Abstract
In this study, the protective effect of sweet potato extract against hydrogen peroxide-induced oxidative stress and cytotoxicity on the pheochromocytoma cell line (PC12) was investigated. The active component of the sweet potato extract was purified and determined to be 2,4-di-tert-butylphenol. The antioxidant capacity of 2,4-di-tert-butylphenol was measured by using 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) radical. To examine the effects of 2,4-di-tert-butylphenol on amyloid-beta peptide (Aβ1-42)-induced learning and memory impairment in mice, in vivo behavioral tests were performed. Administration of 2,4-di-tert-butylphenol increased alternation behavior in mice injected with Aβ1-42. These results suggest that sweet potato extract could be protective against Aβ-induced neurotoxicity, possibly due to the antioxidative capacity of its constituent, 2,4-di-tert-butylphenol.
Key Words: Alzheimer's disease, amyloid beta peptide, oxidative stress, PC12 cells
Introduction
Oxidative stress has been linked to a variety of neurodegenerative disorders such as Alzheimer's disease (AD).1 AD is a progressive brain degenerative disorder that is characterized by neuronal loss, neurofibrillary tangles, and abnormal deposition of senile plaque and amyloid beta peptide (Aβ).2 Aβ overproduction leads to the intracellular accumulation of reactive oxygen species (ROS), which eventually results in peroxidation of the cell membrane, modification of proteins, DNA/RNA damage, and cell death.3–5
Natural antioxidants have been reported to block oxidative stress induced by free radicals and by ROS. The use of natural products to treat chronic diseases, particularly those that require long-term treatment, is gaining popularity.6,7 Many phytochemicals are recognized to be safe and have been shown to possess potential health-promoting and therapeutic effects that include boosting the immune system, detoxifying the body, and providing nutritional benefits.8 The role of phytochemicals in disease prevention has been attributed to the antioxidant properties of their phenolic compounds.9–12 Thus, antioxidants may slow cognitive decline and the progression of AD.13–15
In our previous experiments, sweet potato extract exhibited potent inhibitory activity against oxidative stress in both pheochromocytoma (PC12) cells and Aβ1-42-injected imprinting control region (ICR) mice. Thus, the sweet potato extract effectively reversed the deleterious effects of the oxidative damage in the in vitro and in vivo models. Moreover, biochemical experiments using brain tissues showed lowered oxidative stress levels.16 However, the protectant compound has not been identified. In this study, we sought to identify the active component of the sweet potato extract. The active component was purified sequentially through silica gel column chromatography and high-performance liquid chromatography (HPLC). To obtain the chemical structure of the active component, electron ionization mass spectrometry (EI-MS) and 13C/1H- nuclear magnetic resonance (NMR) were performed. The active component was identified as 2,4-di-tert-butylphenol.
In our previous study, pomegranate extract, which contains 2,4-di-tert-butylphenol, was shown to possess antioxidant and neuronal protective effects. The pomegranate ethanol extract mitigated hydrogen peroxide (H2O2)-induced oxidative stress in PC12 cells. To examine the effects of pomegranate extract on Aβ1-42-induced learning and memory impairment in mice, in vivo behavioral tests were performed. Treatment with pomegranate extract increased step-through latency in mice injected with Aβ1-42.17 This protective effect against learning and memory impairment induced by Aβ1-42 might be due to the antioxidant capacity of the 2,4-di-tert-butylphenol.
In this study, ABTS radical assay was used to confirm the antioxidant capacity of 2,4-di-tert-butylphenol. The vitamin C-equivalent antioxidant capacity (VCEAC) was also calculated. Finally, the protective effect of 2,4-di-tert-butylphenol against oxidative stress-induced learning and memory impairment was measured in Aβ1-42-injected ICR mice.
Materials and Methods
Chemicals
H2O2, amyloid beta peptide (Aβ1-42), dimethyl sulfoxide (DMSO), 2′,7′-dichlorofluorescein diacetate (DCF-DA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS2−), 2,4-di-tert-butylphenol, and ascorbic acid were purchased from Sigma Co. (St. Louis, MO, USA). 2,2′-azobis-(2-amidinopropane) dihydrochloride (AAPH) was purchased from Wako Chemicals USA, Inc. (Richmond, VA, USA). Aβ42-1 was purchased from BACHEM (Bubendorf, Switzerland). The RPMI 1640 medium, horse serum from a donor herd, fetal bovine serum, and antibiotic–antimycotic were purchased from Gibco-BRL™ (Grand Island, NY, USA).
