Abstract
The pedunculopontine tegmental nucleus (PPTg) and laterodorsal tegmental nucleus (LDTg) provide cholinergic afferents to several brain areas. This cholinergic complex has been suggested to play a role in sleep, waking, motor function, learning and reward. To have a better understanding of the neurochemical organization of the PPTg/LDTg we characterized the phenotype of PPTg/LDTg neurons by determining in these cells the expression of transcripts encoding choline acetyltransferase (ChAT), glutamic acid decarboxylase (GAD) or the vesicular glutamate transporters (vGluT1, vGluT2 and vGluT3). Within the PPTg/LDTg complex we found neurons expressing ChAT, vGluT2 or GAD transcripts, these neuronal phenotypes were intermingled, but not homogeneously distributed within the PPTg or LDTg. Previous studies suggested the presence of either glutamate or GABA immunolabeling in a large number of PPTg/LDTg cholinergic neurons, leading to the widespread notion that PPTg/LDTg cholinergic neurons co-release acetylcholine together with either glutamate or GABA. To assess the glutamatergic or GABAergic nature of the PPTg/LDTg cholinergic neurons we combined in situ hybridization (to detect vGluT2 or GAD transcripts) and immunohistochemistry (to detect ChAT), and found that over 95% of all PPTg/LDTg cholinergic neurons lack transcripts encoding either vGluT2 mRNA or GAD mRNA. As the vast majority of PPTg/LDTg cholinergic neurons lack transcripts encoding essential proteins for the vesicular transport of glutamate or for the synthesis of GABA, co-release of acetylcholine with either glutamate or GABA is unlikely to be a major factor in the interactions between acetylcholine, glutamate, and GABA at the postsynaptic site.
Keywords: PPTg, LDTg, ventral tegmental area, substantia nigra, dopamine, reward
INTRODUCTION
The pedunculopontine tegmental nucleus (PPTg) and the laterodorsal tegmental nucleus (LDTg) of the mesopontine tegmentum comprise respectively the cholinergic cell groups Ch5 and Ch6 of the caudal cholinergic column (Mesulam et al., 1983; Satoh et al., 1983; Vincent et al., 1986; Maley et al., 1988; Woolf, 1991). These cholinergic neurons innervate several brain areas (the pontine reticular formation, thalamus, limbic system, superior colliculus, and basal ganglia), and play a role in the rapid eye movement sleep and eye movements, sleep-wake cycle and arousal, stimulus-reward learning, visual orienting and sensory-motor patterns (Sofroniew et al., 1985; Woolf & Butcher, 1986; Hall et al., 1989; Cornwall et al., 1990; Steriade et al., 1990; Garcia-Rill, 1991; Jones 1991; Bechara & van Der Kooy, 1992; Inglis & Winn, 1995; Krauthamer et al., 1995; Oakman et al., 1995, 1999; Imon et al., 1996; Mathur et al., 1997; Williams et al., 1997; Thakkar et al., 1998; Bardo, 1998 and Olmstead et al., 1998; Winn 2006).
In addition to the cholinergic neurons that conventionally define the boundaries of the PPTg and LDTg nuclei, these nuclei contain glutamatergic and GABAergic neurons. Glutamate immunoreactive neurons have been detected in the PPTg and the LDTg in the rat (Clements & Grant, 1990; Clements et al., 1991) and the monkey (Lavoie & Parent, 1994b). While GAD immunoreactive neurons were detected in the rat PPTg and LDTg (Ford et al., 1995), other immunolabeling studies in the rat (Sutin & Jacobowitz, 1988) and the monkey (Lavoie & Parent, 1994b) failed to show GABA immunoreactive neurons in these nuclei. Immunolabeling studies have raised the possibility that some PPTg and LDTg cholinergic neurons co-express glutamate and that some co-express GABA (Clements et al., 1991; Lavoie & Parent, 1994a; Jia et al., 2003). Cholinergic neurons having glutamate immunoreativity were observed in the rat (Clements et al., 1991) and the monkey (Lavoie & Parent, 1994a), and estimated that as many as 40% of cholinergic neurons of the PPTg at the level of the throchlear nucleus expressed glutamate in the monkey (Lavoie & Parent, 1994a). While GABA immunoreactivity has been reported for 50% of the cholinergic neurons in the cat mesopontine tegmentum (Jia et al., 2003), immunoreactivity for GAD (essential enzyme for the synthesis of GABA) was not detected in rat PPTg/LDTg cholinergic neurons (Kosaka et al., 1988; Ford et al., 1995). Based on the reported presence of glutamate or GABA immunoreactivity in cholinergic neurons, the possibility of co-release of acetylcholine with glutamate (Clements et al., 1991) or GABA from the mesopontine tegmentum cholinergic afferents has been suggested (Lavoie & Parent, 1994b).
We characterized the phenotype of PPTg/LDTg neurons using in situ hybridization to detect cellular expression of transcripts encoding ChAT, GAD or the vesicular glutamate transporters (vGluT1, vGluT2 and vGluT3). Contrary to results from immunological studies, by in situ hybridization we found that PPTg/LDTg cholinergic neurons lack either glutamatergic or GABAergic phenotypes, as the vast majority of PPTg/LDTg cholinergic neurons lack either vGluTs or GAD mRNAs. The lack of these transcripts in cholinergic neurons fails to support the widespread hypothesis that PPTg/LDTg cholinergic neurons co-release acetylcholine with either glutamate or GABA.
MATERIALS AND METHODS
Tissue preparation
Seven Sprague-Dawley male rats (200–300 g body weight) were used for these studies. They were single housed on a 12-h light schedule in a temperature-controlled (20°C) animal room and given access to standard rat chow and water ad libitum. All animal procedures were approved by the NIDA IRP local Animal Care and Use Committee. Each rat was first deeply anaesthetized with chloral hydrate (300 mg/kg), and perfused transcardially with 4% (W/V) paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.3. Brains were removed and left in 4% paraformaldehyde for 2 h at 4°C, rinsed with PB and transferred sequentially to 12%, 14%, and 18% sucrose solutions in PB. Three rats were used to prepare coronal cryosections (20 μm in thickness) and 4 rats were used to make sagittal serial cryosections (20 μm in thickness).
Single in situ hybridization
In situ hybridization was performed as described previously (Wang & Morales, 2008). Cryosections were incubated for 10 min in PB containing 0.5% Triton X-100, rinsed 2 × 5 min with PB, treated with 0.2 N HCl for 10 min, rinsed 2 × 5 min with PB and then acetylated in 0.25% acetic anhydride in 0.1 M triethanolamine, pH 8.0 for 10 min. Sections were rinsed 2 × 5 min with PB, post-fixed with 4% paraformaldehyde for 10 min, and after a final rinse with PB, were hybridized for 16 h at 55°C in hybridization buffer (50% formamide; 10% dextran sulfate; 5x Denhardt's solution; 0.62 M NaCl; 50 mM DTT; 10 mM EDTA; 20 mM PIPES, pH 6.8; 0.2% SDS; 250 μg/ml salmon sperm DNA; 250 μg/ml tRNA) containing [35S]- and [33P]-labeled single-stranded antisense or sense of ChAT (nucleotides 271–2247, Accession # 017464), vGluT1 (nucleotides 1391–1976, Accession # NM-053859.1), vGluT2 (nucleotides 1704–2437, Accession # NM-053427) or vGluT3 (nucleotides 1–1619, Accession # 182959) probes at 107 cpm/ml. Material hybridized with [35S]- and [33P]-labeled riboprobes requires shorter exposure times than material hybridized with single [35S]- or [33P]- labeled riboprobes. Plasmids that contained the vGluT1 and vGluT2 were generously provided by Dr. Robert H. Edwards (University of California, San Francisco). Sections were treated with 4 μg/ml RNase A at 37°C for 1h, washed with 1 × SSC, 50% formamide at 55°C for 1h, and with 0.1 × SSC at 68°C for 1 h. After the last SSC wash, sections were rinsed with PB. Finally, slides were dipped in Ilford K.5 nuclear tract emulsion (Polysciences, Inc., Warrington; 1:1 dilution in double distilled water) and exposed in the dark at 4°C for four weeks prior to development.
Preparation of digoxigenin-labeled riboprobes for the detection of GAD65 mRNA and GAD67 mRNA
The antisense digoxigenin riboprobes for GAD65 (nucleotides 1–1758, Accession # NM012563) and GAD67 (nucleotides 1–1782, Accession # NM017007) were obtained by in vitro transcription using digoxigenin-11-UTP labeling mix (Roche Indianapolis, IN), and T3 RNA polymerase. Plasmids that contained GAD65 and GAD67 were generously provided by Dr. Allan Tobin (University of California Los Angeles, Los Angeles, CA).
