Abstract
We have previously found that modest chronic increases in maternal cortisol result in an enlarged fetal heart. To explore the mechanisms of this effect, we used intrapericardial infusions of a mineralocorticoid receptor (MR) antagonist (canrenoate) or of a glucocorticoid receptor (GR) antagonist (mifepristone) in the fetus during maternal infusion of cortisol (1 mg·kg−1·day−1). We have shown that the MR antagonist blocked the increase in fetal heart weight and in wall thickness resulting from maternal cortisol infusion. In the current study we extended those studies and found that cortisol increased Ki67 staining in both ventricles, indicating cell proliferation, but also increased active caspase-3 staining in cells of the conduction pathway in the septum and subendocardial layers of the left ventricle, suggesting increased apoptosis in Purkinje fibers. The MR antagonist blocked the increase in cell proliferation, whereas the GR antagonist blocked the increased apoptosis in Purkinje fibers. We also found evidence of activation of caspase-3 in c-kit-positive cells, suggesting apoptosis in stem cell populations in the ventricle. These studies suggest a potentially important role of corticosteroids in the terminal remodeling of the late gestation fetal heart and suggest a mechanism for the cardiac enlargement with excess corticosteroid exposure.
Keywords: sheep, cardiomyocyte, stress, Purkinje fiber, caspase-3
maternal stress has been associated with changes in metabolism and stress responsiveness in the offspring (reviewed in Refs. 13, 15, 18). In animal models, glucocorticoid exposure in the antenatal period has been demonstrated to reduce birth weight and increase the risk of metabolic, neurological and cognitive, and cardiovascular disorders (reviewed in Ref. 26). Several studies have suggested that exogenous glucocorticoids also impact fetal heart development, producing cardiac enlargement (10, 23, 36, 62). Cardiac enlargement is also observed with maternal Cushing's disease (19), and with high-dose steroid treatment in infants with congenital adrenal hyperplasia (3, 52). We have used an ovine model of chronic maternal stress using exogenous cortisol infusion to increase maternal cortisol concentrations to those comparable to that seen acutely after transport in ewes (31, 57). We have found that chronic elevation of maternal cortisol concentrations caused a slowing of overall fetal growth but an increase in fetal heart weight, ventricular wall thickness, and heart weight-to-body weight ratio (28, 46). These increases in maternal cortisol produce a modest increase in fetal cortisol relative to the concentrations that are achieved near term, without an increase in fetal blood pressure or an increase in cardiac fibrosis (46). Intracardiac infusion of cortisol has been shown to cause an increase in heart weight and entry of myocytes into the cell cycle without any change in myocyte size or binucleation (23); there was also no increase in fetal blood pressure in those studies. On the other hand, a more dramatic increase in fetal cortisol increased fetal blood pressure and caused cardiac hypertrophy and fibrosis (36). We have also observed that the enlargement of the fetal heart caused by the more modest chronic increase in maternal and fetal cortisol in late gestation was completely blocked by intrapericardial infusion of a mineralocorticoid receptor (MR) antagonist (46). To test the hypothesis that the effect of MR on enlargement of the ventricles in our model was associated with effects on cell proliferation in the heart, we characterized the roles of MR and glucocorticoid receptor (GR) in cardiac proliferation and apoptosis in those hearts.
MATERIALS AND METHODS
Experimental procedures.
Sheep with singleton pregnancies were studied. All animal use was approved by the University of Florida Institutional Animal Care and Use Committee and conformed to the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.” Sheep were studied as described previously (46). Pregnant sheep were randomly assigned to one of four groups at surgery (118–123 days gestation): ewes infused with saline (control group, n = 6); ewes infused with cortisol (hydrocortisone hemisuccinate; Sigma; 1 mg·kg−1·day−1 iv; cortisol group; n = 5); ewes infused with cortisol and with fetal intrapericardial infusion of the MR antagonist potassium canrenoate (Sigma; 600 μg/day; n = 7); and ewes infused with cortisol and with fetal intrapericardial infusion of the GR antagonist mifepristone (Sigma; 50 μg/day; n = 4). All pericardial infusions were delivered as previously described (46) from a Silastic catheter (1.65 mm od) at 5 μl/h using an Alzet minipump (model 2ML2; Durect, Cupertino, CA); the minipump was placed subcutaneously on the back of the fetus.
The infusion of cortisol started immediately after surgery. Sheep were studied for 10 days before necropsy (days 128–133 gestation). This is a period during which there is remodeling of the fetal heart; over this period of gestation the number of mononuclear myocytes in the left ventricle is reduced by almost 50% so that by the time of necropsy 65–70% of the left ventricular myocytes are binucleated (30). During the study period, sheep had access to water, food, and salt blocks ad libitum. Sheep were euthanized with an overdose of euthanasia solution containing pentobarbital and phenytoin (Euthasol; Virbac Animal Health; Webster Veterinary Supply). Data on heart weight and hormone concentrations have been previously published (46). The maternal infusion of cortisol increased fetal cortisol concentrations from 4.1 ± 1.7 nM in the control group to an average of 9.4 ± 1.7 nM in the fetuses of cortisol-infused ewes. Heart weight-to-body weight ratio, left ventricular free wall, right ventricular free wall, and intraventricular septal wall thicknesses were significantly increased by the maternal infusion of cortisol, and the MR antagonist blocked the effect of cortisol on these measures. The GR antagonist also significantly attenuated the effect of cortisol on right ventricular free wall thickness.
Immunohistochemistry.
At the time of necropsy, a cross section of the fetal heart including left ventricle, right ventricle, and septum was cut and fixed in 4% buffered paraformaldehyde overnight. Hearts were dehydrated in increasing concentrations of reagent alcohol, cleared in xylene, and embedded in paraffin wax. Sections were cut at 5–10 μm (Zeiss rotary microtome; model HM325) and placed on poly-l-lysine-coated slides.
