Abstract
An enhanced glutamate excitatory function within the hypothalamic supraoptic and paraventricluar nuclei is known to contribute to increased neurosecretory and presympathetic neuronal activity, and hence, neurohumoral activation, during heart failure (HF). Still, the precise mechanisms underlying enhanced glutamate-driven neuronal activity in HF remain to be elucidated. Here, we performed simultaneous electrophysiology and fast confocal Ca2+ imaging to determine whether altered N-methyl-d-aspartate (NMDA) receptor-mediated changes in intracellular Ca2+ levels (NMDA-ΔCa2+) occurred in hypothalamic magnocellular neurosecretory cells (MNCs) in HF rats. We found that activation of NMDA receptors resulted in a larger ΔCa2+ in MNCs from HF when compared with sham rats. The enhanced NMDA-ΔCa2+ was neither dependent on the magnitude of the NMDA-mediated current (voltage clamp) nor on the degree of membrane depolarization or firing activity evoked by NMDA (current clamp). Differently from NMDA receptor activation, firing activity evoked by direct membrane depolarization resulted in similar changes in intracellular Ca2+ in sham and HF rats. Taken together, our results support a relatively selective alteration of intracellular Ca2+ homeostasis and signaling following activation of NMDA receptors in MNCs during HF. The downstream functional consequences of such altered ΔCa2+ signaling during HF are discussed.
Keywords: NMDA, vasopressin, supraoptic, glutamate, Ca2+
sympathohumoral activation involving augmented sympathetic tone and elevated hormonal plasma levels, including vasopressin (VP) and angiotensin II, among others (37, 43, 73), is a key central nervous system pathophysiological process in congestive heart failure (HF). Chronically elevated plasma VP levels have been reported both in animal models and human patients with HF (15, 19, 52, 62) being an important factor contributing to altered fluid/electrolyte balance, as well as detrimental myocardial effects (17, 18, 40, 44, 53). The importance of VP in HF is also underscored by several clinical trials demonstrating that VP receptor antagonism efficiently improves water balance and hemodynamic parameters in HF patients (2, 9, 41). Thus the VP system is currently emerging as a novel therapeutic target for the treatment of HF (14). Despite its major impact on morbidity and mortality in HF patients (7), the precise mechanisms contributing to neurohumoral activation, including elevated VP release in HF, remain incompletely understood.
The hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei are crucial centers involved in autonomic and neuroendocrine regulation of the circulation (23, 60). Within these nuclei, magnocellular neurosecretory cells (MNCs) directly control VP (and oxytocin) release into the circulation, according to their degree and pattern of electrical activity (6, 51). A growing body of evidence supports an enhanced glutamate excitatory action within the SON and PVN in HF rats (27, 29, 32, 48, 49, 71). This includes elevated endogenous glutamate levels (29, 32), increased expression of glutamate N-methyl-d-aspartate (NMDA) receptors (32), and synaptic remodeling involving an increased predominance of glutamate excitatory over GABA inhibitory inputs in HF rats (24, 48). This exacerbated glutamate excitatory strength was shown to contribute to enhanced presympathetic and neurosecretory neuronal activity (24, 48), as well as the concomitant increase in sympathohumoral activation in HF rats (29, 32, 71).
NMDA receptors are one of the key glutamate receptors influencing neuronal activity and sympathohumoral outflow from the hypothalamus (25, 31, 32, 42, 61, 72). NMDA receptor activation results in an influx of Ca2+, which in addition to evoking a direct membrane depolarization, leads to an increase in intracellular free Ca2+ levels (ΔCa2+) (38). This in turn affects a variety of downstream signaling pathways, including activation of Ca2+-sensitive channels (47, 54) and changes in the intracellular kinase/phosphatase balance (8), all of which can in turn further alter neuronal excitability following NMDA receptor activation. Moreover, other signaling mechanisms known to contribute to altered neuronal function in HF rats, including nitric oxide (NO) and reactive oxygen species (ROS) production (4, 22, 26, 68), are strongly dependent on or influenced by NMDA-mediated increases in intracellular Ca2+. The functional consequences of ΔCa2+ are largely dependent on its magnitude and time course. Still, the precise spatiotemporal dynamics of NMDA-ΔCa2+ in key neurons involved in the regulation of sympathohumoral activation has not been investigated yet. Moreover, whether a change in NMDA-ΔCa2+ dynamics contributes to altered neuronal activity and thus neurohumoral activation in HF rats, is presently unknown. In this study, we performed simultaneous patch-clamp electrophysiological recordings and fast confocal Ca2+ imaging to characterize NMDA-ΔCa2+ dynamics in MNCs in sham and HF rats. Our results show an enhanced NMDA-ΔCa2+ signal in MNCs of HF rats, which was independent of the magnitude of the NMDA-mediated current, or of the degree of NMDA-evoked firing activity. Moreover, our results suggest that the enhanced NMDA-ΔCa2+ acts as a positive feedback mechanism contributing to increased MNC membrane excitability in HF rats.
MATERIALS AND METHODS
Animals and induction of HF-Male Wistar rats (150–180 g) were purchased from Harlan Laboratories (Indianapolis, IN). Rats were housed at room temperature (24–26°C) in a 12-h light-dark cycle room and given free access to food and water. In a subset of experiments, we also used male heterozygous transgenic VP-eGFP Wistar rats (5–6 wk old), in which VP neurons are endogenously fluorescent (67). All procedures were carried out in agreement with the Georgia Regents University Institutional Animal Care and Use Committee guidelines. All protocols used for these studies were submitted, reviewed, and approved by an independent committee (IACUC). HF was induced by coronary artery ligation as previously described (4). Briefly, animals were anesthetized with isoflurane 4% and intubated for mechanical ventilation. A left thoracotomy was performed and the heart exteriorized. The ligation was placed on the main diagonal branch of the left anterior descending coronary artery. Buprenorphine (Bruprenex C3 0.3 mg/kg sc; Butler Schein/NLS, Dublin, OH) was given immediately after surgery to minimize postsurgical pain. Sham animals underwent the same procedure but the coronary artery was not occluded. All animals were used 6 to 7 wk after surgery. Transthoracic echocardiography (Vevo 770 system; Visual Sonics) was performed 4 wk after surgery under light anesthesia. The left ventricle internal diameter, as well as the left ventricle posterior and anterior walls diameter, were obtained throughout the cardiac cycle from the short-axis motion imaging mode. Automatic calculation using the parameters measured was obtained for ejection fraction and fractional shortening. Mean cardiac function values obtained from sham and HF rats are summarized in Table 1.
