Abstract
The second most abundant cation in seawater (SW), Mg2+, is present at concentrations of ∼53 mM. Marine teleosts maintain plasma Mg2+ concentration at 1–2 mM by excreting Mg2+ into the urine. Urine Mg2+ concentrations of SW teleosts exceed 70 mM, most of which is secreted by the renal tubular epithelial cells. However, molecular mechanisms of the Mg2+ secretion have yet to be clarified. To identify transporters involved in Mg2+ secretion, we analyzed the expression of fish homologs of the Slc41 Mg2+ transporter family in various tissues of SW pufferfish torafugu (Takifugu rubripes) and its closely related euryhaline species mefugu (Takifugu obscurus). Takifugu genome contained five members of Slc41 genes, and only Slc41a1 was highly expressed in the kidney. Renal expression of Slc41a1 was markedly elevated when mefugu were transferred from fresh water (FW) to SW. In situ hybridization analysis and immunohistochemistry at the light and electron microscopic levels revealed that Slc41a1 is localized to vacuoles in the apical cytoplasm of the proximal tubules. These results suggest that pufferfish Slc41a1 is a Mg2+ transporter involved in renal tubular transepithelial Mg2+ secretion by mediating Mg2+ transport from the cytosol to the vacuolar lumen, and support the hypothesis that Mg2+ secretion is mediated by exocytosis of Mg2+-rich vacuoles to the lumen.
Keywords: magnesium transporter, proximal tubule, renal secretion, seawater fish, gene expression
seawater (sw) contains ∼53 mM Mg2+, 27 mM SO42−, 10 mM Ca2+, and 10 mM K+, as well as ∼450 mM NaCl, Mg2+ being the second most abundant cation. In the hyperosmotic environment of the sea (∼1,000 mOsm/kg), marine teleosts maintain a plasma osmotic pressure of ∼370 mOsm/kg (38). To balance passive water loss and salt gain across the gills and skin, marine teleosts drink seawater (SW), absorb water, and eliminate salts. Divalent ions (Mg2+, SO42−, Ca2+) are excreted by the kidney in a low volume of urine (4, 26, 49), and monovalent ions Na+, Cl−, and K+ are excreted from ionocytes (chloride cell, mitochondrion-rich cell) in the gills (12).
In marine teleosts, plasma Mg2+ concentration is at a level similar to that of freshwater (FW) fish and is maintained between 1 and 2 mM (5, 9). The difference in Mg2+ concentration between plasma and environmental SW is 30–50-fold. Thus, marine teleosts are at the risk of exposure to excess Mg2+, which enters via integuments in the gills and intestine. In the intestine, much of the ingested Mg2+ and Ca2+ are precipitated as carbonates that are rectally excreted (36, 57), while a small part of Mg2+ is absorbed by the intestinal mucosa (7, 8, 25). Bladder urine Mg2+ concentration of marine teleosts is 57–167 mM (4, 26, 49), and urine/plasma ratios for Mg2+ can exceed 100. In glomerular or aglomerular marine teleost, renal tubular fluid secretion accounts for much or all initial urine production, respectively, and the renal proximal tubule is the major site of active fluid secretion (4, 5). Analyses of the fluid secreted in the isolated proximal tubule demonstrated that the fluid contains ∼22 mM Mg2+ (3, 6), and the exceedingly high Mg2+ concentration in the bladder urine is accomplished by volume reduction of the initial urine in the distal nephron and urinary bladder (4–5). Ion microscopy imaging studies on intracellular distribution of the exogenously injected stable isotope 26Mg observed punctate distribution of injected 26Mg in the proximal tubular cells of SW-acclimated killifish (10). These results indicate that Mg2+ is secreted by a transcellular pathway, and Mg2+-rich intracellular vesicles mediate the Mg2+ secretion. Basolateral uptake of Mg2+ from peritubular fluid is considered to be energetically downhill because of the negative membrane potential of the cell, but extrusion from the cytoplasm into the lumen is uphill against concentration and voltage (4, 5). However, little is known about which proteins are involved in renal Mg2+ excretion by SW fish.
Genetic analyses of bacterial strains of Salmonella typhimurium and Escherichia coli, which require high Mg2+ (10–100 mM) for growth, identified CorA, MgtA, MgtB, and MgtE as Mg2+ transport systems (27, 50, 51). Genetic analyses of the same group of Salmonella typhimurium strains that are resistant to Co2+ identified CorB, CorC, and CorD as Mg2+/Co2+ transport systems (14). In bacteria, CorA is the dominant transporter mediating as much as 99% of the total Mg2+ accumulated (27, 51). The yeast and mammalian homolog of bacterial CorA were identified as Mrs2p and MRS2, respectively, and were characterized as the inner mitochondrial membrane Mg2+ channels (35, 47). Mammalian homologs of MgtE consist of three proteins (solute carrier Slc41a1–3) and were characterized as plasma-membrane Mg2+ transporters (19, 20, 33, 34, 45, 46). CorC homologs in mammals were identified as ACDP (ancient conserved domain protein) or CNNM (cyclin M) 1–4 (18, 52). Analyses of two TRP (transient receptor potential) channel family members, TRPM6 (CHAnnel Kinase, CHAK2) and TRPM7 (CHAK1; family of Long TRP Channel, LTRPC7), revealed that they are plasma-membrane divalent cation channels that mediate Mg2+ influx and are inhibited by intracellular Mg2+ (48). Microarray analysis of mouse renal epithelial cells identified novel groups of Mg2+ transporters, namely, MagT (magnesium transporter) (22, 59), NIPA (nonimprinted in Prader-Willi/Angelman syndrome) (15–16), MMgTs (membrane Mg2+ transporters) (21), and HIP14 (Huntingtin-interacting protein) (17) as transcripts whose expression levels are upregulated under a low Mg2+ medium.
To identify candidate transporters that mediate Mg2+ secretion in the renal proximal tubules, two very closely related Takifugu species, the seawater tiger puffer (Takifugu rubripes; torafugu) and the euryhaline river puffer (Takifugu obscures; mefugu), were used (29–31). The genome sequence of torafugu is available (2) and enabled us to identify homologs of ion transporters exhaustively by genome database mining. The nucleotide sequences of mefugu genes are 99% identical to those of torafugu (31, 58). Tissue distribution analyses of SW torafugu, SW mefugu, and FW mefugu enabled us to identify genes that are highly expressed in the kidney of SW-acclimated animals as candidate genes responsible for SW acclimation. As the first study of renal Mg2+ transporters in marine teleosts, we report on the Slc41 family of pufferfishes because one of the family members, Slc41a1, was found to be highly expressed in the renal tubules of the pufferfish kidney by the expression analyses in the present study. Moreover, a recent study by others has shown that human Slc41A1 is a Na+/Mg2+ exchanger involved in Mg2+ efflux system of the cell (34). In this study, we identify Slc41a1 as a renal Mg2+ transporter that is localized to vacuoles in the apical cytoplasm of the proximal tubules and propose Slc41a1 as a candidate that mediates Mg2+ transport from the cytosol to the lumen, which is exocytosed into the urine of marine teleost.
MATERIALS AND METHODS
Experimental animals.