Double in situ hybridization
For double hybridization, cryosections were processed as indicated for single hybridization but hybridization was performed with hybridization buffer containing [35S]- and [33P]-labeled single-stranded antisense rat vGluT2 riboprobes at 107 cpm/ml, together with 600 ng of a mix of GAD65 and GAD67 digoxigenin-labeled antisense riboprobes. Sections were treated with 4 μg/ml RNase A at 37°C for 1h, washed with 1 × SSC, 50% formamide at 55°C for 1h, and with 0.1 × SSC at 68°C for 1 h. After the last SSC wash, sections were rinsed with TBS buffer (20 mM Tris.HCl, 0.5 M NaCl, pH 8.2). Afterwards, sections were incubated with an alkaline phosphatase-conjugated antibody against digoxigenin (Roche Applied Science; Indianapolis, IN) overnight at 4°C; the alkaline phosphatase reaction was developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche Applied Science; Indianapolis, IN) yielding a purple reaction product. Sections were mounted on slides, air dried and dipped in Ilford K.5 nuclear tract emulsion, and exposed in the dark at 4°C for four weeks prior to development.
Combination of in situ hybridization and immunocytochemistry
After single or double in situ hybridization, sections were rinsed with PB and incubated for 1 h in PB supplemented with 4% bovine serum albumin (BSA) and 0.3% Triton X-100. Sections were then incubated with anti-ChAT goat polyclonal antibody (Chemicon, Temecula, CA, code: AB144P; dilution 1:50) overnight at 4°C. After rinsing 3 × 10 min in PB, sections were processed with an ABC kit (Vector Laboratories, Burlingame, CA). The sections were incubated for 1 h at room temperature in 1:200 dilution of the biotinylated secondary antibody, rinsed with PB, and incubated with avidin-biotinylated horseradish peroxidase for 1h. Sections were rinsed and the peroxidase reaction was then developed with 0.05% 3, 3-diaminobenzidine-4 HCl (DAB) and 0.003% hydrogen peroxide (H2O2). Sections were mounted on slides, air-dried, dipped in nuclear track emulsion, and exposed for 4 weeks prior to development. The anti-ChAT goat polyclonal antibody was raised against purified human placental ChAT and shown to label all cells containing ChAT mRNA (see result section).
Data analysis
Sections were viewed, analyzed, and photographed with bright field or epiluminescence microscopy using a Nikon Eclipse E 800 microscope fitted with 4X and 20X objective lenses. Cells expressing ChAT immunoreactivity, VGluT2 mRNA, or GAD mRNA were counted in sagittal sections of 4 different rats, with 140 μm spacing between sections for the PPTg and 60 μm for the LDTg, respectively. Double- and triple-labeled material were analyzed using epiluminescence to increase the contrast of silver grains (neither dark-field nor bright field optics allow clear visualization of silver grains when co-localized with immunoproducts). While silver grains appear as white grains under epiluminesce microscopy, they appear as green grains under epiluminesce and bright field microscopy. To determine the mesopontine tegmentum cytoarchitecture, adjacent cryosections were either processed for detection of mRNAs or stained with cresyl violet (Nissl-stained); mesopontine tegmentum subdivisions were traced according to Rye et al., 1987 and Spann and Grofova, 1989. The two subdivisions of the PPTg, pars compacta and pars dissipata, were traced in sagittal Nissl-stained sections as shown by Spann and Grofova, 1989. Single- and double-labeled neurons were observed within each traced region at high power (20x objective lens) and marked electronically. Using radioactive riboprobes, a cell was considered to express ChAT mRNA, vGluT2 mRNA, or GAD mRNA when its soma contained aggregates of silver particles. Using digoxigenin-labeled riboprobes, a neuron was considered to express GAD mRNA when its soma was clearly labeled as purple. For the calculation of the different cellular subpopulation, we only included labeled cells with at least 5 μm in diameter. To determine cellular co-expression of ChAT immunolabel with either vGluT2 mRNA or GAD mRNA, sections were analyzed as follow: (a) silver grains corresponding to vGluT2 mRNA or GAD mRNA expression were focused under epiluminescence microscopy, (b) the path of epiluminescence light was blocked without changing the focus, and (c) bright field light was used to determine if a brown neuron (ChAT immunopositive) or a purple neuron (GAD mRNA positive) in focus contained the aggregates of silver grains seen under epiluminescence. To avoid underestimation of double-labeled cells, ChAT immunoreactive sections were photographed before slides were dipped in nuclear tract emulsion. Digital photographs of these ChAT immunolabeled neurons were used to determine (after the development of silver grains) for the presence of ChAT immunolabel under the overlaying aggregates of silver grains. Labeled cells were counted twice, each time by a different observer. The background was evaluated from slides hybridized with sense probes. Pictures were adjusted to match contrast and brightness by using Adobe Photoshop (Adobe Systems Incorporated, Seattle, WA).
RESULTS
Identification of cholinergic neurons in the mesopontine tegmentum
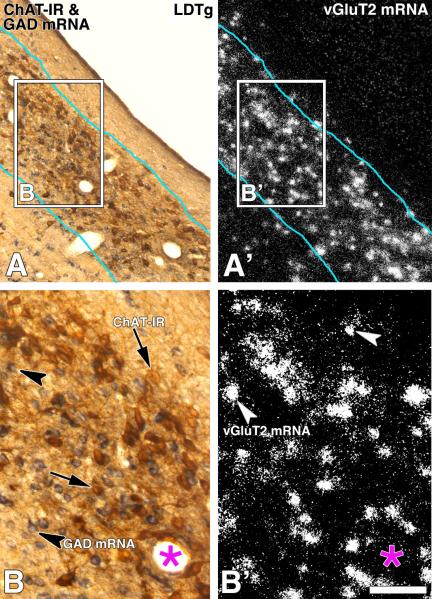
We used a combination of a ChAT radioactive antisense riboprobe and anti-ChAT antibodies to detect cellular co-expression of ChAT mRNA and ChAT protein in the PPTg and the LDTg (Fig. 1). ChAT mRNA was detected in all ChAT immunoreactive neurons, likewise, all neurons containing ChAT mRNA displayed ChAT immunoreactivity (Fig. 1). Since the anti-ChAT antibody used in this study labels only neurons encoding ChAT mRNA, and because the ChAT mRNA was detected only in neurons immunolabeled with the antibody, then, we used the anti-ChAT antibody throughout the rest of the study to identify cholinergic cell bodies.
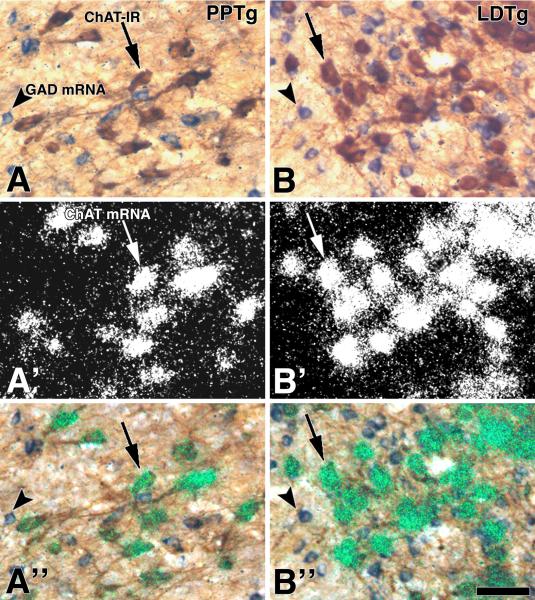
Figure 1. Cellular co-expression of ChAT immunoreactivity and ChAT mRNA in the PPTg and the LDTg (sagittal sections).
Frames A and B: Bright field microscopy showing neurons with ChAT immunoreactivity (DAB: brown cells; ChAT-IR, arrows) or GAD mRNA (purple cells; arrow heads). Frames A' and B': Epiluminescence microscopy showing cellular expression of ChAT mRNA (silver grains seen as white grain aggregates). Note examples of neurons expressing ChAT mRNA (arrows) shown in frames A and B as ChAT immunoreactive. Frames A” and B”. Cellular co-expression of ChAT immunoreactivity and ChAT mRNA under bright field and epiluminescence microscopy. Note detection of ChAT mRNA (silver grains seen as green grain aggregates) in all ChAT immunoreactive neurons (DAB: brown cells). ChAT, choline acetyltransferase; PPTg, pedunculopontine tegmental nucleus; LDTg, laterodorsal tegmental nucleus. Scale bar is 50 μm.