To determine whether cell proliferation was increased in cardiomyocytes of the enlarged, cortisol-treated hearts, sections (10 μm) from fetal hearts were stained with an anti-Ki67 antibody. Hearts from fetuses of control ewes (n = 6), cortisol-treated ewes (n = 5) and from cortisol-treated ewes with intracardiac infusion of a MR antagonist (n = 6) or GR antagonist (n = 4) were analyzed. Ki67 is expressed in all active phases of the cell cycle but is absent in the resting phase; as a result it serves as a marker of cell proliferation. Heart sections were deparaffinized and rehydrated using standard procedures and quenched in hydrogen peroxide (0.3%; Fisher Scientific, Fair Lawn, NJ) to block endogenous peroxidase. Antigen retrieval was performed in 0.1 M sodium citrate buffer at 95°C for 30 min. Heart sections were blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS) for 1 h at room temperature and incubated with anti-Ki67 monoclonal antibody (diluted 1:100 in blocking solution; Dako, Glostrup, Denmark) overnight at 4°C, then incubated with biotinylated goat anti-mouse secondary antibody (Zymed, San Francisco, CA) for 1 h at room temperature, followed by streptavidin-peroxidase (Zymed) incubation. Sections were then incubated with metal enhanced diaminobenzidine (DAB; Pierce DAB substrate kit, ThermoFisher Scientific; Rockford, IL) for 10 min, and counterstained with hematoxylin (Fisher Scientific). Slides were processed in batches containing sections from animals in each experimental group. To assure specificity of the Ki67 staining, negative control slides were included in each batch of slides processed. The negative control slides treated similar to the other slides with the exception that blocking solution without primary antibody was used. Images were taken with a ×40 objective on a BX 41 microscope and recorded with a digital camera DP71 (Olympus, Center Valley, PA). For each heart, three images were taken from each ventricular free wall; all fields were chosen within bundles of cardiomyocytes so as to include predominately cardiomyocytes; there were no fields photographed that included either the endocardial or epicardial layer or discernible blood vessels. The total number of nuclei and the number of KI67-stained nuclei from each image were then counted; the investigators taking the images and performing counts were blinded to the experimental group of the images. The percentage of Ki67-stained nuclei was analyzed by two-way analysis of variance corrected for repeated measures. The criteria for significance was P < 0.05.
To detect cell apoptosis in the fetal hearts, an antibody against active caspase-3 was used. Hearts from fetuses of control ewes (n = 5), cortisol-treated ewes (n = 5), and from cortisol-treated ewes with fetal intracardiac infusion of a MR antagonist (n = 7) or GR antagonist (n = 4) were analyzed. Sections were deparaffinized and rehydrated and quenched in hydrogen peroxide. Antigen retrieval was performed with 0.1 M sodium citrate buffer in a microwave (1,350 W for 5 min). Sections were blocked with 5% goat serum for 1 h, incubated with antibody against active caspase-3 antibody (1:500 dilution in blocking buffer; R&D System, Minneapolis, MN) for 2 h at 4°C, and washed in PBS; negative control slides with no primary antibody application were also included in each batch and analyzed. Activated caspase-3 staining was visualized as described above for Ki67 staining.
Preliminary analysis showed that the pattern of active caspase-3-positive cells varied significantly across areas of the hearts. For the purpose of quantification, each heart section was divided into three different parts: left ventricle, septum, and right ventricle. Each part was further divided into three layers from epicardial to endocardial surfaces in the case of the left and right ventricular free walls. As a result, each heart slide contains nine layers, from left ventricle to right ventricle, including left or right ventricle subepicardial layer (L or R-epi), a layer in the middle consisting primarily of cardiomyocytes (L or R-myo) and the subendocardial layer (L or R-endo); for the septum the layers were similarly identified as the subendocardial layers nearest to the left or right ventricular chambers (S-L-endo or S-R-endo, respectively) or in the middle of the section (S-mid). Five images were taken with a ×10 objective within each of the nine heart layers and analyzed using Image J [Image J 1.43; using the Colour Deconvolution plug-in (50, 51)]. On each image, the area of activated caspase-3-positive nuclei and the area of heart tissue were measured, and the area of active caspase-3-positive staining as a percentage of tissue area was calculated. Activated caspase-3 staining area in control slides was negligible relative to the positively stained slides. The data were analyzed by two-way analysis of variance (treatment group and cardiac layer) corrected for repeated measures (on cardiac layer) using SPSS software (IBM, Armonk, NY).
Based on the morphology and location of some activated caspase-3-positive cells, we hypothesized that Purkinje fibers in the subendocardial layer and septum might be apoptotic. Purkinje fibers are part of the electrical conduction system of the heart. As a result, they expresses some neuronal proteins. Neurofilament medium (NF-M) is expressed in the cardiac conduction system (8), and microtubule-associated protein 2 (MAP2) is a neuronal cytoskeletal protein (11). Heart sections were first stained with active caspase-3 antibody followed by Alexa Fluor 594 goat anti-rabbit IgG secondary antibody (1:500 dilution; Molecular Probes, Invitrogen, Eugene, OR) and then stained with NF-M or MAP-2 antibodies (1:500 dilution; EnCor Biotechnology, Gainesville, FL) followed by Alexa Fluor 488 goat anti-chicken IgG secondary antibody (1:500 dilution; Molecular Probes). Slides were visualized with fluorescence filters. These results confirmed a Purkinje fiber phenotype for a population of caspase-3-positive cells. Purkinje fiber cells contain more glycogen than cardiomyocytes (2, 22), which we detected with Periodic acid-Schiff staining (PAS; Santa Cruz Biotechnology). Thus double staining of caspase-3 and PAS was also performed, and these sections were used to specifically quantitate activation of caspase-3 in Purkinje cells. Photographs were taken using a ×10 objective and analyzed with Image J software. Purkinje fibers were identified by PAS staining, their area measured, and number of active caspase-3 positive staining nuclei within these fibers counted. The number of positively stained nuclei per fiber area was analyzed by two-way analysis of variance (treatment group and cardiac layer) corrected for repeated measures using SPSS. Because the data were not normally distributed, the data were transformed to the logarithm of the raw values before analysis. P < 0.05 was used as the criteria for significance using a one-tailed comparison.
To determine whether stem cells are apoptotic, additional sections were double stained for activated caspase-3 and c-kit. Sections were first stained for activated caspase-3 as described above, except that instead of DAB, sections were incubated with nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (NBT/BCIP) for 2.5 h, then washed, blocked with 10% bovine serum albumin (BSA) for 1 h, followed by incubation with c-kit antibody (H300, 1:100 dilution in blocking buffer; Santa Cruz Biotechnology) for 2 h, and biotinylated goat anti-rabbit secondary antibody (1:200 dilution in 10% BSA; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h and streptavidin-conjugated horseradish peroxidase (1:200 dilution in 10% BSA; Thermo scientific, Rockford, IL) for 30 min. Slides were then stained with the Metal Enhanced DAB substrate Kit (Thermo scientific) for 40 min, washed, and counterstained with methyl green.