Table 1.
Summary data of echocardiography measurements of left ventricular parameters obtained from sham and heart failure rats
| EF, % | FS, % | LVIDd, mm | LVIDs, mm | |
|---|---|---|---|---|
| Sham | 80.1 ± 4.0 | 52.2 ± 4.7 | 7.6 ± 0.3 | 3.7 ± 0.5 |
| HF | 34.8 ± 1.6* | 17.6 ± 0.9* | 10.2 ± 0.2* | 8.5 ± 0.2* |
Values are means ± SE; n = 19 rats for each group. EF, ejection fraction; FS, fractional shortening; LVID,d and s: left ventricle internal dimension during diastole and systole respectively; HF, heart failure.
P < 0.0001 vs. sham.
Hypothalamic slice preparation.
Hypothalamic brain slices were prepared according to methods previously described (48, 58). Briefly, rats were deeply anesthetized with pentobarbital sodium (80 mg/kg ip) and perfused through the heart with an ice-cold sucrose solution [containing in mM: 200 sucrose, 2.5 KCl, 3 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 1 CaCl2, and 2 pyruvic acid (290–310 mosmol/l)]. Rats were then quickly decapitated, brains dissected out, and coronal slices cut (300 μm thick) using a vibroslicer (D.S.K. Microslicer, Ted Pella, Redding, CA). An oxygenated ice-cold artificial cerebrospinal fluid (aCSF) was used during slicing (containing in mM: 119 NaCl, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose, 0.4 ascorbic acid, 2 CaCl2, and 2 pyruvic acid; pH 7.4; 290–310 mosmol/l). Slices were placed in a holding chamber containing aCSF and kept at room temperature until used.
Patch-clamp electrophysiology.
Slices were bathed with solutions (∼2.0 ml/min) that were continuously bubbled with 95% O2-5% CO2 and maintained at 32°C. Thin-walled (1.5 mm od, 1.17 mm id) borosilicate glass (G150TF-3, Warner Instruments, Sarasota, FL) was used to pull patch pipettes (3–4 MΩ) on a horizontal Flaming/Brown micropipette puller (P-97, Sutter Instruments, Novato, CA). The internal solution contained (in mM) 140 potassium gluconate, 0.2 EGTA, 10 HEPES, 10 KCl, 0.9 MgCl2, 4 MgATP, 0.3 NaGTP, and 20 phosphocreatine (Na+); pH 7.2–7.3. For voltage-clamp recordings, a low Mg2+ aCSF (20 μM MgSO4) was used to facilitate measurements of NMDA-mediated currents. Recordings were obtained with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) from SON neurons using infrared differential interference contrast (IR-DIC) videomicroscopy. The voltage output was digitized at 16-bit resolution and 10 kHz and were filtered at 2 kHz (Digidata 1320A, Axon Instruments). Data were discarded if the series resistance was not stable throughout the entire recording (>20% change) (48, 58). The NMDA receptor-mediated current (INMDA) in SON neurons was assessed by measuring the peak and the integrated area of the evoked change in holding current (Iholding) following a focal puff of NMDA into the recorded cell using a picospritzer device (Toohey) connected to a patch pipette positioned around 5–10 μm from the recorded cell, which in most cases was located in the second layer of cells within the slice preparation. A minimal pressure of 5–8 PSI was used. Cell input resistance and cell capacitance were calculated in voltage clamp using a 5-mV pulse while holding the cells at −70 mV. Spike threshold was calculated based on the third derivative of the action potential waveform implemented by MiniAnalysis software (5, 56). Repetitive firing activity was evoked by injecting depolarizing current pulses (80 pA) of progressively increasing durations (0.1–0.7 s), and plots of the number of evoked spikes as a function of the current pulse duration were generated. All drugs were purchased from Sigma-Aldrich (St. Louis, MO).
Confocal calcium imaging.
SON neurons were loaded through the patch pipette with Fluo-5F pentapotassium salt (100 μM; Molecular Probes, Carlsbad, CA), as previously described (13, 57). Once in the whole cell mode, the dye was allowed to dialyze into the cell for at least 15 min before the initiation of the recordings to allow complete dialysis of the dye. Imaging was conducted using the Yokogawa real time live cell laser confocal system combined with a highly sensitive EMCCD camera (iXON+885, Andor Technology, South Windsor, CT). Fluorescence images were obtained using diode-pumped solid-state laser (Melles Griot, Carlsbad, CA) at 488 nm and emitted light at >495. Images were acquired at a rate of 2 Hz. The fractional fluorescence (F/F0) was determined by dividing the fluorescence intensity (F) within a region of interest (6 × 6 pixels ∼ 4.8 × 4.8 μm) by a baseline fluorescence value (F0) determined from 30 images before activation of NMDA receptors. Data were analyzed using Andor IQ software (Andor Technology).
Statistical analysis.
All values are expressed as means ± SE. Between-group differences (sham vs. HF) were compared using unpaired t-tests or analysis of variance, as indicated followed by Bonferroni post hoc tests. Differences were considered statistically significant at P < 0.05, and n refers to the number of cells. All statistical analyses were conducted using GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
Cardiac function values in sham and HF rats.
Mean cardiac function values obtained from sham and HF rats in this study are summarized in Table 1. When compared with sham rats, ligated rats showed a significant increased left ventricle internal dimension throughout the cardiac cycle, a decreased percentage of ejection fraction, and a decreased percentage fractional shortening (P < 0.0001 in all cases).
NMDA receptor activation evokes a similar INMDA, but larger ΔCa2+ in MNCs in HF rats.