The animal protocols and procedures were approved by the Institutional Animal Care and Use Committee of the Tokyo Institute of Technology and conform to the American Physiological Society's Guiding Principles in the Care and Use of Laboratory Animals (1). Mefugu (T. obscurus) were purchased from a local dealer and held in 150-liter indoor tanks containing brackish water (3–14% diluted seawater) until use. For freshwater samples, mefugu were transferred to 150-liter freshwater tanks and held for 7–9 days before sample collection. For seawater samples, mefugu in freshwater tanks were transferred to 150-liter seawater tanks and acclimated for 7–9 days. Torafugu (T. rubripes) were purchased from a local dealer and held in 150-liter indoor tanks containing seawater until use. The water temperature was maintained at 18–22°C. The fish were fed daily with commercial fish pellets. Artificial seawater (Rohto-Marine) was obtained from Rei-Sea (Tokyo, Japan). The experimental animals were anesthetized by immersion in 0.1% ethyl m-aminobenzoate (MS-222; tricaine) before being killed. After decapitation, the tissues were dissected, snap-frozen in liquid nitrogen, and stored at −80°C until use.
Quantitative determination of serum and urine magnesium concentration.
Sera magnesium concentrations of mefugu were determined by the xylysine blue method, as described previously (31). Bladder urine was collected from SW and FW mefugu. Urine (50 μl) was transferred to 8-ml Teflon tubes (Nalgene, Tokyo, Japan), and 1 ml of concentrated nitric acid was added. Samples were digested at 90°C for 30 min then 120°C for 3 h, and left to complete dryness at 90°C. The residues were dissolved in 0.08 M nitric acid containing 5 μg/ml Be. Concentrations of magnesium in bladder urine of SW mefugu and torafugu were measured by inductively coupled plasma atomic emission spectrometry (ICP-AES; model ICPS-8100; Shimazu, Kyoto, Japan) with Be as an internal standard. Concentrations of magnesium in bladder urine of FW mefugu were measured by inductively coupled plasma mass spectrometry (ICP-MS, model ELAN DRC-e; Perkin Elmer, Waltham, MA) with Be as an internal standard. Differences among mean magnesium concentrations were assessed by ANOVA, and the 0.05 level of significance was established by the Tukey-Kramer post hoc test using GraphPad Prism software (GraphPad, San Diego, CA).
RNA isolation and molecular cloning.
Total RNA was isolated from various tissues by acidic guanidinium thiocyanate-phenol-chloroform extraction with Isogen (Nippon Gene, Tokyo, Japan), as previously described (29, 36, 41). Tissues were homogenized in Isogen (1 g of tissue per 10 ml of Isogen) with a Polytron tissue homogenizer, followed by guanidinium thiocyanate-phenol-chloroform extraction, isopropanol precipitation, and 70% (vol/vol) ethanol washing of precipitated RNA. The RNA was dissolved in diethyl pyrocarbonate-treated water, and its concentration was measured spectrophotometrically at 260 nm.
Genes encoding torafugu Slc41s were identified by mining a torafugu genomic database (http://genome.jgi-psf.org/Takru4/Takru4.home.html). Partial and full-length cDNAs of torafugu and mefugu Slc41s were obtained by RT-PCR using primers designed and based on the genomic database (Table 1), and the products were sequenced using BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA).
Table 1.
List of primers used for PCR amplification
| Gene | Species | Sequence | Remark |
|---|---|---|---|
| Slc41a1 | Mefugu/Torafugu | TGGAGGGAACTTGGTTGCAG | RT-PCR(S) |
| Mefugu/Torafugu | ACCAGCACATGTGGAAGCAG | RT-PCR (AS) | |
| Mefugu/Torafugu | AATGGTACCATGGTACTTTGGTCGAAAGAACAAG | Full length cloning (S) | |
| Mefugu/Torafugu | TTACTCGAGTCAGTTTCCCGGGATCGGG | Full length cloning (AS) | |
| Mefugu/Torafugu | CTTCCTCTACACCATCAACTC | Real-time PCR (S) | |
| Mefugu/Torafugu | CCATGTAGAGAAGGATCATCAC | Real-time PCR (AS) | |
| Slc41a2a | Mefugu/Torafugu | CCTCTACAAGTGCCTGGATAC | RT-PCR(S) |
| Mefugu/Torafugu | CCAGTCCGCTATACACAAGAG | RT-PCR (AS) | |
| Torafugu | GCGCATCATGAGTGGTCCAAGACTGGGTGC | Full length cloning (S) | |
| Torafugu | GGGGTTGTCAGTCCCCCACATCGCTGTCCC | Full length cloning (AS) | |
| Mefugu | CAATGTAGCCACGCCCATT | Partial cloning (S) | |
| Mefugu | CGTCAGGTACGGGATGGAA | Partial cloning (AS) | |
| Slc41a2b | Mefugu/Torafugu | TCCAGCCAGTCAGGCTCTAC | RT-PCR(S) |
| Mefugu/Torafugu | CTCCAAGGGCTGTGAGATAGGG | RT-PCR (AS) | |
| Torafugu | TCCTAACATGCCAGAGTACACTGAGGCAGA | Full length cloning (S) | |
| Torafugu | TGTTACTTCATAGACTTCCGTGATCACCAA | Full length cloning (AS) | |
| Mefugu | ATCTCCTCACTCTCGCCTTG | Partial cloning (S) | |
| Mefugu | TCATAGACTTCCGTGATCACCAA | Partial cloning (AS) | |
| Slc41a3a | Mefugu/Torafugu | CTTGGGTGATTTGATCACAC | RT-PCR(S) |
| Mefugu/Torafugu | ATGAAGGCTTCAGAGATAGG | RT-PCR (AS) | |
| Torafugu | TGTCACCATGATTGAAGATAAGCCACCACA | Full length cloning (S) | |
| Torafugu | TGGAGCTTCAGAGGGCAAAGCTCTGAACCA | Full length cloning (AS) | |
| Mefugu | GGTCTGGTGATGATTGCAGTAATC | Partial cloning (S) | |
| Mefugu | GCAAATACGTTTACCTGCAGCA | Partial cloning (AS) | |
| Slc41a3b | Mefugu/Torafugu | CCTGCTTCTGATACCAGCCT | RT-PCR(S) |
| Mefugu/Torafugu | TCACCGAGAGCGGTGAGGTA | RT-PCR (AS) | |
| Torafugu | ATCAGACATGGTGGTCACCCATCTGGCTCT | Full length cloning (S) | |
| Torafugu | ACGCGTGTCAAAAATCCGTCCCGGCAGAAT | Full length cloning (AS) | |
| Mefugu | TCACAGAGACATGTGGTTCCTT | Partial cloning (S) | |
| Mefugu | TCAAAAATCCGTCCCGGCAG | Partial cloning (AS) | |
| GAPDH | Mefugu/Torafugu | GGCCCAATGAAAGGCATTCT | Real-time PCR (S) |
| Mefugu/Torafugu | TGGGTGTCGCCGTTGAA | Real-time PCR (AS) | |
| β-actin | Mefugu/Torafugu | AGCGTGGGTACTCCTTCACTAC | RT-PCR(S) |
| Mefugu/Torafugu | TCGTACTCCTGCTTGCTGATCC | RT-PCR (AS) |
S, sense primer; AS, antisense primer.
Phylogenetic analysis.
Genes encoding Slc41s of other species were identified by mining Ensembl gene predictions (http://www.ensembl.org/index.html). The phylogenic relationship between the amino acid sequences of the fish and mammalian Slc41s was analyzed using ClustalW software (55). A phylogenetic tree was constructed using MEGA software based on the maximum likelihood method with 200 bootstrap replicates (53).
Semiquantitative RT-PCR.
