Abstract
Agaricus blazei Murrill (ABM), a mushroom native to Brazil, is a basidiomycete brown fungus, which is popularly known as “Cogumelo do Sol” in Brazil or “Himematsutake” in Japan, and there has been a prominent increase in the use of ABM for therapeutic and medicinal purposes. ABM is useful against a variety of diseases like cancer, tumor, chronic hepatitis, diabetes, atherosclerosis, hypercholesterolemia, and so on. In this review, we demonstrated various pharmacological effects of ABM, so that we can use different effects of ABM against different diseases and provide reference for the study of ABM in the future.
1. Introduction
Mushrooms have been used in humans' food since ancient times, which are low in calories and high in minerals, vitamins, fibers, and essential amino acids. In recent years, there has been a significant increase in the consumption of mushroom due to an increasing number of studies identifying the therapeutic properties of the substances isolated from various species of these fungi [1].
Agaricus blazei Murrill (or A. brasiliensis), a mushroom of Brazilian origin, is widely used for nonprescript, medicinal purposes, both as an edible mushroom and in the form of extracts, which has been used as a health care product for the prevention of a wide range of illnesses including cancer, tumor, chronic hepatitis, diabetes, atherosclerosis, and hypercholesterolemia (Table 1). With the development of scientific researches, more and more scientific research have studied the chemical constituents and pharmacological effects of A. brasiliensis. The major chemical compounds include polysaccharide, protein, lectins, amino acid, vitamin, and sterols.
Table 1.
Medicinal values and active compounds of A.brasiliensis.
| Medicinal value | Active compounds | References |
|---|---|---|
| Anticancer activity | Polysaccharides agaritine | [2–10] |
| Antiviral activity | Polysaccharides Protein polysaccharide |
[11–13] |
| Liver protection | Aqueous extract | [14–20] |
| Immunomodulating effect | Polysaccharides | [21–24] |
| Antidiabetic effect | β-glucans and their enzymatically hydrolyzed oligosaccharides (AO) | [25–28] |
| Antileishmaniasis effect | Aqueous extract | [29–31] |
After the in vivo and in vitro studies, the pharmacological effects of A. brasiliensis including antitumor, antiviral, anti-inflammatory, liver protection, antidiabetic, antihyperlipidemic, antiatherosclerosis, antiallergic, and immunomodulating were found. Although the clinical research about pharmacological effects of A. brasiliensis is less, A. brasiliensis as a complementary and alternative medicine is widely used.
2. Medicinal Values of A. brasiliensis
2.1. Anticancer Activity
Agaricus brasiliensis, a medicinal edible fungus, is widely studied and used because of its significant anticancer activity. In Japan, researchers demonstrated anticancer and immunostimulant effects of A. brasiliensis extracts experimentally, and due to the improving consumption of this mushroom in recent years, a considerable effort investigated the putative effects with interesting but still insufficient clinical studies. Some reports showed that polysaccharide is the main component of A. brasiliensis for antitumor [2–4].
2.1.1. Studies in Animals
Polysaccharide antitumoral activity has been evaluated most often against allogenic sarcoma 180 in CD-I mice [5]. A. brasiliensis (Himematsutake) has stronger antitumor activity against Sarcoma 180 in mice than do polysaccharides from Ganoderma lucidum, Lentinus edodes, and Coriolus versicolor [6]. Mizuno et al. [6] studied the against antitumor polysaccharide Sarcoma 180 from the mycelium of liquid-cultured A. brasiliensis. But the isolated polysaccharide did not react with antibodies of antitumor polysaccharides such as lentinan, gliforan, and FIII-2-b, which is one of the antitumor polysaccharides from A. brasiliensis. Moreover, the analyses of 13C-NMR and GC-MS suggested that this polysaccharide was preliminarily glucomannan with a main chain of β-l,2-1inked D-mannopyranosyl residues and β-D-glucopyranosyl-3-O-13-D-glucopyranosyl residues as a side chain [7].
