cost per patient per year from default of $932 (but the mean increased to $1,314; range $162 to $4212).
Third, we replaced the country income classification according to each country’s 2009 income level by a classification using each country’s income level in the year of cost data collection (while in both cases applying World Bank’s 2009 income thresholds). This shifted five countries that had grown richer between the costing study year and 2009 (Brazil, India, Lesotho, Nigeria, and South Africa) into a lower income category. This country regrouping reduced the median ART per-patient cost among LIC (from $792 to $646), increased the estimate for LMIC (from $932 to $1,454) and decreased the estimate for UMIC (from $1,454 to $1,241).
4. Discussion
Empirical data on ART delivery unit costs in low- and middle-income countries has increased significantly in recent years, as access to ART has expanded. The current review identifies several more good-quality costing studies of both outpatient and home-based ART than earlier reviews [11, 12, 14], and provides cost estimates for the components ARVs, personnel and laboratory costs separately.
The results have several implications for program planning and budgeting, and for seeking efficiency improvement. For ART, within health facilities the key cost drivers are ARV procurement, laboratory tests, and staff. ARV prices have declined substantially over the last decade, with average declines of 12–39% over 2006–2009 for the first-line regimens most commonly used in low- and middle-income countries.[54] Lacking specification of regimens and drug sources in sites studied, it was not possible to adjust cost estimates for price declines. Only 6 out of the 24 ART costing studies reported whether the program used generic or innovator drugs (see SDC version of tables I, II and III). Of 14 studies that specified ARV regimens, the majority used lamivudine (3TC) + nevirapine (NVP) + stavudine (d4T) as the most common first-line regimen, the regimen for which recent price declines have been smallest: 9–12% per year. [54] The predominance of this regimen may explain why over 2004–2009, the average proportion of overall ART cost covered by ARV drugs was relatively stable, at 48–52% every year, rather than declining.
The observed large variations between countries and sites in laboratory costs point to possible opportunities for efficiency seeking. Part of variations may relate to differing clinical stage of patients and response to treatment [45], with high laboratory costs during the first 6 months after ART initiation.[22] Further, laboratory costs tend to be lower in established programs or in hospitals, for example, $15.7 or 6% of overall cost in Ethiopia in 2004–5 [25], compared to starting programs or clinics that are investing more in laboratory equipment.[26]
Available data (tables I—III) were limited in the specification of laboratory tests performed, test-specific costs as well as in the specification of precise laboratory testing practices and frequencies. We could therefore not analyze whether sites with routine, systematic CD4 and viral load monitoring were more expensive than sites with selective viral load monitoring, sites with only (or less frequent) CD4, or sites with just clinical monitoring, so we cannot estimate the likely savings from shifting testing patterns. In the trial of clinically-driven versus laboratory-guided ART (DART) in Uganda and Zimbabwe, hematology and biochemistry added little benefit; 12-weekly CD4 monitoring improved clinical outcomes from the 2nd year from treatment initiation onwards. Based on minimal lab monitoring (i.e., CD4 12-weekly after the second year), the cost of CD4 testing would have to drop to $3.8 (from current $175) in order to make laboratory monitoring cost-effective. [55] [56]
Another approach to improving ART program efficiency may be personnel task shifting. [57–59] In an NGO-led program in rural Uganda, costs to the provider were similar for clinic-based and home-based ART ($834 and $789 per patient-year, respectively), at comparable patient retention and clinical outcomes [28]. The study suggests an opportunity to scale-up ART access in settings with restrained health system capacity. The markedly higher cost of inpatient compared to outpatient ART (in 2 studies in South Africa, table III) furthermore demonstrates the financial importance of preventing hospitalizations.