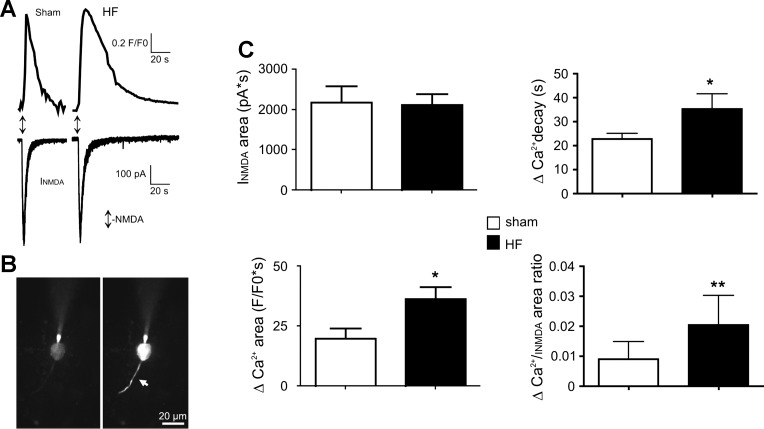
Patch-clamp electrophysiological and/or imaging recordings were obtained from a total of 95 SON MNCs obtained from sham (n = 48 MNCs from 19 rats) and HF rats (n = 47 MNCs from 19 rats). To study NMDA receptor-mediated changes intracellular Ca2+ dynamics (NMDA-ΔCa2+) in MNCs from sham and HF rats, simultaneous patch-clamp recordings (voltage-clamp mode) and fast confocal Ca2+ imaging measurements were obtained from neurons loaded with Fluo-5F (100 μM) through the patch pipette. Focal application of NMDA (50 μM, 1 s, 5–8 PSI) onto the recording neurons evoked a transient inward current (INMDA) and a concomitant ΔCa2+ (Fig. 1, A and B). As shown in Fig. 1C, the overall magnitude of INMDA was not different between MNCs in sham and HF rats (n = 9 and 13, respectively, P = 0.8). Conversely, the magnitude of the NMDA-ΔCa2+ was significantly larger in MNCs from HF rats (∼85%, P < 0.05). When Ca2+ responses were normalized to the underlying INMDA within each cell, a larger ΔCa2+/unit of INMDA was still observed in HF compared with sham rats (∼125%, P < 0.01, Fig. 1C). Given similar cell capacitance values between MNCs in sham and HF rats (Table 2), similar results were observed when INMDA and ΔCa2+ values were normalized by cell capacitance (not shown). Thus, despite similar INMDA-evoked currents, a larger increase in intracellular Ca2+ was observed in MNCs from HF rats.
Fig. 1.
N-methyl-d-aspartate (NMDA) receptor activation in magnocellular neurosecretion cells (MNCs) of heart failure (HF) rats evokes a similar NMDA receptor-mediated current (INMDA) but a larger NMDA-mediated ΔCa2+. A: representative examples showing INMDA (top traces), and the concomitant increase in ΔCa2+ (F/F0, bottom traces), following a focal application of NMDA (50 μM, 1 s, arrows) to a MNC in a sham and HF rats. B: example of the Fluo5-loaded neuron in the sham rat before (left) and after (right) NMDA application. Note the increases Fluo-5 fluorescence following NMDA receptor activation both in the soma and a proximal dendrite (arrow). C: summary data showing the mean INMDA area (top left), ΔCa2+ area (lower left), ΔCa2+ decay time (top right) and mean ΔCa2+ area-to-INMDA area ratio (bottom right) in MNCs from sham and HF rats (n = 9 and 13, respectively). *P < 0.05 and **P < 0.01 vs. respective sham, unpaired t-tests.
Table 2.
Summary data of basic intrinsic membrane properties of MNCs in sham and HF rats
| Resting Vm, mV | Input Resistance, GΩ | Cell Capacitance, pF | Spike Threshold, mV | |
|---|---|---|---|---|
| Sham | −72.9 ± 0.8 (n = 30) | 0.51 ± 0.05 (n = 30) | 25.7 ± 0.9 (n = 48) | −45.0 ± 1.2 (n = 30) |
| HF | −73.4 ± 0.9 (n = 24) | 0.52 ± 0.04 (n = 24) | 27.3 ± 1.1 (n = 47) | −44.7 ± 1.0 (n = 24) |
Values are means ± SE; n = number of rats. Vm, membrane potential.
A major factor influencing the shape and time course of intracellular Ca2+ signal is buffering by the endoplasmic reticulum via the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump (66). Thus to determine whether the prolonged NMDA-ΔCa2+ in HF rats was due to a diminished ER Ca2+ buffering capacity, we repeated a set of experiments in slices preincubated with thapsigargin (2 μM, 45 min), an endoplasmic reticulum SERCA pump blocker (35). We found that thapsigargin significantly prolonged the duration of the NMDA-ΔCa2+, in MNCs from both sham and HF rats (ΔCa2+ decay time: sham-thapsigargin: 43.4 ± 4.9 s; HF-thapsigargin: 56.9 ± 7.4 s; n = 11 and 6, P < 0.05 vs. the respective sham and HF groups in control ACSF, see Fig. 1C for comparison). No changes in other ΔCa2+ parameters were observed (not shown).
NMDA receptor activation evokes an enhanced firing activity along with larger ΔCa2+ per action potential in HF rats.