However, some reports indicated that agaritine and its derivatives exerted antitumor activity against leukemic cells, mainly U937 cells [8, 9]. Agaritine was fractionated by HPLC from a hot water extract of ABM powder, and the structure was determined by NMR and MS analyses. This compound inhibited the proliferation of leukemic cell lines, especially suppressing the viability of U937 cells in vitro [8]. Therefore, the antitumor substances of ABM remain to be further researched.
2.1.2. Clinical Studies
It has been reported that 100,000–300,000 kg of the dried body of A. blazei is produced every year in Japan, and about 300,000–500,000 persons assume the 3–5 g three times a day by a typical hot water extract of A. blazei as an adjuvant with cancer chemotherapy drugs for the prevention or treatment of cancer.
2.2. Antiviral Activity
The pharmacological effects of Agaricus brasiliensis have been mainly related to the presence of polysaccharides and protein polysaccharide complexes [11]. Faccin et al. [12] reported that the extracts of fruiting bodies of ABM, including aqueous and alcohol extracts, and an isolated against polysaccharide from this species displayed antiviral activity poliovirus type 1. de Sousa Cardozo et al. [13] reported the chemical modification of a polysaccharide extracted from A. brasiliensis mycelia to obtain its sulfated derivative (MI-S), which presented a promising inhibitory activity against HSV-1 (KOS and 29R (acyclovir-resistant) strains) and HSV-2 strain 333. Furthermore, the sulfated polysaccharide also presented synergistic antiviral effect with acyclovir.
2.3. Liver Protection
Hepatic fibrosis is caused by chronic damage to the liver in union with the progressive accumulation of fibrillar extracellular matrix proteins [32–34]. The main causes of hepatic fibrosis in humans include infection by hepatitis B and C, alcohol abuse, and nonalcohol steatohepatitis, and liver cirrhosis can be induced by carbon tetrachloride (CCL4) [35, 36]. A few studies have researched that A. brasiliensis extract could ameliorate or abrogate CCL4-induced liver injury in rats [14, 15].
2.3.1. Studies in Animals
Chen et al. [16] have demonstrated that A. brasiliensis extract might serve as an adjuvant in improving the efficacy of hepatitis B vaccines in vivo. The results showed that not only a significant increase in the HBcAg-specific antibody response was observed, but also T cell proliferation was observed in mice which received HBcAg DNA vaccine plus A. brasiliensis extract [16].
2.3.2. Clinical Studies
Hsu et al. [17] performed a 1-year open-label pilot study to observe whether A. brasiliensis extract improves liver function in patients with hepatitis B. They gave the four enrolled patients A. brasiliensis extract of 1500 mg daily for 12 months and measured the level of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). At the end of the study, the mean level of AST and ALT decreased from 246.0 (± standard deviation (SD) 138.9) to 61.3 (± SD 32.6) IU/L and 151.0 (± SD 86.9) to 46.1 (± SD 22.5) IU/L, respectively [17]. Although the result of this study showed that A. brasiliensis extract can normalize liver function of the 4 patients, this study is just a small sample research.
In addition, Grinde et al. [18] reported the effect on gene expression in peripheral blood cells from four chronic hepatitis C patients, using global (29 k) oligo-based, single channel microarrays. After dates being analyzed, the results suggested that the β-glucan part of the A. brasiliensis extract was not transported into the blood in appreciable quantities. And although the average (n = 5) titre of virus was slightly lower after one week on A. brasiliensis, the difference was clearly not significant [18]. So, this result of the study showed that β-glucan of the A. brasiliensis extract cannot treat HCV. However, one study evaluated the clinical effects and safety on 20 volunteers (50% of men) with elevated γ-GTP activity of A. blazei condensed liquid (Agaricus mushroom extract, ABCL) in the treatment of C-hepatitis. Decreasing effect for serum γ-GTP activity was found in 80% of the patients in both sexes after these patients received the ABCL orally, twice a day, for 8 weeks, without any toxicological findings and other side effects [19]. Additionally, Mukai et al. [20] reported three cases of patients with advanced cancer who showed severe hepatic damage, and two of whom died of fulminant hepatitis after taking A. brasiliensis extract. Reporters demonstrated that a strong causal relationship between the A. brasiliensis extract and liver damage was suggested and, at least, taking the A. brasiliensis extract made the clinical decision-making process much more complicated, although several other factors cannot be completely ruled out as the causes of liver damage [20].