Detection of neurons expressing transcripts encoding either GAD or vGluT2 in the mesopontine tegmentum
We found cellular expression of GAD mRNA within the PPTg (Fig. 2A') and the LDTg (Fig. 2B'). We did not detect in the PPTg or the LDTg transcripts encoding either vGluT1 (Fig. 3A) or vGluT3 (Fig. 3B). In contrast, we identified a prominent expression of vGluT2 mRNA in many neurons within the PPTg (Figs. 4C and 4C') and the LDTg (Figs. 5C and 5C'). Signal for vGluT2 mRNA was not seen when brain sections were hybridized with the corresponding vGluT2 sense riboprobe (Figs. 4 D' and 5 D').
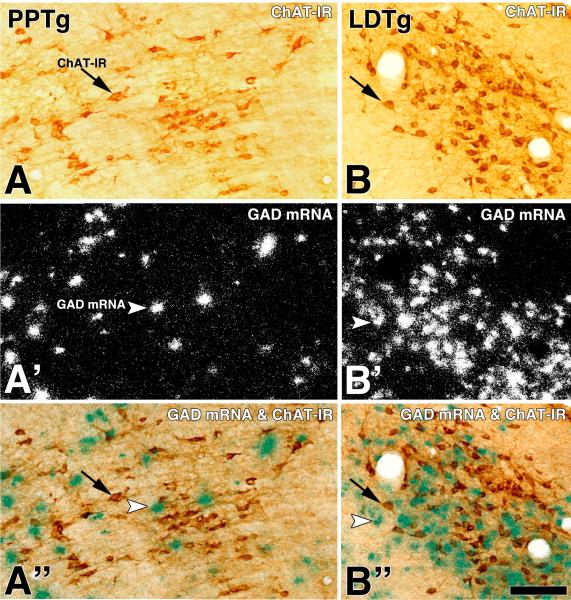
Figure 2. Cellular expression of GAD mRNA in the PPTg and the LDTg (sagittal sections).
Frames A and B: Bright field microscopy showing ChAT immunoreactive (DAB: brown cells; ChAT-IR, arrows) neurons in the PPTg (A) and the LDTg (B). Frames A' and B': Same sections under epiluminescence microscopy show cellular expression of GAD mRNA (silver grains seen as white grain aggregates, arrow heads). Frames A” and B”: Note under bright field and epiluminescence microscopy cells expressing GAD mRNA (silver grains seen as green grain aggregates) intermingle with ChAT immunoreactive neurons (DAB: brown cells). Scale bar is 135 μm.
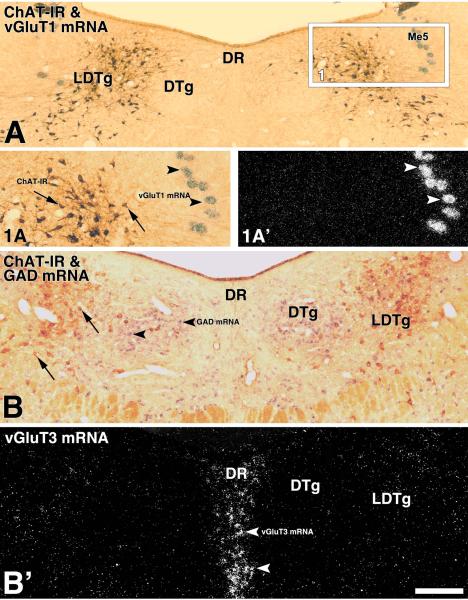
Figure 3. Lack of expression of vGluT1mRNA or vGluT3 mRNA in the LDTg (coronal sections).
Frame A: Bright field microscopy showing ChAT immunoreactive cells (DAB: brown). Rectangle in frame A delimitates low magnification area shown at higher magnification in 1A (bright field microscopy) and 1A' (epiluminescence microscopy). Cellular expression of vGluT1 mRNA is present within the mesencephalic trigeminal nucleus, Me5 (arrow heads), note lack of vGluT1 mRNA within the LDTg. Frame B: Epiluminescence microscopy, vGluT3 mRNA is clearly seen within the Dorsal Raphe (DR), note lack of vGluT3 mRNA in the dorsal tegmental nucleus (DTg) and in the LDTg. Frame B': Bright field microscopy of same section shown in frame B, ChAT immunoreactive neurons (DAB: brown cells) are prominent in the LDTg while those with GAD mRNA (purple cells) appear to be concentrated in the DTg. Scale bar is 610 μm for A, 360 μm for 1A and 1A', and 265 μm for B and B'.
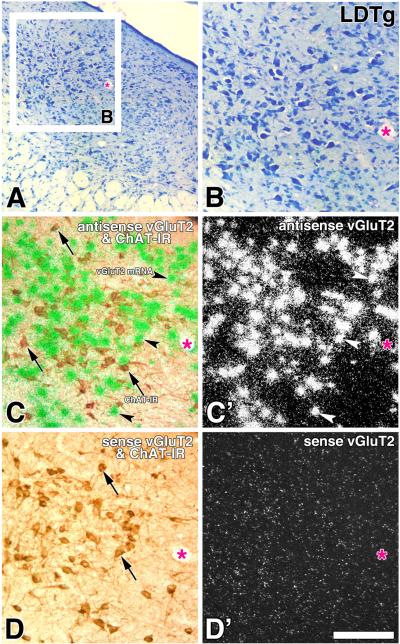
Fig. 4. Expression of vGluT2 mRNA in the PPTg (coronal sections).
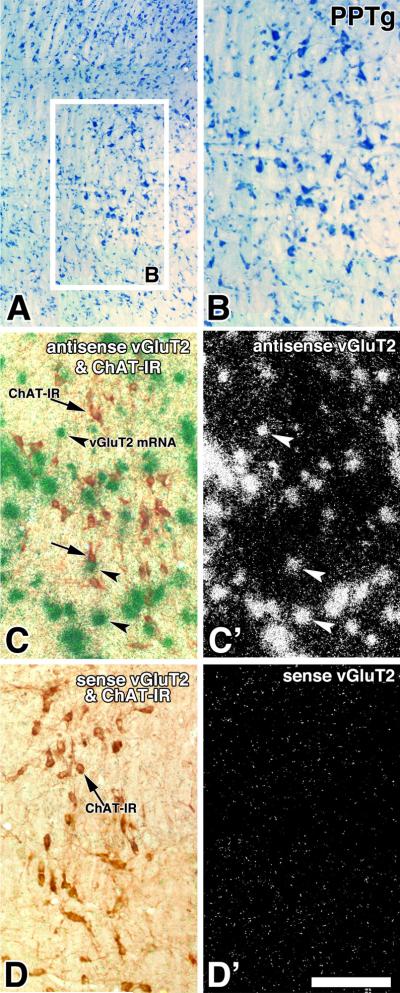
Frame A and B: Low (A) and high magnification (B) of a Nissl stained section. Frame C and C': Adjacent section to the one showed in frame B, section was hybridized with vGluT2 antisense riboprobe. Note in frame C expression of ChAT immunoreactive neurons (DAB: brown cells, arrows) and vGluT2 mRNA (silver grains seen as green aggregates, arrow-heads) seen under bright field and epiluminescence microscopy. Cellular expression of vGluT2 mRNA is seen as white aggregates under epiluminescence microscopy in frame C'. Frames D and D': Adjacent section to the one showed in frames C and C', section was hybridized with a sense radioactive vGluT2 riboprobe, note lack of green aggregates in frame D and white aggregates in frame D'. Scale is 250 μm for A and 180 μm for B, C, C', D and D'.
Fig. 5. Expression of vGluT2 mRNA in the LDTg (coronal sections).
Frame A and B: Low (A) and high magnification (B) of a Nissl stained section. Frame C and C': Adjacent section to the one showed in frame B, section was hybridized with vGluT2 antisense riboprobe. Note in frame C expression of ChAT immunoreactive neurons (DAB: brown cells, arrows) and vGluT2 mRNA (silver grains seen as green aggregates, arrow-heads) seen under bright field and epiluminescence microscopy. Cellular expression of vGluT2 mRNA is seen as white aggregates under epiluminescence microscopy in frame C'. Frames D and D': Adjacent section to the one showed in frames C and C', section was hybridized with a sense radioactive vGluT2 riboprobe, note lack of green aggregates in frame D and white aggregates in frame D'. Scale bar is 325 μm for A and 165 μm for B, C, C', D and D'.