4.1 Limitations
This review reflects the state of evidence in the first decade of global ART scale-up. Given the limited number of studies, from selected countries and settings, the available data could hardly claim global representativeness. Despite the recent increase in good-quality studies (as defined in our inclusion criteria), costing information remains extremely limited in South Asia (N=2 studies), Latin America and the Caribbean (N=5) and East Asia and the Pacific (N=1). Also, in sub-Saharan Africa, 8 out of the 16 studies were conducted in South Africa, which by its relatively high income level is the country least representative of the region. The lack of regional representativeness is one reason why our first sensitivity analysis found higher median ART costs in low-income countries compared to lower-middle-income countries, a result that seems contrary to intuition and to observed patterns in ARV prices.[54] Despite the inclusion of studies from the gray literature (of which 7 were found eligible [18, 21, 22, 26, 32, 33, 38]), we cannot exclude the possibility of publication bias in available costing studies. Several recent, well-executed ART and PMTCT cost studies that are recognized for influencing donor funding policies have not been published in books, peer-reviewed journals, or made otherwise publically available, so were unfortunately not included in our meta-analyses.
Innovative Monitoring & Evaluation strategies are needed to address the gaps in good-quality evidence, especially concerning above-facility and program-level costs. We intended to analyze all relevant cost components, including program–level (above-facility-level) activities and expenses including managerial overheads, administration, monitoring and evaluation and training borne at district, province and national levels. Only three ART studies however provided such programmatic costs, without sufficient description of the relevant activities or cost items to analyze the determinants of these potentially significant contributions to overall delivery cost.
Among available costing studies, quality and completeness varied. Many studies did not explicitly report all cost components, missing information on important cost determinants such as ARV drugs, regimens, type and frequency of laboratory testing used. Most studies also lacked details about variation in costs between different types of facilities and patient populations studied (in terms of e.g., age and CD4 cell count at treatment initiation).[22] Basic information on cost components was lacking in many studies; often either capital costs (such as those related to setting up laboratory equipment), or selected recurrent costs (like utilities) were omitted. In particular, definitions of overhead costs varied, with some studies not even including any overheads. Only three studies [32, 33, 35] mentioned explicitly that their data collection instruments had been validated or piloted before application. The best studies reviewed use a micro-costing approach, where expenditures on each component of providing ART or PMTCT delivery are separately listed and costed out. Even when an ingredients approach was utilized, most studies in the last decade do not provide the type of ART used (not even generic vs. patent, or first-line as distinct from second-line). In many studies sample sizes appear to have been driven by clinical outcomes, rather than by costing-related statistical considerations. Sample sizes varied between 209 (median for LIC, range 122–218) and 430 patients (median for UMIC, range 22 to 2,835), which may be considered appropriate for an overall estimate from one single locality. However, convenience sampling limits the precision and power to evaluate costs for relevant strata of sites, patient age groups, treatment regimens and modality and phases of treatment.
Heterogeneity in unit denominators, notably the calculation of patient-years, further complicates comparisons among studies. For illustration, a study in India [29] reported 1,094 clients who “ever started ART” (as average across sites), 939 clients “at the end of study” and 503 clients who had been “on therapy for the entire study period”. These authors selected the corresponding 9,460 client-months of ART as appropriate denominator unit; however the large differences between patients starting and retained illustrates the critical influence that varying denominator calculations will have on unit cost estimates, especially in programs with low patient retention and/or rapid scale-up with many new patients initiating ART.
4.2. Way forwards
The limitations in availability and comparability of existing cost data described above highlight an urgent need for development and dissemination of standardized cost measurement methodologies and for capacity building. Awaiting such guidance and improved data quality and standardization, any cost comparisons between sites and studies should only be done with extreme caution and attention to methodological differences; interpretations on relative efficiency will typically be limited to within-study determinants. Future strategic use of cost data for improving the efficiency of ART delivery will in particular benefit from more careful costing and reporting of ARV drugs sources and regimens, laboratory costs by type and frequency of tests, and of staff costs by type of personnel including those above the health facility level).
Future assessments should link costs to health outcomes, including cost differentials associated with clinical response[45], drug toxicity and resistance [43]. This is ever more important in view of the WHO’s revised 2010 guidelines for adult ART in resource-limited settings, which recommend an expanded access to ART starting at CD4 cell count below 350/uL instead of the former 200/uL. In addition, a gradual phasing-out is now recommended of stavudine (d4T) in first-line regimens, to be replaced by less toxic but more costly efavirenz and/or tenofovir-based first-line regimens [60]. As countries will over coming years gradually adopt and implement the new treatment guidelines, it is imperative that costing studies document the mix of patients evaluated in terms of CD4 cell counts at treatment initiation and regimens used, to facilitate interpretation of cost findings and cost determinants.
