Abstract
Methods for the in vitro culture of primary small intestinal epithelium have improved greatly in recent years. A critical barrier for the translation of this methodology to the patient's bedside is the ability to grow intestinal stem cells using a well-defined extracellular matrix. Current methods rely on the use of Matrigel™, a proprietary basement membrane-enriched extracellular matrix gel produced in mice that is not approved for clinical use. We demonstrate for the first time the capacity to support the long-term in vitro growth of murine intestinal epithelium in monoculture, using type I collagen. We further demonstrate successful in vivo engraftment of enteroids co-cultured with intestinal subepithelial myofibroblasts in collagen gel. Small intestinal crypts were isolated from 6 to 10 week old transgenic enhanced green fluorescent protein (eGFP+) mice and suspended within either Matrigel or collagen gel; cultures were supported using previously reported media and growth factors. After 1 week, cultures were either lysed for DNA or RNA extraction or were implanted subcutaneously in syngeneic host mice. Quantitative real-time polymerase chain reaction (qPCR) was performed to determine expansion of the transgenic eGFP-DNA and to determine the mRNA gene expression profile. Immunohistochemistry was performed on in vitro cultures and recovered in vivo explants. Small intestinal crypts reliably expanded to form enteroids in either Matrigel or collagen in both mono- and co-cultures as confirmed by microscopy and eGFP-DNA qPCR quantification. Collagen-based cultures yielded a distinct morphology with smooth enteroids and epithelial monolayer growth at the gel surface; both enteroid and monolayer cells demonstrated reactivity to Cdx2, E-cadherin, CD10, Periodic Acid-Schiff, and lysozyme. Collagen-based enteroids were successfully subcultured in vitro, whereas pure monolayer epithelial sheets did not survive passaging. Reverse transcriptase-polymerase chain reaction demonstrated evidence of Cdx2, villin 1, mucin 2, chromogranin A, lysozyme 1, and Lgr5 expression, suggesting a fully elaborated intestinal epithelium. Additionally, collagen-based enteroids co-cultured with myofibroblasts were successfully recovered after 5 weeks of in vivo implantation, with a preserved immunophenotype. These results indicate that collagen-based techniques have the capacity to eliminate the need for Matrigel in intestinal stem cell culture. This is a critical step towards producing neo-mucosa using good manufacturing practices for clinical applications in the future.
Introduction
There has been considerable progress in our understanding of intestinal stem cell biology in recent years.1,2 Different groups have identified putative intestinal stem cells at or near the base of the intestinal crypt.3,4 Following the description of a novel method for their long-term in vitro culture,5 there have been reports of successful culture of the various mucosal epithelia of murine stomach, murine colon, human small intestine, both benign and neoplastic human colon, and human Barrett's esophagus.6–8 Previous methods for in vivo implantation relied on initial isolation of stem cell clusters surrounded by neighboring mesenchyme,9–12 whereas the current culture techniques appear to have obviated this requirement.13 In vitro cultured small intestinal units have been variously termed enteroids, spheroids, and organoids by different groups. In accordance with recently proposed nomenclature,14 we will refer to cultured intestinal epithelial units as enteroids and aggregates of enteroids co-cultured with myofibroblasts as reconstituted intestinal organoids.
The majority of investigations in the current era have employed Matrigel™, a proprietary three-dimensional (3D) extracellular matrix gel which is rich in laminin, collagen IV, and entactin.15,16 Matrigel contains several growth factors, including basic fibroblast growth factor, epidermal growth factor (EGF), insulin-like growth factor 1, transforming growth factor beta, platelet-derived growth factor, and nerve growth factor,17 which are present even in its growth-factor-reduced variant. A recent proteomic analysis found only 53% concordance in proteins from distinct growth-factor-reduced Matrigel lots.18 As it is derived from mouse sarcoma, Matrigel will provoke immune responses in other species. Taken together, these features portend poorly on its role as a matrix for future translational therapies given stringent requirements instituted by the U.S. Food and Drug Administration (FDA).19
We considered well-defined, nonimmunogenic systems and identified collagen gel as a natural alternative with several appealing properties. Collagen is readily available, can be purified, is minimally immunogenic, and has been approved by the FDA since the 1970's for several applications.20–22 Several attempts were made in the 1980's and 1990's to culture intestinal epithelium in vitro using collagen-based approaches23–25; cultured cells invariably underwent autolysis within 1 week unless crypts were harvested together with a shell of mesenchyme or co-cultured with feeder cells. The apparent requirement during that era for stromal cells or a feeder layer is not surprising, as the requisite growth factor cocktail was not described until 2009.5
We sought in this investigation to test the hypothesis that collagen gel can be used for the in vitro and in vivo growth of mouse small intestinal epithelium.
Materials and Methods
All animals used in this study were approved by the UCLA Institutional Animal Care and Use Committee, under protocol #2005-169.
Isolation of small intestinal crypts
Six to ten week old transgenic C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) with ubiquitous enhanced green fluorescent protein (eGFP) expression were used as small intestinal crypt donors. Animals were euthanized, a laparotomy was performed, and the entire small intestine was harvested from duodenum to terminal ileum. The lumen was flushed with ice-cold phosphate buffered saline (PBS) (Fisher Scientific, Pittsburgh, PA), and the intestine was cut into 5 mm fragments and washed multiple times with ice-cold PBS until the supernatant returned clear. The fragments were then incubated in 2.5 mM ethylenediaminetetraacetic acid (Sigma, St. Louis, MO) with gentle shaking at 4°C for 30 min.