First-strand complementary DNA was synthesized by reverse transcribing 5 μg total RNA using an oligo(dT) primer and the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA), and then diluted eight times with nuclease-free water. The cDNA (1 μl of the SuperScript III reaction) was used as the template for PCR with the specific set of primers of each gene (Table 1). These primers anneal to cDNAs encoding mefugu and torafugu Slc41s. Each reaction consisted of 1 μl of cDNA, 1.5 μM of each primer, 12.5 μl of GoTaq Green Master Mix (2×; Promega, Madison, WI) and nuclease-free water in a final volume of 25 μl. The PCR reaction conditions were as follows: 27 or 32 cycles of initial denaturation (94°C, 2 min), denaturation (94°C, 15 s), annealing (55°C, 30 s), extension (72°C, 1 min), and a final extension (72°C, 10 min). After PCR amplification, 5 μl of each reaction mixture was run on a 1.5% agarose gel in Tris·HCl/acetic acid/EDTA buffer. The gel was stained with 0.5 μg/ml ethidium bromide, and the fluorescence image was analyzed with an Image Station 2000R system (Eastman Kodak, Rochester, NY).
Quantitative real-time PCR.
For the quantification of mRNA levels by real-time PCR, total RNAs were extracted from the kidney of mefugu acclimated to seawater and freshwater (n = 5 for each group). RNA (5 μg) was used as a template for the reverse transcription using oligo(dT) primer and the SuperScript III first-strand synthesis system (Invitrogen). After reverse transcription, the cDNAs were amplified by Slc41a1 and GAPDH primers. Reference gene GAPDH sequence was identified by mining the torafugu genome database (http://genome.jgi-psf.org/Takru4/Takru4.home.html) and Slc41a1 primers obtained from the mefugu sequence. Reactions were performed with the SYBR Green method using SYBR Premix Ex Taq II Kit (Takara Bio, Otsu, Japan) on a Thermal Cycler Dice real-time system (Takara Bio). The optimized 25 μl PCR mixture contained SYBR Premix Ex Taq II (12.5 μl), 900 nM forward and reverse primers (Table 1), and template DNA (1 μl), and the reactions performed in a 96-well plate (Applied Biosystems). Thermal cycling conditions included predenaturation for 5 min at 95°C, followed by 40 cycles of 15 s denaturation at 95°C, annealing at 57°C for 30 s, and final extension at 95°C for 30 s. Melt curve analysis was implemented on SYBR Green real-time PCR assays to verify specificity by ramping the temperature from 65°C to 95°C at a rate of 0.1°C/s. For each assay, the threshold cycle (Ct) value, defined as the PCR cycle at which the fluorescence signal increases above the background threshold, was determined to quantify each mRNA product. The reference gene, GAPDH mRNA was stably expressed and mRNA concentrations of Slc41a1 were normalized to GAPDH levels. Experiments were performed in duplicate. Data are expressed as means ± SE, and statistically analyzed by Student's t-test.
Antibody production and specificity.
Polyclonal antisera were made in rabbits that had been immunized with BSA-conjugated synthetic peptides corresponding to parts of torafugu/mefugu Slc41a1 (amino acid residues 92–106, CRANAKGQREEDALL; amino acid residues 417–431, NGVPMGDPNPTSRKC). Antibody specificity was established by staining COS7 cells exogenously expressing mefugu (mf)Slc41a1. For expression in mammalian cells, full-length cDNA of mfSlc41a1 was subcloned into the KpnI and XhoI site of pcDNA3.1 vector (Invitrogen). COS7 cells were transfected with plasmids encoding mfSlc41a1 or an empty vector (mock transfection) using Lipofectamine LTX (Invitrogen), as described previously (29, 37). For immunofluorescence experiments, at 36 h after transfection, the cells were fixed and stained with anti-mfSlc41a1 or preimmune serum (1:1,000) for 1 h at room temperature, as described previously (29, 37). Cells were then treated with Alexa Fluor 488-labeled secondary antibody (1:2,000; Invitrogen) and Hoechst 33342 (100 ng/ml; Invitrogen) for 1 h at room temperature. Fluorescence images were acquired with a laser confocal microscope (model TCS-SPE; Leica, Wetzlar, Germany) by using a fixed setting and processed with LAS AF software (Leica).
Western blotting of the membrane fractions from mfSlc41a1-overexpressing or mock transfected COS7 cells was performed, as described previously (29, 40). The membrane proteins (1 μg) were separated by SDS-PAGE using 10% polyacrylamide gel and electroblotted onto a PVDF membrane. The PVDF membrane was probed with anti-mfSlc41a1 or preimmune serum at 1:1,000 dilution for 12 h at 4°C. Bound antibodies were detected using peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and chemiluminescent substrate solution (Immobilon Western, Millipore, Billerica, MA) and the signals were captured using Image Station 2000R.
In situ hybridization.
Kidney from SW mefugu was perfused and fixed with 10% buffered neutral formalin (Muto Pure Chemicals, Tokyo, Japan), harvested, embedded in paraffin, and sectioned (4 μm). cDNA of mfSlc41a1 (129–609 nucleotides, 481 bp) was used as the template to prepare digoxigenin (DIG)-labeled riboprobes. A DIG RNA-labeling mix (Roche Diagnostics, Mannheim, Germany) was used to synthesize DIG-labeled sense and antisense probes. Alkaline phosphatase-conjugated anti-DIG antibodies, nitro blue tetrazolium chloride, and 5-bromo-4-chloro-3-indolyl phosphate substrates were used to visualize the signal; the sample was then counterstained with Kernechtrot (Muto Pure Chemicals, Tokyo, Japan). Serial sections were stained with hematoxylin and eosin or anti-Na+/K+-ATPase antibodies, as previously described (32). Images were acquired using TOCO automatic virtual slide system (Claro, Hirosaki, Japan).
Immunohistochemistry.
Immunohistochemical analyses of frozen sections (6 μm) of SW mefugu kidney were performed, as described previously (29). The sections were reacted with anti-mfSlc41a1 or preimmune serum (1:2,000) for 8 h at room temperature. Sections were then washed with PBS and stained with Alexa Fluor 488-labeled anti-rabbit secondary antibodies (1:2,000; Invitrogen), tetramethylrhodamine isothiocyanate (TRITC)-labeled phalloidin (0.15 μM; Sigma-Aldrich), and Hoechst 33342 (100 ng/ml; Invitrogen). The fluorescence images were obtained as described above. Immunohistochemistry staining was also performed using the avidin-biotin-peroxidase complex (ABC) technique, as described previously (40) using anti-mfSlc41a1 or preimmune serum (1:10,000). The bound peroxidase was demonstrated using 3, 3'-diaminobenzidine (DAB).
Transmission electron microscopy.
Kidney from SW mefugu was perfused, fixed with 2% (wt/vol) paraformaldehyde, and postfixed with 0.5% glutaraldehyde. The kidney sections were treated with anti-mfSlc41a1 or preimmune serum (1:10,000), and the specific signals were visualized using an ABC kit (Vector Laboratories, Burlingame, CA) and DAB staining. After washing, the sections were incubated in 1% OsO4, washed, dehydrated in an ethanol series, and flat-embedded in epoxy resin. Ultrathin sections were cut and examined with an electron microscope (model H-7500; Hitachi, Tokyo, Japan).
Expression of Slc41a1 in Xenopus oocytes and immunohistochemistry.
The entire coding region of mfSlc41a1 cDNA was inserted into the pGEMHE Xenopus laevis expression vector. The plasmid was linearized with NotI, and cRNAs were transcribed in vitro using the T7 mMessage mMachine kit (Ambion, Austin, TX). X. laevis oocytes were dissociated with collagenase and injected with 50 nl of water or a solution containing cRNA at 0.5 μg/μl (25 ng/oocyte), as previously described (44). Oocytes were incubated at 16°C in OR3 medium, and studied 3 or 4 days after injection. Frozen sections (6 μm) of oocytes were prepared and incubated with anti-mfSlc41a1 or preimmune serum (1:1,000), as described previously (29, 37), and the bound antibody was detected with Alexa Fluor 488-labeled secondary antibody. Fluorescence images were obtained as described above.