2.4. Immunomodulating Effect
Polysaccharide is an immunologic adjuvant, which not only can activate the activity of T cells, B cells, NK cells, and other immune cells but also can promote the synthesis of IL-1, IL-2, TNF-α, IFN-γ, and NO, regulating the formation of body's antibodies and complement. A. brasiliensis contains compounds such as (1→3), (1→6)-β-glucans (Figure 1), (1→3)-α-glucans, and protein-polysaccharide complexes, which can enhance in vivo and in vitro cell-mediated immune responses and act as biological response modifiers [21, 22].
Figure 1.
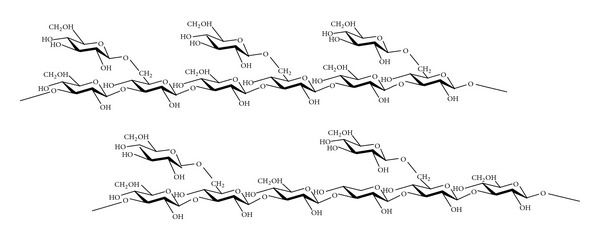
β-(1,3-1,6)-D-Glucan.
2.4.1. Studies in Animals
Lin et al. [23] have established leukemia mice through the injection of WEHI-3 cells and chronically treated mice with A. brasiliensis. In their study, results showed that A. brasiliensis can promote immune responses in leukemia mice in vivo and also can promote T cell proliferation. Furthermore, the A. brasiliensis extract significantly enhanced both NK cell activities and phagocytosis of macrophages [23].
2.4.2. Clinical Studies
However, there was a randomized clinical trial on elderly women to ascertain the effects of AbM intake on serum levels of IL-6, IFN-γ, and TNF-α in community-living seniors [24]. After the study period, no changes from baseline were detectable for any parameter in either group, receiving placebo or AbM dry extract with 900 mg/day for 60 days. Therefore, it showed that AbM extract had no modulating effect on IL-6, IFN-c, or TNF-a levels in elderly females [24].
2.5. Antidiabetic Effect
Agaricus brasiliensis is rich in polysaccharides and protein, especially β-glucans. Kim et al. [25] demonstrated that β-glucans and their enzymatically hydrolyzed oligosaccharides (AO) from A. brasiliensis show the activities of antihyperglycemic, antihypertriglyceridemic, antihypercholesterolemic, and antiarteriosclerotic indicating antidiabetic activity as a whole in diabetic rats. In this study, diabetic rats were divided into four groups, including normal control, diabetic control, treated group I (β-glucans), and treated group II (AO), to contrast the different changes of their body weights. The data suggested that both β-glucans and AO might promote insulin secretion from islets as well as the viability and proliferation of islets in diabetic or normal rats.
In addition, several studies demonstrated that A. brasiliensis had an effect on streptozotocin-induced diabetic rats [26–28]. Oxidative stress induced by hyperglycemia possibly causes the dysfunction of pancreatic β-cells and various forms of tissue damage in patients with diabetes mellitus. Niwa et al. [28] researched the antidiabetic efficacy and hypoglycemic mechanisms of Ipomoea batatas and A. brasiliensis in streptozotocin-induced diabetic rats. The results of the study suggested hypoglycemic effects of Ipomoea batatas or A. brasiliensis due to their suppression of oxidative stress and proinflammatory cytokine production followed by improvement of pancreatic β-cells mass [28].