The supernatant was removed, fragments were washed with 15 mL of cold PBS, and the sample was vortexed for 30 s with 3 s pulses. The fragments were allowed to settle, and the supernatant was set aside on ice as fraction 1. This process was repeated five more times for a total of six fractions. The fractions were centrifuged at 100 g for 2 min, the supernatant was removed, and each pellet was resuspended in 1 mL of cold PBS, to which 100 μL of fetal bovine serum (FBS) was added (Invitrogen, Carlsbad, CA). The fractions were then pooled and filtered first through 100 μm pore followed by 70 μm pore cell strainers (BD Biosciences, Bedford, MA). The filtrate was centrifuged again at 100 g for 2 min to yield a crypt pellet which was then resuspended in 5 mL of basic medium, consisting of advanced Dulbecco's modified Eagle medium (DMEM)/Ham's F12 (Invitrogen) with 2 mM GlutaMAX (Invitrogen), 10 mM HEPES (Invitrogen), and 1× Antibiotic-Antimycotic (Invitrogen).
Intestinal subepithelial myofibroblast isolation and culture
Intestinal subepithelial myofibroblasts (ISEMF) were isolated as previously described from syngeneic 5-day-old wild-type C57BL/6 mouse pups.7 The ISEMF were cultured using myofibroblast medium consisting of DMEM/Low Glucose/GlutaMAX (Invitrogen), 10% FBS (Invitrogen), 1× Antibiotic-Antimycotic (Invitrogen), 0.25 U/mL insulin (Sigma), 20 ng/mL recombinant murine EGF (PeproTech, Rocky Hill, NJ), and 10 μg/mL transferrin (Sigma).
Crypt culture technique
Standard formulation Matrigel (BD Biosciences) was thawed on ice, and a type I collagen gel was prepared in the following manner. Cellmatrix type I-A porcine tendon collagen (Component A; Nitta Gelatin, Inc., Osaka, Japan), was mixed with 10× Ham's F12 (Component B; Sigma) and a buffer solution of 2.2 g NaHCO3 in 100 mL of 0.05 N NaOH and 200 mM HEPES (Component C) in an 8:1:1 (A:B:C) volume ratio. Components A and B were mixed first; Component C was mixed into the gel after final crypt pellets (see below) were prepared.
The number of crypts in the final basic medium suspension was counted using a Leica DM IL microscope (Leica Microsystems, Wetzlar, Germany). Individual aliquots were microcentrifuged to yield final crypt pellets; these were suspended at a concentration of 10 crypts per μL of either Matrigel or collagen. They were then placed into 48-well Costar cell culture plates (Fisher Scientific) with 25 μL per well of either crypt-gel suspension. For co-cultures, the crypt suspension was placed onto a confluent layer of Mitomycin C (Sigma) inactivated ISEMF; in two such experiments, ISEMF were left mitotically active for subsequent in vivo implantation. Crypt-gel suspensions were allowed to solidify for 10 min in a 37°C incubator.
Once the gels were set, each well was treated with 250 μL of complete medium consisting of basic medium, 1 mM N-Acetylcysteine (Sigma), 100 ng/mL recombinant murine Noggin (PeproTech), 50 ng/mL recombinant murine EGF (PeproTech), 1× N2 supplement (Invitrogen), 1× B27 supplement (Invitrogen), and 500 ng/mL recombinant human R-spondin 1 (R&D Systems, Minneapolis, MN). Fresh culture medium was replaced every 4 days, and growth factors (Noggin, EGF, and R-spondin 1) were supplemented 2 days after each new medium delivery. Cultures were followed with inverted light microscopy on a Nikon Eclipse Ti-U (Nikon Instruments, Inc., Melville, NY). One culture was allowed to continue to grow ad libitum with media and growth factor replacement as above to determine the maximum length of in vitro viability without subculturing. Three experiments included subculturing at 1 week intervals as described below. In the remainder of experiments, cultures were terminated after 1 week and underwent fixation with 10% buffered formalin for histologic analysis, lysis with DNA or RNA stabilization buffer, or in vivo implantation.
Sub-culture technique
Three experiments were initiated in collagen gel in a 3D monoculture configuration as described above. Enteroids were subcultured at 1 week intervals without splitting, using a 1:1 passaging ratio. Cultures were treated with 20 μg/mL of type XI-S collagenase (Sigma) for 25 min until the collagen matrix was digested. The suspension was collected and microcentrifuged to yield an enteroid pellet, and the supernatant was discarded. The enteroid pellet was gently resuspended in 55% FBS (a 1:1 mixture of FBS with myofibroblast media). The spin-resuspension process was repeated once more, followed by a final spin. Enteroids were then resuspended in collagen gel and re-plated.
Monolayer-only cultures were prepared in these experiments by allowing crypts suspended in complete media to gravity sediment above a pregelled layer of collagen to confine cultures to a two dimensional (2D) initial configuration. Monolayers were passaged identically to the 3D enteroid-containing cultures and gravity sedimented above a new pre-gelled layer of collagen.
Subcutaneous implantation
Nonwoven polyglycolic acid (PGA) felt discs (Synthecon, Houston, TX) were prepared as scaffolds for in vivo implantation. Following alcohol sterilization, scaffolds were rinsed with Dulbecco's PBS (Invitrogen) and coated four times with 50 μL of 3 mg/mL PureCol® bovine collagen solution (Advanced BioMatrix, San Diego, CA) for 15 min each.