RESULTS
Cortisol treatment increases cell proliferation in fetal sheep heart.
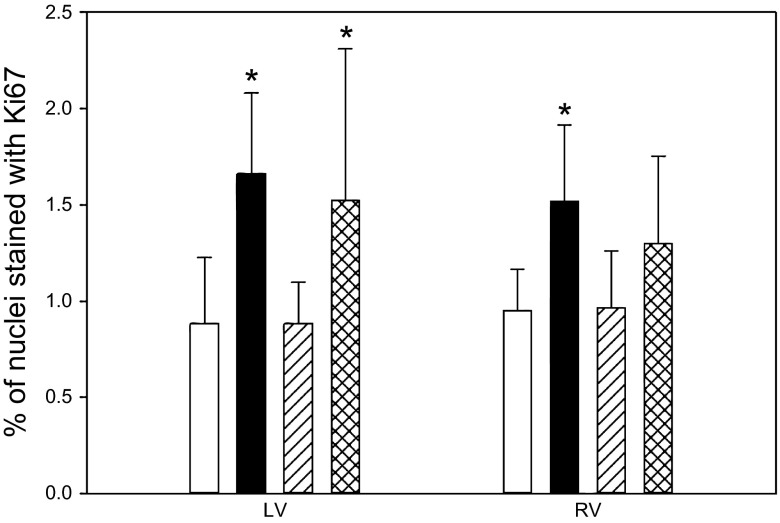
The percentage of nuclei positively stained for Ki67 in the left ventricle was increased in the cortisol group and the cortisol-infused group treated with a GR antagonist compared with that in the control or cortisol-infused group treated with the MR antagonist (Fig. 1). In the right ventricle, the cortisol-infused group had significantly more Ki67-stained nuclei than the control and cortisol-infused group treated with the MR antagonist.
Fig. 1.
The percentage of Ki67-stained nuclei in the left ventricle (LV) and right ventricle (RV) of late-gestation fetal hearts. Open bars, control fetuses; solid bars, fetuses of cortisol-infused ewes; hatched bars, fetuses of cortisol-infused ewes with mineralocorticoid receptor (MR) blocker administered into the fetal heart; cross-hatched bars, fetuses of cortisol-infused ewes with GR blocker administered into the fetal heart. Data are expressed as means ± SE. *Significantly different from control.
Cortisol treatment increases apoptosis in fetal sheep heart.
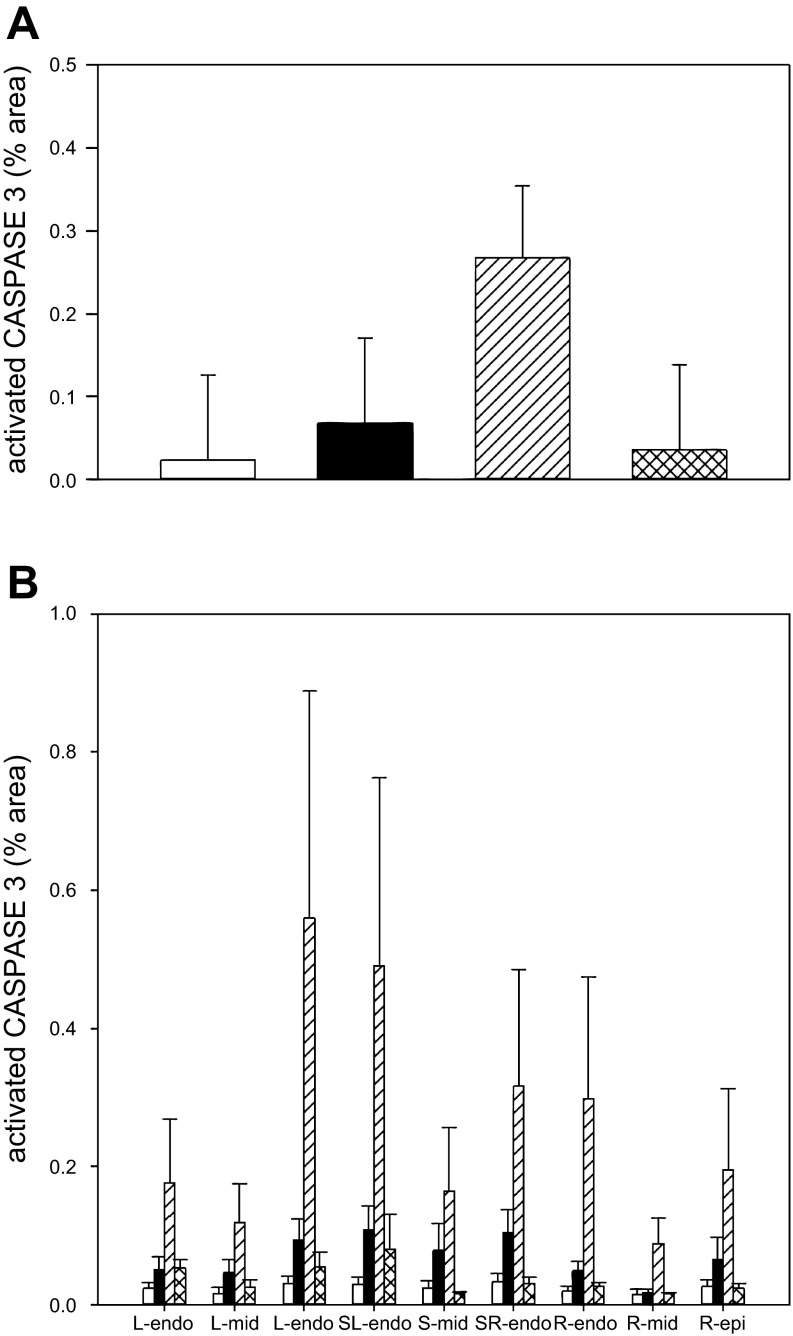
The hearts of the fetuses whose mothers were infused with cortisol tended to have a higher percentage of active caspase-3-positive staining (0.068 ± 0.103%) compared with control sheep (0.024 ± 0.103%) or fetal hearts from cortisol-infused ewes that were treated with the GR antagonist (0.036 ± 0.115%) (overall group means ± SE from two-way ANOVA; Fig. 2A). However, there were no overall differences among the treatment groups in the staining for activated caspase-3. Although the group treated with the MR antagonist tended to have the greatest staining for activated caspase-3 (0.267 ± 0.088%), this difference was not statistically significant as a result of the high variability in staining among regions and subjects within this group.