Extraction
Dried sample was placed in a flask with five volumes of ethanol. Sample was incubated on a shaker for 24 h at 1.57 g and then filtered through a filter paper No. 42 (Whatman International Ltd., Maidstone, United Kingdom). The sample residue was collected, and the above-mentioned extraction process was repeated five times. The supernatant of the ethanol extract was separated from the residual sample material, and then concentrated in a rotary evaporator (EYELA, Tokyo, Japan) under reduced pressure at 37°C.
Isolation of 2,4-di-tert-butylphenol
The sweet potato extract (214 g) was dissolved in distilled water and was sequentially partitioned using n-hexane, chloroform, and ethyl acetate, three times in each. Among the nine fractions obtained from the solvent partitioning, the first chloroform fraction (1.2 g) was analyzed by using thin layer chromatography (TLC). The first chloroform fraction was evaporated and then dissolved in absolute ethanol (400 mg/mL), followed by separation on the silica gel TLC plate (30×30 cm; Merck, Darmstadt, Germany). To isolate the active compound from the band (Rf value=0.28) of the preparative TLC, the HPLC system coupled with the 2996 photodiode array detector was utilized (Waters 2690 apparatus, Milford, MA, USA). A C18 μ-Bondapak™ column (reverse-phase column, size: 3.9×150 mm) was employed at 23°C, with a flow rate of 0.5 mL/min and wavelength of 200–800 nm. Separation was conducted using a 95-min linear gradient of 0–100% ethanol (Merck).
Cell cultures
The rat PC12 cell line was obtained from American Type Culture Collection (ATCC, CRL-1721) and was cultured in the RPMI 1640 medium supplemented with 10% horse serum (v/v), 5% fetal bovine serum (v/v), and 1% antibiotic–antimycotic (v/v). The cells were cultured in 100-mm tissue culture dishes (Falcon™; DB Biosciences, Franklin Lakes, NJ, USA) and maintained in a water-saturated incubator at 37°C with 5% CO2 atmosphere. The cells were subcultured when a confluency of 80–90% (split ratio 1:4) was reached. The medium was replaced approximately three times a week.
Measurement of cellular oxidative stress
Cellular oxidative stress, as indicated by ROS levels, was measured by using a DCF-DA assay. The PC12 cells were pretreated with the sample for 48 h and then exposed to H2O2 for 2 h. At the end of the treatment, the cells were incubated with 50 μM DCF-DA for 50 min, and DCF was quantified with a fluorometer (GENios, Tecan Ltd., Männedorf, Switzerland) using 485 nm excitation and 535 nm emission filters. The results are given as a percent relative to the oxidative stress of the control cells, which were set to 100%.18
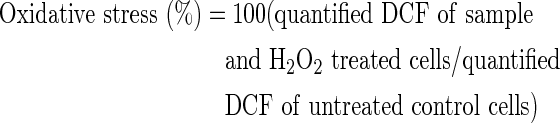 |
Measurement of cell viability
Cell viability was evaluated using MTT reduction. Cells were preincubated with the sample for 48 h before H2O2 was added. They were then treated with H2O2 for 2 h. MTT reduction was initiated by adding 10 μL of MTT stock solution (2.5 mg/mL) per well, and the plates were incubated at 37°C. After a 3-h incubation, the reaction was stopped by adding 150 μL of DMSO. The absorbance was measured at 570 nm. The reference wavelength was then determined at 630 nm using a microplate reader (Model 550; BIO-RAD Laboratories, Hercules, CA, USA).18
VCEAC assay using ABTS radicals
ABTS radical anion was used as described previously.19 Briefly, 1.0 mM AAPH was mixed with 2.5 mM ABTS2− in phosphate-buffered saline (pH 7.4). The mixed solution was heated in a water bath at 70°C for 30 min. The resulting ABTS solution was diluted to absorbance of 0.650±0.020 at 734 nm. Twenty microliters of the sample was mixed with 980 μL of the ABTS radical solution. The mixture was incubated in a water bath at 37°C for 10 min. The decrease of absorbance at 734 nm was measured at the end of 10 min.