To improve the knowledge base on efficient ART and PMTCT delivery, collaboration between countries could be very important: First, to increase costing capacity and resources, and second, to exchange experiences in increasing service efficiency. Multi-country exercises should help to improve and standardize costing methodologies and tools for comprehensive data collection. Economies of scale as apparent in some settings [29] are worth investigating, to establish more clearly if these are achieved merely by allocating fixed costs over a larger number of patients, or by improved technical efficiency (i.e. transformation of inputs to outputs) associated with program maturation and learning.
Whenever possible (conditional on the availability of data on patient wages and other opportunity costs), both financial and economic costs should be collected. Financial costs are relevant for programmatic budgeting, while economic costs (including opportunity costs, productivity losses, transport and out-of-pocket expenditure by patients) will determine cost-effectiveness and prioritization of resource allocations. For example, if an ART service package includes in-kind contributions such as food subsidies or other resources donated from external funding sources, these will influence future planning and sustainability. Ignoring such external assistance would distort the picture of overall costs and incentives structures in which an ART program operates.
5. Conclusion
There is a paucity of information about the delivery unit costs of ART and PMTCT in different HIV/AIDS programs, particularly in low-income countries. Future evaluations of program-level ART and PMTCT unit costs will benefit from international guidance on standardized expenditure definitions and categories, standardized formats for specifying ARV regimen mixes and laboratory testing practices (including type of tests and frequency) and for human resource disaggregation (facility-level vs. above-facility and program-level); as well as standardized service unit denominators (possibly including a component of service quality or of patient retention). The large differences in ART unit costs observed in settings with similar epidemiologic and economic characteristics deserve additional assessments focusing on cost determinants and opportunities for efficiency gain in program implementation and scale-up. To scale-up ART and PMTCT to universal access globally, innovative options are needed to contain costs while maintaining or improving quality and health gain.
Supplementary Material
Fig. 1. Literature review on ART and PMTCT per-person year delivery unit costs: flowchart of search and review process.
* Seven studies included for in-depth analysis were from the gray literature
Acknowledgments
We thank Daniel Acuña, who conducted the initial bibliographic search, as well as Lazarus Muchabaiwa and Jesse Kigozi for their research assistance. We acknowledge Sergio Bautista, Lisa DeMaria, Steven Forsythe, Lori Bollinger, Megan O’Brien and various participants at the first Latin America & the Caribbean Conference on Global Health in Cuernavaca, Mexico and at the XVIII International AIDS Conference in Vienna, Austria for helpful comments. The research was partially funded by the Global Fund to fight AIDS, Tuberculosis and Malaria (PO#2008301) through INSP/Consortium for Research on HIV/AIDS and Tuberculosis (CISIDAT1). Additional funding for Omar Galárraga was provided by U.S. National Institutes of Health (NIH)/Fogarty International Center (K01-TW008016-02) through the Institute of Business and Economic Research (IBER) at the University of California, Berkeley. The opinions expressed in the paper do not reflect the views of any of the funding or the other organizations that supported or facilitated the study.
Footnotes
CISIDAT (www.cisidat.org.mx): Consortium for HIV/AIDS and Tuberculosis Research is a non-profit organization to support research in various academic and health care institutions in Mexico, among them the National Institute of Public Health (INSP).
The authors are solely responsible for the contents; they report no conflicts of interest.
References
- 1.WHO/UNAIDS/UNICEF. Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector: Progress Report. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Beck EJ, Santas XM, Delay PR. Why and how to monitor the cost and evaluate the cost-effectiveness of HIV services in countries. Aids. 2008 Jul;22(Suppl 1):S75–85. doi: 10.1097/01.aids.0000327626.77597.fa. [DOI] [PubMed] [Google Scholar]
- 3.The Global Fund to fight AIDS Tuberculosis and Malaria third replenishment 2011–2013. Improving value for money in Global Fund supported programs. 2010 [cited 28 March 2010]; available from: http://www.theglobalfund.org/documents/replenishment/2010/Improving%20Value%20for%20Money%20in%20Global%20Fund%20Supported%20Programs.pdf.