Fig. 2.
A: overall activated caspase-3 staining in sections of fetal hearts expressed as percent area stained with caspase-3. Groups are indicated as in Fig. 1. Data are expressed as means ± SE. There were no differences among groups. B: activated caspase-3 staining in fetal hearts showed a pattern dependent on location. Hearts were separated into 9 layers for analysis: from left ventricle (L) to right ventricle (R), including left ventricle subepicardial layer (L-epi), a layer in the middle consisting primarily of cardiomyocytes (L-mid) and the subendocardial layer (L-endo); for the septum the layers were similarly identified as the subendocardial layers nearest to the left or right ventricular chambers (L-sep, R-sep) or the middle portion of the septum (S-mid); 3 sections of right ventricular free wall were similarly analyzed (R-endo, R-mid, R-epi). Analysis by two-way ANOVA indicated difference by tissue layer; there was significantly more activated caspase-3-positive staining in the subendocardial layers of ventricles and septum than in the left or right ventricular myocardial layer.
There were overall significant differences in active caspase-3 staining among the regions of the walls of the cardiac chambers (Fig. 2B), which were visually evident. When these differences were analyzed, there were significant location-dependent differences in the amount of activated caspase-3 staining. The subendocardial layers of the left ventricular free wall (L-endo) and of the septum proximal to the left and right chambers (L-sep and R-sep) had significantly more activated caspase-3 staining than any of the layers of the right ventricle or than the middle portions of the left ventricular free wall or the septum. However, there was no overall difference among groups in this pattern.
Cortisol induces apoptosis in Purkinje fibers.
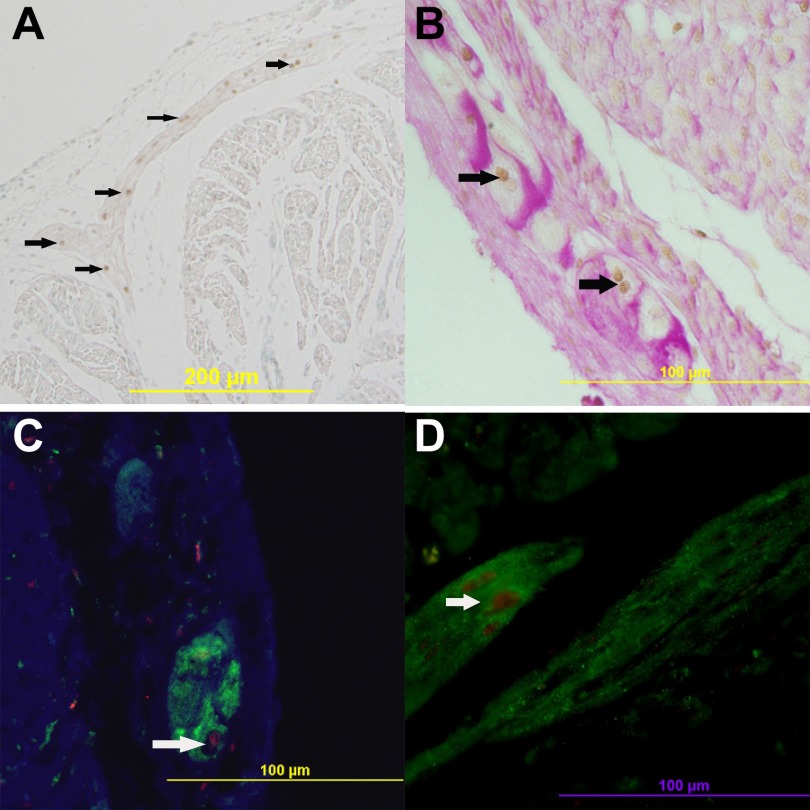
Activated caspase-3 staining was predominately located in the subendocardial regions, in which Purkinje fiber cells are a prominent cell type. As seen in Fig. 3A, activated caspase-3-positive cells in fetal hearts of cortisol-treated ewes are primarily located between the endocardium and myocardium with morphology consistent with that of Purkinje fibers: large cells with large, round nuclei or double nuclei, and projection of the fibers into the ventricle walls. Double staining of activated caspase-3 and PAS demonstrates that those apoptotic cells are Purkinje fiber cells (Fig. 3B).
Fig. 3.
Activated caspase-3 nuclei is detected in Purkinje fibers in the fetal heart. A: caspase-3-positive cells (brown nuclei) project into the ventricle wall. B: costaining of periodic and Schiff (PAS) (pink) and caspase-3 (brown nuclei). C: costaining of neurofilament medium (NF-M) (green) and caspase-3 (red). D: costaining of microtubule-associated protein 2 (MAP2) (green) and caspase-3 (red). Arrows show caspase-3-positive Purkinje fibers.
Double staining of activated caspase-3 and NF-M or MAP-2 also confirms that these fibers are Purkinje fiber cells (Fig. 3, C and D).
Cortisol treatment increased Purkinje fiber apoptosis in fetal sheep heart (Fig. 4A). Intracardiac administration of the MR antagonist did not attenuate the increase in apoptosis resulting from maternal cortisol infusion; however, intrapericardial infusion of the GR antagonist significantly attenuated the increase in activated caspase-3 staining in PAS-positive cells resulting from maternal infusion of cortisol. The apoptosis caused by cortisol was greater in the subendocardial spaces of septum (SL, SR) and left ventricle (LV) than in the right ventricular free wall (RV), although the apoptosis in the septum on the side adjoining the LV chamber (SL) was greater than that in the LV free wall adjoining the same chamber (LV) (Fig. 4B).
Fig. 4.