One week old 3D co-cultures were removed from their culture plates and resuspended within 50 μL of either Matrigel or Cellmatrix collagen, according to the original gel used. The resuspended co-cultures were placed into the prepared PGA scaffolds and allowed to solidify at 37°C for 10 min.
Syngeneic adult wild-type C57BL/6 mice were anesthetized with inhaled isoflurane and given a subcutaneous injection of 0.05 mg/kg buprenorphine. The abdomen was shaved, prepared, and draped sterilely. A ventral midline incision was made and skin flaps were raised laterally to create subcutaneous pockets. The PGA scaffolds were sutured to the abdominal wall using 6-0 Prolene suture (Ethicon, Somerville, NJ), and the incision was closed using 3-0 silk suture (Ethicon). Mice were sacrificed 5 weeks postoperatively, and the scaffolds were recovered and fixed in 10% buffered formalin overnight, followed by processing for histologic analysis.
Histologic analysis
In vitro cultures or recovered in vivo explants were fixed in 10% buffered formalin overnight and embedded in paraffin blocks. Serial 5 μm sections were cut; every fifth slide was stained with hematoxylin and eosin, and unstained sections were prepared for immunohistochemical staining on the Dako automated FLEX system. Slides were dewaxed with xylene and rehydrated with serial dilutions of ethanol. After treatment with antigen-retrieval and blocking solutions, sections were incubated with primary antibodies to Cdx2, E-cadherin, CD10, lysozyme, and smooth muscle actin at manufacturer-provided concentrations. Visualization was achieved with horseradish peroxidase-linked secondary antibodies and exposure to diaminobenzidine tetrahydrochloride chromogen solution. Mucin was visualized using the Periodic Acid-Schiff (PAS)-Green stain kit. All antibodies and reagents were obtained from Dako (Carpinteria, CA).
Quantitative real-time polymerase chain reaction
DNA and mRNA were isolated from 1 week old cultures with the DNeasy Blood and Tissue Kit and RNeasy Mini Kit (Qiagen, Valencia, CA). Quantitative real-time polymerase chain reaction (PCR) was performed to quantify the amount of transgenic eGFP-DNA (forward primer ACTACAACAGC CACAACGTCTATATCA, reverse primer GGCGGATCTTG AAGTTCACC, probe [6-FAM]-CCGACAAGCAGAAGAA CGGCATCA-[Tamra-Q], Eurofins MWG Operon, Huntsville, AL). mRNA samples were prepared using the Quantitect Probe RT-PCR Kit (Qiagen). Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed to determine gene expression levels in RNA samples, using Taqman Gene Expression Assays (Applied Biosystems, Carlsbad, CA) for villin 1, lysozyme 1, Cdx2, chromogranin A, mucin 2, Lgr5, and GAPDH. PCR reactions were performed on a LightCycler 480 Real-Time PCR System (Roche, Indianapolis, IN).
Statistical analysis
Two-tailed unpaired student's t-test was used to compare results, and associated p-values are reported.
Results
In vitro growth
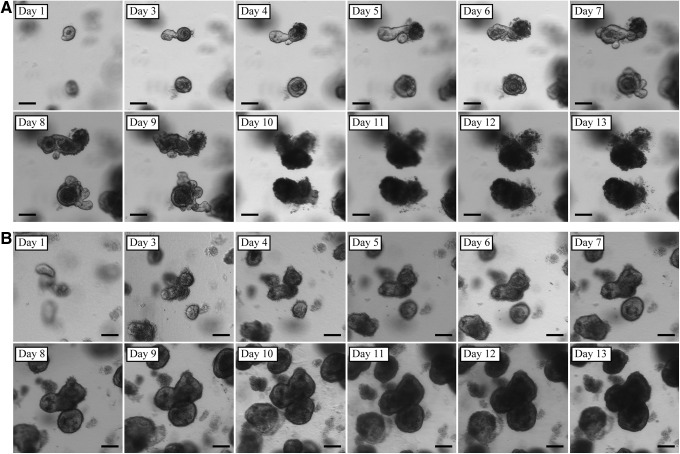
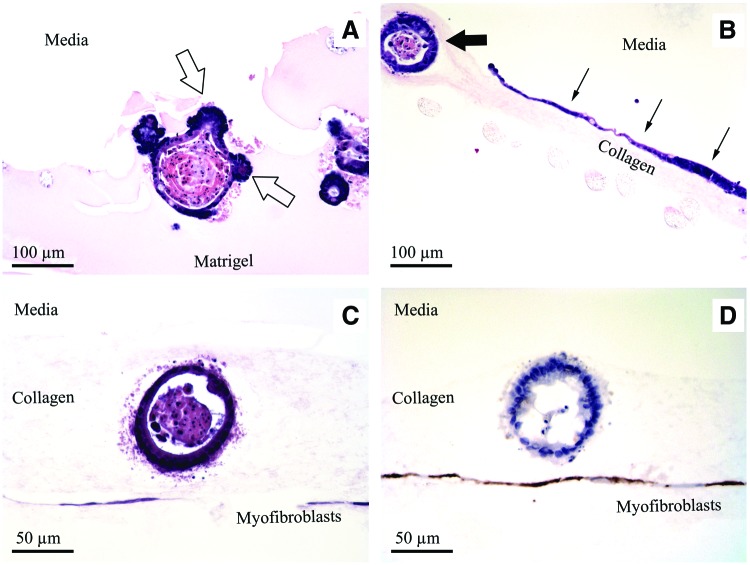
Crypts suspended in either Matrigel or collagen reliably expanded in vitro and grew to form enteroid structures. When grown within Matrigel without ISEMF (n=12), enteroid morphology exhibited the familiar crypt-like budding domains (Fig. 1A) described by Sato et al.5 Crypts suspended in collagen gel similarly expanded in vitro without ISEMF (n=11); however, they exhibited a different morphology. Rather than forming buds, collagen gel promoted the growth of smooth enteroids (Fig. 1B). In addition to the smooth enteroid morphology, there was consistent evidence of a second domain composed of a monolayer epithelial sheet growing at the collagen gel surface (Fig. 2). These observations were correlated histologically—enteroids grown in collagen (Fig. 3B) had a simple, cystic appearance with extruded cells in the lumen, and the epithelial sheet was seen as a monolayer at the gel–medium interface.