- 4.UNAIDS. What countries need: investments needed for 2010 targets. 2009 [cited 15 June 2010]; available from: http://data.unaids.org/pub/Report/2009/jc1681_what_countries_need_en.pdf.
- 5.UNAIDS and The World Bank Global HIV/AIDS program. The global economic crisis and HIV prevention and treatment programmes: vulnerabilities and impact. Washington, DC: The World Bank; 2009. [Google Scholar]
- 6.United States Presidential Emergency Plan for AIDS Relief (PEPFAR) An act to authorize appropriations for fiscal years 2009 through 2013 to provide assistance to foreign countries to combat HIV/AIDS, tuberculosis, and malaria, and for other purposes, in 2nd session. One Hundred Tenth Congress of the United States of America; Washington, DC. 2009. [Google Scholar]
- 7.United Kingdom Department for International Development. Achieving Universal Access – the UK’s strategy for halting and reversing the spread of HIV in the developing world. Monitoring performance and evaluating impact. 2008 [cited 29 July 2010]; available from: http://www.dfid.gov.uk/Documents/publications/achving-uni-access-mon-eval.pdf.
- 8.Global Fund. The Global Fund 2010: Innovation and Impact. 2010 [cited 10 July 2010]; available from: http://www.theglobalfund.org/documents/replenishment/2010/Global_Fund_2010_Innovation_and_Impact_en.pdf.
- 9.Izazola-Licea JA, Wiegelmann J, Aran C, Guthrie T, De Lay P, Avila-Figueroa C. Financing the response to HIV in low-income and middle-income countries. J Acquir Immune Defic Syndr. 2009 Dec;52(Suppl 2):S119–26. doi: 10.1097/QAI.0b013e3181baeeda. [DOI] [PubMed] [Google Scholar]
- 10.WHO. New progress and guidance on HIV treatment. WHO Fact Sheet. 2010 [cited 20 July 2010]; available from: http://data.unaids.org/pub/FactSheet/2010/20100721_art_factsheet_en.pdf.
- 11.Beck EJ, Miners AH, Tolley K. The cost of HIV treatment and care. A global review. Pharmacoeconomics. 2001 Jan;19(1):13–39. doi: 10.2165/00019053-200119010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bollinger L, Stover J. Financial resources required to achieve universal access to HIV prevention, treatment, care and support. Methodology for Care and Treatment Interventions. 2007 [cited 26 April 2010]; available from: http://data.unaids.org/pub/Report/2007/20070925_annex_iii_treatment_care_methodology_en.pdf.
- 13.Ades AE, Ratcliffe J, Gibb DM, Sculpher MJ. Economic issues in the prevention of vertical transmission of HIV. Pharmacoeconomics. 2000 Jul;18(1):9–22. doi: 10.2165/00019053-200018010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Beck EJ, Harling G, Gerbase S, DeLay P. The cost of treatment and care for people living with HIV infection: implications of published studies, 1999–2008. Curr Opin HIV AIDS. 2010 May;5(3):215–24. doi: 10.1097/COH.0b013e32833860e9. [DOI] [PubMed] [Google Scholar]
- 15.PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2010 [cited 17 February 2009]; available from: http://www.prisma-statement.org/statement.htm.
- 16.World Bank. World Bank List of Economies (July 2009) 2009 [cited 17 July 2009]; available from: http://siteresources.worldbank.org/DATASTATISTICS/Resources/CLASS.XLS.