RESULTS
Determination of magnesium in urine and serum of mefugu and torafugu.
Bladder urine and serum magnesium concentrations from SW torafugu, SW mefugu, and FW mefugu were shown in Table 2. Bladder urine magnesium concentrations of SW pufferfish were 94–140 Mm, while that of FW mefugu was ∼4 mM. Serum magnesium concentrations of SW torafugu, SW mefugu, and FW mefugu were as low as 1–2 mM.
Table 2.
Magnesium concentrations of sera and bladder urine from mefugu
| Torafugu (SW) | Mefugu (SW) | Mefugu (FW) | |
|---|---|---|---|
| Serum Mg, mM | 1.0 ± 0.1 | 1.8 ± 0.1 | 1.3 ± 0.1 |
| Urine Mg, mM | 140.0 ± 4.4 | 93.9 ± 12.6 | 3.9 ± 1.1* |
Values are expressed as means ± SE; n = 3 or 4. Serum magnesium concentrations of mefugu were measured as previously described by Kato et al. (31).
P < 0.05.
Identification of pufferfish Slc41 family members and phylogenetic analysis.
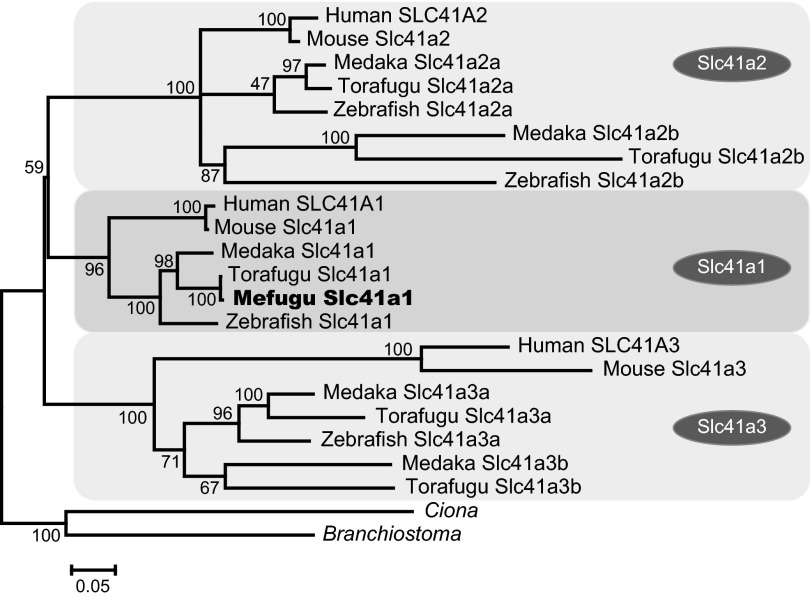
A phylogenetic tree was constructed to analyze the relationship between the torafugu Slc41 genes and related genes from other organisms (Fig. 1). In the torafugu genome, one Slc41a1 gene, two Slc41a2 genes, and two Slc41a3 genes were identified. Paralogs for Slc41a2 and Slc41a3 were also identified in the genome databases of Tetraodon nigroviridis (data not shown), Gasterosteus aculeatus (stickleback) (data not shown), and Oryzias latipes (medaka) (Fig. 1), but not in those of mammals, Gallus gallus and Xenopus tropicalis (data not shown), suggesting that those paralogs had been generated by fish-specific, whole-genome duplication or gene duplication. Zebrafish genome database had paralogs for Slc41a2 but not for Slc41a3, suggesting that zebrafish lacked Slc41a3b after the speciation.
Fig. 1.
Identification of Takifugu Slc41 family. A: phylogenetic analysis of the Slc41 family. The tree was generated using the maximum likelihood (ML) method with ClustalW (55) and MEGA5 (53). The numbers show bootstrap values (200 replications). The scale bar represents a genetic distance of 0.05 amino acid substitutions per site. Mefugu (Takifugu obscurus) Slc41a1 is indicated by a black circle. The GenBank accession numbers of the amino acid sequences of Slc41a1 are as follows: human Slc41A1, NM_173854.4; human Slc41A2, NM_032148; human Slc41A3, NM_001008485; mouse Slc41a1, NM_173865.3; mouse Slc41a2, NM_177388; mouse Slc41a3, NM_027868; zebrafish Slc41a1, XM_002663821; zebrafish Slc41a2a, XM_689511; zebrafish Slc41a2b, XM_688250; zebrafish Slc41a3, XM_002666507; torafugu (Takifugu rubripes) Slc41a1, AB700621; torafugu Slc41a2a, AB700622; torafugu Slc41a2b, AB700623; torafugu Slc41a3a, AB700624; torafugu Slc41a3b, AB700625; medaka (Oryzias latipes) Slc41a1, ENSORLG00000012106; medaka Slc41a2a, ENSORLG00000011939; medaka Slc41a2b, ENSORLG00000016413; medaka Slc41a3a, ENSORLG00000001156; medaka Slc41a3b, ENSORLG00000011740; Ciona intestinalis, XM_002122016; Lancelet (Branchiostoma floridae), XM_002606624; and mefugu Slc41a1, AB700626.
Full-length cDNA for torafugu Slc41s were isolated by RT-PCR from the kidney (Slc41a1, Slc41a2b), gill (Slc41a2a), skeletal muscle (Slc41a3a), and heart (Slc41a3b) (DDBJ/GenBank/EMBL accession numbers: AB700621-AB700625). Multiple sequence alignments of torafugu and human Slc41s are shown in Fig. 2. We also isolated full-length or partial cDNAs for mefugu Slc41s and determined the sequences (DDBJ/GenBank/EMBL numbers: AB700626-AB700630). Eleven transmembrane domains are conserved among Slc41s (overlines in Fig. 2). In the cytoplasmic regions of all Slc41s, there were sequences that match the consensus sequence for the PKC phosphorylation site [-S/T-X-R/K-, where X is any amino acid (42)] (red filled boxes in Fig. 2). The consensus sequences for PKA [-R/K-R/K-X-S/T-, where X is any amino acid (54)] were observed in the cytoplasmic region of fugu Slc41a3a only (blue-filled boxes in Fig. 2). Amino-terminal cytoplasmic regions were not well conserved among the family members except for a 40-amino-acid residue region of Slc41a1 (open green boxes in Fig. 2). PX6GN and P(D/A)X4PX6D motifs (56) were conserved among all fugu Slc41 members (green filled boxes in Fig. 2).
Fig. 2.
Multiple alignments of Takifugu and human Slc41s. Conserved or semiconserved amino acid residues are shaded. Putative phosphorylation sites for PKC (-S/T-X-R/K-) and PKA (-R/K-R/K-X-S/T-) are shown in red and blue filled boxes, respectively. Transmembrane domains are overlined and numbered.
Tissue distribution of pufferfish Slc41s and selection of renal magnesium transporter.