Either vGluT2 mRNA or GAD mRNA were rarely found in the PPTg/LDTg cholinergic neurons
We prepared serial sagittal brain sections through the mesopontine tegmentum and used one set of sections for the simultaneous detection of ChAT immunoreactivity and vGluT2 mRNA. Another set of serial sections was used for the simultaneous detection of ChAT immunoreactivity and GAD mRNA. We evaluated co-expression of ChAT immunoreactivity [ChAT (+)] with either vGluT2 mRNA or GAD mRNA at the cellular level in the PPTg (Fig. 6) and the LDTg (Fig. 7). The vGluT2 mRNA and GAD mRNA were rarely observed in ChAT (+) neurons in the PPTg and LDTg (Table 1 and Table 2, respectively). Only 2.1 ± 0.2 % (mean ± sem) of the ChAT (+) cells appeared to co-express vGluT2 mRNA in the PPTg [n = 1513 ChAT (+) cells] and only 1.1 ± 0.1 % appeared to co-express vGluT2 mRNA in the LDTg [n = 1809 ChAT (+) cells]. Similarly, only 0.7 ± 0.2% of the ChAT (+) cells appeared to co-express GAD mRNA in the PPTg [n = 1565 ChAT (+) cells] and 1.2 ± 0.1 % in the LDTg [n = 2137 ChAT (+) cells].
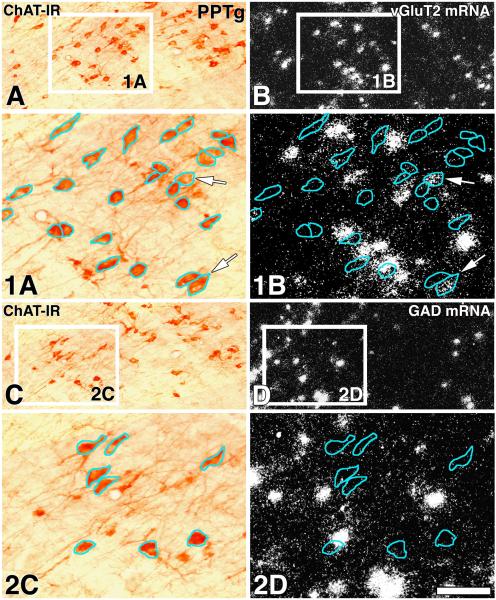
Figure 6. ChAT immunoreactive neurons of the PPTg lack either vGluT2 mRNA or GAD mRNA (sagittal sections).
Frames A and B correspond to a low magnification view of the same section showing ChAT immunoreactivity under bright field microscopy (A) or vGluT2 mRNA under epiluminescence microscopy (B). Rectangles in frames A and B delimitate low magnification areas shown at higher magnification in 1A and 1B. In frames 1A and 1B, the blue outlines demarcate the profile of ChAT (+) neurons; note in frame 1B lack of concentricity of white aggregates (vGluT2 mRNA) within most of the blue outlines, concentricity is apparent in two cells (arrows). An adjacent section to the one shown in frames A and B is displayed in frames C and D. Frames C and D correspond to a low magnification view of the same section showing ChAT immunoreactivity (C) or GAD mRNA (D). Rectangles in frames C and D delimitate low magnification areas shown at higher magnification in frames 2C and 2D. In frames 2C and 2D, the blue outlines demarcate the profile of ChAT (+) neurons; note in frame 2D lack of concentricity of white aggregates (GAD mRNA) within the blue outlines. Scale bar is 60 μm for 1A, 1B, 2C and 2D, and 150 μm for A, B, C and D.
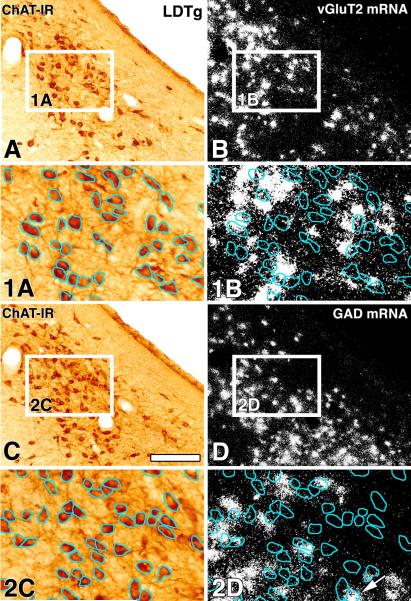
Figure 7. ChAT immunoreactive neurons of the LDTg lack either vGluT2 mRNA or GAD mRNA (sagittal sections).
Frames A and B correspond to a low magnification view of the same section showing ChAT immunoreactivity (A) or vGluT2 mRNA (B). Rectangles in frames A and B delimitate low magnification areas shown at higher magnification in frames 1A and 1B. In frames 1A and 1B, the blue outlines demarcate the profile of ChAT (+) neurons; note in frame 1B the lack of concentricity of white aggregates (vGluT2 mRNA) within the blue outlines. An adjacent section to the one shown in frames A and B is displayed in frames C and D. Frames C and D correspond to low magnification view of the same section showing ChAT immunoreactivity (C) or GAD mRNA (D). Rectangles in C and D delimitate a low magnification areas shown at higher magnification in frames 2C and 2D. In frames 2C and 2D, the blue outlines demarcate the profile of ChAT (+) neurons; note in 2D lack of concentricity of white aggregates (GAD mRNA) within the blue outlines, concentricity is clearly seen in one cell (arrow). Scale bar is 63 μm for 1A, 1B, 2C and 2D, and 164 μm for A, B, C and D.
Table 1.
Frequency of ChAT immunolabeled neurons co-expressing either vGluT2 mRNA or GAD mRNA in the PPTg
| Percentage of neurons co-expressing ChAT immunolabel and vGluT2 mRNA in the total population of ChAT immunolabeled neuronsa | Percentage of neurons co-expressing ChAT immunolabel and GAD65/GAD67 mRNAs in the total population of ChAT immunolabeled neuronsb | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Subject | ChAT (+) | ChAT (+)/vGluT2 (+) | % | Subject | ChAT (+) | ChAT (+)/GADs (+) | % |
| #1 | n = 146 | n = 3 | 2.0 | #1 | n = 333 | n = 1 | 0.3 |
| #2 | n = 365 | n = 6 | 1.6 | #2 | n = 504 | n = 4 | 0.8 |
| #3 | n = 579 | n = 14 | 2.4 | #3 | n = 465 | n = 6 | 1.3 |
| #4 | n = 423 | n = 10 | 2.4 | #4 | n = 265 | n = 1 | 0.4 |
|
| |||||||
| Total | n = 1513 | n =33 | 2.1 ± 0.2 (mean ± sem) | Total | n = 1565 | n = 12 | 0.7 ± 0.2 (mean ± sem) |
Sagittal sections were treated simultaneously with anti-ChAT antibodies and a radioactive antisense vGluT2 riboprobe. Cells expressing ChAT immunoreactivity or vGluT2 mRNA were counted every seventh 20 μm section; 16 sections were counted from four rats.
Sagittal sections were treated simultaneously with anti-ChAT antibodies and radioactive antisense GAD65 and GAD67 riboprobes. Cells expressing ChAT immunoreactivity or GAD65/GAD67 mRNAs were counted every seventh 20 μm section; 16 sections were counted from four rats.
Table 2.
Frequency of ChAT immunolabeled neurons co-expressing either vGluT2 mRNA or GAD mRNA in the LDTg
| Percentage of neurons co-expressing ChAT immunolabel and vGluT2 mRNA in the total population of ChAT immunolabeled neuronsa | Percentage of neurons co-expressing ChAT immunolabel and GAD65/GAD67 mRNAs in the total population of ChAT immunolabeled neuronsb | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Subject | ChAT (+) | ChAT (+)/vGluT2 (+) | % | Subject | ChAT (+) | ChAT (+)/GADs (+) | % |
| #1 | n = 524 | n = 6 | 1.1 | #1 | n = 546 | n = 8 | 1.5 |
| #2 | n = 448 | n = 5 | 1.1 | #2 | n = 635 | n = 6 | 0.9 |
| #3 | n = 444 | n = 6 | 1.4 | #3 | n = 438 | n = 5 | 1.1 |
| #4 | n = 393 | n = 3 | 0.8 | #4 | n = 518 | n = 6 | 1.2 |
|
| |||||||
| Total | n = 1809 | n = 20 | 1.1 ± 0.1 (mean ± sem) | Total | n = 2137 | n = 25 | 1.2 ± 0.1 (mean ± sem) |
Sagittal sections were treated simultaneously with anti-ChAT antibodies and a radioactive antisense vGluT2 riboprobe. Cells expressing ChAT protein or vGluT2 mRNA were counted every third 20 μm section; 16 sections were counted from four rats.