Animals
The ICR mice (5-week-old males) were purchased from DBL (Eumseong, Korea) and behavioral studies were performed to assess whether the Aβ1-42-induced neurotoxicity could be mitigated by supplementing the diet with 2,4-di-tert-butylphenol. The mice were housed in a room maintained at 23°C±2°C with a 12-h light/12-h dark cycle and fed a commercial diet (Purina Korea, Seoul, Korea) supplemented with 2,4-di-tert-butylphenol for 4 weeks ad libitum. The 2,4-di-tert-butylphenol was mixed with the commercial diet at concentrations of 5, 10, 20, and 40 mg/kg of body weight per day. Subsequently, Aβ1-42 was administered by means of intracerebroventricular (ICV) injection. The control group was injected with the nontoxic reverse fragment, Aβ42-1, and the Aβ group was injected with 410 pmol of Aβ1-42 per mouse. For each injection, the Aβ was dissolved in a 0.85% (v/v) sodium chloride solution. Each mouse was injected at the bregma with a Hamilton microsyringe (depth: 2.5 mm, injection volume: 5 μL, dose: 410 pmol/mouse). The sample groups (Bp5, Bp10, Bp20, and Bp40) were injected with Aβ1-42 after their diets were supplemented with 2,4-di-tert-butylphenol.
Y-maze test
A Y-maze test was carried out 3 days after Aβ injection. The maze was made of black plastic, and each arm of the maze was 33 cm long, 15 cm high, 10 cm wide, and positioned such that the arms were equidistant from each other. Each mouse was placed at the end of one arm and allowed to move freely through the maze during an 8-min period. The sequence of arm entries was recorded manually. Possible alternation was defined as entry into all three arms consecutively by choice in overlapping triplet sets. The percentage of spontaneous alternation behavior was calculated as the ratio of actual to possible alternations.
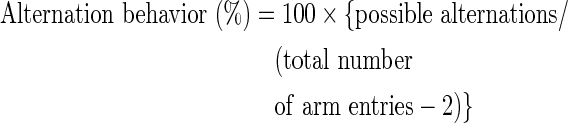 |
Passive avoidance test
The passive avoidance test was carried out 7 days after the Aβ injection. The apparatus consisted of an illuminated chamber connected to a dark chamber. On the first day, an acquisition trial was performed. Each mouse was placed in the apparatus and left for a 1-min period with no light or shock followed by a 2-min period with light and no shock to habituate the mice to the apparatus. Subsequently, the mice were individually placed in the illuminated chamber. Immediately after entering the dark chamber, an inescapable scrambled electric shock (0.5 mA, 1 sec) was delivered through the floor grid. The mice were then returned to their cages. Twenty-four hours later, each mouse was again placed in the illuminated chamber (retention trial). The interval between placement into the illuminated chamber and entry into the dark chamber was measured as the step-through latency. The maximum testing limit for step-through latency was 300 sec.
Statistical analysis
Data are expressed as mean±SD. The data were analyzed by using one-way ANOVA followed by the post hoc Tukey's multiple comparison test.
Results
Measurement of cellular oxidative stress
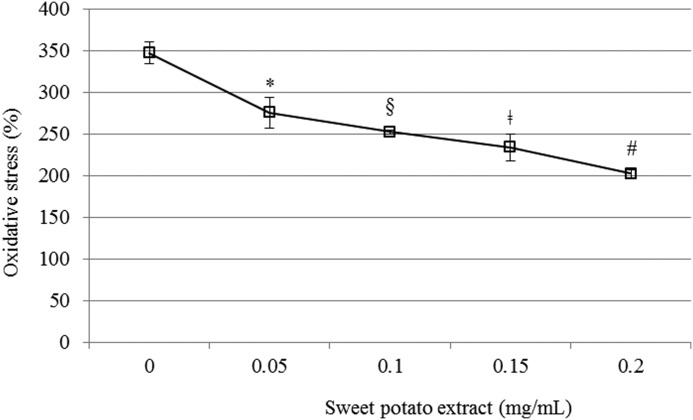
To evaluate the effect of sweet potato extract on the oxidative stress levels in PC12 cells, ROS levels were measured by using DCF-DA assay. The intracellular ROS levels resulting from H2O2 treatment were significantly lower in cells that were pretreated with the sweet potato extract, than those in the H2O2-treated cells. In addition, the pretreated cells showed a dose-dependent decrease in ROS levels (Fig. 1). As expected, the cells exposed to H2O2 alone, exhibited a drastic increase (250%) in ROS levels.
FIG. 1.
Effect of sweet potato extract on oxidative stress in PC12. All groups were treated with 100 μM H2O2. Sample groups were preincubated with the extract (0.05, 0.1, 0.15, or 0.2 mg/mL) for 48 h before H2O2 treatment. Data represented as mean±SD (n=4). *, §, ‡, #P<.01 versus control group. H2O2, hydrogen peroxide.
