Abstract
Preeclampsia is a complication of pregnancy that is marked by hypertension, proteinuria, and maternal endothelial dysfunction. A central factor in the etiology of the disease is the development of placental hypoxia/ischemia, which releases pathogenic soluble factors. There is currently no effective treatment for preeclampsia, but the phosphodiesterase-5 (PDE-5) inhibitor sildenafil has been suggested, as PDE-5 is enriched in the uterus, and its antagonism could improve uteroplacental function. Here, we report in the reduced uterine perfusion pressure (RUPP) rat model that administration of oral sildenafil is effective in attenuating placental ischemia-induced hypertension during gestation. RUPP animals have significantly elevated arterial pressure compared with control animals (132 ± 3 vs. 100 ± 2 mmHg; P < 0.05). Administration of oral sildenafil (45 mg·kg−1·day−1) had no effect on blood pressure in control rats but decreased pressure in RUPP rats (115 ± 1 mmHg; P < 0.05). RUPP induced changes in placental sFlt-1, and vascular endothelial growth factor (VEGF) was unaffected by sildenafil administration, as was the decrease in free plasma VEGF. RUPP animals had a significant increase in medullary PDE-5/β-actin ratio (1 ± 0.14 vs. 1.63 ± 0.18; P < 0.05) expression with a resulting reduction in renal medullary cGMP (1.5 ± 0.15 vs. 0.99 ± 0.1 pmol/μg protein, P < 0.05) compared with controls. Although sildenafil had no effect on renal medullary cGMP in control animals, it significantly increased cGMP in RUPP animals (1.3 ± 0.1 pmol/μg protein; P < 0.05). These data suggest that sildenafil might provide an effective therapeutic option for the management of hypertension during preeclampsia.
Keywords: phosphodiesterase-5, preeclampsia, pregnancy, soluble fms-like tyrosine kinase, vascular endothelial growth factor
one of the most common obstetrical complications is preeclampsia, affecting ∼5–8% of all pregnancies, with higher incidence in certain ethnic subgroups (31). Preeclampsia typically presents after the 20th wk of gestation and is classically characterized by new-onset hypertension, proteinuria, and edema, although these last two indications are not universally observed (38). Preeclampsia is a major health concern, as it is one of the leading causes of maternal and fetal morbidity and mortality, also being implicated in up to 15% of all preterm births (25). One of the most pressing problems in managing the preeclampsia patient is a relative dearth of effective therapeutic approaches. Current interventions include prophylactic administration of magnesium sulfate to help prevent seizure, and in severe cases, administration of various antihypertensive agents. With these courses of treatment, however, it is typically not possible to restore blood pressure to nonpathological levels. Ultimately, as the hypertension reaches dangerous levels, the only effective intervention is induction of labor and delivery of both the fetus and placenta. The treatment goal, then is essentially to prolong pregnancy as long as possible to allow for maximum fetal development (38).
Although the initiating events that underpin the development of preeclampsia remain obscure, research over the past 15 years has highlighted the role of placental ischemia and hypoxia in the production of pathogenic factors during preeclampsia. During normal gestation, the blood vessels that supply the developing fetoplacental unit undergo a complicated series of remodeling events, which cause the normally high-resistance vessels to expand into high-capacitance vessels; thereby allowing for enhanced blood flow. In the preeclamptic pregnancy, this remodeling often fails to occur, causing underperfusion of the placenta, resulting in chronic hypoxia and ischemia (17). In response, the placenta secretes factors that enter the maternal bloodstream and cause the symptomatic phase of the disorder (12). This effect can be mimicked in various animal models by artificial restriction of blood to the placenta, which mimics in many ways the human disorder (13, 23). In theory, a therapeutic approach that could cause artificial dilation of the uterine spiral arteries, the vessels that supply the placenta, could restore placental function and ameliorate the symptoms of the disorder.