- 17.IMF. International exchange rates. Washington, DC: International Monetary Fund; 2009. [Google Scholar]
- 18.Cleary S, Tshehlo R, Jouquet G, Makakole L. Ensuring Access to Free HIV/AIDS Care and Treatment in Lesotho. Capetown and Maseru: University of Cape Town, Ministry of Health & Social Welfare, Kingdom of Lesotho, Doctors Without Borders/Médecins Sans Frontières, Scott Hospital Health Service Area; 2007. [Google Scholar]
- 19.Lesotho Central Bank. Official exchange rates. 2009 [cited 15 December 2009]; available from: http://www.centralbank.org.ls/statistics/2006%20Annual%20Report%20Tables1(final).pdf.
- 20.BLS. Consumer Price Index: Historical Dataset. Washington, DC: Bureau of Labor Statistics; 2009. [Google Scholar]
- 21.Vella V, Govender T, Dlamini S, et al. Evaluation of the antiretroviral therapy in KwaZulu-Natal, South Africa. 2008 [cited 12 December 2009]; available from: http://www.kznhealth.gov.za/italian/arv.pdf.
- 22.PEPFAR ART Costing Project Team. The costs of comprehensive HIV treatment in Ethiopia: report of a cost study of PEPFAR-supported HIV treatment programs in Ethiopia. Atlanta, GA: CDC and PEPFAR; 2009. [Google Scholar]
- 23.Marques HH, Couttolenc BF, do Latorre MR, Aquino MZ, Aveiro MI, Pluciennik AM. Costs of care provided in a university hospital for children exposed to or infected with the HIV/AIDS. Cad Saude Publica. 2007;23 (Suppl 3):S402–13. doi: 10.1590/s0102-311x2007001500008. [DOI] [PubMed] [Google Scholar]
- 24.Hounton SH, Akonde A, Zannou DM, Bashi J, Meda N, Newlands D. Costing universal access of highly active antiretroviral therapy in Benin. AIDS Care. 2008 May;20(5):582–7. doi: 10.1080/09540120701868303. [DOI] [PubMed] [Google Scholar]
- 25.Bikilla AD, Jerene D, Robberstad B, Lindtjorn B. Cost estimates of HIV care and treatment with and without anti-retroviral therapy at Arba Minch Hospital in southern Ethiopia. Cost Eff Resour Alloc. 2009;7:6. doi: 10.1186/1478-7547-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kombe G, David G, Raj G, et al. Human and Financial Resource Requirements for Scaling Up HIV/AIDS Services in Ethiopia. Bethesda, MD: The Partners for Health Reformplus Project, Abt Associates; 2004. [Google Scholar]
- 27.Koenig SP, Riviere C, Leger P, Severe P, Atwood S, Fitzgerald DW, et al. The cost of antiretroviral therapy in Haiti. Cost Eff Resour Alloc. 2008;6:3. doi: 10.1186/1478-7547-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaffar S, Amuron B, Foster S, Birungi J, Levin J, Namara G, et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009 Dec 19;374(9707):2080–9. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta I, Trivedi M, Kandamuthan S. Recurrent costs of India’s free ART program. In: Haacker M, Claeson M, editors. HIV and AIDS in South Asia: An Economic Development Risk. Washington, D.C: World Bank; 2009. p. xxvi.p. 244. [Google Scholar]
- 30.John KR, Rajagopalan N, Madhuri KV. Brief communication: economic comparison of opportunistic infection management with antiretroviral treatment in people living with HIV/AIDS presenting at an NGO clinic in Bangalore, India. MedGenMed. 2006;8(4):24. doi: 10.1186/1758-2652-8-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loubiere S, el Filal KM, Sodqi M, Loundou A, Luchini S, Cleary S, et al. When to initiate highly active antiretroviral therapy in low-resource settings: the Moroccan experience. Antivir Ther. 2008;13(2):241–51. [PubMed] [Google Scholar]
- 32.Kombe G, Galaty D, Nwagbara C. Scaling-up antiretroviral treatment in the public sector in Nigeria: a comprehensive analysis of resource requirements. Washington, DC: Partners for Health Reform plus/Abt Associates; 2004. [Google Scholar]