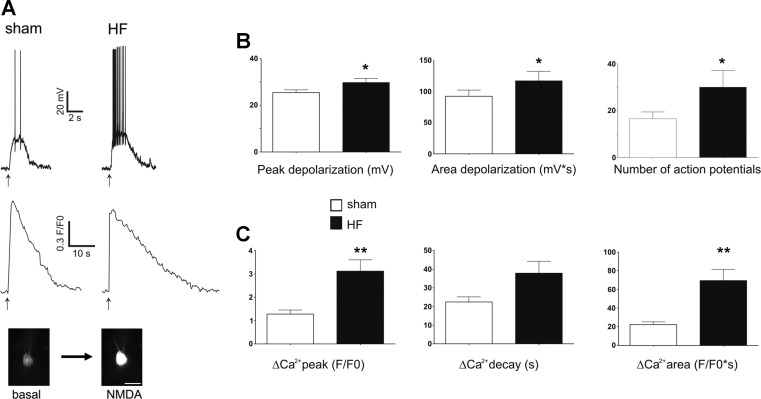
To study NMDA responses under more physiological conditions, experiments were performed also in the current-clamp mode, a condition in which NMDA receptor activation evokes membrane depolarization and firing discharge. As summarized in Table 2, no differences in resting membrane potential, input resistance, or action potential threshold were observed between sham and HF groups. Focal application of NMDA to the recording cell (10 μM, 1 s) evoked a transient membrane depolarization, an increased firing rate, and a concomitant increase in intracellular Ca2+ (Fig. 2). The degree of NMDA receptor-mediated membrane depolarization and firing discharge was significantly larger in MNCs from HF rats (P < 0.05 in both cases, see Fig. 2B, n = 30 and 24 in sham and HF rats, respectively). In a proportion of these cells, we were able to reliably quantify ΔCa2+ responses. In these MNCs (n = 16 and 18 in sham and HF rats, respectively), the magnitude and time course of the NMDA receptor-evoked ΔCa2+ was significantly larger in HF rats. Thus both the ΔCa2+ peak amplitude and area were significantly larger in HF compared with sham rats (∼145 and 210%, respectively, P < 0.001) (Fig. 2C). While a strong tendency for a slower monoexponential decay time course was observed in MNCs from HF rats, differences did not reach statistical significance (P = 0.08). Conversely, no differences in the ΔCa2+ rise time were observed between experimental groups (not shown).
Fig. 2.
NMDA receptor activation in current-clamp mode evokes an enhanced membrane depolarization, firing discharge, and ΔCa2+ in MNCs from HF rats. A: representative examples showing NMDA-mediated depolarization and firing activity (top traces) and the concomitant increase in ΔCa2+ (bottom traces), following a focal application of NMDA (10 μM, 1 s, arrows) to a MNC in a sham and HF rats. The bottom insets show the Fluo5-loaded neuron in the sham rat before and after NMDA application. B: summary data showing the mean depolarizing peak amplitude, depolarizing area, and number of evoked action potentials, respectively, following a focal NMDA application in MNCs from sham and HF rats (n = 30 and 24, respectively). C: summary data showing the mean peak ΔCa2+ amplitude, decay time, and area following a focal NMDA application in MNCs from sham and HF rats (n = 16 and 18, respectively). *P < 0.05 and **P < 0.01 vs. respective sham, unpaired t-tests. Scale bar in inset = 20 μm.
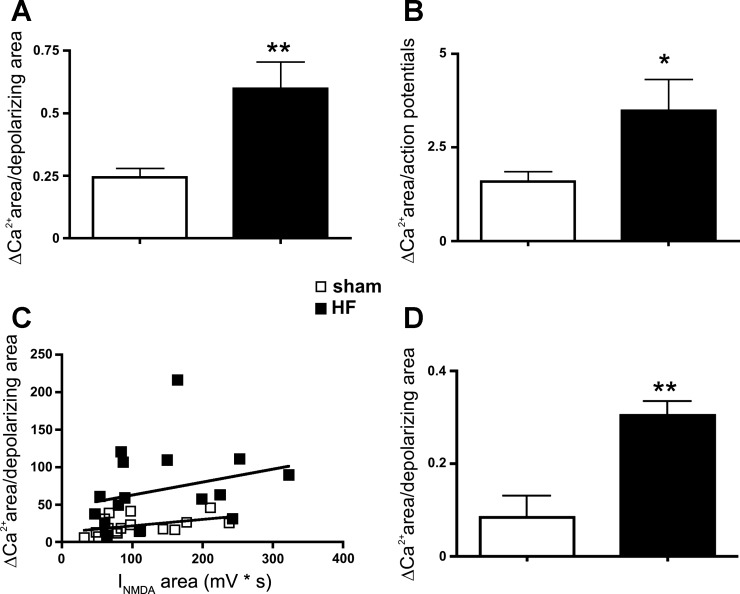
The larger NMDA-ΔCa2+ observed in HF rats could be the result of the larger depolarization and/or number of action potentials triggered by NMDA receptor activation in these rats. However, when we normalized within each MNC, the overall magnitude of the evoked ΔCa2+ by either the area of the membrane depolarization or the total number of spikes evoked, a significantly larger ΔCa2+ response was still observed in MNCs of HF rats (P < 0.01 and P = 0.05, respectively, n = 16 and 18 in sham and HF rats, respectively, Fig. 3, A and B). In addition, no significant correlation was observed between the magnitude of the NMDA-ΔCa2+ and the NMDA-ΔmV (R2 = 0.19 and 0.08 for sham and HF rats, respectively). Moreover, the enhanced NMDA-ΔCa2+ in HF rats persisted when action potentials were blocked in slices pretreated with tetrodotoxin (TTX, 1 μM) (P < 0.05, n = 7 and 4 in sham and HF rats, respectively, Fig. 3D). Finally, to verify that these changes occur in vasopressin neurons, we obtained a few recordings from endogenously fluorescent eGFP-VP MNCs. Similar to nonidentified cells, we found a significantly larger NMDA-ΔCa2+ in identified eGFP-VP neurons in HF rats [ΔCa2+ area (F/F0 × s): sham = 20.1 ± 8.9; HF: 63.3 ± 11.2, P < 0.05, n = 4 and 11, respectively], as well as larger ΔCa2+/unit of membrane depolarization (sham = 0.12 ± 0.04; HF: 0.65 ± 0.16, P < 0.05, n = 4 and 11, respectively).
Fig. 3.
Enhanced NMDA-mediated ΔCa2+ per unit of membrane depolarization or evoked action poetential in MNCs of HF rats. Summary data showing a significant increase in NMDA-mediated ΔCa2+ in MNCs from HF rats, when data were normalized either by the total area of the NMDA-mediated depolarization (A) or by the total number of evoked action potentials (B). In C, a plot of the NMDA-evoked ΔCa2+ area as a function of the NMDA-evoked depolarizing area is shown (n = 16 and 18 in sham and HF rats, respectively). D: summary data showing that the larger increase in NMDA-mediated ΔCa2+ per unit of membrane depolarization in HF rats persisted in the presence of tetrodotoxin (1 μM, n = 7 and 4 in sham and HF rats, respectively). *P = 0.05 and **P < 0.01 vs. respective sham.
Taken together, these results indicate that the enhanced NMDA-ΔCa2+ in MNCs in HF rats was not due to the larger NMDA-mediated depolarization/firing discharge. Conversely, they support an overall increased ΔCa2+ entry per unit of NMDA-mediated membrane excitation.