2.6. Antileishmanial Effect
Leishmaniasis is a flock of vector-transmitted diseases that are endemic in many tropical and subtropical countries. The current treatment for leishmaniasis has certain side effects, and some drugs are of high cost for the majority of patients. In recent years, A. brasiliensis was demonstrated to have antileishmanial activity, and thereinto an in vitro antileishmanial activity against L. amazonens is, L. chagasi, and L. major was demonstrated for an A. blazei water extract [29]. Valadares et al. [30] studied the therapeutic efficacy induced by the oral administration of A. brasiliensis against Leishmania amazonensis. The results showed that mice treated with the A. brasiliensis presented a 60% reduction in the inflammation of infected footpads as compared to untreated control-infected mice. These treated animals produced significantly higher levels of interferon gamma (IFN-γ) and nitric oxide (NO), higher levels of parasite-specific IgG2a isotype antibodies, and lower levels of IL-4 and IL-10 in the spleen and lymph node cell cultures than did the controls [30]. In addition, Valadares et al. [31] used five fractions obtained from A. brasiliensis water extract to treat BALB/c mice infected with Leishmania chagasi in vivo (Figure 2). The results suggested that the use of Fab5 (molecules >100,000 Da) or A. brasiliensis, as compared to control groups, resulted in significant reduced parasite burdens in the liver, spleen, and draining lymph nodes of the infected animals.
Figure 2.
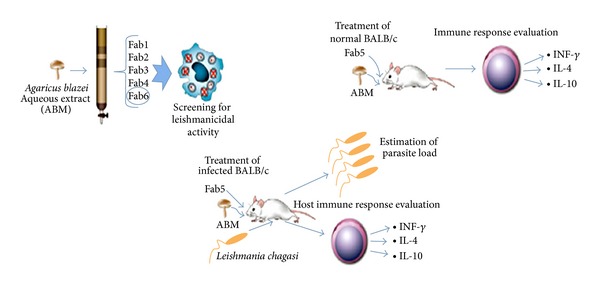
Reference schematic diagram of Valadares et al. using the five fractions obtained from ABM water extract to treat BALB/c mice infected with Leishmania chagasi.
3. Other Effects
There are not only these pharmacological effects of A. brasiliensis, which are described previously, but also other beneficial effects. The chloroform-soluble extract of A. brasiliensis inhibited IL-6 production in PMA plus A23187-induced BMMCs (bone marrow-derived mast cells) to express the anti-inflammatory and antiallergic effects [37]. One study, the first in vivo study, showed that A. brasiliensis can enhance local and systemic inflammation, upregulate proinflammatory molecules, and enhance leukocyte homing to atherosclerosis sites without affecting the lipoprotein profile [38]. The polysaccharides of A. brasiliensis have antitumor, antiviral, and immunomodulating effects as described above. Besides, one study indicated that A. brasiliensis polysaccharides could be useful in promoting burn wound healing [39].
There is a clinical study stating that Administration of γ-aminobutyric acid (GABA) enriched A. blazei (AG-GABA) to mild hypertensive human subjects showed that both systolic and diastolic blood pressure values decreased to statistically significant levels [40]. Maybe there are also many other effects of A. brasiliensis, which are not known so far. Therefore, it needs to be further researched.
4. Conclusion
Agaricus blazei Murrill, a mushroom of biomedical importance, contains a number of bioactive components, many of them biological are response modifiers which activate our immune systems for a multitude of defensive functions (Figure 3). Polysaccharides of A. brasiliensis have been known to have anticancer, antiviral, and immunomodulatory effects, and other substances are probably involved as well. Moreover, β-glucans and their enzymatically hydrolyzed oligosaccharides (AO) from A. brasiliensis show antihyperglycemic, antihypertriglyceridemic, antihypercholesterolemic, and antiarteriosclerotic activities [25]. Therefore, the pharmacological effects and health function of A. brasiliensis are more and more focused on in the world. Although there seems to be clear evidences that ABM extract are rich in β-glucans, which presumably contribute to the observed pharmacological activities, isolation, and dose response studies, as well as chemical identification and quantification of specific compounds responsible for the potential benefit from ABM, mushroom ingestion should be fully developed. Careful clinical studies comparing the activity of the whole mushroom extracts, isolated compounds, and epidemiological data still need to determine whether A. brasiliensis provides real clinical benefits.
Figure 3.