- 33.PHR. Nigeria: rapid assessment of HIV/AIDS care in the public and private sectors. Bethesda, MD: The Partners for Health Reformplus (PHR) Project, DELIVER, POLICY Project, Abt Associates; 2004. [Google Scholar]
- 34.Kitajima T, Kobayashi Y, Chaipah W, Sato H, Chadbunchachai W, Thuennadee R. Costs of medical services for patients with HIV/AIDS in Khon Kaen, Thailand. Aids. 2003 Nov 7;17(16):2375–81. doi: 10.1097/00002030-200311070-00013. [DOI] [PubMed] [Google Scholar]
- 35.de Acurcio FA, Puig-Junoy J, de Bonolo PF, Braga Ceccato MG, Guimaraes MD. Cost-effectiveness of initial adherence to antiretroviral therapy among HIV infected patients in Belo Horizonte, Brazil. Rev Esp Salud Publica. 2006 Jan-Feb;80(1):41–54. doi: 10.1590/s1135-57272006000100005. [DOI] [PubMed] [Google Scholar]
- 36.Aracena-Genao B, Navarro JO, Lamadrid-Figueroa H, Forsythe S, Trejo-Valdivia B. Costs and benefits of HAART for patients with HIV in a public hospital in Mexico. Aids. 2008 Jul;22(Suppl 1):S141–8. doi: 10.1097/01.aids.0000327635.74919.fd. [DOI] [PubMed] [Google Scholar]
- 37.Bautista-Arredondo S, Dmytraczenko T, Kombe G, Bertozzi SM. Costing of scaling up HIV/AIDS treatment in Mexico. Salud Publica Mex. 2008;50 (Suppl 4):S437–44. doi: 10.1590/s0036-36342008001000004. [DOI] [PubMed] [Google Scholar]
- 38.Bautista-Arredondo S, Dmytraczenko T, Kombe G, Bertozzi S. Costing of HIV/AIDS Treatment in Mexico. Bethesda, MD: Partners for Health Reformplus Project, Abt Associates Inc; 2003. Report No.: 020. [Google Scholar]
- 39.Cleary SM, McIntyre D, Boulle AM. The cost-effectiveness of antiretroviral treatment in Khayelitsha, South Africa--a primary data analysis. Cost Eff Resour Alloc. 2006;4:20. doi: 10.1186/1478-7547-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deghaye N, Pawinski RA, Desmond C. Financial and economic costs of scaling up the provision of HAART to HIV-infected health care workers in KwaZulu-Natal. S Afr Med J. 2006 Feb;96(2):140–3. [PubMed] [Google Scholar]
- 41.Harling G, Bekker LG, Wood R. Cost of a dedicated ART clinic. S Afr Med J. 2007 Aug;97(8):593–6. [PubMed] [Google Scholar]
- 42.Harling G, Wood R. The evolving cost of HIV in South Africa: changes in health care cost with duration on antiretroviral therapy for public sector patients. J Acquir Immune Defic Syndr. 2007 Jul 1;45(3):348–54. doi: 10.1097/QAI.0b013e3180691115. [DOI] [PubMed] [Google Scholar]
- 43.Kevany S, Meintjes G, Rebe K, Maartens G, Cleary S. Clinical and financial burdens of secondary level care in a public sector antiretroviral roll-out setting (G. F. Jooste Hospital) S Afr Med J. 2009 May;99(5):320–5. [PubMed] [Google Scholar]
- 44.Martinson N, Mohapi L, Bakos D, Gray GE, McIntyre JA, Holmes CB. Costs of providing care for HIV-infected adults in an urban HIV clinic in Soweto, South Africa. J Acquir Immune Defic Syndr. 2009 Mar 1;50(3):327–30. doi: 10.1097/QAI.0b013e3181958546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen S, Long L, Sanne I. The outcomes and outpatient costs of different models of antiretroviral treatment delivery in South Africa. Trop Med Int Health. 2008 Aug;13(8):1005–15. doi: 10.1111/j.1365-3156.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 46.Dandona L, Kumar SG, Ramesh YK, Rao MC, Marseille E, Kahn JG, et al. Outputs, cost and efficiency of public sector centres for prevention of mother to child transmission of HIV in Andhra Pradesh, India. BMC Health Serv Res. 2008;8:26. doi: 10.1186/1472-6963-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desmond C, Franklin L, Steinberg M. The prevention of mother-to-child HIV transmission: costing the service in four sites in South Africa. Durban: Health Systems Trust; 2004. [Google Scholar]