A: activated caspase-3 staining in Purkinje fibers calculated as number of positive-stained nuclei per Purkinje fiber area; groups are as indicated in Fig. 1. Data are expressed as means ± SE. *Significantly different from control. B: activated caspase-3 staining in Purkinje cells in fetal hearts showed a distribution pattern depending on the location within the ventricular free walls or septum. Activation of caspase-3 in Purkinje fibers was greater in the subendocardial spaces of septum (SL, SR) and left ventricle (LV) than in the right ventricular free wall (RV), although the apoptosis in the septum on the side adjoining the LV chamber was greater than that in the LV free wall adjoining the same chamber.
Double staining of active caspase-3 and c-kit also showed the presence of non-Purkinje fiber c-kit-positive apoptotic cells, indicating apoptosis of stem cells or progenitor cells in the heart (Fig. 5). Because of the relative scarcity of these cells in the heart at this gestational age, we did not quantify the relative apoptosis in the c-kit-positive cells among the treatment groups.
Fig. 5.
Coexpression of c-kit (brown) and activated caspase-3 (blue) in cell types in the fetal heart. Sections A–D are from left ventricle. Arrows indicate c-kit (brown)- and caspase-3 (blue)-positive staining. Large binucleate cell indicated by yellow arrow in D has Purkinje fiber morphology.
DISCUSSION
Our study has demonstrated a striking remodeling of the fetal heart by cortisol. Maternal cortisol infusion increases fetal heart size, cell proliferation, and apoptosis in the late gestation fetal heart. Our results indicate that the increased wall thickness caused by chronic elevation of maternal cortisol involves an increase in myocyte proliferation, but with accompanying apoptosis of Purkinje cells. Whereas previous studies have suggested a proliferative effect of somewhat higher levels of cortisol (23), these studies suggest even relatively small increases in fetal cortisol can induce increased proliferation when those increases occur chronically during the period in which the fetal heart is normally transitioning from proliferative mononuclear cells to terminally differentiated binucleate cells (30). The proliferative and apoptotic effects of cortisol appear to be mediated by different receptors: blockade of MR attenuated the proliferation induced by cortisol, whereas blockade of GR attenuated the apoptosis. Thus these results suggest the two receptors mediate changes in different pathways in the late gestation heart: MR stimulates proliferation and GR stimulates apoptosis.
MR and GR are the high- and low-affinity receptors, respectively, for cortisol. In many epithelial tissues, MRs are protected from binding by cortisol because of dehydrogenase activity of 11β-hydroxysteroid dehydrogenase type II (11βHSD2) (42, 45). However, we have previously found (47) that the fetal heart expresses both MR and GR and has relatively low expression of 11βHSD2 and relatively high expression of the complementary enzyme 11βHSD1, which has predominant reductase activity; in comparison fetal kidney at 130 days of gestation had 13-fold lower expression of MR and 750-fold higher expression of 11βHSD2 (47). Therefore, it would be expected that cortisol action in the fetal heart could be mediated by both MR and GR. In this sense the fetal heart is similar to the adult hippocampus, a tissue in which cortisol binds to both MR and GR (16, 45). In the rat hippocampus MR expression is increased in response to neuron injury and is associated with neuronal survival (14, 32, 49), whereas overexpression of GR can enhance the toxic effects of exogenous insults and cause cell apoptosis (4, 58). Our studies similarly demonstrate that cortisol enlarged the fetal heart by increasing cell proliferation through activation of the MR. In contrast, the apoptosis in Purkinje fibers with maternal cortisol administration can be blocked by a GR antagonist, but not a MR antagonist, indicating that GR mediates cell apoptosis in the cardiac conduction system. Indeed, the cells of the cardiac conduction system share some similarities with neurons, and the differential roles of MR and GR we found in Purkinje fibers are consistent with the hippocampal findings.
In newborn rats systemic treatment with a MR antagonist decreased cardiomyocyte proliferation due to endogenous corticosteroids (53), although the MR blocker also decreased apoptosis in the cardiomyocytes. That study did not determine effects on cell types other than myocytes and did not examine the conduction system in the hearts. It is also likely that the systemic administration of a blocker early in neonatal life, a time of relatively low cardiac MR expression in most species (38), may have different effects than chronic intracardiac administration over a longer period of cardiac maturation.
Although a role of MR in cell proliferation in the fetus is not well characterized in any tissues, a proliferative role of MR has been identified in neuronal progenitor cells and in the developing brain and the neonatal kidney (5, 56, 60). In contrast, in adult hearts and kidney, MR mediate fibrosis in response to injury (25, 61) and blockade of MR receptors by drugs such as eplerenone block the fibrosis after injury or cardiac ischemia (44). However, in our model there is no increase in cardiac collagen in the left or right ventricle after maternal cortisol administration (46), nor did we find changes in genes associated with collagen deposition (COL1A1, COL3A; unpublished data; E. Richards and M. Keller-Wood). In these hearts there was also no increase in ANP mRNA in the left ventricle, nor were BNP or CNP mRNAs altered (unpublished data; X. Fang and M. Keller-Wood); in adult hearts these are considered markers of cardiac failure (reviewed in Ref. 20). Thus the effect of cortisol via MR on these fetal hearts appears to be distinct from the hypertrophic, pro-fibrotic effect of MR in the ischemic adult heart. The in vivo effects of MR and GR on the developing fetal heart also differ from the effects of glucocorticoids seen in cardiomyocyte cultures. In cultures derived from embryonic heart (H9C2 cells) or primary cultures of neonatal cardiomyocytes (48), synthetic glucocorticoids caused hypertrophy in the presence of serum but inhibited cardiomyocyte apoptosis in response to TNF-β or serum deprivation; these effects were mediated by GR. These results do not contradict our findings, because of the difference in both steroid dose between the two studies and the differences between the environment of the in vivo heart and the cultured cells. The anti-apoptotic effect of glucocorticoid receptor activation observed by Ren and co-workers (48) occurred in cardiomyocytes, while the pro-apoptotic effect of GR we observed was in Purkinje fibers. Furthermore, the developmental age of the hearts may play a role; for example, endothelin has been shown to induce cardiomyocytes to develop into Purkinje fibers at earlier stages of chick heart development but causes cardiomyocyte hypertrophy at later stages (39). Thus different cell types, different developmental ages, and different cellular mileu might determine the effects of glucocorticoids.