FIG. 1.
In vitro growth patterns seen with enteroids monocultured without intestinal subepithelial myofibroblasts (ISEMF) within Matrigel (A) and collagen gel (B). Scale bar is 100 μm (×40 magnification).
FIG. 2.
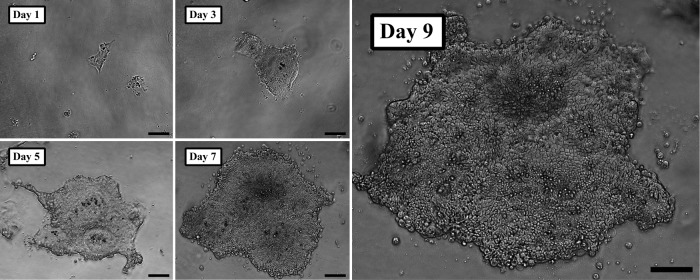
Monolayer epithelial sheets observed with in vitro monocultures using collagen gel. Monolayer sheets were observed with both two (2D) and three dimensional (3D) initial configurations (3D initial configuration illustrated here). Note the cobblestone pattern evident by day 7. Scale bar is 100 μm (×100 magnification).
FIG. 3.
Histology of intestinal epithelium after 1 week of in vitro monoculture without ISEMF (A, B) and in vitro coculture with ISEMF (C, D). Empty arrows signify crypt-like budding, seen within Matrigel (A). Thick arrow indicates typical cystic enteroid domain and thin arrows indicate sheet-like planar domain, seen with collagen gel (B). Reconstituted intestinal organoids grown in collagen gel are seen in vitro with a hematoxylin and eosin stain (C). Anti-smooth muscle actin immunohistochemical staining is evident in the ISEMF layer (D). (A, B ×200 magnification; C, D ×400 magnification). Color images available online at www.liebertpub.com/tec
Enteroids grown in collagen gel remained viable with distinct borders and continuous expansion for up to 23 days without subculturing. Co-cultures (n=6 for Matrigel, n=4 for collagen) demonstrated larger enteroids with the same respective morphologies growing over a confluent layer of ISEMF, comprising reconstituted intestinal organoids (Fig. 3C, D).
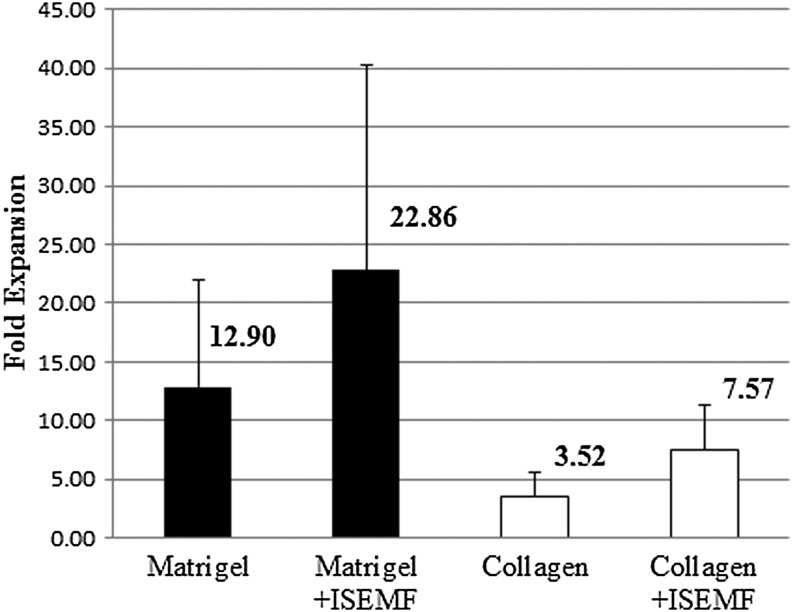
We assessed the degree of in vitro epithelial expansion by quantifying the transgenic eGFP-DNA present after 1 week normalized to the amount present at the outset of each experiment (Fig. 4). In the absence of ISEMF, Matrigel supported a 13-fold expansion of the cell mass in crypts, and collagen gel supported a 4-fold expansion (p=0.003 compared with Matrigel). When co-cultured with Mitomycin C inactivated ISEMF, Matrigel supported a 23-fold expansion, and collagen supported an 8-fold expansion (p=0.13 compared with Matrigel).
FIG. 4.
Epithelial expansion after 1 week of in vitro culture, in the absence and presence of ISEMF. Data derived from quantitative real-time polymerase chain reaction (qPCR) quantification of transgenic enhanced green fluorescent protein (eGFP) DNA. n=12 for Matrigel, n=6 for Matrigel+ISEMF, n=11 for collagen, n=4 for collagen+ISEMF. Error bars denote standard deviation. A statistically significant difference was noted between Matrigel and collagen monocultures (p=0.003).