One existing therapeutic class that has been proposed for the management of preeclampsia is phosphodiesterase type 5 (PDE-5) inhibitors. PDE-5 normally catalyzes the hydrolysis of the nitric oxide (NO) secondary messenger cGMP, thus having a vasoconstrictive effect. Interesting preliminary work in sheep demonstrated that PDE-5 was specifically localized to the maternal portion of the uteroplacental unit (4). Intriguing ex vivo studies demonstrated that myometrial arteries from preeclampsia patients exhibit enhanced endothelium-dependent relaxation when exposed to a PDE-5 inhibitor (39). Although initial trials in human patients were disappointing, various critiques of the design of the studies have left open the possibility that PDE-5 inhibitors could be beneficial to the preeclamptic patient (8, 32). The objective of this study was to examine the possible beneficial effects of the PDE-5 inhibitor sildenafil citrate in a rodent model of placental ischemia-induced hypertension, the reduced uterine perfusion pressure (RUPP) rat, thereby shedding light on the future potential use of PDE-5 inhibitors in the preeclampsia patient.
METHODS
Animals.
Timed pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN) were received on gestational day 11. All protocols were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee, and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Rats were maintained on a 12:12-h light-dark cycle, at 23° C constant temperature and were provided food and water ad libitium.
Experimental treatment.
For RUPP rats, on gestational day 14, animals were suggested to aortic and bilateral ovarian artery constriction. Briefly, rats were anesthetized and maintained on 3% isoflurane and a midline abdominal incision was made. After externalization of both uterine horns, one single 0.023-mm silver surgical clip was placed on the abdominal aorta above the iliac bifurcation. One 0.100-mm silver surgical clip was placed on both the left and right ovarian arteries that supply the uterus to prevent compensatory flow. Total reabsorption of pups on gestational day 19 led to exclusion of the animal from the study. Sildenafil was administered in drinking water at a dose of 45 mg·kg−1·day−1, to be within the therapeutic range used experimentally in rodents to treat pulmonary hypertension, and fluid intake was monitored throughout the study to ensure adequate drug delivery (6, 40).
Measurement of mean arterial pressure.
On gestational day 18, rats were anesthetized as above and implanted with indwelling carotid catheters consisting of V-3 tubing (Scientific Commodities, Lake Havasu City, AZ), which were tunneled under the skin and externalized at the back of the neck. The following day, rats were placed in individual restraining cages and acclimatized. Mean arterial pressure was measured consciously for 1 h via Cobe III pressure transducers (CDX Sema), and data were collected and analyzed using receivers, amplifiers, and PowerLab software from ADInstruments. Each experimental group had n = 10–12.
Tissue harvest.
Rats were anesthetized as above. The uterus was externalized through a ventral midline incision, and blood was collected by cannulation of the abdominal aorta. Records were made of the viable and reabsorbed pups present in each animal, and individual pups and placentas were weighed and recorded. The largest and smallest placental samples from each horn, the thoracic aorta, and the liver were flash frozen in liquid nitrogen and stored at −80°C for later analysis.
Measurement of sFlt-1 and vascular endothelial growth factor.
Placental protein was extracted from a random placenta from individual rats. Briefly, the frozen tissue was mechanically ground by mortar and pestle in liquid nitrogen. Tissue fragments were resuspended in radioimmunoassay buffer with protease inhibitor cocktail, PMSF, and sodium orthovanadate (Santa Cruz Biotechnology). Homogenization was performed in glass tissue Dounces, and the solution was cleared by centrifugation 12,000 g for 20 min. Resulting protein concentration was measured by the bicinchoninic acid method (Pierce Biotechnology). Vascular endothelial growth factor (VEGF) and sFlt-1 were both measured by sandwich ELISA (R&D Systems) in duplicate, according to manufacturer's protocols. Measurements of free plasma VEGF were performed with the same kit. Each group had n = 6.
Measurement of placental and renal PDE-5.
Western blot detection of PDE-5 expression has been performed many times, as previously described (33). In brief, placental, cortical, and medullary tissue from experimental rats was pulverized in liquid nitrogen, and protein was isolated in RIPA buffer, as described above. One-hundred micrograms of total protein was run on 4–20% gradient SDS-PAGE gels and transferred by semi-dry transfer onto nitrocellulose. The primary antibody for PDE-5 (H-120; Santa Cruz Biotechnology) was incubated at 1:200 at 4°C overnight and detected by incubation by fluorescently labeled secondary (Rockland Immunochemicals, Boyertown, PA) and observed on a Li-Cor Odyssey membrane scanner. Bands were normalized to β-actin and normalized to control pregnant rat levels. n = 4 in each group.
cGMP determination.