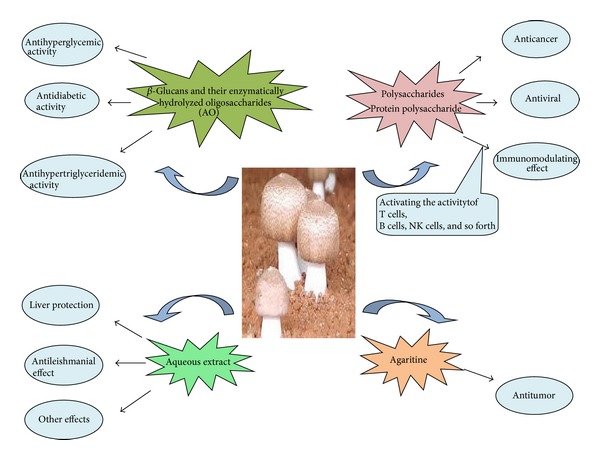
Medicinal values and active compounds of A. brasiliensis.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgment
This work was supported by the Foundation of Jinan Science and Technology Development Program (201303055).
References
- 1.Lima CU, Cordova CO, Nóbrega OD, Funghetto SS, Karnikowski MG. Does the Agaricus blazei Murill mushroom have properties that affect the immune system? an integrative review. Journal of Medicinal Food. 2011;14(1-2):2–8. doi: 10.1089/jmf.2010.0017. [DOI] [PubMed] [Google Scholar]
- 2.Gonzaga MLC, Ricardo NMPS, Heatley F, Soares SDA. Isolation and characterization of polysaccharides from Agaricus blazei Murill. Carbohydrate Polymers. 2005;60(1):43–49. [Google Scholar]
- 3.Kawagishi H, Kanao T, Inagaki R, et al. Formolysis of a potent antitumor (1 → 6)-β-d-glucan-protein complex from Agaricus blazei fruiting bodies and antitumor activity of the resulting products. Carbohydrate Polymers. 1990;12(4):393–403. [Google Scholar]
- 4.Shu C-H, Wen B-J. Enhanced shear protection and increased production of an anti-tumor polysaccharide by Agaricus blazei in xanthan-supplemented cultures. Biotechnology Letters. 2003;25(11):873–876. doi: 10.1023/a:1024056910417. [DOI] [PubMed] [Google Scholar]
- 5.Gonzaga ML, Bezerra DP, Alves AP, et al. In vivo growth-inhibition of Sarcoma 180 by an α-(1 → 4)-glucan-β-(1 → 6)-glucan-protein complex polysaccharide obtained from Agaricus blazei Murill. Journal of Natural Medicines. 2009;63(1):32–40. doi: 10.1007/s11418-008-0286-4. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno T, Hagiwara T, Nakamura T, et al. Antitumor activity and some properties of water-soluble polysaccharides from ‘Himematsutake’, the fruiting body of Agaricus blazei Murill. Agricultural and Biological Chemistry. 1990;54:2889–2896. [Google Scholar]
- 7.Mizuno M, Minato K-I, Ito H, Kawade M, Terai H, Tsuchida H. Anti-tumor polysaccharide from the mycelium of liquid-cultured Agaricus blazei mill. Biochemistry and Molecular Biology International. 1999;47(4):707–714. doi: 10.1080/15216549900201773. [DOI] [PubMed] [Google Scholar]
- 8.Endo M, Beppu H, Akiyama H, et al. Agaritine purified from Agaricus blazei Murrill exerts anti-tumor activity against leukemic cells. Biochimica et Biophysica Acta. 2010;1800(7):669–673. doi: 10.1016/j.bbagen.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama H, Endo M, Matsui T, et al. Agaritine from Agaricus blazei Murrill induces apoptosis in the leukemic cell line U937. Biochimica et Biophysica Acta. 2011;1810(5):519–525. doi: 10.1016/j.bbagen.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Takaku T, Kimura Y, Okuda H. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. Journal of Nutrition. 2001;131(5):1409–1413. doi: 10.1093/jn/131.5.1409. [DOI] [PubMed] [Google Scholar]
- 11.Firenzuoli F, Gori L, Lombardo G. The medicinal mushroom Agaricus blazei murrill: review of literature and pharmaco-toxicological problems. Evidence-Based Complementary and Alternative Medicine. 2008;5(1):3–15. doi: 10.1093/ecam/nem007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faccin LC, Benati F, Rincão VP, et al. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Letters in Applied Microbiology. 2007;45(1):24–28. doi: 10.1111/j.1472-765X.2007.02153.x. [DOI] [PubMed] [Google Scholar]