Measurement of cell viability
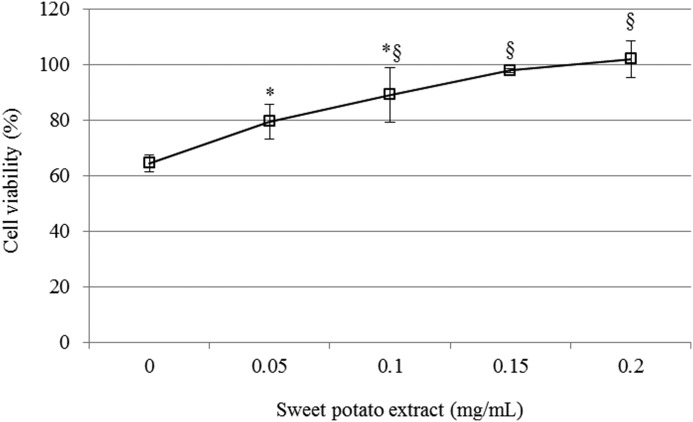
To evaluate the protective effect of the extract against H2O2-induced neurotoxicity, a MTT reduction assay was used to measure the cell viability. As shown in Figure 2, cell viability diminished due to H2O2 exposure (60%). The data show that the cytotoxicity reduction was proportional to the extract concentration. These data clearly demonstrate the potent protective effect of the extract against H2O2.
FIG. 2.
Protective effect of sweet potato extract against H2O2-induced neurotoxicity. All groups were treated with 100 μM H2O2. Sample groups were preincubated with the extract (0.05, 0.1, 0.15, 0.2 mg/mL) for 48 h before H2O2 treatment. Data represented as mean±SD (n=4). *, §P<.01 versus control group.
Isolation of 2,4-di-tert-butylphenol from sweet potato
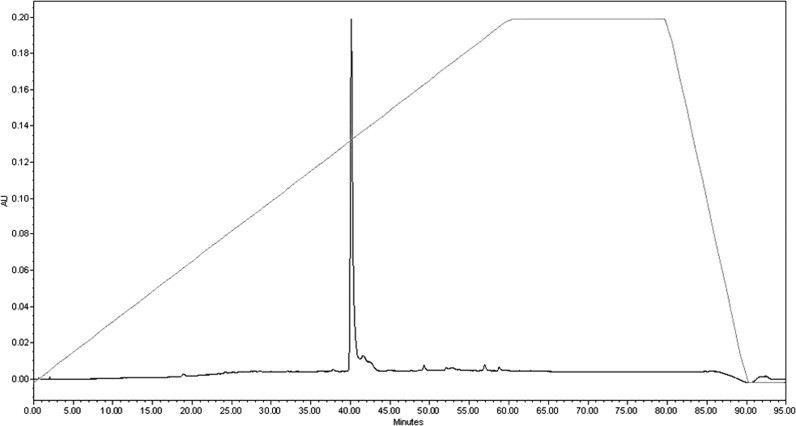
Solvent partitioning followed by TLC and HPLC were used to separate the active compound from the sweet potato extract. The extract was first fractioned using three solvents (n-hexane, chloroform, and ethyl acetate) sequentially. The first chloroform fraction was further separated using TLC. The separated band (Rf=0.28) showed the strongest effect. The selected band was extracted and evaporated under reduced pressure at 39°C, and the obtained sample was dissolved in ethanol and analyzed with HPLC using a C18 μ-bondapak™ column. The data were monitored over the range 200–800 nm, and the detection wavelength was 251.2 nm. A significant peak appeared at 40 min (Fig. 3). The sample collected using HPLC was dissolved in ethanol. In the EI-MS, the molecular weight of the putative active component was 206 m/z. This peak was then analyzed by EI-MS and 13C/1H-NMR, and the active compound was finally identified as 2,4-di-tert-butylphenol.
FIG. 3.
Isolation of 2,4-di-tert-butylphenol from sweet potato extract using HPLC. A C18 μ-Bondapak column (reverse-phase column; 3.9×150 mm) was used at a flow rate of 0.5 mL/min and a wavelength of 200–800 nm. The monitoring wavelength was 251.2 nm. HPLC, high-performance liquid chromatography.