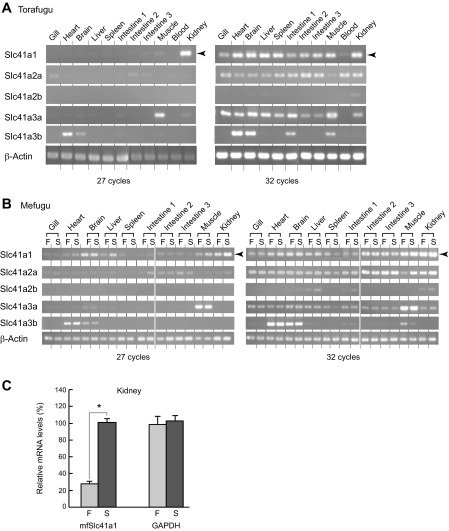
Tissue distribution of torafugu Slc41s was analyzed by semiquantitative RT-PCR (Fig. 3). The primers were designed to anneal to Slc41 cDNAs of both mefugu and torafugu (Table 1). In the torafugu kidney, only Slc41a1 was highly expressed (Fig. 3A, left, 27 cycles). Slc41a3a and Slc41a3b were highly expressed in the skeletal muscle and heart, respectively (Fig. 3A, left). Slc41a1, Slc41a2a, and Slc41a3a were ubiquitously expressed at low levels (Fig. 3A, right, 32 cycles).
Fig. 3.
Expression of Slc41 family in seawater and euryhaline pufferfishes. A and B: expressions of mRNAs encoding Slc41s were analyzed in the seawater pufferfish (torafugu, Takifugu rubripes) and euryhaline pufferfish (mefugu, Takifugu obscurus) using semiquantitative RT-PCR. β-actin was used as an internal control. F, freshwater; S, seawater. C: real-time PCR quantification of Slc41a1 expression in the kidney of freshwater- and seawater-acclimated mefugu. Values are expressed as means ± SE of the relative expression levels compared with GAPDH (*P < 0.05).
To compare the expression of Slc41s in fish acclimated in SW and FW, we analyzed tissue distribution of Slc41s of SW- and FW-acclimated mefugu (Fig. 3B). Tissue distribution patterns of Slc41s were essentially similar between torafugu and mefugu, and high-level expression of Slc41a1 was also observed in the kidney of mefugu (Fig. 3B, left, 27 cycles). Quantitative real-time PCR analysis demonstrated that renal expression of Slc41a1 in SW mefugu was 3.6 times higher than those detected in FW mefugu (P < 0.05; n = 5) (Fig. 3C), making it a good candidate transporter that mediates Mg2+ secretion in the renal tubule.
Expression of Slc41a1 in the proximal tubule.
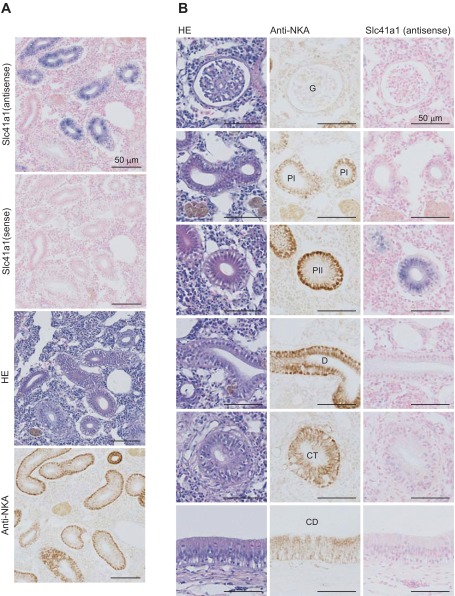
In situ hybridization was performed to determine the localization of Slc41a1 in the kidney of SW mefugu. A DIG-labeled antisense cRNA probe for mfSlc41a1 produced strong signals in the renal tubule, and no hybridization was observed with the sense probe (Fig. 4).
Fig. 4.
In situ hybridization analysis of Slc41a1 in the seawater (SW) mefugu kidney. Paraffin-embedded kidney sections were stained with anti-Na+/K+-ATPase antibodies (NKA), hematoxylin-and-eosin (HE), and DIG-labeled antisense and sense cRNA probes for mefugu Slc41a1. Low (A) and high (B) magnification images are shown. P, proximal tubule, I and II, segment I and II; D, distal tubule; CD, collecting duct; CT, collecting tubule; G, glomerulus; P, proximal tubule. Scale bars, 50 μm.
Expression of Slc41a1 in the second segment of the proximal tubule was confirmed by staining serial sections with hematoxylin or anti-Na+/K+-ATPase antibody. Proximal tubules were characterized by the presence of a brush border and shallow basolateral infoldings that are labeled by anti-Na+/K+-ATPase antibody. The proximal tubule consisted of two segments based on their Na+/K+-ATPase content (32): segment I (PI) that was weakly stained with anti-Na+/K+-ATPase antibody and segment II (PII) that was strongly positive for anti-Na+/K+-ATPase antibody. Distal tubule and collecting duct were identified by the lack of a brush border at the apical membrane and deeply invaginated basal infoldings that express Na+/K+-ATPase at high levels (32). Expression of Slc41a1 was observed in PII but not the glomeruli, PI, distal tubule, and collecting duct.
Immunofluorescence of Slc41a1 expressed in COS7 cells and Xenopus oocytes.
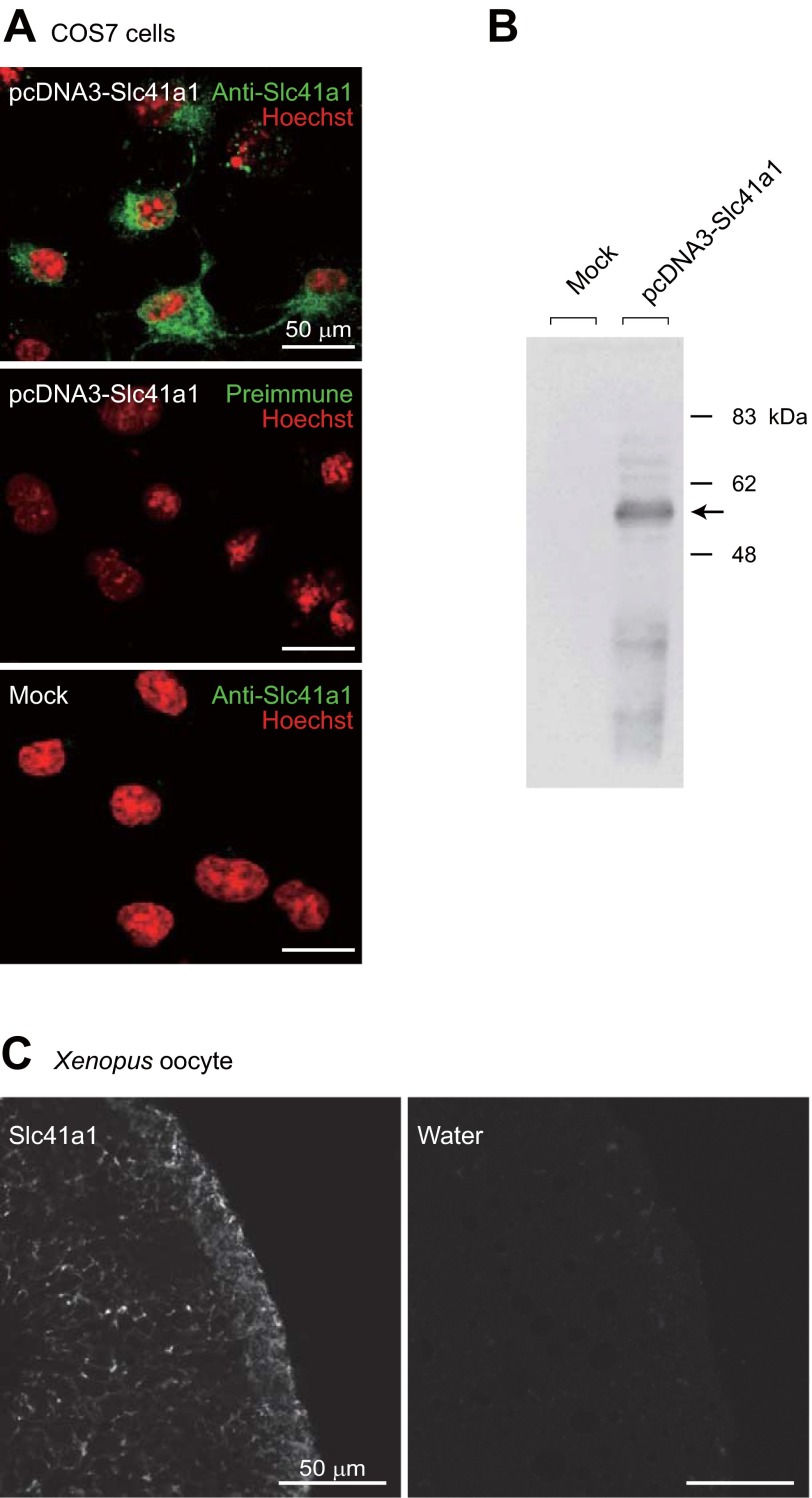
Antisera of Slc41a1 were prepared by immunizing rabbits with synthetic peptides of mfSlc41a1. Immunocytochemical analyses showed that anti-mfSlc41a1 antiserum strongly stained COS7 cells expressing mfSlc41a1 (Fig. 5A). No signal was detected with preimmune serum in COS7 cells expressing mfSlc41a1, and mock-transfected COS7 cells were not stained with the antiserum (Fig. 5A). Bands of ∼60 kDa were detected by Western blot analysis using anti-mfSlc41a1 antiserum in the membrane preparation of COS7 cells expressing mfSlc41a1 but not in that of mock-transfected COS7 cells (Fig. 5B). The antibody also labeled Xenopus oocytes injected with cRNA for mfScl41a1, but not water-injected oocytes (Fig. 5C). These results indicated that anti-mfSlc41a1 antiserum specifically recognizes mfSlc41a1. In both COS7 cells and Xenopus oocytes, the major part of exogenously expressed mfSlc41a1 is localized intracellularly (Fig. 5, A and C).