- 13.de Sousa Cardozo FTG, Camelini CM, Mascarello A, et al. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antiviral Research. 2011;92(1):108–114. doi: 10.1016/j.antiviral.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Wu M-F, Hsu Y-M, Tang M-C, et al. Agaricus blazei Murill extract abrogates CCl14-induced liver injury in rats. In Vivo. 2011;25(1):35–40. [PubMed] [Google Scholar]
- 15.Chang J-B, Wu M-F, Yang Y-Y, et al. Carbon tetrachloride-induced hepatotoxicity and its amelioration by Agaricus blazei Murrill extract in a mouse model. In Vivo. 2011;25(6):971–976. [PubMed] [Google Scholar]
- 16.Chen L, Shao HJ, Su YB. Coimmunization of Agaricus blazei Murill extract with hepatitis B virus core protein through DNA vaccine enhances cellular and humoral immune responses. International Immunopharmacology. 2004;4(3):403–409. doi: 10.1016/j.intimp.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Hsu C-H, Hwang K-C, Chiang Y-H, Chou P. The mushroom Agaricus blazei Murill extract normalizes liver function in patients with chronic hepatitis B. Journal of Alternative and Complementary Medicine. 2008;14(3):299–301. doi: 10.1089/acm.2006.6344. [DOI] [PubMed] [Google Scholar]
- 18.Grinde B, Hetland G, Johnson E. Effects on gene expression and viral load of a medicinal extract from Agaricus blazei in patients with chronic hepatitis C infection. International Immunopharmacology. 2006;6(8):1311–1314. doi: 10.1016/j.intimp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Inuzuka H, Yoshida T. Clinical utility of ABCL (Agalicus Mushroom Extract) treatment for C-type hepatitis. Japanese Pharmacology and Therapeutics. 2002;30(2):103–107. [Google Scholar]
- 20.Mukai H, Watanabe T, Ando M, Katsumata N. An alternative medicine, Agaricus blazei, may have induced severe hepatic dysfunction in cancer patients. Japanese Journal of Clinical Oncology. 2006;36(12):808–810. doi: 10.1093/jjco/hyl108. [DOI] [PubMed] [Google Scholar]
- 21.Ooi VEC, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Current Medicinal Chemistry. 2000;7(7):715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno M, Morimoto M, Minato K-I, Tsuchida H. Polysaccharides from Agaricus blazei stimulate lymphocyte T-cell subsets in mice. Bioscience, Biotechnology and Biochemistry. 1998;62(3):434–437. doi: 10.1271/bbb.62.434. [DOI] [PubMed] [Google Scholar]
- 23.Lin JG, Fan MJ, Tang NY, et al. An extract of Agaricus blazei Murill administered orally promotes immune responses in murine leukemia BALB/c mice in vivo. Integrative Cancer Therapies. 2012;11:29–36. doi: 10.1177/1534735411400314. [DOI] [PubMed] [Google Scholar]
- 24.Lima CUJO, Souza VC, Morita MC, Chiarello MD, de Oliveira Karnikowski MG. Agaricus blazei Murrill and inflammatory mediators in elderly women: a randomized clinical trial. Scandinavian Journal of Immunology. 2012;75(3):336–341. doi: 10.1111/j.1365-3083.2011.02656.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y-W, Kim K-H, Choi H-J, Lee D-S. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei . Biotechnology Letters. 2005;27(7):483–487. doi: 10.1007/s10529-005-2225-8. [DOI] [PubMed] [Google Scholar]
- 26.Oh TW, Kim YA, Jang WJ, et al. Semipurified fractions from the submerged-culture broth of Agaricus blazei Murill reduce blood glucose levels in streptozotocin-induced diabetic rats. Journal of Agricultural and Food Chemistry. 2010;58(7):4113–4119. doi: 10.1021/jf9036672. [DOI] [PubMed] [Google Scholar]