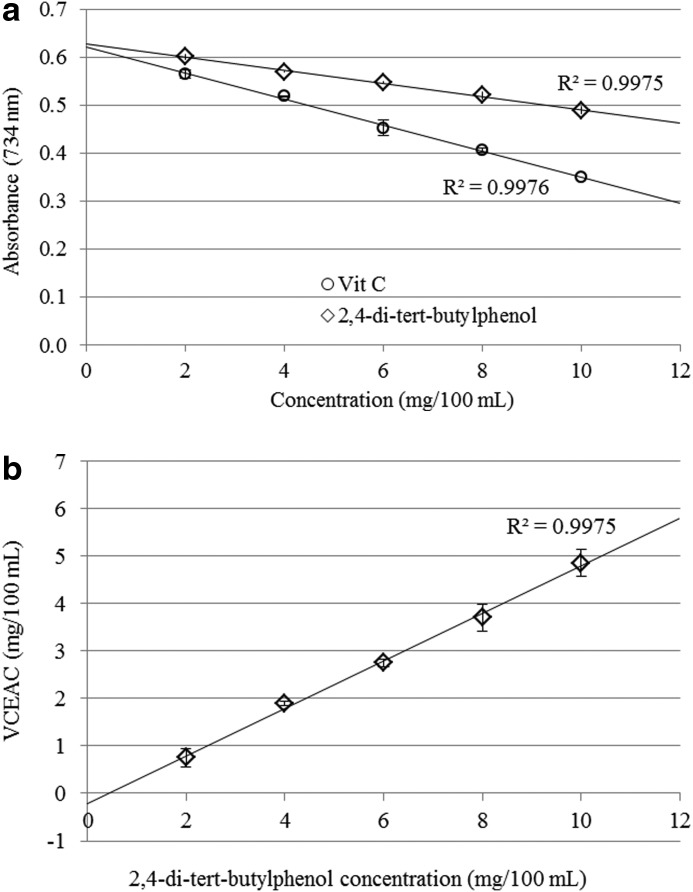
Vitamin C equivalent antioxidant capacity
The antioxidant potential of the 2,4-di-tert-butylphenol was evaluated by using ABTS radical anions. The VCEAC standard curve was calculated by using the amount of absorbance reduction and concentration of vitamin C. 2,4-Di-tert-butylphenol and vitamin C scavenged ABTS radical anions in a dose-dependent manner (Fig. 4a). Antioxidant capacity of 2,4-di-tert-butylphenol was converted to VCEAC on a weight basis. As shown in Figure 4b, antioxidant capacity 2,4-di-tert-butylphenol is half of that of vitamin C. The correlation between 2,4-di-tert-butylphenol and VCEAC was calculated (R2=0.9975).
FIG. 4.
Antioxidant potential of 2,4-di-tert-butylphenol. (a) Relationship between 2,4-di-tert-butylphenol, vitamin C, and absorbance reduction of the ABTS radical at 734 nm. (b) Correlation between 2,4-di-tert-butylphenol and vitamin C-equivalent antioxidant capacity (VCEAC). Values represented as mean±SD (n=4).
Y-maze test
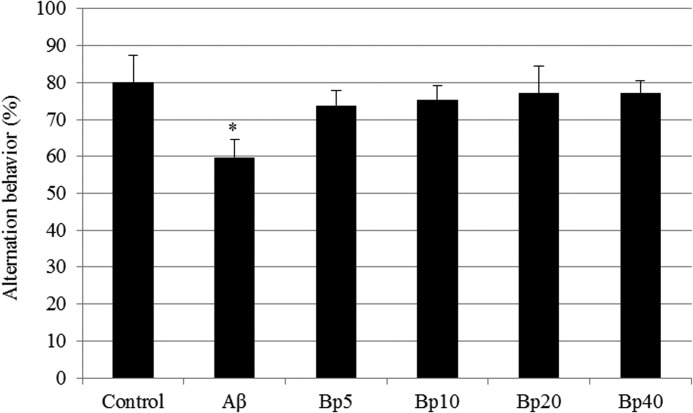
The Y-maze test was carried out 3 days after the Aβ injection. The control group was injected with the nontoxic reverse fragment, Aβ42-1. The Aβ group was injected with 410 pmol of Aβ1-42 per mouse. The Aβ1-42-injected mice (group Aβ) exhibited a significantly impaired spatial working memory (20% decrease in alternation behavior) as compared to that of the control group. Administration of diet supplemented with 2,4-di-tert-butylphenol increased the spontaneous alternation behavior in the Aβ1-42-injected mice (Fig. 5). In contrast, the number of arm entries and brain weight did not change among all the experimental groups (Table 1).
FIG. 5.
Effect of 2,4-di-tert-butylphenol on mice subjected to the Y-maze test. Control, injected with 410 pmol of Aβ42-1; Aβ, injected with 410 pmol of Aβ1-42 per mouse; BP5, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (5 mg/kg of body weight per day); BP10, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (10 mg/kg of body weight per day); BP20, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (20 mg/kg of body weight per day); BP40, injected with 410 pmol of Aβ1-42 per mouse and few with 2,4-di-tert-butylphenol diet (40 mg/kg of body weight per day). Values represented as mean±SD (n=8). *P<.01 versus control group. Aβ, amyloid beta peptide.