Evoked repetitive action potential firing activity results in similar ΔCa2+ in MNCs from sham and HF rats.
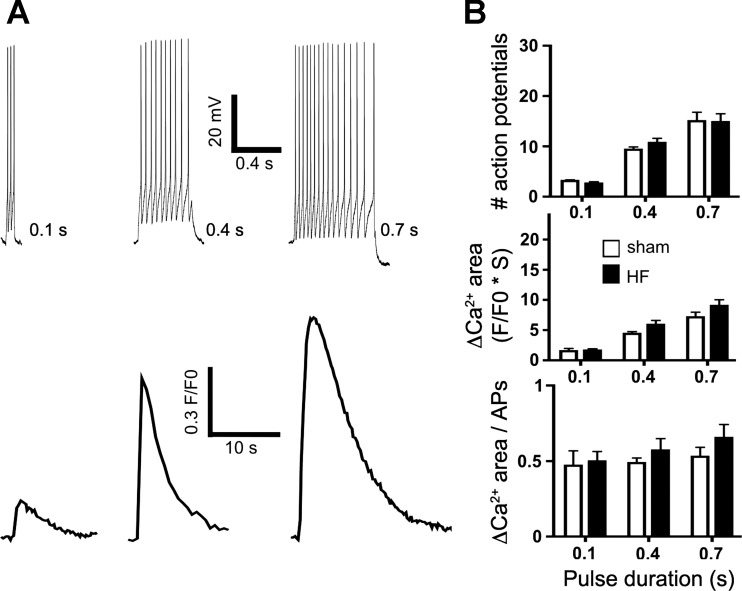
The results above suggest that NMDA receptor activation per se, independent of action potential firing, is sufficient to evoke differences in NMDA-ΔCa2+ dynamics between MNCs in sham and HF rats. To study in more details the relationship between repetitive action potential firing and Ca2+ dynamics in MNCs, repetitive firing was evoked by injecting depolarizing current pulses of incremental durations (80 pA, 0.1, 0.4, and 0.7 s), while monitoring the magnitude of the evoked ΔCa2+ (n = 9 and 10 in sham and HF, respectively). As shown in Fig. 4, the firing discharge in MNCs increased progressively in both groups as a function of the injected current (P < 0.0001, two-way ANOVA). However, no differences in the number of evoked actions potentials were observed between sham and HF rats (P = 0.5, two-way ANOVA). Similarly, the mean ΔCa2+ area increased progressively in both groups as a function of the injected current (P < 0.0001, two-way ANOVA), but no differences between groups were observed (P = 0.8, two-way ANOVA). Finally, when the magnitude of the evoked ΔCa2+ was normalized to the number of evoked action potentials for each current step, no differences were observed between experimental groups (P = 0.2, two-way ANOVA).
Fig. 4.
Repetitive action potential firing evoked by direct current injection resulted in similar ΔCa2+ in MNCs from sham and HF rats. A: representative examples of 3 bursts of action potentials evoked by progressively increasing current pulse duration (0.1, 0.4, and 0.7 s, respectively, top traces), and the respective ΔCa2+ (bottom traces) obtained from a MNCs in a HF rat. B: summary data showing mean number of evoked action potentials (top), mean changes in ΔCa2+ area (middle), and mean ΔCa2+ area/number of action potential (APs, bottom) in MNCs from sham and HF (n = 9 and 10 in sham and HF, respectively). Note the lack of differences between sham and HF rats in any of the parameters measured.
DISCUSSION
Using a combination of patch-clamp electrophysiology with simultaneous fast confocal Ca2+ imaging, we characterized NMDA-ΔCa2+ in MNCs from sham and HF rats. We show that: 1) while the magnitude of INMDA was similar between MNCs in the two experimental groups, a larger ΔCa2+ was evoked in HF rats; 2) in current-clamp mode, NMDA receptor activation evoked an enhanced membrane depolarization and firing discharge, along with a larger ΔCa2+ in HF, compared with sham rats; 3), the NMDA-ΔCa2+ per unit of membrane depolarization or per individual action potential was larger in HF rats; and 4) the number of action potentials and concomitant ΔCa2+ evoked by direct delivery of depolarizing steps of increasing durations was similar between sham and HF rats.
While an enhanced glutamate function within the SON/PVN of HF rats was previously reported (27, 29, 32, 48, 71), the precise underlying mechanisms and cellular consequences of the exacerbated glutamate function in HF rats remain to be fully elucidated. In this study, we focused on the functional efficacy of NMDA receptors and their actions on membrane excitability and intracellular Ca2+ dynamics. We report here that focal and transient activation of NMDA receptors evoked a similar NMDA-mediated inward current in MNCs from sham and HF rats. Despite a similar INMDA magnitude between the two experimental groups, a larger NMDA-ΔCa2+ was observed in HF rats. Multiple factors shape the magnitude and waveform of a Ca2+ transient such as those following NMDA receptor activation. These include the total number of NMDA receptors, the single-channel Ca2+ permeability of NMDA receptors, release of Ca2+ from intracellular stores, as well as intracellular Ca2+ buffering and cytosolic clearance mechanisms. The fact that a similar INMDA current was evoked in sham and HF rats would argue against an increased in NMDA receptor numbers or increased in Ca2+ permeability in this conditions, since Ca2+ influx is a major component mediating INMDA (38). This is in agreement with a previous study showing lack of changes in NMDA receptor NR1 subunit mRNA expression in the SON in HF rats (32).
MNCs possess numerous Ca2+ buffering/clearance mechanisms, including plasmalemmal (PMCa) and endoplasmic reticulum (ER-SERCa) Ca2+ transport ATPases, and the mitochondrial Ca2+ selective uniporter (10), all of which have been shown to efficiently shape somatic Ca2+ transients in these neurons (28, 30). These Ca2+ buffering mechanisms slowly decrease the levels of cytosolic free Ca2+, resulting in a slow decaying Ca2+ time course following the initial transient rise. Thus changes in the efficacy of Ca2+ buffering mechanisms typically affect not only the peak, but mostly the decay phase, of the Ca2+ transient. In the present study, we found that both the peak and duration of the NMDA-ΔCa2+ in MNCs from HF rats were significantly enhanced compared with sham rats, suggesting a compromised Ca2+ buffering.