Fig. 5.
Specificity of anti-Slc41a1 antibody. A: COS7 cells were transfected with pcDNA3-Slc41a1 or empty vector (mock), and stained with anti-Slc41a1 or preimmune serum (green). Nuclei were stained with Hoechst (red). Scale bars: 50 μm. B: Western blot analysis. COS7 cells were transfected with pcDNA3-Slc41a1 or empty vector (mock), and the membrane fractions of the cells were analyzed by Western blot analysis using anti-Slc41a1 antiserum. C: immunofluorescence staining of Xenopus oocytes injected with Slc41a1 cRNA or water (negative control). Scale bars: 50 μm.
Vacuolar localization of Slc41a1 in the apical cytoplasm of the proximal tubule.
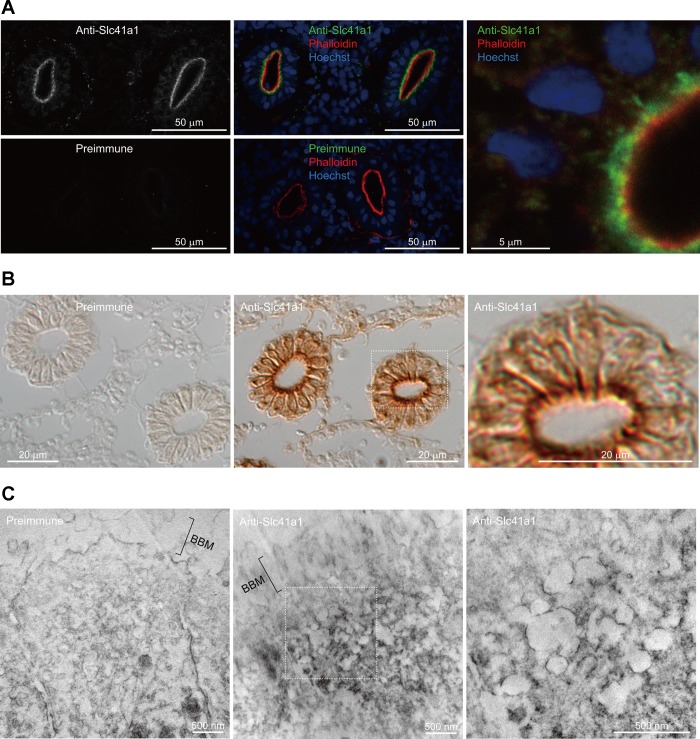
To determine the subcellular localization of Slc41a1, immunohistochemical analyses were performed on frozen sections of the SW mefugu kidney using the anti-mfSlc41a1 antiserum. Slc41a1 was detected near the apical membrane of the proximal tubules (Fig. 6, A and B). The proximal tubules were identified by staining the apical brush borders with F-actin marker phalloidin-TRITC (32). No signals were observed in the negative controls stained with preimmune serum (Fig. 6, A and B) or antigen-absorbed antiserum (data not shown).
Fig. 6.
Localization of Slc41a1 in the proximal tubules of SW mefugu. A: Immunofluorescence of the proximal tubules. Kidney sections were stained with anti-Slc41a1 or preimmune serum (green). F-actin was visualized with fluorescently labeled phalloidin (red), and nuclei were stained with Hoechst (blue). High-magnification view is shown on the right. B: differential interference contrast image of the proximal tubules. Kidney sections were stained with anti-Slc41a1 or preimmune serum, and localization of the antibody was visualized with DAB (brown reaction product). High-magnification view is shown on the right. C: immunoelectron microscopy of the proximal tubule. The kidney sections were treated with anti-Slc41a1 or preimmune serum, and the reaction product of DAB was observed by transmission electron microscopy. Figure on the right side shows a higher-magnification image of Slc41a1 distribution. Scale bars: 500 nm. BBM, brush border membrane.
A highly magnified image of the proximal tubule showed that Slc41a1 is localized to intracellular vesicles (right panels of Fig. 6, A and B). Immunoelectron microscopy indicated that the Slc41a1 immunosignals are associated with intracellular vacuoles in the apical cytoplasm but not with the brush-border membrane (Fig. 6C).
DISCUSSION
Since the initial findings that urine of SW fish contains ∼150 mM Mg2+ in the 1930s (28) and the proximal tubular cells secrete fluids rich in Mg2+ (3), the molecular mechanism whereby Mg2+ is secreted by renal tubular epithelial cells has not been clarified. The present study is the first report describing the identification of an Mg2+ transporter that is likely to be involved in renal Mg2+ excretion by a marine teleost.
As reported for other SW fishes, torafugu and mefugu in SW concentrated Mg2+ into the urine, confirming that torafugu and mefugu have a system, whereby Mg2+ is secreted into the kidney as do other marine teleosts. Taking advantage of the Takifugu species, whose genome sequence is known, we identified homologous genes of Slc41 Mg2+ transporters in the fugu genome database and selected genes that are expressed in the renal tubule at high levels by RT-PCR and in situ hybridization. We found that Slc41a1 is highly expressed in the proximal tubules, and the expression is upregulated when mefugu is transferred from FW to SW when Mg2+ excretion is essential for survival. As the proximal tubule is the site of transcellular secretion of Mg2+, Slc41a1 is expected to be involved in the transcellular Mg2+ secretion by the proximal tubules.