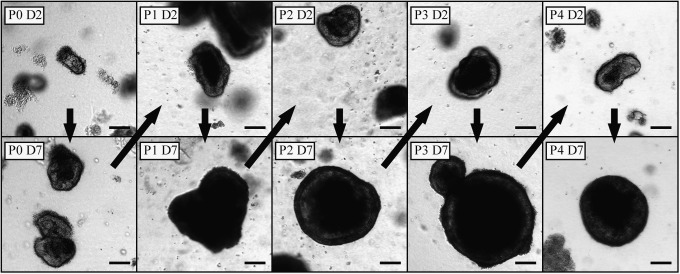
Collagen-based 3D monocultures grown without ISEMF were successfully sub-cultured (n=3, Fig. 5). They were maintained through multiple passages and consistently yielded both enteroids and monolayer epithelial sheet morphologies at all passages. While enteroids in primary culture were noted to autolyze by day 23 after harvest, subcultured enteroids remained viable at least 35 days after initial crypt harvest.
FIG. 5.
Enteroids grown in 3D collagen monoculture were successfully passaged in vitro. P, passage number; D, days since most recent passage. Scale bar is 100 μm (×40 magnification). Arrows denote passage of time.
The 2D initial configuration yielded viable monolayer-only cultures without cystic enteroids after 1 week in primary culture using collagen gel. Cells were noted to attach to the collagen substrate within hours of passaging; they uniformly underwent autolysis or detachment 1–2 days thereafter.
In vitro cell lineage differentiation
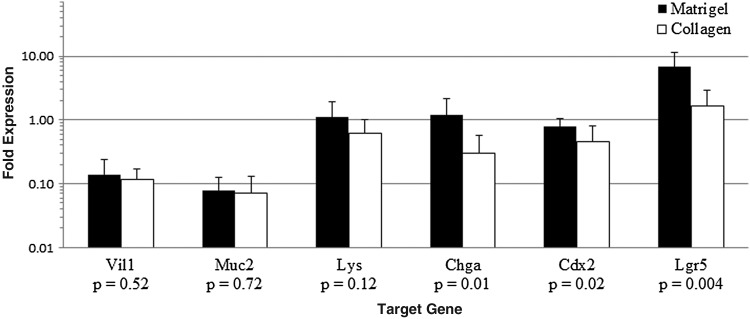
RT-PCR demonstrated expression of several target genes in both culture systems (Fig. 6). Both Matrigel and collagen-based cultures showed expression patterns characteristic of stem (Lgr5) and differentiated (Chga, Lys, Muc2, Vil1) cells of intestinal (Cdx2) origin.
FIG. 6.
Gene expression relative to murine whole bowel after 1 week of in vitro monoculture without ISEMF. RNA qPCR was performed using GAPDH as a housekeeping gene, and relative mRNA expression levels are plotted on a logarithmic scale. Statistically significant differences were noted for chromogranin A, Cdx2, and Lgr5. n=11 for Matrigel and n=10 for collagen. Error bars denote standard deviation.
Immunohistochemical and PAS stains of formalin-fixed, paraffin-embedded sections revealed evidence of cell lineage differentiation in collagen-based monocultures. There was evidence of equivalent staining in both enteroids and monolayer sheets (Fig. 7). We observed a similar pattern of staining in the co-cultures with ISEMF.
FIG. 7.
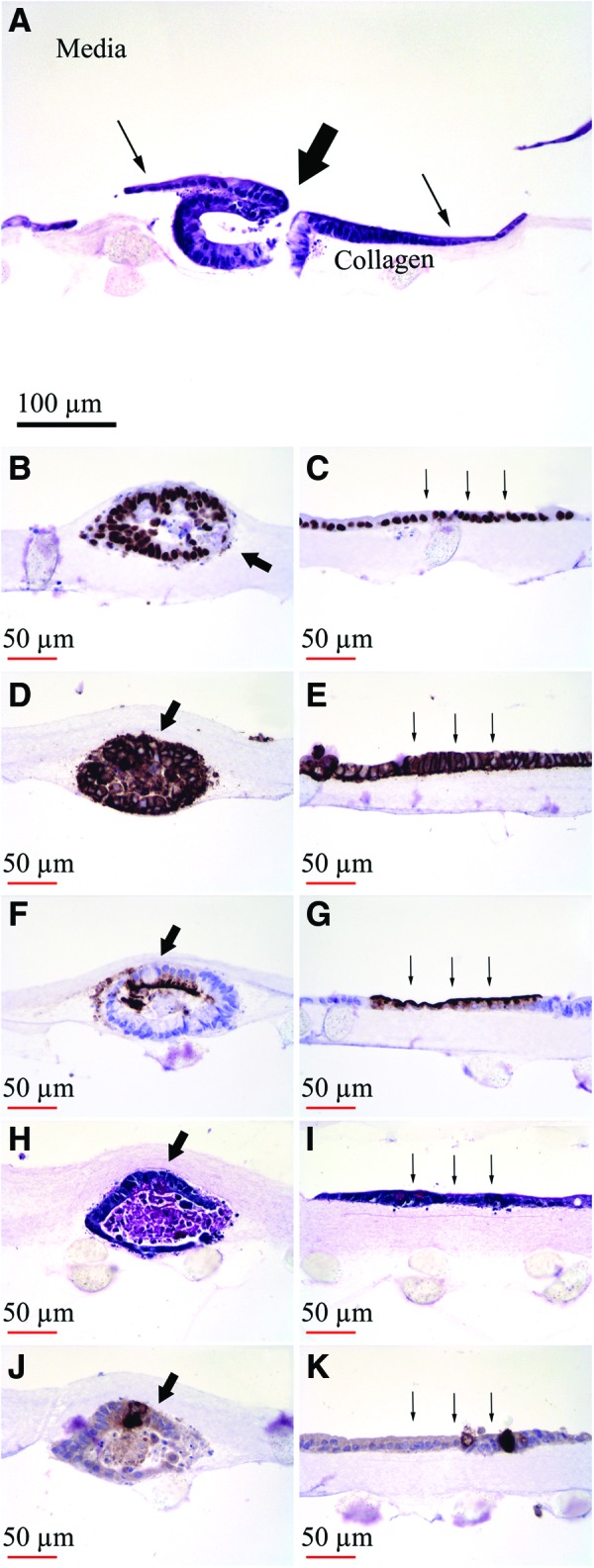
Histology of intestinal epithelium after in vitro monoculture without ISEMF in collagen gel for 1 week. Thick arrow indicates typical cystic enteroid domain and thin arrows indicate monolayer domain. (A) Hematoxylin and eosin. (B, C) Cdx2. (D, E) E-cadherin. (F, G) CD10. (H, I) Periodic Acid-Schiff (PAS). (J, K) Lysozyme. (A ×200 magnification; B–K ×400 magnification). Color images available online at www.liebertpub.com/tec
In vivo engraftment and differentiation
Co-cultures of enteroids over a confluent layer of actively dividing ISEMF were implanted subcutaneously in mice (n=2). Samples were retrieved after 5 weeks. Histologic analysis of recovered explants revealed viable reconstituted intestinal organoids with crypt-like buds and continued immunohistochemical evidence of differentiated lineages (Fig. 8).
FIG. 8.
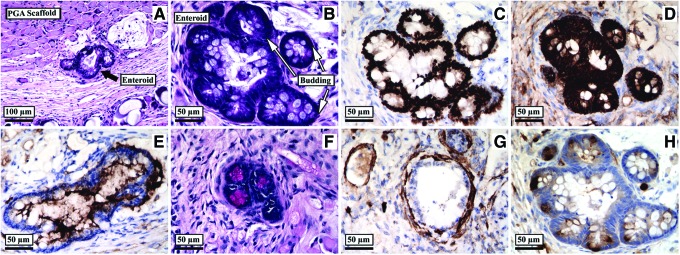
Histology of recovered reconstituted intestinal organoid after in vitro co-culture with ISEMF in collagen gel for 1 week, followed by subcutaneous implantation in vivo for 5 weeks. (A, B) Hematoxylin and eosin. (C) Cdx2. (D) E-cadherin. (E) CD10. (F) PAS. (G) Smooth muscle actin. (H) Lysozyme. (A ×200 magnification; B–H ×400 magnification). Color images available online at www.liebertpub.com/tec