To determine whether a blunted ER-SERCa buffering mechanism contributed to the prolonged NMDA-ΔCa2+ in HF rats, we compared the effects of thapsigargin (TG), a SERCa blocker, between sham and HF rats. Our results showing that TG prolonged the NMDA-ΔCa2+ signal both in sham and HF rats, indicate that the ER indeed acts as an important intracellular Ca2+ buffering mechanism shaping the NMDA-ΔCa2+ waveform. This is in agreement with previous studies showing that blockade of the ER-SERCa prolonged the decay of a K+-induced increase in Ca2+ in MNCs (30). However, the fact that TG prolonged the NMDA-ΔCa2+ waveform in both experimental groups to a similar extent would argue against a blunted ER-SERCa function during HF. Finally, in addition to acting as a Ca2+ buffering organelle, the ER can also release stored Ca2+, contributing to the overall NMDA-ΔCa2+ waveform (12). Prolonged blockade of the SERCa with TG, as performed in our study, also leads to the depletion of ER Ca2+ store (45). Thus our results showing that TG resulted in an enhancement, rather than a reduction, in the NMDA-ΔCa2+ signal indicate that Ca2+ release from the ER does not contribute to the ΔCa2+ signals following NMDA receptor activation in MNCs. Clearly, future studies will be needed to elucidate alternative mechanisms contributing to the altered NMDA receptor-mediated Ca2+ waveform in HF rats.
We observed a similar enhanced NMDA-ΔCa2+ in the current clamp mode, in which NMDA receptor activation evoked also a larger membrane depolarization along with a more pronounced firing discharge in MNCs of HF rats. Still, the larger NMDA-ΔCa2+ response persisted when action potentials were blocked by TTX, indicating that activation of NMDA receptors per se (and not the Ca2+ influx associated with action potential firing) was associated to the prolonged Ca2+ signal in HF rats. The implications of the more robust NMDA-mediated membrane depolarization and firing discharge in MNCs of HF rats is discussed further below. Notably, no differences in ΔCa2+ dynamics between sham and HF rats were observed following trains of action potentials evoked by direct membrane depolarization. In this case, most of the Ca2+ contributing to the ΔCa2+ originates from influx via voltage-gated Ca2+ channels. Thus these results suggest that Ca2+ homeostasis is not globally affected in MNCs from HF rats, but rather, that the source of Ca2+ (i.e., NMDA receptors), and their spatial proximity to specific buffering mechanism are key determining factors (46).
What are the possible downstream consequences of the enhanced ΔCa2+ following NMDA receptor activation in HF? Critical inhibitory signals within the SON and PVN, such as NO and GABA, are both Ca2+ dependent and can be influenced directly or indirectly by NMDA receptor activation (3, 50). Moreover, the efficacy of both NO and GABA actions have been shown to be diminished in HF rats, contributing in turn to neurohumoral activation in this disease. For example, a blunted NO production within the PVN is recognized as a key mechanism contributing to sympathohumoral activation in HF rats (4, 68, 73). While a diminished neuronal NO synthase and endothelial NO synthase expression has been reported in the SON/PVN of HF rats (4, 70), the possibility that an blunted NMDA-ΔCa2+ response contributes to diminished NOS activity and consequently, NO production, has not been explored. While not directly tested in this study, our results showing an enhanced, rather than a blunted, Ca2+ response would argue against this possibility. Similarly, a blunted GABAergic inhibitory function has been reported in the hypothalamus of HF rats (24, 48, 69). We recently demonstrated, both in SON MNCs and presympathetic PVN neurons, an NMDA-mediated, Ca2+-dependent potentiation of GABAA receptor function, which serves as a counterbalancing inhibitory feedback mechanism to restrain overexcitation following NMDA receptor activation (50). Importantly, this NMDA-Ca2+-GABAA coupling was blunted in MNCs from HF rats, likely contributing to NMDA-driven neurohumoral activation in HF (50). While the precise mechanisms underlying the blunted NMDA-GABAA receptor coupling are still unknown, the present results argue against a diminished ability of NMDA receptors to evoke a sufficiently large change in intracellular Ca2+ as a contributing factor.
Another important finding in this study is that NMDA receptor activation in HF, despite evoking a similar underlying INMDA current than in sham rats, resulted in a more pronounced membrane depolarization and firing discharge in the former. One possibility is that the blunted NO and GABA inhibitory mechanisms previously reported to occur during HF (4, 24, 68, 69) enabled a more robust NMDA excitatory response to be elicited in this condition. Alternatively, it is also reasonable to speculate that the enhanced NMDA-ΔCa2+ could itself contribute to altered MNCs neuronal excitability in HF rats, via interactions with Ca2+ sensitive ion channels, including Ca2+-activated K+ channels (e.g., SK and BK channels) or Ca2+-activated, nonselective cation channels (CAN) (47, 54). Both types of Ca2+-sensitive channels are expressed in MNCs and play important though opposing roles in regulating membrane excitability and firing properties in these neurons. Thus, whereas SK channels mediate an afterhyperpolarization (AHP) that acts to inhibit repetitive firing (21, 59, 64), CAN channels promote firing activity, in part via generation of fast depolarizing after potentials (fDAPs) (16, 63, 65). Importantly, both AHPs and DAPs temporally overlap, being their balance then a critical factor that determines the influence of ΔCa2+ on membrane excitability (1). Thus it is possible that in HF rats, the exacerbated NMDA-ΔCa2+ could tip the balance toward a predominant activation of CAN channels, resulting in an enhanced NMDA-mediated membrane excitability in HF rats. While such interaction between NMDA receptors and CAN channels has been recently shown in substantia nigra neurons (39), future studies addressing the coupling of NMDA-ΔCa2+ to downstream Ca2+-sensitive channels in MNCs are needed to more conclusively test their involvement in increased membrane excitability during HF.