The expression of Ki67, indicative of cell proliferation, is increased by cortisol in the ventricular free wall in areas in which cardiomyocytes predominate; this result is consistent with the observation of Giraud and co-workers (23), who found increased Ki67 labeling of isolated cardiomyocytes after infusion of cortisol into the circumflex artery of the fetus. They found a doubling in the percentage of Ki67-positive nuclei in mononuclear cardiomyocyes from 2.7 ± 0.4% to 5.5 ± 0.1%, consistent with the increase from 0.9 ± 0.1% to 1.6 ± 0.2% observed in the fields of left ventricular free wall in the present study. In contrast, although some caspase-3 was observed in myocyte bundles, the caspase-3 staining was more concentrated in the portions of the ventricular wall near the cardiac chambers.
The effects of chronic fetal exposure to cortisol appears to differ from the effect of fetal stress induced by placental restriction or placental embolization (35, 41); placental restriction increases the proportion of mononuclear myocytes at 135 days without producing heart enlargement or myocyte proliferation, and placental embolization decreases myocyte proliferation and the transition to binucleated myocytes. However, in these models the fetuses are hypoxemic so that factors other than cortisol likely contribute to the observed changes in myocyte maturation.
The predominant apoptotic cell type identified in the fetal heart was that forming Purkinje fibers, suggesting that increased maternal cortisol could impair electrical conduction in the fetal heart. Although our data suggest GR activates the apoptotic pathways in the conduction system of the late gestation heart, conditional overexpression of MR in the heart in mice leads to life threatening ventricular arrhythmias both in utero and shortly after birth, with few mice surviving to more than 3 wk of age (43). However, since MR and GR are 94% homologous in the DNA binding domain (7), MR overexpression may mimic excess GR activation, resulting in disruption of the conduction in the developing heart. One possible explanation for the sensitivity of cells in the conduction pathway to corticosteroid exposure is that like neurons, Purkinje fibers are susceptible to cellular stress. Studies in rat embryos showed that maternal hyperthermia caused malformations in both the central nervous system and cardiac conduction system (6). Although Purkinje fiber development has not been as thoroughly studied in the ovine fetus in terms of factors inducing Purkinje fiber maturation, structural studies of the ovine heart show that the time of our treatment corresponds to the end of the development of a fibrous sheath around the septal Purkinje fibers and the beginning of the ensheathment of the ventricular Purkinje fibers; formation of this sheath is complete, well organized, and dense by gestational day 140, but nonexistent at day 115 (12).
We also saw apoptotic cells that were c-kit-positive; these cells are a source of cardiogenesis in the developing heart (21). Although the c-kit-positive cells were relatively sparse, they appeared to fall into two distinct morphologies: cells with big, round nuclei, scattered among cardiomyocytes, cells with long, thin nuclei with little cytoplasm located between muscle bundles and inside connective tissue. Their morphological differences suggest they may be distinct progenitor cell populations. The first phenotype is consistent with previous studies in which patterns of apoptosis in the developing heart were examined; in those studies apoptotic cells were scattered throughout the myocardium, although these reports did not conclusively identify the dying cells (29, 37). Recent studies suggest that c-kit labels a heterogeneous population of cells in the heart arising from different precursors and consisting of cardiac stem cells, mast cells, hematopoietic cells, cells derived by epithelial to mesenchymal transition, and possibly others. Several of these populations are progenitor cells in the developing heart (27, 59). A stem cell population derived from the epicardium (epicardially derived cells, EPDC) is c-kit positive (17). EPDC can differentiate into fibroblasts and smooth muscle vascular cells; their contribution to cardiomyocytes is controversial, but studies suggest they may participate in the development or maturation of Purkinje fibers (24, 54). The spindle shape c-kit-positive cells are scattered between muscle bundles (Fig. 5, B and C), suggesting that they might be fibroblasts derived from EPDC; we also found c-kit-positive cells in close proximity to Purkinje fibers (Fig. 5D). In the chick heart EPDCs indirectly affect Purkinje fiber formation by contributing to formation of blood vessels adjacent to the Purkinje fibers and by becoming the interstitial fibroblasts that form the fibrous heart skeleton (55). Further studies will be required to quantitate apoptosis in progenitor cell populations; these studies would be more appropriately performed using techniques that allow separation of these subpopulations of cells.
The hearts of the fetuses of cortisol-infused ewes were larger than those of control hearts, and the increase in apoptosis we observed may reflect remodeling rather than pathology. This awaits further study, however, the pronounced activation of apoptotic pathways in the cardiac conduction fibers suggests the possibility that cortisol induced pathological changes in the hearts. In a subsequent study in which ewes with this treatment were studied to term, 3 of 4 lambs born from 143–146 days gestation in cortisol-treated ewes were stillborn or died at birth; in the 6 control ewes, 5 delivered live lambs and 1 lamb died during delivery due to dystocia (unpublished results; M. Keller-Wood and X. Fang).
Perspectives and Significance
In this study, we have observed potentially adverse effects of the maternal stress hormone cortisol on cells within the fetal cardiac conduction system. We also demonstrated differential roles of MR and GR on cell proliferation and apoptosis. Before late gestation, fetal cortisol concentrations are in the range for activation of MR with only partial activation of GR. The activation of MR may support proliferation during most of gestation. The normal late gestation increase in cortisol dramatically increases plasma cortisol concentrations (9) and result in levels that would more completely activate GR. These GR-mediated effects play an important role in fetal development and organ maturation in late gestation, especially in terms of lung, liver, and gastrointestinal maturation (1, 33). The GR-mediated effects of cortisol are normally limited to the immediate peripartal period, occurring at a time at which the Purkinje fibers should normally be mature, and the number of left ventricular myocytes in the cell cycle is dramatically decreasing (30). Since Liggins and Howie (34) started clinical trials in the 1970s, short-term prenatal glucocorticoids treatment has become a standard treatment for promoting fetal lung maturation in the event of preterm delivery. A recent examination of the use of prenatal glucocorticoids suggested that there is no risk for cardiac enlargement at birth after repeated courses for threatened prematurity (40), suggesting that the repeated acute treatment paradigm does not produce long-term changes in the heart. In contrast, maternal Cushing's disease or steroid treatment of the neonate with congenital adrenal hyperplasia results in cardiac enlargement (3, 19, 52). We studied relatively mature fetuses, at an age at which terminal differentiation begins in the myocardium (30) and the conduction system is maturing (12). Our results suggest a vulnerability of the late-gestation fetal or term heart to chronic stress or to excess corticosteroid administration during this period. Thus our results suggest a potential perinatal consequence of maternal stress but also indicate dangers to chronic therapeutic steroid administration during this period of gestation, which may represent a critical period in maturation of the cardiac conduction pathway.