Discussion
Recent advances in characterizing the intestinal stem cell niche include a rapidly growing recognition of the role of supportive cells in fostering “stemness.”26–28 The field has also benefited greatly from leveraging an increasing degree of control over intercellular signaling pathways29; in particular, use of the Wnt agonist R-spondin and the bone morphogenetic protein antagonist Noggin has ushered in a new era in intestinal stem cell biology research. The recent progress has considerable implications for intestinal tissue engineering, which in turn, has the potential for the development of as yet untold therapeutic applications. In this context, the development and characterization of a fully defined cell culture system is critically important in the field of regenerative medicine. We have endeavored in this work to develop such a system.
We have observed that collagen gel supports the long-term growth and expansion of murine small intestinal epithelium in vitro without the need for supportive cell lines. In the absence of low concentrations of growth factors, which necessarily accompany Matrigel, this observation strengthens the evidence that the growth factor cocktail defined by Sato et al.5 is in fact sufficient for the in vitro expansion of small intestinal epithelium.
There are several important differences between the phenotypes of intestinal epithelium grown in Matrigel versus collagen. In particular, enteroids grown in vitro in collagen had a smooth appearance without buds, and they continuously enlarged over a longer period of time than is allowed by the standard method using Matrigel (Fig. 1). Further, we consistently observed a monolayer epithelial sheet at the surface of the collagen gel which emerged by day 1–2 and expanded over the course of at least 9 days in primary in vitro culture (Fig. 2); we never observed a monolayer arise from crypts suspended within Matrigel. The monolayer sheet pattern was reminiscent of a cobblestone floor when viewed under the light microscope. The majority of cells grown three dimensionally in collagen were found in the enteroid pattern. This remained the case even if the crypt-collagen suspension was spread over the bottom of the well rather than placed as a droplet; however, there was a greater propensity towards growth of the monolayer with the spread arrangement. With 2D initial configurations, only monolayers were observed.
Both enteroids and monolayer sheets exhibited evidence of intestinal epithelial lineage differentiation (Fig. 7B–K). The RT-PCR results (Fig. 6) also suggested a differentiated epithelium of intestinal origin (Cdx2 expression), with elaboration of brush border containing enterocyte (Vil1), goblet (Muc2), enteroendocrine (Chga), Paneth (Lys), and stem (Lgr5) cell lineages. Matrigel- and collagen-based cultures showed statistically significant differences in Lgr5, Cdx2, and chromogranin A expression; a trend towards significance was seen with lysozyme. These genes are all most strongly expressed in the lower crypt in the native intestine. Their relatively lower expression in collagen-based cultures compared with Matrigel correlates to the absence of crypt-like buds seen in collagen.