Determination of renal medullary cGMP was determined by a commercially available competitive EIA (Cayman Chemical, Ann Arbor, MI). Briefly, frozen medullary samples were homogenized in 750 μl of 5% trichloroacetic acid (TCA), and the tissue debris was removed by centrifugation at 1,500 g for 10 min. The resulting supernatant was triple extracted with water-saturated ether, and the residual ether was removed by incubation at 70°C for 10 min. The assay standards were resuspended in similarly prepared TCA, which was also ether extracted. The remainder of the assay was performed according to the manufacturer's instructions. Total protein concentration was determined by bicinchoninic acid method (Pierce Biotechnology), and cGMP levels were normalized to total protein concentration; n = 5 for each group.
Statistics.
All statistical analysis was carried out with GraphPad Prism 6. Data from all groups were analyzed by one-way ANOVA using Tukey's multiple-comparison test at a P value cutoff of less than 0.05.
RESULTS
Sildenafil significantly attenuates placental ischemia-induced hypertension but has little effect on placental or pup weight.
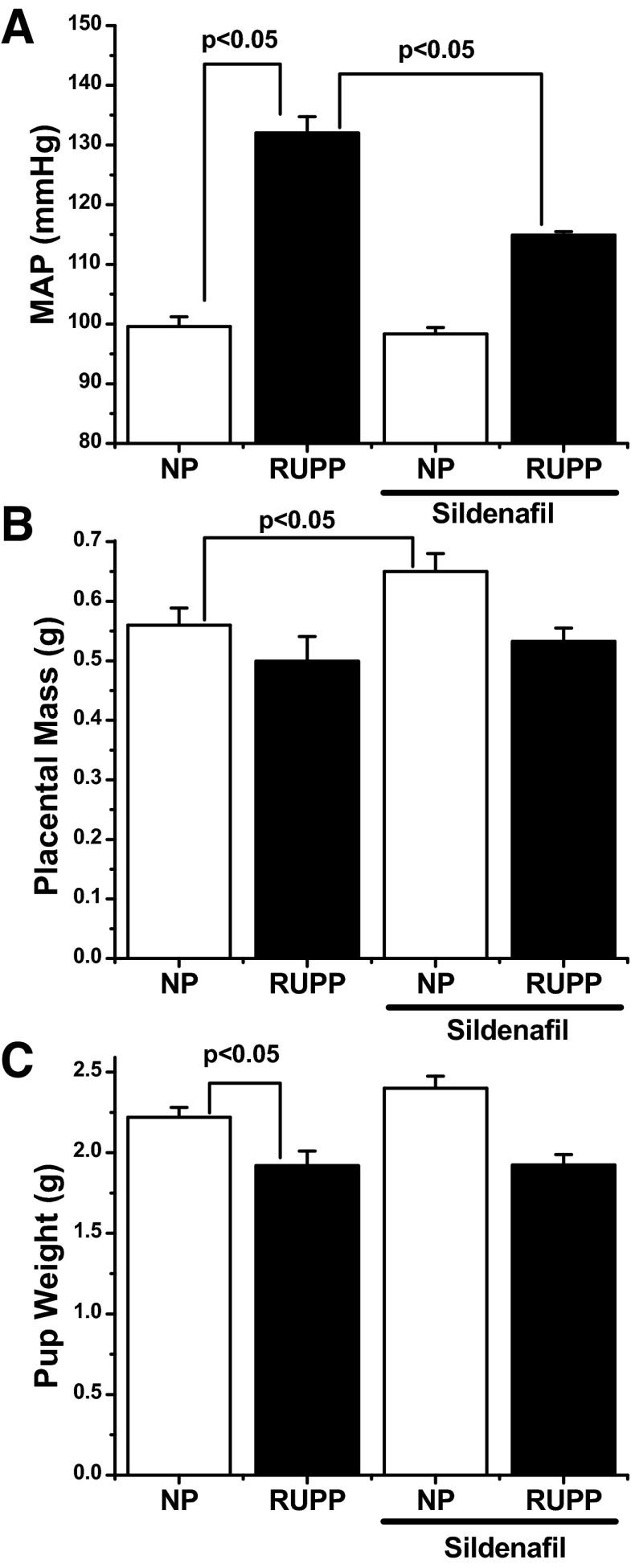
In response to the RUPP procedure, mean arterial pressure (MAP) on gestational day 19 (GD19) was significantly increased (100 ± 1.6 vs. 132 ± 2.7 mmHg; P < 0.05) by direct pressure measurements (Fig. 1A). Sildenafil administration alone had no significant effect in control animals (99 ± 3 mmHg). However, RUPP animals who received sildenafil had a significantly lower MAP (115 ± 1 mmHg; P < 0.05) compared with the RUPP-untreated group. The RUPP procedure did not significantly decrease placental mass compared with the control group, although there was a trend for a decrease in the RUPP animals (0.56 ± 0.1 vs. 0.50 ± 0.04; P = 0.24). Sildenafil administration caused a significant increase in placental mass in control animals (0.65 ± 0.03; P < 0.05) but did not significantly alter placental weight in RUPP animals (0.53 ± 0.02; P = 0.34) compared with the untreated RUPP group (Fig. 1B). Finally, the RUPP procedure caused a significant decrease in fetal weight compared with control animals (2.22 ± 0.06 vs. 1.92 ± 0.09; P < 0.05). Sildenafil had no significant effect on fetal weight in either normal pregnant or RUPP animals compared with their respective controls (Fig. 1C) or on the number of viable offspring (data not shown).