Sagittal sections were treated simultaneously with anti-ChAT antibodies and radioactive antisense GAD65 and GAD67 riboprobes. Cells expressing ChAT protein or GAD65/GAD67 mRNAs were counted every third 20 μm section; 16 sections were counted from four rats.
Differential distribution of cholinergic, glutamatergic and GABAergic neurons within the PPTg
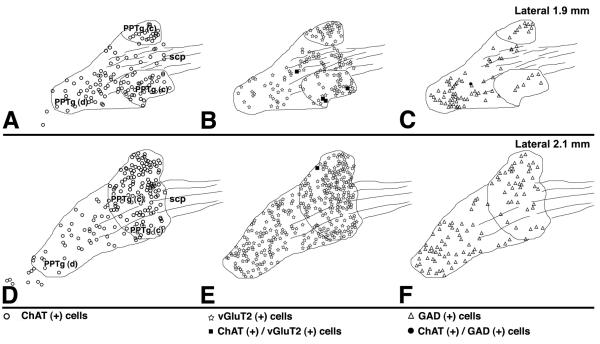
Once we determined that cholinergic, glutamatergic, and GABAergic neurons of the PPTg/LDTg complex belong mostly to three distinct neuronal subpopulations, we then analyzed the density and pattern of distribution of these neuronal subpopulations using sagittal sections. Observations from triple label material clearly showed that the cholinergic, glutamatergic and GABAergic neurons are intermingled in the pars compacta (Figs. 8B and 8B') and the pars dissipata (Figs. 8C and 8C') of the PPTg. Although cholinergic, glutamatergic and GABAergic neurons are intermingled, they have different distribution in the pars compacta and the pars dissipata, as seen clearly from double labeled material processed for detection of either ChAT immunoreactivity with vGluT2 mRNA (Figs. 9A' and 9B') or ChAT immunoreactivity with GAD mRNA (Fig. 9 C' and 9D'). In the pars compacta the glutamatergic neurons were the major neuronal subpopulation (50 ± 4 %, Table 3) with the cholinergic (31 ± 3 %) greater than the GABAergic (19 ± 2 %) subpopulation. In contrast, in the pars dissipata, the GABAergic neurons were slightly more concentrated (40 ± 4 %) than the glutamatergic neurons (37 ± 2 %), but were almost twice more concentrated than the cholinergic neurons (23 ± 3 %).
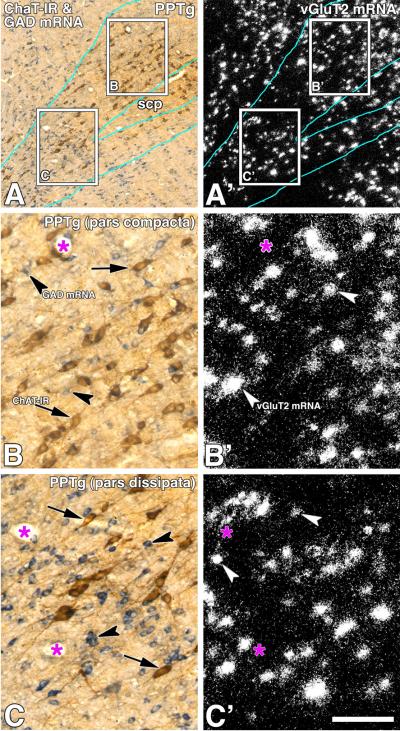
Figure 8. ChAT immunoreactive neurons in the PPTg are intermingled with neurons expressing either vGluT2 mRNA or GAD mRNA (triple labeling).
Frame A: Bright field microscopy of a sagittal section showing ChAT immunoreactive neurons (DAB: brown cells) and cells expressing GAD mRNA (purple neurons). Frame A': Epiluminescence microscopy of the section showed in frame A, note cellular expression of vGluT2 mRNA (silver grains seen as white aggregates). Frames B, B', C and C' correspond to higher magnification areas delimited by rectangles in frames A and A' of the pars compacta (B and B') and the pars dissipata (C and C') of the PPTg. scp, superior cerebellar peduncle. Scale bar is 346 μm for A and A', and 95 μm for B, B', C and C'.
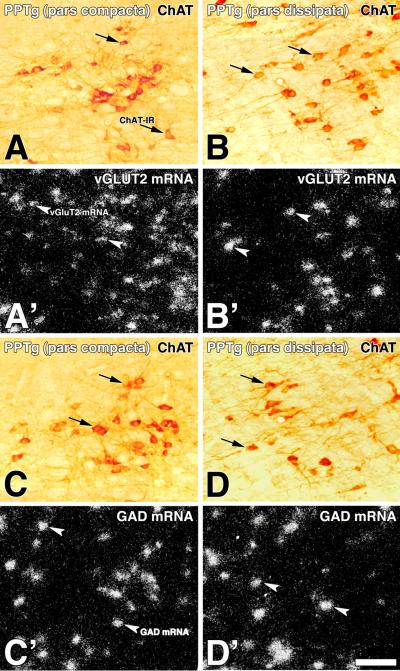
Figure 9. Distribution of neurons expressing transcripts encoding either vGluT2 or GAD within the PPTg (double labeling).
Frames A and B: Bright field microscopy of a sagittal section showing ChAT immunoreactive neurons (DAB: brown cells) in the pars compacta (A) and pars dissipata (B) of the PPTg. Frame A' and B': Epiluminescence microscopy of the section showed in frame A and B, note cellular expression of vGluT2 mRNA (silver grains seen as white aggregates) within both sub-divisions of the PPTg. Frames C and D: Bright field microscopy of a sagittal section, adjacent to the one in frames A and B, showing ChAT immunoreactive neurons (DAB: brown cells) in the pars compacta (C) and pars dissipata (D) of the PPTg. Frame C' and D': Epiluminescence microscopy of the section showed in frame C and D, note cellular expression of GAD mRNA (silver grains seen as white aggregates) within both subdivisions of the PPTg. Scale bar is 84 μm.
Table 3.
Frequency of three distinct subpopulations of neurons in the PPTg: neurons containing ChAT immunoreactivity, vGluT2 mRNA or GAD mRNAa
| % of neurons containing ChAT immunoreactivity (mean ± sem) | % of neurons expressing vGluT2 mRNA (mean ± sem) | % of neurons expressing GAD mRNA (mean ± sem) | |
|---|---|---|---|
| PPTg | |||
| Pars compacta (100%; n = 2093) | 31 ± 3 (n= 652) | 50 ± 4 (n= 912) | 19 ± 2 (n= 372) |
|
| |||
| PPTg | |||
| Pars dissipata (100%; n = 3789) | 23 ± 3 (n= 760) | 37 ± 2 (n= 1392) | 40 ± 4 (n= 1637) |
| Total in the PPTg (n = 5725) | 27 ± 2 (n= 1412) | 43 ± 2 (n= 2304) | 31 ± 3 (n= 2009) |
Sagittal sections were treated simultaneously with anti-ChAT antibodies, a radioactive antisense vGluT2 riboprobe, and digoxigenin antisense GAD65 and GAD67 riboprobes. Cells expressing ChAT immunoreactivity, vGluT2 mRNA, or GAD mRNA were counted every seventh 20 μm section. A total of 12 sections were counted from three rats. n = counted cells. The percentage values of all sections were used to calculate the mean and standard error of the mean (sem).
In summary, we found cholinergic, glutamatergic, and GABAergic neurons at all levels and subdivisions of the PPTg (Fig. 10). Notably, the cholinergic neurons were not the major neuronal subpopulation in either the pars compacta or pars dissipata. In the pars compacta, the glutamatergic neurons were 1.5 times more concentrated than the cholinergic neurons, and in the pars dissipate, the GABAergic neurons were almost twice as concentrated as the cholinergic neurons.
Figure 10. Summary diagram of the distribution of cells containing ChAT immunoreactivity (circles in frames A and D), vGluT2 mRNA (stars in frames B and E) or GAD mRNA (triangles in frames C and F) in the PPTg.