The recovered in vivo explants contained viable reconstituted intestinal organoids after a total of 6 weeks following initial crypt harvest (1 week in vitro followed by 5 weeks in vivo during which no exogenous growth factors were delivered), providing strong evidence of the recapitulation of an autonomous experimental stem cell niche. Our implantation scheme relies on co-culture with myofibroblasts. This limitation is not unique to the collagen system; we have observed that both murine (unpublished results) and human7 crypts grown in Matrigel require myofibroblasts for successful subcutaneous in vivo engraftment.
Other investigators have described the use of collagen gels in intestinal epithelial culture applications. Ootani et al. cultured murine small intestine and colon in Cellmatrix collagen, beginning with minced whole intestinal fragments.30 Yui et al. recently reported the use of type I collagen gel for primary culture of colonic stem cells.13 Another recent report describes the use of basement-membrane-enriched collagen gel for the in vitro culture of immortalized HCT-8 cells in the presence of fibroblasts, endothelial cells, and lymphocytes.31 Several groups have reported the capacity to grow Caco-2 cells using collagen gel.32–34 The results reported herein are novel. They are the first demonstration of the long-term in vitro growth of nontransformed small intestinal crypts using a completely defined cell culture system, to which the addition of ISEMF allows for in vivo applications.
The mechanisms behind the smooth enteroid appearance as well as the emergence of the monolayer sheet in collagen remain unknown. Interestingly, the in vivo morphology was characterized by budding structures, whereas this was never observed in vitro. The reasons for this are unclear but may relate to the distinct microenvironment in the animal host. It is also unclear if the Matrigel phenotype can be recaptured by exposing the cells to specific basement membrane constituents, such as collagen IV and laminin, or whether the differences are attributable to biomechanical properties of collagen gel. Further investigation is warranted to address these questions.
In primary and passaged 3D cultures, all monolayers were closely associated with enteroids near the surface of the gel (seen out of the plane of focus in Fig. 2). It is likely, though still unproven, that the stem cells responsible for monolayer expansion reside within the enteroids rather than within the monolayer itself in the 3D configuration. Monolayer-only cultures did not survive passaging, suggesting that they were populated in the 2D configuration by transit-amplifying or other short-lived predifferentiated cells. On the other hand, subcultured enteroids continued to grow in vitro (Fig. 5) and yielded both further enteroids and monolayer sheets using defined reagents without a feeder cell layer.
The differential morphology-specific behavior of collagen-based epithelium may be related to the distinct local tissue curvature within enteroids compared with flat sheets.35 Future research should address this hypothesis and will carry significant design implications in searching for the optimal system. Nonetheless, our results suggest that the 3D collagen culture is a fully defined system for supporting long-term in vitro intestinal stem cell growth and differentiation.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases U01 Intestinal Stem Cell Consortium (DK085535-01 and DK085535-02S2), DK083762, and DK083319 and the National Institute of Allergy and Infectious Diseases. We sincerely thank Renee Bowers (UCLA) and Nazlin Sharif (West Los Angeles, VA) for their expert consultation and generous technical assistance with all elements of histology work.
Disclosure Statement
No competing financial interests exist.
References
- 1.van der Flier L.G. Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 2.Neal M.D. Richardson W.M. Sodhi C.P. Russo A. Hackam D.J. Intestinal stem cells and their roles during mucosal injury and repair. J Surg Res. 2011;167:1. doi: 10.1016/j.jss.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N. van Es J.H. Kuipers J. Kujala P. van den Born M. Cozijnsen M. Haegebarth A. Korving J. Begthel H. Peters P.J. Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 4.Potten C.S. Gandara R. Mahida Y.R. Loeffler M. Wright N.A. The stem cells of small intestinal crypts: where are they? Cell Prolif. 2009;42:731. doi: 10.1111/j.1365-2184.2009.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato T. Vries R.G. Snippert H.J. van de Wetering M. Barker N. Stange D.E. van Es J.H. Abo A. Kujala P. Peters P.J. Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 6.Barker N. Huch M. Kujala P. van de Wetering M. Snippert H.J. van Es J.H. Sato T. Strange D.E. Begthel H. van den Born M. Danenberg E. van den Brink S. Korving J. Abo A. Peters P.J. Wright N. Poulsom R. Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Lahar N. Lei N.Y. Wang J. Jabaji Z. Tung S.C. Joshi V. Lewis M. Stelzner M. Martín M.G. Dunn J.C.Y. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One. 2011;6:e26898. doi: 10.1371/journal.pone.0026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T. Stange D.E. Ferrante M. Vries R.G. van Es J.H. van den Brink S. van Houdt W.J. Pronk A. van Gorp J. Siersema P.D. Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Avansino J.R. Chen D.C. Hoagland V.D. Woolman J.D. Haigh W.G. Stelzner M. Treatment of bile acid malabsorption using ileal stem cell transplantation. J Am Coll Surg. 2005;201:710. doi: 10.1016/j.jamcollsurg.2005.06.270. [DOI] [PubMed] [Google Scholar]