GRANTS
This work was supported by National Institutes of Health Grants DK-62080 and HD-057871 and a predoctoral award from the Southeastern Affiliate of the American Heart Association to S. A. Reini.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.F., S.A.R., E.R., C.E.W., and M.K.-W. conception and design of research; X.F., S.A.R., C.E.W., and M.K.-W. performed experiments; X.F., S.A.R., and M.K.-W. analyzed data; X.F., S.A.R., E.R., C.E.W., and M.K.-W. interpreted results of experiments; X.F., S.A.R., and M.K.-W. prepared figures.; X.F. and E.R. drafted manuscript; X.F., S.A.R., E.R., C.E.W., and M.K.-W. edited and revised manuscript; X.F., S.A.R., E.R., C.E.W., and M.K.-W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gerry Shaw, Department of Neuroscience, University of Florida, for supplying the MAP2 antibody.
REFERENCES
- 1. Abel MH, Bass FG, Krane EJ, Thomas AL, Liggins GC. Pituitary stalk-section and some of its effects on endocrine function in the fetal lamb. Q J Exp Physiol Cogn Med Sci 63: 211– 219, 1978 [DOI] [PubMed] [Google Scholar]
- 2. Airey JA, Almeida-Porada G, Colletti EJ, Porada CD, Chamberlain J, Movsesian M, Sutko JL, Zanjani ED. Human mesenchymal stem cells form Purkinje fibers in fetal sheep heart. Circulation 109: 1401– 1407, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Al Jarallah AS. Reversible cardiomyopathy caused by an uncommon form of congenital adrenal hyperplasia. Pediatr Cardiol 25: 675– 676, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Almeida OF, Conde GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J 14: 779– 790, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38: 872– 883, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aoyama N, Yamashina S, Poelmann RE, Gittenberger-De Groot AC, Izumi T, Soma K, Ohwada T. Conduction system abnormalities in rat embryos induced by maternal hyperthermia. Anat Rec 267: 213– 219, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237: 268– 275, 1987 [DOI] [PubMed] [Google Scholar]
- 8. Atkinson A, Inada S, Li J, Tellez JO, Yanni J, Sleiman R, Allah EA, Anderson RH, Zhang H, Boyett MR, Dobrzynski H. Anatomical and molecular mapping of the left and right ventricular His-Purkinje conduction networks. J Mol Cell Cardiol 51: 689– 701, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Bassett JM, Thorburn GD. Foetal plasma corticosteroids and the initiation of parturition in sheep. J Endocrinol 44: 285– 286, 1969 [DOI] [PubMed] [Google Scholar]
- 10. Boeuf B, Maragnes P, Belzic I, Lacotte J, Bonte JB, Guillois B. [Glucocorticoid-induced hypertrophic cardiomyopathy in premature infants: apropos of 4 cases]. Arch Pediatr 4: 152– 157, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Caceres A, Payne MR, Binder LI, Steward O. Immunocytochemical localization of actin and microtubule-associated protein MAP2 in dendritic spines. Proc Natl Acad Sci USA 80: 1738– 1742, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canale E, Smolich JJ, Campbell GR. Differentiation and innervation of the atrioventricular bundle and ventricular Purkinje system in sheep heart. Development 100: 641– 651, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3: 19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry 10: 790– 798, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry 52: 119– 129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev 19: 269– 301, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Di Meglio F, Castaldo C, Nurzynska D, Romano V, Miraglia R, Bancone C, Langella G, Vosa C, Montagnani S. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J Mol Cell Cardiol 49: 719– 727, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Entringer S, Buss C, Swanson JM, Cooper DM, Wing DA, Waffarn F, Wadhwa PD. Fetal programming of body composition, obesity, and metabolic function: the role of intrauterine stress and stress biology. J Nutr Metab 2012: 632548, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fayol L, Masson P, Millet V, Simeoni U. Cushing's syndrome in pregnancy and neonatal hypertrophic obstructive cardiomyopathy. Acta Paediatr 93: 1400– 1402, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Federico C. Natriuretic Peptide system and cardiovascular disease. Heart Views 11: 10– 15, 2010 [PMC free article] [PubMed] [Google Scholar]
- 21. Ferreira-Martins J, OgÃ3rek B, Cappetta D, Matsuda A, Signore S, D'Amario D, Kostyla J, Steadman E, Ide-Iwata N, Sanada F, Iaffaldano G, Ottolenghi S, Hosoda T, Leri A, Kajstura J, Anversa P, Rota M. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res 110: 701– 715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Forsgren S, Thornell LE. The development of Purkinje fibres and ordinary myocytes in the bovine fetal heart. An ultrastructural study. Anat Embryol (Berl) 162: 127– 136, 1981 [DOI] [PubMed] [Google Scholar]
- 23. Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol Stimulates Cell Cycle Activity in the Cardiomyocyte of the Sheep Fetus. Endocrinology 147: 3643– 3649, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gittenberger-De Groot AC, Winter EM, Bartelings MM, Goumans MJ, Deruiter MC, Poelmann RE. The arterial and cardiac epicardium in development, disease and repair. Differentiation 84: 41– 53, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology 147: 5363– 5373, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 59: 279– 289, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Hou J, Wang L, Jiang J, Zhou C, Guo T, Zheng S, Wang T. Cardiac stem cells and their roles in myocardial infarction. Stem Cell Rev 2012 [DOI] [PubMed] [Google Scholar]
- 28. Jensen E, Wood CE, Keller-Wood M. Chronic alterations in ovine maternal corticosteroid levels influence uterine blood flow and placental and fetal growth. Am J Physiol Regul Integr Comp Physiol 288: R54– R61, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Jerse M, Zidar N. Apoptosis in the developing human heart resembles apoptosis in epithelial tissues. Cell Tissue Res 343: 537– 543, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol 102: 1130– 1142, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Keller-Wood M. Inhibition of stimulated and basal ACTH by cortisol during ovine pregnancy. Am J Physiol Regul Integr Comp Physiol 271: R130– R136, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Lai M, Horsburgh K, Bae SE, Carter RN, Stenvers DJ, Fowler JH, Yau JL, Gomez-Sanchez CE, Holmes MC, Kenyon CJ, Seckl JR, Macleod MR. Forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischaemia. Eur J Neurosci 25: 1832– 1842, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Liggins GC. Adrenocortical-related maturational events in the fetus. Am J Obstet Gynecol 126: 931, 1976 [DOI] [PubMed] [Google Scholar]