Since most of the mfSlc41a1 expressed in culture cells and Xenopus oocytes were intracellularly localized, further study is required to analyze the activity of mfSlc41a1. Slc41 families are homologs of the bacterial MgtE Mg2+ channel that is involved in Mg2+ uptake of bacterium. The cytosolic domain of MgtE acts as a Mg2+ sensor that changes the “opened form” to the “closed form” when intracellular Mg2+ is elevated (23), but vertebrate Slc41s lack the sensor domain. Mouse distal convoluted tubule cells cultured in low-magnesium media and the kidney and heart of mice maintained on low-magnesium diets were found to have elevated Slc41a1 expressions (56). Xenopus oocytes expressing mammalian Slc41a1 exhibited Mg2+ channel-like activity when Mg2+-elicited current was measured (19, 22). More recently, however, another group demonstrated that HEK293 cells expressing human Slc41a1 reduce intracellular [Mg2+] and increase extracellular Na+-dependent Mg2+ efflux activity and proposed that Slc41a1 is a plasma membrane electroneutral Na+/Mg2+ exchanger mediating cellular Mg2+ efflux (34). Although the activity of mfSlc41a1 has not been clarified yet, it is highly likely that mfSlc41a1 also has Na+/Mg2+ exchanger activity, which is advantageous to excrete Mg2+.
In the proximal tubules of SW-acclimated mefugu, Slc41a1 was localized to the vacuoles in the apical cytoplasm. Vacuoles rich in Mg2+ were found in the proximal tubules of SW-acclimated killifish (Fundulus heteroclitu) (10) and shark (Scyliorhinus caniculus) (24). In shark, small apical vacuoles contained Mg2+ at an equivalent concentration of 229 mmol/kg water (24). Peritubular uptake of the radioisotope 28Mg by renal tubules isolated from winter flounder (Pseudopleuronectes americanus) was stimulated by cytochalasin B, which inhibits actin filament assembly and modulates vesicular trafficking (43). These findings are evidence of vesicular trafficking-mediated Mg2+ secretion that was initially proposed by Hickman and Trump based on ultrastructural evidence of marine teleost nephrons (26). Vacuolar localization of Slc41a1 strongly suggests that Slc41a1 mediates vacuolar accumulation of Mg2+, i.e., transporting Mg2+ from the cytosol to the vacuolar lumen, which is later exocytosed into the urine. In contrast, brush border membrane vesicles of the proximal tubules of fresh water rainbow trout (Oncorhynchus mykiss) and winter flounder indicated an electrical gradient-driven Mg2+ transport (13, 43), which is advantageous for Mg2+ absorption. Therefore, the brush border is the likely site of Mg2+ reabsorption but not the major site of Mg2+ secretion, and the vacuole is the major site of Mg2+-efflux activity in the proximal tubule.
In mammals, TRPM7 is a Mg2+-permeable cation channel. Using a negative membrane potential as a driving force, TRPM7 absorbs extracellular Mg2+ and elevates intracellular [Mg2+] (48). In the apical membrane of renal tubular cells of mammalian kidney, TRPM7 forms a complex with TRPM6, whose mutation causes failure of renal Mg2+ reabsorption resulting in hypomagnesemia (11, 48). CNNM family members are homologs of bacterial CorC protein, which is involved in Mg2+ and Co2+ efflux (52). Human mutation of CNNM2 and CNNM3 is also associated with hypomagnesemia (39, 52). In contrast to TRPM7, CNNM2 localizes to the basolateral membrane of renal tubular cells. Although the mode of Mg2+ transport by CNNM2 and CNNM3 is under debate, the basolateral membrane localization suggests that CNNM2 mediates or stimulates Mg2+ efflux from the cytosol to the tissue fluid, the failure of which results in reduced renal reabsorption of Mg2+. Future comparative analyses of fish homologs of these Mg2+ transporters will reveal the whole image of renal tubular Mg2+ secretion by marine teleost.
Perspectives and Significance
In marine teleosts, the proximal tubule is the site of divalent ion secretion. In SW mefugu, transporters that mediate efflux of SO42− (Slc26a6A; Cl−/SO42− exchanger) and Ca2+ (NCX2a; Na+/Ca2+ exchanger) are present in the brush border region of the proximal tubule (29, 30). The vacuolar localization of Slc41a1 shown in the present study is a notable contrast to their normal localization. The reason why Mg2+ is not directly secreted via the brush border should be clarified in a future study. Since Mg2+ efflux system is still largely unknown in mammals, our results suggest that vacuolar Slc41a1-mediated Mg2+ efflux system is also present in mammals.
GRANTS
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) Grants-in-Aid for Scientific Research (21770077 and 22370029), the 21st Century and Global Center of Excellence Program of MEXT, and the Sumitomo Foundation (100535). Work in the Romero laboratory was supported by the National Institutes of Health (EY-017732, DK-083007, and DK-090728).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.I., M.F.R., S.H., and A.K. conception and design of research; Z.I., N.H., Y.Y., H.D., and A.K. performed experiments; Z.I., N.H., and A.K. analyzed data; Z.I., N.H., M.F.R., S.H., and A.K. interpreted results of experiments; Z.I., N.H., and A.K. prepared figures; Z.I., N.H., and A.K. drafted manuscript; Z.I., M.F.R., S.H., and A.K. edited and revised manuscript; Z.I., N.H., H.D., M.F.R., S.H., and A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Min-Hwang Chang, Dr. Hana Inoue, Dr. Michiko Tashiro, and Dr. Masato Konishi for discussion, Heather L. Holmes, Elyse M. Scileppi, Ayako Takada, Nana Shinohara, Yuuri Kimura, Takahiro Umezawa, and Noriko Isoyama for their technical assistance, Shinpei Nakamura for the ICP-AES and ICP-MS experiments, and Yuriko Ishii and Tomoko Okada for their secretarial assistance.