- 10.Avansino J.R. Chen D.C. Hoagland V.D. Woolman J.D. Stelzner M. Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery. 2006;140:423. doi: 10.1016/j.surg.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen D.C. Agopian V.G. Avansino J.R. Lee J.K. Farley S.M. Stelzner M. Optical tissue window: a novel model for optimizing engraftment of intestinal stem cell organoids. J Surg Res. 2006;134:52. doi: 10.1016/j.jss.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Agopian V.G. Chen D.C. Avansino J.R. Stelzner M. Intestinal stem cell organoid transplantation generates neomucosa in dogs. J Gastrointest Surg. 2009;13:971. doi: 10.1007/s11605-009-0806-x. [DOI] [PubMed] [Google Scholar]
- 13.Yui S. Nakamura T. Sato T. Nemoto Y. Mizutani T. Zheng X. Ichinose S. Nagaishi T. Okamoto R. Tsuchiya K. Clevers H. Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 14.Stelzner M. Helmrath M. Dunn J.C.Y. Henning S.J. Houchen C.W. Kuo C. Lynch J. Li L. Magness S.T. Martín M.G. Wong M.H. Yu J. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1359. doi: 10.1152/ajpgi.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinman H.K. McGarvey M.L. Liotta L.a. Robey P.G. Tryggvason K. Martin G.R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 16.Kleinman H.K. McGarvey M.L. Hassell J.R. Star V.L. Cannon F.B. Laurie G.W. Martin G.R. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 17.Vukicevic S. Kleinman H.K. Luyten F.P. Roberts A.B. Roche N.S. Reddi A.H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 18.Hughes C.S. Postovit L.M. Lajoie G.A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 19.Lee M.H. Arcidiacono J.A. Bilek A.M. Wille J.J. Hamill C.A. Wonnacott K.M. Wells M.A. Oh S.S. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng Part B Rev. 2010;16:41. doi: 10.1089/ten.TEB.2009.0449. [DOI] [PubMed] [Google Scholar]
- 20.Ramshaw J.A. Werkmeister J.A. Glattauer V. Collagen-based biomaterials. Biotechnol Genet Eng Rev. 1996;13:335. doi: 10.1080/02648725.1996.10647934. [DOI] [PubMed] [Google Scholar]
- 21.Pachence J.M. Collagen-based devices for soft tissue repair. J Biomed Mater Res. 1996;33:35. doi: 10.1002/(SICI)1097-4636(199621)33:1<35::AID-JBM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 22.Matarasso S.L. The use of injectable collagens for aesthetic rejuvenation. Semin Cutan Med Surg. 2006;25:151. doi: 10.1016/j.sder.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Kaeffer B. Mammalian intestinal epithelial cells in primary culture: a mini-review. In Vitro Cell Dev Biol Anim. 2002;38:123. doi: 10.1290/1071-2690(2002)038<0123:MIECIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Wildrick D.M. Lointier P. Nichols D.H. Roll R. Quintanilla B. Boman B.M. Isolation of normal human colonic mucosa: comparison of methods. In Vitro Cell Dev Biol Anim. 1997;33:18. doi: 10.1007/s11626-997-0017-2. [DOI] [PubMed] [Google Scholar]
- 25.Whitehead R.H. Brown A. Bhathal P.S. A method for the isolation and culture of human colonic crypts in collagen gels. In Vitro Cell Dev Biol. 1987;23:436. doi: 10.1007/BF02623860. [DOI] [PubMed] [Google Scholar]
- 26.Yeung T.M. Chia L.A. Kosinski C.M. Kuo C.J. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci. 2011;68:2513. doi: 10.1007/s00018-011-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T. van Es J.H. Snippert H.J. Stange D.E. Vries R.G. van den Born M. Barker N. Shroyer N.F. van de Wetering M. Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell D.W. Mifflin R.C. Valentich J.D. Crowe S.E. Saada J.I. West A.B. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277(2 Pt 1):C183. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 29.Farin H.F. Van Es J.H. Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of paneth cells. Gastroenterology. 2012;143:1518. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Ootani A. Li X. Sangiorgi E. Ho Q.T. Ueno H. Toda S. Sugihara H. Fujimoto K. Weissman I.L. Capecchi M.R. Kuo K.J. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salerno-Goncalves R. Fasano A. Sztein M.B. Engineering of a multicellular organotypic model of the human intestinal mucosa. Gastroenterology. 2011;141:e18. doi: 10.1053/j.gastro.2011.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J. Peng S. Luo D. March J.C. In vitro 3D human small intestinal villous model for drug permeability determination. Biotechnol Bioeng. 2012;109:2173. doi: 10.1002/bit.24518. [DOI] [PubMed] [Google Scholar]
- 33.Takezawa T. Ozaki K. Nitani A. Takabayashi C. Shimo-Oka T. Collagen vitrigel: a novel scaffold that can facilitate a three-dimensional culture for reconstructing organoids. Cell Transplant. 2004;13:463. doi: 10.3727/000000004783983882. [DOI] [PubMed] [Google Scholar]
- 34.Sung J.H. Yu J. Luo D. Shuler M.L. March J.C. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip. 2011;11:389. doi: 10.1039/c0lc00273a. [DOI] [PubMed] [Google Scholar]
- 35.Buske P. Przybilla J. Loeffler M. Sachs N. Sato T. Clevers H. Galle J. On the biomechanics of stem cell niche formation in the gut - modelling growing organoids. FEBS J. 2012;279:3475. doi: 10.1111/j.1742-4658.2012.08646.x. [DOI] [PubMed] [Google Scholar]