- 34. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50: 515– 525, 1972 [PubMed] [Google Scholar]
- 35. Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol 580: 639– 648, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lumbers ER, Boyce AC, Joulianos G, Kumarasamy V, Barner E, Segar JL, Burrell JH. Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am J Physiol Regul Integr Comp Physiol 288: R567– R574, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Manasek FJ. Myocardial cell death in the embryonic chick ventricle. J Embryol Exp Morphol 21: 271– 284, 1969 [PubMed] [Google Scholar]
- 38. Martinerie L, Munier M, Le MD, Meduri G, Viengchareun S, Lombes M. The mineralocorticoid signaling pathway throughout development: Expression, regulation and pathophysiological implications. Biochimie 95: 148– 157, 2013 [DOI] [PubMed] [Google Scholar]
- 39. Mikawa T, Gourdie RG, Takebayashi-Suzuki K, Kanzawa N, Hyer J, Pennisi DJ, Poma CP, Shulimovich M, Diaz KG, Layliev J, Prasad A. Induction and patterning of the Purkinje fibre network. Novartis Found Symp 250: 142– 153, 2003 [PubMed] [Google Scholar]
- 40. Mildenhall L, Battin M, Bevan C, Kuschel C, Harding JE. Repeat prenatal corticosteroid doses do not alter neonatal blood pressure or myocardial thickness: randomized, controlled trial. Pediatrics 123: e646– e652, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 293: R306– R313, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Murphy BE, Clark SJ, Donald IR, Pinsky M, Vedady D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am J Obstet Gynecol 118: 538– 541, 1974 [DOI] [PubMed] [Google Scholar]
- 43. Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, Perrier R, Soukaseum C, Nguyen Dinh CA, Royer A, Le QK, Charpentier F, Demolombe S, Mechta-Grigoriou F, Beggah AT, Maison-Blanche P, Oblin ME, Delcayre C, Fishman GI, Farman N, Escoubet B, Jaisser F. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation 111: 3025– 3033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pitt B, Williams G, Remme W, Martinez F, Lopez-Sendon J, Zannad F, Neaton J, Roniker B, Hurley S, Burns D, Bittman R, Kleiman J. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther 15: 79– 87, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Rashid S, Lewis GF. The mechanisms of differential glucocorticoid and mineralocorticoid action in the brain and peripheral tissues. Clin Biochem 38: 401– 409, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Reini SA, Dutta G, Wood CE, Keller-Wood M. Cardiac corticosteroid receptors mediate the enlargement of the ovine fetal heart induced by chronic increases in maternal cortisol. J Endocrinol 198: 419– 427, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reini SA, Wood CE, Jensen E, Keller-Wood M. Increased maternal cortisol in late gestation ewes decreases fetal cardiac expression of 11β-HSD2 mRNA and the ratio of AT1 to AT2 receptor mRNA. Am J Physiol Regul Integr Comp Physiol 291: R1708– R1716, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Ren R, Oakley RH, Cruz-Topete D, Cidlowski JA. Dual role for glucocorticoids in cardiomyocyte hypertrophy and apoptosis. Endocrinology 153: 5346– 5360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rogalska J, Kang P, Wotherspoon W, Macleod MR, Lai M. Effect of hyperthermia and anoxia on glucocorticoid and mineralocorticoid receptor expression in neonatal rat hippocampus. Neurosci Lett 450: 196– 200, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23: 291– 299, 2001 [PubMed] [Google Scholar]
- 51. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671– 675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scire G, D'Anella G, Cristofori L, Mazzuca V, Cianfarani S. Marked left ventricular hypertrophy mimicking hypertrophic cardiomyopathy associated with steroid therapy for congenital adrenal hyperplasia. J Cardiovasc Med (Hagerstown) 8: 465– 467, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Sohn HJ, Yoo KH, Jang GY, Lee JH, Choi BM, Lee JH, Bae IS, Yim HE, Son CS, Lee JW. Aldosterone modulates cell proliferation and apoptosis in the neonatal rat heart. J Korean Med Sci 25: 1296– 1304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Winter EM, Gittenberger-De Groot AC. Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci 64: 692– 703, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Winter EM, Grauss RW, Hogers B, van Tuyn J, van der Geest R, Lie-Venema H, Steijn RV, Maas S, Deruiter MC, deVries AA, Steendijk P, Doevendans PA, Van Der Laarse A, Poelmann RE, Schalij MJ, Atsma DE, Gittenberger-De Groot AC. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation 116: 917– 927, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Wong EY, Herbert J. Roles of mineralocorticoid and glucocorticoid receptors in the regulation of progenitor proliferation in the adult hippocampus. Eur J Neurosci 22: 785– 792, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wood CE. Negative-feedback inhibition of fetal ACTH secretion by maternal cortisol. Am J Physiol Regul Integr Comp Physiol 252: R743– R748, 1987 [DOI] [PubMed] [Google Scholar]
- 58. Woolley CS, Gould E, Sakai RR, Spencer RL, McEwen BS. Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res 554: 312– 315, 1991 [DOI] [PubMed] [Google Scholar]
- 59. Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell 132: 537– 543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yim HE, Yoo KH, Bae IS, Jang GY, Hong YS, Lee JW. Aldosterone regulates cellular turnover and mitogen-activated protein kinase family expression in the neonatal rat kidney. J Cell Physiol 219: 724– 733, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Young MJ, Lam EY, Rickard AJ. Mineralocorticoid receptor activation and cardiac fibrosis. Clin Sci (Lond) 112: 467– 475, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Yunis KA, Bitar FF, Hayek P, Mroueh SM, Mikati M. Transient hypertrophic cardiomyopathy in the newborn following multiple doses of antenatal corticosteroids. Am J Perinatol 16: 17– 21, 1999 [DOI] [PubMed] [Google Scholar]