- 27.Di Naso FC, de Mello RN, Bona S, et al. Effect of Agaricus blazei Murill on the pulmonary tissue of animals with streptozotocin-induced diabetes. Experimental Diabetes Research. 2010;2010:p. 543926. doi: 10.1155/2010/543926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa A, Tajiri T, Higashino H. Ipomoea batatas and Agarics blazei ameliorate diabetic disorders with therapeutic antioxidant potential in streptozotocin-induced diabetic rats. Journal of Clinical Biochemistry and Nutrition. 2011;48(3):194–202. doi: 10.3164/jcbn.10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valadares DG, Duarte MC, Oliveira JS, et al. Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitology International. 2011;60(4):357–363. doi: 10.1016/j.parint.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Valadares DG, Duarte MC, Ramírez L, et al. Therapeutic efficacy induced by the oral administration of Agaricus blazei Murill against Leishmania amazonensis . Parasitology Research. 2012;111:1807–1816. doi: 10.1007/s00436-012-3028-1. [DOI] [PubMed] [Google Scholar]
- 31.Valadares DG, Duarte MC, Ramírez L, et al. Prophylactic or therapeutic administration of Agaricus blazei Murill is effective in treatment of murine visceral leishmaniasis. Experimental Parasitology. 2012;132(2):228–236. doi: 10.1016/j.exppara.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Friedman SL. Seminars in medicine of the Beth Isreal Hospital, Boston, the cellular basis of hepatic fibrosis, mechanismas and treatment strategies. The New England Journal of Medicine. 1993;328(25):1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 33.Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. Journal of Hepatology. 1995;22(2):28–36. [PubMed] [Google Scholar]
- 34.Lieber CS. Prevention and treatment of liver fibrosis based on pathogenesis. Alcoholism. 1999;23(5):944–949. [PubMed] [Google Scholar]
- 35.Brattin WJ, Glende EA, Jr., Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. Journal of Free Radicals in Biology and Medicine. 1985;1(1):27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 36.Boulton RA, Alison MR, Golding M, Selden C, Hodgson HJF. Augmentation of the early phase of liver regeneration after 70% partial hepatectomy in rats following selective Kupffer cell depletion. Journal of Hepatology. 1998;29(2):271–280. doi: 10.1016/s0168-8278(98)80013-5. [DOI] [PubMed] [Google Scholar]
- 37.Song HH, Chae HS, Oh SR, Lee HK, Chin YW. Anti-Inflammatory and anti-allergic effect of Agaricus blazei extract in bone marrow-derived mast cells. The American Journal of Chinese Medicine. 2012;40(5):1073–1084. doi: 10.1142/S0192415X12500796. [DOI] [PubMed] [Google Scholar]
- 38.Gonçalves JL, Roma EH, Gomes-Santos AC, et al. Pro-inflammatory effects of the mushroom Agaricus blazei and its consequences on atherosclerosis development. European Journal of Nutrition. 2012;51:927–937. doi: 10.1007/s00394-011-0270-8. [DOI] [PubMed] [Google Scholar]
- 39.Sui Z, Yang R, Liu B, et al. Chemical analysis of Agaricus blazei polysaccharides and effect of the polysaccharides on IL-1β mRNA expression in skin of burn wound-treated rats. International Journal of Biological Macromolecules. 2010;47(2):155–157. doi: 10.1016/j.ijbiomac.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe T, Kawashita A, Ishi S, et al. Antihypertensive effect of γ-aminobutyric acid-enriched Agaricus blazei on mild hypertensive human subjects. Nippon Shokuhin Kagaku Kogaku Kaishi. 2003;50(4):167–173. [Google Scholar]