Table 1.
Effect of 2,4-Di-tert-butylphenol Diet on Total Arm Entries and Brain Weight
| Total arm entries | Brain weight (g) | |
|---|---|---|
| Control | 0.47±0.01 | 0.47±0.01 |
| Aβ | 0.46±0.02 | 0.46±0.02 |
| BP5 | 0.46±0.03 | 0.46±0.03 |
| BP10 | 0.48±0.01 | 0.48±0.01 |
| BP20 | 0.47±0.02 | 0.47±0.02 |
| BP40 | 0.47±0.02 | 0.47±0.02 |
Values represent the mean±SD (n=8). Total arm entries and brain weight did not change significantly. P<.01 versus control group.
Control, injected with 410 pmol of Aβ42-1; Aβ, injected with 410 pmol of Aβ1-42 per mouse; BP5, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (5 mg/kg of body weight per day); BP10, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (10 mg/kg of body weight per day); BP20, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (20 mg/kg of body weight per day); BP40, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (40 mg/kg of body weight per day).
Passive avoidance test
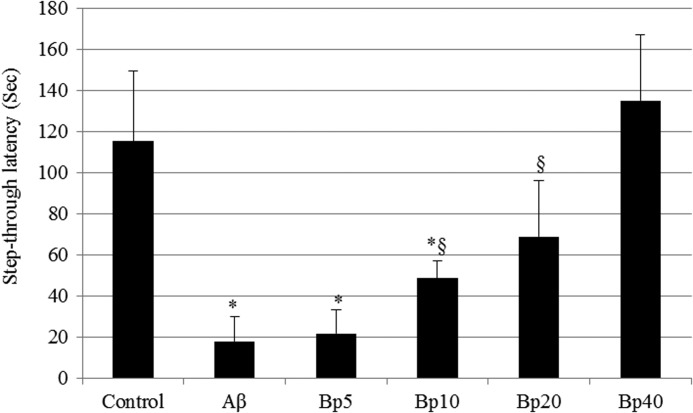
The passive avoidance test was carried out 7 days after Aβ injection. The Aβ1-42-injected mice displayed a significant reduction in the step-through latency time (98-sec decrease) as compared to that of the control group. Thus, the 2,4-di-tert-butylphenol diet attenuated the Aβ1-42-induced impairment of mice in the passive avoidance test (Fig. 6).
FIG. 6.
Effect of 2,4-di-tert-butylphenol on step-through latency in the passive avoidance test. Control, injected with 410 pmol of Aβ42-1; Aβ, injected with 410 pmol of Aβ1-42 per mouse; BP5, injected with 410 pmol of Aβ1-42 per mouse and 2,4-di-tert-butylphenol diet (5 mg/kg of body weight per day); BP10, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (10 mg/kg of body weight per day); BP20, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (20 mg/kg of body weight per day); BP40, injected with 410 pmol of Aβ1-42 per mouse and fed with 2,4-di-tert-butylphenol diet (40 mg/kg of body weight per day). Values represented as mean±SD (n=8). *, §P<.01 versus control group.
Discussion
Amyloid β accumulation in the brain is a pathological hallmark of AD and promotes the disease progression.20 In AD, Aβ is one of the major factors causing activation, whereas the ROS produced by activated microglial cells plays a critical role in the AD pathogenesis. Some phytochemicals have been reported to prevent hydroxyl radical-induced apoptosis in cultured neurons and to decrease oxidative stress.7,21,22 In vitro studies using cell cultures have demonstrated the neurotoxic and the apoptotic effects of ROS in brain regions affected by AD.23,24
Phenolic compounds are commonly found in plants. The potential protective role of phenolic compounds against oxidative damage-induced diseases that can be provided by the consumption of fruits, vegetables, and herbs has drawn considerable research interest. The importance of antioxidant activity of phenolic compounds and their possible use in processed foods as a natural antioxidant, notably, phenolic compounds have been shown to exert potent antioxidant effects.10,25–27 In addition, some compounds have been found to inhibit neurotoxicity through their antioxidant properties.14,28 Thus, some phenolic acids have been suggested to be useful in managing oxidative stress-induced disorders such as AD.7,29,30
In our previous study, sweet potato extract administration to mice effectively reversed Aβ-induced cognitive deficits in the passive avoidance test without any acute toxicity. Moreover, the extract administration reduced the lipid peroxidation level and increased the catalase activities, which was demonstrated using biochemical studies of the brain tissue of mice.16 A limitation of the above-mentioned study was that the active compound was not isolated.