Very few ChAT (+) cells co-express either vGluT2 mRNA (solid squares in frames B and E) or GAD mRNA (solid circles in frames C). PPTg (c), pars compacta; PPTg (d), pars dissipata.
Differential distribution of cholinergic, glutamatergic and GABAergic neurons within the LDTg
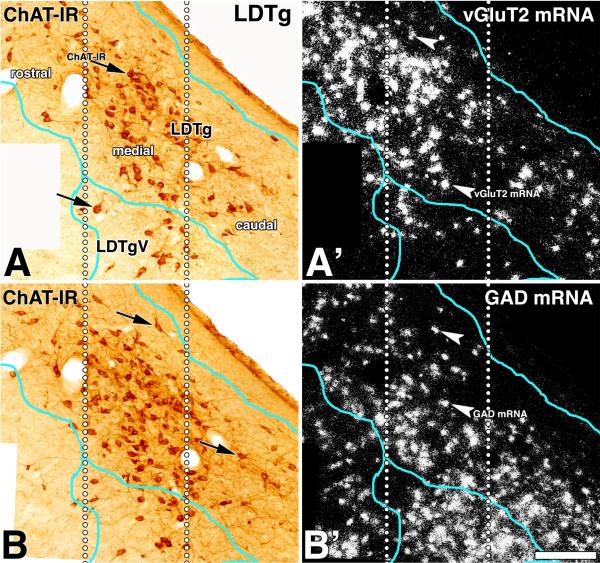
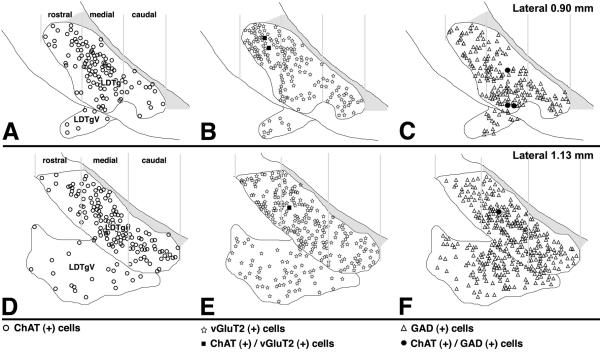
Similar to observations made in the PPTg, cholinergic, glutamatergic, and GABAergic neurons are intermingled (Fig. 11), but not homogenously distributed throughout the LDTg, as seen clearly from double labeled material processed for detection of either ChAT immunoreactivity with vGluT2 mRNA (Fig. 12 A') or ChAT immunoreactivity with GAD mRNA (Fig. 12 B'). While neurons expressing vGluT2 mRNA were more concentrated in the rostral aspect of the LDTg (Fig. 12 A'), those expressing GAD mRNA were more concentrated in the caudal portion of the LDTg (Fig. 12 B'). Based on these observations, we estimated the percentage of cholinergic, glutamatergic, and GABAergic neurons within three subdivisions of the LDTg: rostral, medial, and caudal. In the rostral LDTg, the glutamatergic neurons were more concentrated (54 ± 3 %, Table 4) than the cholinergic (19 ± 2 %) and GABAergic (27 ± 3 %) neurons. Of the three subdivisions of the LDTg, the medial subdivision had the highest concentration of cholinergic neurons; however, the density of the cholinergic neuronal subpopulation (32 ± 2 %) was comparable to the cellular density of glutamatergic (32 ± 2 %) and GABAergic (36 ± 2 %) subpopulations. The caudal portion of the LDTg had the highest concentration of GABAergic neurons (58 ± 2 %), with low concentration of cholinergic (14 ± 1 %) and glutamatergic neurons (28 ± 2 %).
Figure 11. ChAT (+) neurons in the LDTg are intermingled with neurons expressing either vGluT2 mRNA or GAD mRNA (triple labeling).
Frame A: Bright field microscopy of a sagittal section showing ChAT immunoreactive neurons (DAB: brown cells) and cells expressing GAD mRNA (purple neurons). Frame A': Epiluminescence microscopy of the section showed in frame A, note cellular expression of vGluT2 mRNA (silver grains seen as white aggregates). Frames B and B', correspond to higher magnification areas delimited by rectangles in frames A and A'. Scale bar is 225 μm for A and A', and 88 μm for B and B'.
Figure 12. Distribution of neurons expressing either vGluT2 mRNA or GAD mRNA within the LDTg (double labeling).
Frames A and B: Bright field microscopy of two consecutive sections showing ChAT immunoreactive neurons (brown cells) within the LDTg (sagittal sections). Note the highest concentration of ChAT (+) cells in the medial portion of the LDTg. Frame A': Epiluminescence microscopy of section showed in frame A. Note an apparent descending rostral-caudal gradient of expression of vGluT2 mRNA (silver grains seen as white aggregates). Frame B': Epiluminescence microscopy of section showed in frame B. Note an apparent ascending rostral-caudal gradient of expression of GAD mRNA (silver grains seen as white aggregates). LDTg, dorsal LDTg; LDTgV, ventral LDTg. Scale bar is 285 μm.
Table 4.
Frequency of three distinct subpopulations of neurons in the LDTg: neurons containing ChAT immunoreactivity, vGluT2 mRNA or GAD mRNAa
| % of neurons containing ChAT immunoreactivity (mean ± sem) | % of neurons expressing vGluT2 mRNA (mean ± sem) | % of neurons expressing GAD mRNA (mean ± sem) | |
|---|---|---|---|
| Rostral LDTg (100%) (n = 2038) | 19 ± 2 (n= 373) | 54 ± 3 (n= 1094) | 27 ± 3 (n= 571) |
| Medial LDTg (100%) (n = 2981) | 32 ± 2 (n= 955) | 32 ± 2 (n= 966) | 36 ± 3 (n= 1060) |
| Caudal LDTg (100%) (n = 3479) | 14 ± 1 (n= 483) | 28 ± 2 (n= 986) | 58 ± 2 (n= 2010) |
| Total in the LDTg (n = 8498) | 22 ± 2 (n= 1811) | 38 ± 2 (n= 3046) | 40 ± 3 (n= 3641) |
Sagittal sections were treated simultaneously with anti-ChAT antibodies, a radioactive antisense vGluT2 riboprobe, and digoxigenin antisense GAD65 and GAD67 riboprobes. Cells expressing ChAT immunoreactivity, vGluT2 mRNA, or GAD mRNA were counted every third 20 μm section. A total of 12 sections were counted from three rats. n = counted cells. The percentage values of all sections were used to calculate the mean and standard error of the mean (sem).
In summary, we found that the cholinergic, glutamatergic, and GABAergic neurons were not homogenously distributed within the LDTg (Fig. 13). Similar to observations made in the PPTg, the cholinergic neurons were not the major neuronal subpopulation within the LDTg. The highest concentration of cholinergic neurons was found in the medial aspect of the LDTg, where cholinergic, glutamatergic and GABAergic neuronal subpopulations had comparable neuronal density.
Figure 13. Summary diagram of the distribution of cells containing ChAT immunoreactivity (circles in frames A and D), vGluT2 mRNA (stars in frames B and E) or GAD mRNA (triangles in frames C and F) in the LDTg.
Very few ChAT (+) cells co-express either vGluT2 mRNA (solid squares in frames B and E) or GAD mRNA (solid circles in frames C and F).
DISCUSSION
Our study provides anatomical evidence that the PPTg and LDTg are comprised of at least three subpopulations of neurons: cholinergic, glutamatergic and GABAergic. These distinctively neuronal phenotypes are intermingled, but not homogeneously distributed. More than 95% of the PPTg and LDTg cholinergic neurons lack co-expression of either GAD mRNA or vGLuT2 mRNA. We conclude that co-release of acetylcholine with either glutamate or GABA, if it occurs at all, occurs in very few PPTg and LDTg efferents; thus co-release is not a major factor in the interactions between acetylcholine, glutamate, and GABA at the postsynaptic site.