Perspectives and Significance
Neurohumoral activation is a critical pathological process contributing to morbidity and mortality in patients with heart failure (7). Thus elucidating underlying mechanisms contributing to neurohumoral activation in this disease is of high clinical relevance. While an enhanced glutamate excitatory action has been recognized as a crucial factor in HF, the precise mechanisms contributing to elevated glutamate excitatory function remain to be determined. Activity-dependent changes in neuronal intracellular Ca2+ levels act as a critical signal capable of affecting multiple neuronal functions including regulation of membrane excitability, neurotransmitter release, neuroplasticity, and gene expression, among others (36). Glutamate NMDA receptors (NMDA receptors) are pivotal molecules that translate activity-dependent signaling between neurons into complex changes intracellular Ca2+, being thus a major source of Ca2+ in neurons, including MNCs. Results from the present study provide evidence for an enhanced NMDA-mediated increase in Ca2+ in MNCs during HF. In addition to contributing to increased membrane excitability, as supported in this study, the exacerbated NMDA-ΔCa2+ is expected to affect other critical functions in MNCs, including dendritic excitation-secretion coupling. It is well recognized that in addition to releasing their peptide content from axonal terminal in the neurohypophysis into the general circulation, MNCs can also release VP and oxytocin from their dendrites in an activity-dependent manner (34). This intranuclear dendritic peptide release serves as an efficient autocrine mechanism by which MNCs optimize their own firing activity in response to specific physiological challenges (20, 33). Moreover, recent studies from our laboratory indicate that dendritic release of VP can also act in a diffusible manner to stimulate the activity of neighboring presympathetic neurons, leading to an increased renal sympathetic nerve activity (55). Activation of NMDA receptor, and the subsequent increase in Ca2+, is a powerful mechanism that stimulates dendritic peptide release from MNCs (11). Thus it is reasonable to speculate that the enhanced NMDA-ΔCa2+ reported here during HF may lead to an exacerbated dendritic VP release, resulting in turn not only in further increased VP neuronal activity, but also in a more prominent recruitment of presympathetic neurons, both ultimately contributing to neurohumoral activation in HF. Future studies assessing on one hand the precise mechanisms underlying exacerbated NMDA-ΔCa2+ (e.g., intracellular Ca2+ buffering capacity), and on the other hand the overall impact of such changes to sympathohumoral activation at the systems level, are warranted.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute R01 HL-090948 (to J. E. Stern).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.E.S. conception and design of research; J.E.S. and E.S.P. analyzed data; J.E.S. interpreted results of experiments; J.E.S. drafted manuscript; J.E.S. edited and revised manuscript; J.E.S. approved final version of manuscript; E.S.P. performed experiments; E.S.P. prepared figures.
REFERENCES
- 1. Armstrong WE, Wang L, Li C, Teruyama R. Performance, properties and plasticity of identified oxytocin and vasopressin neurones in vitro. J Neuroendocrinol 22: 330– 342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnolda L, McGrath BP, Cocks M, Johnston CI. Vasoconstrictor role for vasopressin in experimental heart failure in the rabbit. J Clin Invest 78: 674– 679, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol 499: 733– 746, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biancardi VC, Son SJ, Sonner PM, Zheng H, Patel KP, Stern JE. Contribution of central nervous system endothelial nitric oxide synthase to neurohumoral activation in heart failure rats. Hypertension 58: 454– 463, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Botta P, de Souza FM, Sangrey T, De Schutter E, Valenzuela CF. Alcohol excites cerebellar Golgi cells by inhibiting the Na+/K+ ATPase. Neuropsychopharmacology 35: 1984– 1996, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol 369: 45– 60, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819– 823, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol 14: 318– 327, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Creager MA, Faxon DP, Cutler SS, Kohlmann O, Ryan TJ, Gavras H. Contribution of vasopressin to vasoconstriction in patients with congestive heart failure: comparison with the renin-angiotensin system and the sympathetic nervous system. J Am Coll Cardiol 7: 758– 765, 1986 [DOI] [PubMed] [Google Scholar]
- 10. Dayanithi G, Forostyak O, Ueta Y, Verkhratsky A, Toescu EC. Segregation of calcium signalling mechanisms in magnocellular neurones and terminals. Cell Calcium 51: 293– 299, 2012 [DOI] [PubMed] [Google Scholar]
- 11. de Kock CP, Burnashev N, Lodder JC, Mansvelder HD, Brussaard AB. NMDA receptors induce somatodendritic secretion in hypothalamic neurones of lactating female rats. J Physiol 561: 53– 64, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emptage N, Bliss TV, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron 22: 115– 124, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Filosa JA, Naskar K, Perfume G, Iddings JA, Biancardi VC, Vatta MS, Stern JE. Endothelin-mediated calcium responses in supraoptic nucleus astrocytes influence magnocellular neurosecretory firing activity. J Neuroendocrinol 24: 378– 392, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Finley JJt, Konstam MA, Udelson JE. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation 118: 410– 421, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 82: 1724– 1729, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Ghamari-Langroudi M, Bourque CW. Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. J Physiol 545: 537– 542, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldsmith SR, Francis GS, Cowley AW., Jr Arginine vasopressin and the renal response to water loading in congestive heart failure. Am J Cardiol 58: 295– 299, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Goldsmith SR, Francis GS, Cowley AW, Jr, Goldenberg IF, Cohn JN. Hemodynamic effects of infused arginine vasopressin in congestive heart failure. J Am Coll Cardiol 8: 779– 783, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Goldsmith SR, Francis GS, Cowley AW, Jr., Levine TB, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol 1: 1385– 1390, 1983 [DOI] [PubMed] [Google Scholar]