In this study, the protective effect of the sweet potato extract against oxidative stress was first investigated in vitro. The changes in the intracellular oxidative stress were measured using a DCF-DA assay. H2O2 is known to increase free radical production in PC12 cells, leading to apoptosis and cell death. The intracellular oxidative stress, induced by H2O2 treatment, was significantly lower in the extract-treated PC12 cells than that in the cells treated with H2O2 only. Furthermore, the sweet potato extract was shown to protect PC12 cells from oxidative toxicity in a dose-dependent manner. To further evaluate oxidative stress-induced neurotoxicity, an MTT reduction assay was performed. The assay determines the cell viability based on the redox activity of living cells that convert MTT into a purple formazan; a decrease in cellular MTT reduction could be an index of cell damage. Corroborating the previous findings, cells pretreated with the extract displayed decreased H2O2-induced cytotoxicity. Moreover, the extract dose dependently protected PC-12 cells from oxidative stress-induced cell death. Taken together, the data indicated that the extract contained the active protectant against oxidative stress-induced cytotoxicity. To identify the protectant compound, sweet potato extract was purified by using a sequential purification process. The active compound in the sweet potato extract was identified as 2,4-di-tert-butylphenol, which is one of the phenolic antioxidants. Antioxidant activities of this compound have been previously studied. 2,4-Di-tert-butylphenol is routinely used as an intermediate for the preparation of antioxidants and UV stabilizers, and in the manufacturing of pharmaceuticals and fragrances. 2,4-Di-tert-butylphenol exhibits a significant antioxidant activity.31 In a previous study, we confirmed that the antiapoptotic effect of the pomegranate extract was attributable to its inhibition of oxidative stress-induced toxicity.17
The antioxidant capacity of 2,4-di-tert-butylphenol was confirmed using the ABTS radical assay. There was a high level of correlation between 2,4-di-tert-butylphenol and vitamin C equivalent antioxidant capacity. This implied that 2,4-di-tert-butylphenol scavenged the ABTS radical anions in a dose-dependent manner. 2,4-Di-tert-butylphenol from sweet potato may supply substantial amounts of antioxidants, which may provide health-promoting effects. The effect of dietary administration of 2,4-di-tert-butylphenol extract on behavioral abilities was examined using an AD animal model based on an ICV Aβ1-24 injection. A Y-maze test was used to evaluate memory, learning abilities, and spontaneous alternation behavior, which is regarded as a measure of spatial memory. As shown in the Y-maze test and passive avoidance test, Aβ1-24–induced cognitive dysfunction was attenuated in mice treated with the 2,4-di-tert-butylphenol diet. Thus, the 2,4-di-tert-butylphenol diet mediated a significant anti-amnesic effect in the mouse model.
In conclusion, the sweet potato extract, which contains 2,4-di-tert-butylphenol, was shown to have a protective effect against Aβ1-24 by decreasing neuronal cell damage. This protective effect might be due to the antioxidant capacity of the 2,4-di-tert-butylphenol. Overall, we conclude that increased consumption of sweet potato may inhibit, prevent, or retard oxidative stress-induced disorders such as AD.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science, ICT & Future Planning) (NRF-2010-0005094).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Klein JA. Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest. 2003;111:785–793. doi: 10.1172/JCI18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz DG. Feldman H. Causes of Alzheimer's disease. CMAJ. 2000;162:65–72. [PMC free article] [PubMed] [Google Scholar]
- 3.Chauhan V. Chauhan A. Oxidative stress in Alzheimer's disease. Pathophysiology. 2006;13:195–208. doi: 10.1016/j.pathophys.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Moreira PI. Nunomura A. Nakamura M, et al. Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield DA. Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 6.Wickens AP. Ageing and the free radical theory. Respir Physiol. 2001;128:379–391. doi: 10.1016/s0034-5687(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 7.Esposito E. Rotilio D. Di Matteo V. Di Giulio C. Cacchio M. Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23:719–735. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 8.Surh Y-J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 9.Rice-Evans C. Miller N. Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 10.Jang HD. Chang KS. Huang YS. Hsu CL. Lee SH. Su MS. Principal phenolic phytochemicals and antioxidant activities of three Chinese medicinal plants. Food Chem. 2007;103:749–756. [Google Scholar]