PPTg/LDTg identification of glutamatergic neurons by in situ hybridization
We used detection of transcripts encoding vGluTs as a reliable method to label cell bodies of glutamatergic neurons. vGluTs is a distinct family of glutamate transporters that transport glutamate into synaptic vesicles at presynaptic terminals (Bellocchio et al., 1998, 2000; Fujiyama et al., 2001; Fremeau et al., 2002; Gras et al., 2002; Hayashi et al., 2001; Takamori et al., 2000, 2001; Bai et al 2001; Fremeau et al., 2001; Herzog et al., 2001; Schafer et al., 2002; Varoqui et al., 2002). Although there are antibodies developed against vGluTs that can detect vGluTs at the axon terminals, they do not detect the low levels of the transporters in cell bodies and dendrites of glutamatergic neurons. A common method for identification of cell bodies of glutamatergic neurons in the PPTg/LDTg has been based on the immunodetection of glutamate. However, glutamate has a ubiquitous cellular distribution due to its role in the synthesis of proteins and production of energy. We chose not to use glutamate immunodetection because it does not differentiate the metabolic and transmitter pools of glutamate. As an alternative to glutamate immunolabeling, we identified glutamatergic neurons in the PPTg/LDTg by their expression of vGluTs mRNA using radioactive in situ hybridization, and conclude that all glutamatergic neurons within these PPTg/LDTg selectively contained vGluT2. Neither vGluT1 nor vGluT3 were found in the mesopontine tegmentum. The lack of vGluT3 mRNA within the mesopontine tegmentum indicates that these cholinergic neurons differ from striatal cholinergic interneurons which are shown to contain functional vGluT3 for the synergetic vesicular uptake of acetylcholine (Gras et al., 2008).
PPTg/LDTg identification of GABAergic neurons by in situ hybridization
GAD is the enzyme responsible for the synthesis of GABA; however, most of the GABAergic neurons contain undetectable levels of GAD or GABA in their cell bodies. The accumulation of GAD or GABA in the cell bodies of GABAergic neurons is often induced by the blockage of cellular trafficking by intraventricular injection of colchicine. The importance of the use of colchicine to allow detection of GABA or GAD in the PPTg/LDTg cells is underscored by immunolabeling studies showing GABAergic neurons in these brain areas when colchicine treatment was included (Kosaka et al., 1988; Ford et al., 1995), as opposed to the lack of immunodetection of GABAergic neurons in either the PPTg or LDTg when colchicine pre-treatment was not performed (Sutin and Jacowitz, 1988; Lavoie & Parent 1994b). As an alternative to the use of colchicine followed by immunodetection of GAD, we identified GABAergic neurons in the PPTg/LDTg by their expression of transcripts encoding the two isoforms of GAD (GAD65 and GAD67) using either radioactive or non-radioactive riboprobes. Regardless of the applied riboprobe, we detected by in situ hybridization GABAergic neurons in the PPTg/LDTg.
The vast majority of mesopontine cholinergic neurons lack either glutamatergic or GABAergic phenotypes
By using a combination of in situ hybridization for detection of vGluTs mRNA and immunohistochemistry to label cholinergic neurons, we found that only 2.1 % of the PPTg and 1.1 % of the LDTg cholinergic neurons co-express vGluT2 mRNA. Since the vast majority of PPTg/LDTg cholinergic neurons lack transcripts encoding essential proteins for the vesicular transport of glutamate, we conclude that the vast majority of cholinergic neurons of the mesopontine tegmentum are unlikely to use glutamate as a co-transmitter.
The GABAergic nature of cholinergic neurons is controversial (Kosaka et al., 1988; Ford et al., 1995; Jia et al., 2003); in two studies where colchicine was used followed by double immunolabeling, none of the cholinergic mesopontine tegmentum neurons showed GAD immunoreactivity (Kosaka et al., 1988; Ford et al., 1995). In contrast, in a study where colchicine was not used, GABA immunoreactivity was observed in 50% of the cholinergic neurons in the cat mesopontine tegmentum (Jia et al., 2003). Jia et al. suggested that the detection of GABA immunoreactivity in cholinergic neurons of the mesopontine tegmentum was achieved by intensification of the immunolabel signal with tyramide combined with analysis by fluorescence confocal microscopy (Jia et al., 2003). Although this approach is becoming very popular, controls testing the specificity of the obtained signal are not often provided, making difficult to determine the specificity of reported labeling. By using a combination of in situ hybridization for detection of GAD mRNA and immunohistochemistry to label chlolinergic neurons, we found that only 0.7% of ChAT cells in PPTg and 1.2% of ChAT cells in LDTg appeared to co-express GAD mRNA. Our observations are in agreement with previous studies showing that cholinergic neurons of the mesopontine tegmentum lack a GABAergic phenotype (Kosaka et al., 1988; Ford et al., 1995), and do not support the hypothesis that cholinergic neurons of the mesopontine tegmentum use GABA as a co-transmitter.
Cholinergic, glutamatergic, and GABAergic neurons in the PPTg
Our data showing heterogeneous composition of the PPTg pars compacta are in line with previous studies in which cholinergic and non-cholinergic neurons were detected in this subdivision of the PPTg (Spann & Grova, 1992; Steininger et al., 1997); however they do not support the claims of Rye et al., (1987) who concluded that the pars compacta of the rat PPTg is composed entirely of cholinergic neurons. We conclude that cholinergic, glutamatergic, and GABAergic neurons are present at all levels and subdivisions of the PPTg. In the pars compacta, the glutamatergic neurons are 1.5 times more concentrated than the cholinergic neurons and in the pars dissipata, the GABAergic neurons are almost twice more concentrated than the cholinergic neurons. Cellular heterogeneity in the PPTg is supported further by electrophysiological studies from in vitro preparations showing that (based on electrophysiological membrane properties and phenotypic cellular characterization) the PPTg has at least one subpopulation of cholinergic neurons and two of non-cholinergic neurons (Takakusaki et al., 1996). In addition, in vivo studies demonstrated that different PPTg neuronal subtypes fire in opposite phases during the slow oscillations in the cortex of anesthetized rats (Mena-Segovia et al., 2008).
The neurocircuitry of the different mesopontine tegmentum neurons remains to be determined. Some afferents to the different PPTg neuronal subpopulations may arise from common brain structures, as electrophysiological evidence indicates that both cholinergic and non-cholinergic cells of the PPTg receive monosynaptic inhibitory inputs from the substantia nigra reticulata (Kang & Kitai, 1990). The different PPTg neuronal subpopulations are also likely to target similar sites, as indicated by a basal ganglia retrograde tracer study in the monkey in which only 25% of the retrograde labeled neurons in the PPTg were cholinergic (Lavoie & Parent, 1994b); in all likelihood the remaining retrogradely labeled cells are either GABAergic or glutamatergic. In this regard, in addition to the mesopontine tegmentum cholinergic afferents, glutamate immunoreactive afferents innervate the subthalamic nucleus, the substantia nigra and the ventral tegmental area (VTA) (Lavoie & Parent 1994b; Bevan & Bolam, 1995; Charara et al., 1996). Moreover, the mesopontine tegmentum afferents immunoreactive for GABA also innervate the subthalamic nucleus, the entopeduncular nucleus, the substantia nigra, and the VTA (Lavoie & Parent 1994b; Bevan & Bolam, 1995; Charara et al., 1996; Clarke et al., 1997; Omelchenko & Sesack, 2005). Similarly, GABAergic neurons of the mesopontine tegmentum, identified by GAD immunoreactivity, together with cholinergic neurons target the laterodorsal hypothalamus (Ford et al., 1995). In addition, electrophysiological evidence indicates that both cholinergic and glutamatergic afferents from the PPTg converge on a single DA neuron (Futami et al., 1995).
Cholinergic, glutamatergic, and GABAergic neurons in the LDTg
We conclude that in the LDTg as in the PPTg, there are three distinct subpopulations of neurons: cholinergic, glutamatergic, and GABAergic neurons. Notably, the cholinergic neurons are not the major neuronal subpopulation within the LDTg. Our finding that neurons expressing GAD mRNA were twice more concentrated than the ChAT immunoreactive neurons is in agreement with a previous double immunohistochemical study suggesting that the GAD immunoreactive neurons are 1.8 more concentrated than the cholinergic immunoreactive neurons (Ford et al., 1995).
LDTg cholinergic, GABAergic, and putative glutamatergic afferents synapse on VTA neurons (Charara et al., 1996; Omelchenko & Sesack, 2005, 2006), and on dopaminergic and GABAergic neurons (Omelchenko and Sesack, 2005, 2006); it remains to be determined if mesopontine tegmentum afferents are also established with recently identified VTA glutamatergic neurons (Kawano et al., 2006; Yamaguchi et al., 2007; Nair-Roberts et al., 2008). While accumulating evidence suggests a role for LDTg cholinergic and glutamatergic inputs in regulating VTA dopamine neurotransmission (Grace, 1991; Chergui et al., 1994; Overton and Clark, 1997; Paladini et al., 1999; Forster and Blaha, 2000; Floresco et al., 2003; Lodge and Grace, 2006), the contribution of LDTg GABAergic afferents on VTA neurotransmission remains to be explored.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse.