REFERENCES
- 1. American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Aparicio S, Chapman J, Stupka E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MD, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith SF, Clark MS, Edwards YJ, Doggett N, Zharkikh A, Tavtigian SV, Pruss D, Barnstead M, Evans C, Baden H, Powell J, Glusman G, Rowen L, Hood L, Tan YH, Elgar G, Hawkins T, Venkatesh B, Rokhsar D, Brenner S. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science 297: 1301–1310, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Beyenbach KW. Direct demonstration of fluid secretion by glomerular renal tubules in a marine teleost. Nature 299: 54–66, 1982 [DOI] [PubMed] [Google Scholar]
- 4. Beyenbach KW. Kidneys sans glomeruli. Am J Physiol Renal Physiol 286: F811–F827, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Beyenbach KW. Renal handling of magnesium in fish: from whole animal to brush border membrane vesicles. Front Biosci 5: D712–D719, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Beyenbach KW. Secretory NaCl and volume flow in renal tubules. Am J Physiol Regul Integr Comp Physiol 250: R753–R763, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Bijvelds MJ, Flik G, Kolar ZI. Cellular magnesium transport in the vertebrate intestine. Magnes Res 11: 315–322, 1998 [PubMed] [Google Scholar]
- 8. Bijvelds MJ, Kolar ZI, Flik G. Electrodiffusive magnesium transport across the intestinal brush border membrane of tilapia (Oreochromis mossambicus). Eur J Biochem 268: 2867–2872, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Bijvelds MJ, Velden JA, Kolar ZI, Flik G. Magnesium transport in freshwater teleosts. J Exp Biol 201: 1981–1990, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Chandra S, Morrison GH, Beyenbach KW. Identification of Mg-transporting renal tubules and cells by ion microscopy imaging of stable isotopes. Am J Physiol Renal Physiol 273: F939–F948, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci USA 101: 2894–2899, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85: 97–177, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Freire CA, Kinne RK, Kinne-Saffran E, Beyenbach KW. Electrodiffusive transport of Mg across renal membrane vesicles of the rainbow trout Oncorhynchus mykiss. Am J Physiol Renal Fluid Electrolyte Physiol 270: F739–F748, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Gibson MM, Bagga DA, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol Microbiol 5: 2753–2762, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Goytain A, Hines RM, El-Husseini A, Quamme GA. NIPA1(SPG6), the basis for autosomal dominant form of hereditary spastic paraplegia, encodes a functional Mg2+ transporter. J Biol Chem 282: 8060–8068, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Goytain A, Hines RM, Quamme GA. Functional characterization of NIPA2, a selective Mg2+ transporter. Am J Physiol Cell Physiol 295: C944–C953, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Goytain A, Hines RM, Quamme GA. Huntingtin-interacting proteins, HIP14 and HIP14L, mediate dual functions, palmitoyl acyltransferase and Mg2+ transport. J Biol Chem 283: 33365–33374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goytain A, Quamme GA. Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol Genomics 22: 382–389, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Goytain A, Quamme GA. Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiol Genomics 21: 337–342, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Goytain A, Quamme GA. Functional characterization of the mouse solute carrier, SLC41A2. Biochem Biophys Res Commun 330: 701–705, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Goytain A, Quamme GA. Identification and characterization of a novel family of membrane magnesium transporters, MMgT1 and MMgT2. Am J Physiol Cell Physiol 294: C495–C502, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics 6: 48, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. Crystal structure of the MgtE Mg2+ transporter. Nature 448: 1072–1075, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Hentschel H, Zierold K. Morphology and element distribution of magnesium-secreting epithelium: the proximal tubule segment PII of dogfish, Scyliorhinus caniculus (L.). Eur J Cell Biol 63: 32–42, 1994 [PubMed] [Google Scholar]
- 25. Hickman CP., Jr Ingestion, intestinal absorption, and elimination of seawater and salts in the southern flounder, Paralichthys lethostigma. Can J Zool 46: 457–466, 1968 [DOI] [PubMed] [Google Scholar]
- 26. Hickman CP, Jr, Trump BF. The kidney. In: Fish Physiology, edited by Hoar WS, Randall DJ. New York: Academic Press, 1969, p. 91–239 [Google Scholar]
- 27. Hmiel SP, Snavely MD, Florer JB, Maguire ME, Miller CG. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J Bacteriol 171: 4742–4751, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holmer WS, Nicholas F, Anne B. The absorption and excretion of water and salts by marine teleosts. Am J Physiol 93: 480–505, 1930 [Google Scholar]
- 29. Islam Z, Kato A, Romero MF, Hirose S. Identification and apical membrane localization of an electrogenic Na+/Ca2+ exchanger NCX2a likely to be involved in renal Ca2+ excretion by seawater fish. Am J Physiol Regul Integr Comp Physiol 301: R1427–R1439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato A, Chang MH, Kurita Y, Nakada T, Ogoshi M, Nakazato T, Doi H, Hirose S, Romero MF. Identification of renal transporters involved in sulfate excretion in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 297: R1647–R1659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kato A, Doi H, Nakada T, Sakai H, Hirose S. Takifugu obscurus is a euryhaline fugu species very close to Takifugu rubripes and suitable for studying osmoregulation. BMC Physiol 5: 18, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kato A, Muro T, Kimura Y, Li S, Islam Z, Ogoshi M, Doi H, Hirose S. Differential expression of Na+-Cl− cotransporter and Na+-K+-Cl− cotransporter 2 in the distal nephrons of euryhaline and seawater pufferfishes. Am J Physiol Regul Integr Comp Physiol 300: R284–R297, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Kolisek M, Launay P, Beck A, Sponder G, Serafini N, Brenkus M, Froschauer EM, Martens H, Fleig A, Schweigel M. SLC41A1 is a novel mammalian Mg2+ carrier. J Biol Chem 283: 16235–16247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kolisek M, Nestler A, Vormann J, Schweigel-Rontgen M. The human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. Am J Physiol Cell Physiol 302: C318–C326, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Kolisek M, Zsurka G, Samaj J, Weghuber J, Schweyen RJ, Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J 22: 1235–1244, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 294: R1402–R1412, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Li S, Kato A, Takabe S, Chen AP, Romero MF, Umezawa T, Nakada T, Hyodo S, Hirose S. Expression of a novel isoform of Na+/H+ exchanger 3 (NHE3) in the kidney and intestine of banded houndshark Triakis scyllium. Am J Physiol Regul Integr Comp Physiol 304: R865–R876, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marshall WS, Grosell M. Ion transport, osmoregulation, and acid-base balance. In: The Physiology of Fishes, edited by Evans DH, Claiborne JB. New York: CRC, 2005, p. 177–224 [Google Scholar]
- 39. Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, Ehret GB, Boerwinkle E, Felix JF, Leak TS, Harris TB, Yang Q, Dehghan A, Aspelund T, Katz R, Homuth G, Kocher T, Rettig R, Ried JS, Gieger C, Prucha H, Pfeufer A, Meitinger T, Coresh J, Hofman A, Sarnak MJ, Chen YD, Uitterlinden AG, Chakravarti A, Psaty BM, van Duijn CM, Kao WH, Witteman JC, Gudnason V, Siscovick DS, Fox CS, Kottgen A. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet 6: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mistry AC, Chen G, Kato A, Nag K, Sands JM, Hirose S. A novel type of urea transporter, UT-C, is highly expressed in proximal tubule of seawater eel kidney. Am J Physiol Renal Physiol 288: F455–F465, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Nakada T, Westhoff CM, Kato A, Hirose S. Ammonia secretion from fish gill depends on a set of Rh glycoproteins. FASEB J 21: 1067–1074, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol 200: 62–81, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Renfro JL, Shustock E. Peritubular uptake and brush border transport of 28Mg by flounder renal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 249: F497–F506, 1985 [DOI] [PubMed] [Google Scholar]
- 44. Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Sahni J, Nelson B, Scharenberg AM. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem J 401: 505–513, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sahni J, Scharenberg AM. The SLC41 family of MgtE-like magnesium transporters. Mol Aspects Med 34: 620–628, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schindl R, Weghuber J, Romanin C, Schweyen RJ. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys J 93: 3872–3883, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7—Gatekeepers of human magnesium metabolism. Biochim Biophys Acta 1772: 813–821, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Smith HW. The absorption and excretion of water and salts by marine teleosts. Am J Physiol 93: 480–505, 1930 [Google Scholar]
- 50. Smith RL, Thompson LJ, Maguire ME. Cloning and characterization of MgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J Bacteriol 177: 1233–1238, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Snavely MD, Florer JB, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol 171: 4761–4766, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stuiver M, Lainez S, Will C, Terryn S, Gunzel D, Debaix H, Sommer K, Kopplin K, Thumfart J, Kampik NB, Querfeld U, Willnow TE, Nemec V, Wagner CA, Hoenderop JG, Devuyst O, Knoers NV, Bindels RJ, Meij IC, Muller D. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet 88: 333–343, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem 59: 971–1005, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wabakken T, Rian E, Kveine M, Aasheim HC. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem Biophys Res Commun 306: 718–724, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Wilson RW, Wilson JM, Grosell M. Intestinal bicarbonate secretion by marine teleost fish–why and how? Biochim Biophys Acta 1566: 182–193, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Yamanoue Y, Miya M, Matsuura K, Miyazawa S, Tsukamoto N, Doi H, Takahashi H, Mabuchi K, Nishida M, Sakai H. Explosive speciation of Takifugu: another use of fugu as a model system for evolutionary biology. Mol Biol Evol 26: 623–629, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci USA 106: 15750–15755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]