- 11.Wei X. Liu Y. Xiao J. Wang Y. Protective effects of tea polysaccharides and polyphenols on skin. J Agric Food Chem. 2009;57:7757–7762. doi: 10.1021/jf901340f. [DOI] [PubMed] [Google Scholar]
- 12.Aftab N. Vieira A. Antioxidant activities of curcumin and combinations of this curcuminoid with other phytochemicals. Phytother Res. 2010;24:500–502. doi: 10.1002/ptr.2960. [DOI] [PubMed] [Google Scholar]
- 13.Shah RS. Lee H-G. Xiongwei Z. Perry G. Smith MA. Castellani RJ. Current approaches in the treatment of Alzheimer's disease. Biomed Pharmacother. 2008;62:199–207. doi: 10.1016/j.biopha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Jeong C-H. Kwak JH. Kim JH. Choi GN. Kim D-O. Heo HJ. Neuronal cell protective and antioxidant effects of phenolics obtained from Zanthoxylum piperitum leaf using in vitro model system. Food Chem. 2011;125:417–422. [Google Scholar]
- 15.Cuevas E. Limón D. Pérez-Severiano F, et al. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25–35 in rats. Eur J Pharmacol. 2009;616:122–127. doi: 10.1016/j.ejphar.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Kim JK. Choi SJ. Cho HY, et al. Ipomoea batatas attenuates amyloid β peptide-induced neurotoxicity in ICR mice. J Med Food. 2011;14:304–309. doi: 10.1089/jmf.2010.0047. [DOI] [PubMed] [Google Scholar]
- 17.Choi SJ. Lee J-H. Heo HJ, et al. Punica granatum protects against oxidative stress in PC12 cells and oxidative stress-induced alzheimer's symptoms in mice. J Med Food. 2011;14:695–701. doi: 10.1089/jmf.2010.1452. [DOI] [PubMed] [Google Scholar]
- 18.Heo HJ. Cho HY. Hong BS, et al. Protective effect of 4′,5-dihydroxy-3′,6,7-trimethoxyflavone from Artemisia asiatica against Aβ-induced oxidative stress in PC12 cells. Amyloid. 2001;8:194–201. doi: 10.3109/13506120109007362. [DOI] [PubMed] [Google Scholar]
- 19.Kim D-O. Lee KW. Lee HJ. Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- 20.Butterfield DA. Drake J. Pocernich C. Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 21.Eastwood MA. Interaction of dietary antioxidants in vivo: how fruit and vegetables prevent disease? QJM. 1999;92:527–530. doi: 10.1093/qjmed/92.9.527. [DOI] [PubMed] [Google Scholar]
- 22.Li SP. Zhao KJ. Ji ZN, et al. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73:2503–2513. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 23.Ni Y. Zhao B. Hou J. Xin W. Preventive effect of Ginkgo biloba extract on apoptosis in rat cerebellar neuronal cells induced by hydroxyl radicals. Neurosci Lett. 1996;214:115–118. doi: 10.1016/0304-3940(96)12897-4. [DOI] [PubMed] [Google Scholar]
- 24.Xin W. Wei T. Chen C. Ni Y. Zhao B. Hou J. Mechanisms of apoptosis in rat cerebellar granule cells induced by hydroxyl radicals and the effects of EGb761 and its constituents. Toxicology. 2000;148:103–110. doi: 10.1016/s0300-483x(00)00200-6. [DOI] [PubMed] [Google Scholar]
- 25.Harish Nayaka MA. Sathisha UV. Dharmesh SM. Cytoprotective and antioxidant activity of free, conjugated and insoluble-bound phenolic acids from swallow root (Decalepis hamiltonii) Food Chem. 2010;119:1307–1312. [Google Scholar]
- 26.Alothman M. Bhat R. Karim AA. Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends Food Sci Technol. 2009;20:201–212. [Google Scholar]
- 27.Cevallos-Casals BA. Cisneros-Zevallos L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010;119:1485–1490. [Google Scholar]
- 28.Crispo JAG. Piché M. Ansell DR, et al. Protective effects of methyl gallate on H2O2-induced apoptosis in PC12 cells. BBRC. 2010;393:773–778. doi: 10.1016/j.bbrc.2010.02.079. [DOI] [PubMed] [Google Scholar]
- 29.Heo HJ. Lee CY. Phenolic phytochemicals in cabbage inhibit amyloid β protein-induced neurotoxicity. LWT—Food Sci Technol. 2006;39:331–337. [Google Scholar]
- 30.Butterfield DA. Castegna A. Pocernich CB. Drake J. Scapagnini G. Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer's disease. J Nutr Biochem. 2002;13:444–461. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 31.Yoon MA. Jeong TS. Park DS, et al. Antioxidant effects of quinoline alkaloids and 2,4-di-tert-butylphenol isolated from Scolopendra subspinipes. Biol Pharm Bull. 2006;29:735–739. doi: 10.1248/bpb.29.735. [DOI] [PubMed] [Google Scholar]