ABBREVIATIONS
- ChAT
Choline acetyltransferase
- GABA
γ-amino butyric acid
- GAD
glutamic acid decarboxylase
- LDTg
laterodorsal tegmental nucleus
- PPTg
pedunculopontine tegmental nucleus
- vGluT1
vesicular glutamate transport 1
- vGluT2
vesicular glutamate transport 2
- vGluT3
vesicular glutamate transport 3
- VTA
ventral tegmental area
REFERENCES
- Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J. Biol. Chem. 2001;276:36764–36769. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit. Rev. Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Bechara A, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus: effects on the locomotor activity induced by morphine and amphetamine. Pharmacol. Biochem. Behav. 1992;42:9–18. doi: 10.1016/0091-3057(92)90438-l. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J. Neurosci. 1998;18:8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr., Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Bolam JP. Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J. Neurosci. 1995;15:7105–7120. doi: 10.1523/JNEUROSCI.15-11-07105.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J. Comp. Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Chergui K, Suaud-Chagny MF, Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–645. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Clarke NP, Bevan MD, Cozzari C, Hartman BK, Bolam JP. Glutamate-enriched cholinergic synaptic terminals in the entopeduncular nucleus and subthalamic nucleus of the rat. Neuroscience. 1997;81:371–385. doi: 10.1016/s0306-4522(97)00247-9. [DOI] [PubMed] [Google Scholar]
- Clements JR, Grant S. Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci. Lett. 1990;120:70–73. doi: 10.1016/0304-3940(90)90170-e. [DOI] [PubMed] [Google Scholar]
- Clements JR, Toth DD, Highfield DA, Grant SJ. Glutamate-like immunoreactivity is present within cholinergic neurons of the laterodorsal tegmental and pedunculopontine nuclei. Adv. Exp. Med..Biol. 1991;295:127–142. doi: 10.1007/978-1-4757-0145-6_5. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res. Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J. Comp. Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur. J. Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl. Acad. Sci. U.S.A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J. Comp. Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci. Res. 1995;21:331–342. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. The pedunculopontine nucleus. Prog Neurobiol. 1991;36:363–389. doi: 10.1016/0301-0082(91)90016-t. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat. Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J. Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J. Comp. Neurol. 1989;287:495–514. doi: 10.1002/cne.902870408. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Otsuka M, Morimoto R, Hirota S, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y. Differentiation-associated Na+-dependent inorganic phosphate cotransporter (DNPI) is a vesicular glutamate transporter in endocrine glutamatergic systems. J. Biol. Chem. 2001;276:43400–43406. doi: 10.1074/jbc.M106244200. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J. Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imon H, Ito K, Dauphin L, McCarley RW. Electrical stimulation of the cholinergic laterodorsal tegmental nucleus elicits scopolamine-sensitive excitatory postsynaptic potentials in medial pontine reticular formation neurons. Neuroscience. 1996;74:393–401. doi: 10.1016/0306-4522(96)00134-0. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog. Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Jones BE. Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience. 1991;40:637–656. doi: 10.1016/0306-4522(91)90002-6. [DOI] [PubMed] [Google Scholar]
- Kang Y, Kitai ST. Electrophysiological properties of pedunculopontine neurons and their postsynaptic responses following stimulation of substantia nigra reticulata. Brain Res. 1990;535:79–95. doi: 10.1016/0006-8993(90)91826-3. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J. Comp. Neurol. 2006;498:581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Tauchi M, Dahl JL. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp. Brain Res. 1988;70:605–617. doi: 10.1007/BF00247609. [DOI] [PubMed] [Google Scholar]
- Krauthamer GM, Grunwerg BS, Krein H. Putative cholinergic neurons of the pedunculopontine tegmental nucleus projecting to the superior colliculus consist of sensory responsive and unresponsive populations which are functionally distinct from other mesopontine neurons. Neuroscience. 1995;69:507–517. doi: 10.1016/0306-4522(95)00265-k. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J. Comp. Neurol. 1994a;344:232–241. doi: 10.1002/cne.903440205. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J. Comp. Neurol. 1994b;344:190–209. doi: 10.1002/cne.903440203. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley BE, Frick ML, Levey AI, Wainer BH, Elde RP. Immunohistochemistry of choline acetyltransferase in the guinea pig brain. Neurosci. Lett. 1988;84:137–142. doi: 10.1016/0304-3940(88)90397-7. [DOI] [PubMed] [Google Scholar]
- Mathur A, Shandarin A, LaViolette SR, Parker J, Yeomans JS. Locomotion and stereotypy induced by scopolamine: contributions of muscarinic receptors near the pedunculopontine tegmental nucleus. Brain Res. 1997;775:144–155. doi: 10.1016/s0006-8993(97)00928-1. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Sims HM, Magill PJ, Bolam JP. Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J Physiol. 2008;586:2947–2960. doi: 10.1113/jphysiol.2008.153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Cozzari C, Hartman BK. Characterization of the extent of pontomesencephalic cholinergic neurons' projections to the thalamus: comparison with projections to midbrain dopaminergic groups. Neuroscience. 1999;94:529–547. doi: 10.1016/s0306-4522(99)00307-3. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J. Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Munn EM, Franklin KB, Wise RA. Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J. Neurosci. 1998;18:5035–5044. doi: 10.1523/JNEUROSCI.18-13-05035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J. Comp. Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J. Comp. Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res. Brain Res. Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Iribe Y, Tepper JM. GABAA receptor stimulation blocks NMDA-induced bursting of dopaminergic neurons in vitro by decreasing input resistance. Brain Res. 1999;832:145–151. doi: 10.1016/s0006-8993(99)01484-5. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J. Comp. Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Satoh K, Armstrong DM, Fibiger HC. A comparison of the distribution of central cholinergic neurons as demonstrated by acetylcholinesterase pharmacohistochemistry and choline acetyltransferase immunohistochemistry. Brain Res. Bull. 1983;11:693–720. doi: 10.1016/0361-9230(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J. Bio.l Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Priestley JV, Consolazione A, Eckenstein F, Cuello AC. Cholinergic projections from the midbrain and pons to the thalamus in the rat, identified by combined retrograde tracing and choline acetyltransferase immunohistochemistry. Brain Res. 1985;329:213–223. doi: 10.1016/0006-8993(85)90527-x. [DOI] [PubMed] [Google Scholar]
- Spann BM, Grofova I. Origin of ascending and spinal pathways from the nucleus tegmenti pedunculopontinus in the rat. J. Comp. Neurol. 1989;283:13–27. doi: 10.1002/cne.902830103. [DOI] [PubMed] [Google Scholar]
- Spann BM, Grofova I. Cholinergic and non-cholinergic neurons in the rat pedunculopontine tegmental nucleus. Anat. Embryol. (Berl) 1992;186:215–227. doi: 10.1007/BF00174143. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Wainer BH, Rye DB. Ultrastructural study of cholinergic and noncholinergic neurons in the pars compacta of the rat pedunculopontine tegmental nucleus. J. Comp. Neurol. 1997;382:285–301. doi: 10.1002/(sici)1096-9861(19970609)382:3<285::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J. Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin EL, Jacobowitz DM. Immunocytochemical localization of peptides and other neurochemicals in the rat laterodorsal tegmental nucleus and adjacent area. J. Comp. Neurol. 1988;270:243–270. doi: 10.1002/cne.902700206. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Shiroyama T, Yamamoto T, Kitai ST. Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J. Comp. Neurol. 1996;371:345–361. doi: 10.1002/(SICI)1096-9861(19960729)371:3<345::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2) J. Neurosci. 2001;21:RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J. Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J. Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, Satoh K, Armstrong DM, Panula P, Vale W, Fibiger HC. Neuropeptides and NADPH-diaphorase activity in the ascending cholinergic reticular system of the rat. Neuroscience. 1986;17:167–182. doi: 10.1016/0306-4522(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J. Comp. Neurol. 2008;509:302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Vincent SR, Reiner PB. Nitric oxide production in rat thalamus changes with behavioral state, local depolarization, and brainstem stimulation. J. Neurosci. 1997;17:420–427. doi: 10.1523/JNEUROSCI.17-01-00420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J. Neurol. Sci. 2006;248:234–250. doi: 10.1016/j.jns.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog. Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res. Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]