- 20. Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. J Neurosci 18: 1879– 1885, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greffrath W, Martin E, Reuss S, Boehmer G. Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. J Physiol 513: 493– 506, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-α blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol 293: H599– H609, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335– 346, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Han TH, Lee K, Park JB, Ahn D, Park JH, Kim DY, Stern JE, Lee SY, Ryu PD. Reduction in synaptic GABA release contributes to target-selective elevation of PVN neuronal activity in rats with myocardial infarction. Am J Physiol Regul Integr Comp Physiol 299: R129– R139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu B, Bourque CW. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol 458: 667– 687, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 106: 1763– 1774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res 83: 737– 746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim MH, Lee SH, Park KH, Ho WK. Distribution of K+-dependent Na+/Ca2+ exchangers in the rat supraoptic magnocellular neuron is polarized to axon terminals. J Neurosci 23: 11673– 11680, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863– R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Komori Y, Tanaka M, Kuba M, Ishii M, Abe M, Kitamura N, Verkhratsky A, Shibuya I, Dayanithi G. Ca(2+) homeostasis, Ca(2+) signalling and somatodendritic vasopressin release in adult rat supraoptic nucleus neurones. Cell Calcium 48: 324– 332, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol 586: 1637– 1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990– 997, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci 9: 2532– 2540, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7: 126– 136, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266: 17067– 17071, 1991 [PubMed] [Google Scholar]
- 36. Malenka RC, Kauer JA, Perkel DJ, Nicoll RA. The impact of postsynaptic calcium on synaptic transmission–its role in long-term potentiation. Trends Neurosci 12: 444– 450, 1989 [DOI] [PubMed] [Google Scholar]
- 37. Mancia G. Sympathetic activation in congestive heart failure. Eur Heart J 11, Suppl A: 3–11, 1990 [DOI] [PubMed] [Google Scholar]
- 38. McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiol Rev 74: 723– 760, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Mrejeru A, Wei A, Ramirez JM. Calcium-activated non-selective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J Physiol 589: 2497– 2514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakamura Y, Haneda T, Osaki J, Miyata S, Kikuchi K. Hypertrophic growth of cultured neonatal rat heart cells mediated by vasopressin V(1A) receptor. Eur J Pharmacol 391: 39– 48, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Nicod P, Waeber B, Bussien JP, Goy JJ, Turini G, Nussberger J, Hofbauer KG, Brunner HR. Acute hemodynamic effect of a vascular antagonist of vasopressin in patients with congestive heart failure. Am J Cardiol 55: 1043– 1047, 1985 [DOI] [PubMed] [Google Scholar]
- 42. Nissen R, Hu B, Renaud LP. Regulation of spontaneous phasic firing of rat supraoptic vasopressin neurones in vivo by glutamate receptors. J Physiol 484: 415– 424, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77: 721– 730, 1988 [DOI] [PubMed] [Google Scholar]
- 44. Packer M, Lee WH, Kessler PD, Gottlieb SS, Bernstein JL, Kukin ML. Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation 75: IV80– IV92, 1987 [PubMed] [Google Scholar]
- 45. Paschen W, Doutheil J, Gissel C, Treiman M. Depletion of neuronal endoplasmic reticulum calcium stores by thapsigargin: effect on protein synthesis. J Neurochem 67: 1735– 1743, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Peng TI, Greenamyre JT. Privileged access to mitochondria of calcium influx through N-methyl-d-aspartate receptors. Mol Pharmacol 53: 974– 980, 1998 [PubMed] [Google Scholar]
- 47. Petersen OH. Cation channels: homing in on the elusive CAN channels. Curr Biol 12: R520– R522, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Potapenko ES, Biancardi VC, Florschutz RM, Ryu PD, Stern JE. Inhibitory-excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J Neurophysiol 106: 1545– 1557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate trasporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol 303: R291– R300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Astrocytes modulate a postsynaptic NMDA-GABAA-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci 33: 631– 640, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience 7: 773– 808, 1982 [DOI] [PubMed] [Google Scholar]
- 52. Riegger GA, Liebau G, Bauer E, Kochsiek K. Vasopressin and renin in high output heart failure of rats: hemodynamic effects of elevated plasma hormone levels. J Cardiovasc Pharmacol 7: 1– 5, 1985 [DOI] [PubMed] [Google Scholar]
- 53. Rouleau JL, Packer M, Moye L, de Champlain J, Bichet D, Klein M, Rouleau JR, Sussex B, Arnold JM, Sestier F, Parker JO, McEwan P, Bernstein V, Cuddy TE, Lamas G, Gottlieb SS, McCans J, Nadeau C, Delage F, Wun CC, Pfeffer MA. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: effect of captopril. J Am Coll Cardiol 24: 583– 591, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345– 353, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Son SJ, Stern JE. Locally released vasopressin increases presympathetic PVN neuronal activity. FASEB J 22: 952– 918, 2008 [Google Scholar]
- 56. Sonner PM, Filosa JA, Stern JE. Diminished A-type potassium current and altered firing properties in presympathetic PVN neurones in renovascular hypertensive rats. J Physiol 586: 1605– 1622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sonner PM, Lee S, Ryu PD, Lee SY, Stern JE. Imbalanced K+ and Ca2+ subthreshold interactions contribute to increased hypothalamic presympathetic neuronal excitability in hypertensive rats. J Physiol 589: 667– 683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537: 161– 177, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. J Physiol 488: 701– 708, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410– 417, 1980 [DOI] [PubMed] [Google Scholar]
- 61. Swenson KL, Badre SE, Morsette DJ, Sladek CD. N-methyl-d-aspartic acid stimulation of vasopressin release: role in osmotic regulation and modulation by gonadal steroids. J Neuroendocrinol 10: 679– 685, 1998 [DOI] [PubMed] [Google Scholar]
- 62. Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med 305: 263– 266, 1981 [DOI] [PubMed] [Google Scholar]
- 63. Teruyama R, Armstrong WE. Calcium-dependent fast depolarizing afterpotentials in vasopressin neurons in the rat supraoptic nucleus. J Neurophysiol 98: 2612– 2621, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol 566: 505– 518, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Teruyama R, Sakuraba M, Kurotaki H, Armstrong WE. Transient receptor potential channel m4 and m5 in magnocellular cells in rat supraoptic and paraventricular nuclei. J Neuroendocrinol 23: 1204– 1213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Toescu EC, Verkhratsky A. Principles of Calcium Signalling. New York: Plenum, 1998 [Google Scholar]
- 67. Ueta Y, Fujihara H, Serino R, Dayanithi G, Ozawa H, Matsuda K-i, Kawata M, Yamada J, Ueno S, Fukuda A, Murphy D. Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology 146: 406– 413, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol 281: H995– H1004, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006– R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 288: H2332– H2341, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension 58: 966– 973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-d-aspartate receptors. J Comp Neurol 484: 43– 56, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol 84: 217– 232, 2004 [DOI] [PubMed] [Google Scholar]