- 48.McMennamin T, Fellow A. Cost and revenue analysis in six Rwandan health centers: 2005 cost and revenues. Boston, MA: Management Sciencies for Health (MSH); 2007. [Google Scholar]
- 49.McMennamin T, Fellow A. Cost and revenue analysis in six Rwandan health centers: 2005 cost and revenues. Boston, MA: Management Sciencies for Health (MSH); 2007. [Google Scholar]
- 50.Guinness L, Arthur G, Bhatt SM, Achiya G, Kariuki S, Gilks CF. Costs of hospital care for HIV-positive and HIV-negative patients at Kenyatta National Hospital, Nairobi, Kenya. Aids. 2002 Apr 12;16(6):901–8. doi: 10.1097/00002030-200204120-00010. [DOI] [PubMed] [Google Scholar]
- 51.Calmy A, Klement E, Teck R, Berman D, Pecoul B, Ferradini L. Simplifying and adapting antiretroviral treatment in resource-poor settings: a necessary step to scaling-up. Aids. 2004 Dec 3;18(18):2353–60. [PubMed] [Google Scholar]
- 52.Ford N, Wilson D, Costa Chaves G, Lotrowska M, Kijtiwatchakul K. Sustaining access to antiretroviral therapy in the less-developed world: lessons from Brazil and Thailand. Aids. 2007 Jul;21(Suppl 4):S21–9. doi: 10.1097/01.aids.0000279703.78685.a6. [DOI] [PubMed] [Google Scholar]
- 53.Over M, Marseille E, Sudhakar K, Gold J, Gupta I, Indrayan A, et al. Antiretroviral therapy and HIV prevention in India: modeling costs and consequences of policy options. Sex Transm Dis. 2006 Oct;33(10 Suppl):S145–52. doi: 10.1097/01.olq.0000238457.93426.0d. [DOI] [PubMed] [Google Scholar]
- 54.WHO. World Health Organization AIDS Medicines and Diagnostics Services (AMDS) Price Report. Global Price Reporting Mechanism (GPRM) 2010 [cited 28 July 2010]; available from: http://www.who.int/hiv/pub/amds/GPRMsummary_report_may2010.pdf.
- 55.Mugyenyi P, Walker AS, Hakim J, Munderi P, Gibb DM, Kityo C, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010 Jan 9;375(9709):123–31. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medina-Lara A, Kigozi J, Amurwon J, et al. Cost effectiveness analysis of routine laboratory or clinically driven strategies for monitoring anti-retroviral therapy in Uganda and Zimbabwe (DART Trial). 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa. 2009. [Google Scholar]
- 57.Majchrowicz M. Beyond antiretroviral access: low-cost laboratory tests needed for the developing world. Aids. 2003;17 (Suppl 4):S13–5. [PubMed] [Google Scholar]
- 58.Schneider H, Blaauw D, Gilson L, Chabikuli N, Goudge J. Health systems and access to antiretroviral drugs for HIV in Southern Africa: service delivery and human resources challenges. Reprod Health Matters. 2006 May;14(27):12–23. doi: 10.1016/S0968-8080(06)27232-X. [DOI] [PubMed] [Google Scholar]
- 59.Hirschhorn LR, Oguda L, Fullem A, Dreesch N, Wilson P. Estimating health workforce needs for antiretroviral therapy in resource-limited settings. Hum Resour Health. 2006;4:1. doi: 10.1186/1478-4491-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.WHO. Rapid Advice: Antiretroviral therapy for HIV infection in adults and adolescents. 2009 [cited 27 December 2009]; available from: http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf.
- 61.United Nations. Purchasing power parities (PPP) conversion factor, local currency unit to international dollar. Millennium Development Goals Indicators. 2010 [cited 17 February 2010]; available from: http://unstats.un.org/unsd/mdg/Metadata.aspx?IndicatorId=0&SeriesId=699.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.