Fig. 1.
A: in response to the reduced uterine perfusion pressure (RUPP) procedure on gestational day 14, mean arterial pressure (MAP) on gestational day 19 was increased compared with normal pregnant (NP) control animals (100 ± 1.6 vs. 132 ± 2.7 mmHg). Sildenafil administration alone had no net effect on MAP, but significantly attenuated RUPP-induced hypertension (115 ± 1 mmHg). B: RUPP procedure had no significant effect on placental mass, but sildenafil caused a slight, but significant, increase in placental mass on gestational day 19. C: fetal weights were significantly decreased in response to the RUPP procedure (2.22 ± 0.06 vs. 1.92 ± 0.09). Sildenafil administration had no effect on either control or RUPP animals. n = 10–12 for each experimental group. Statistically significant (P < 0.05) comparisons are indicated by brackets.
RUPP animals exhibit significantly elevated placental PDE-5 expression, but sildenafil does not correct ischemia-induced changes in angiogenic balance.
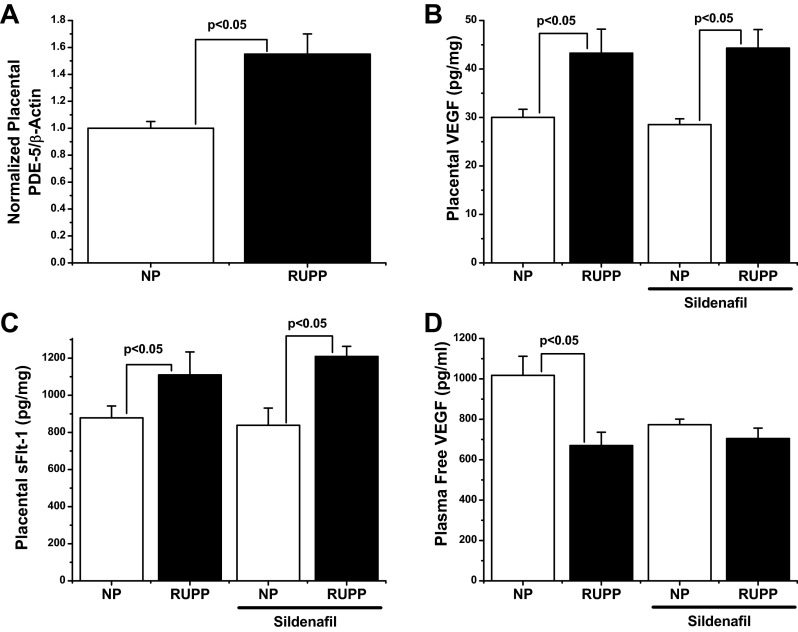
To determine the effect of placental ischemia on placental PDE-5 expression, placental lysates were examined by Western blot analysis for the presence of PDE-5 protein. As seen in Fig. 2A, RUPP rats demonstrated a ∼50% increase in their placental PDE/β-actin ratio (1 ± 0.05 vs. 1.55 ± 0.15, P < 0.05), suggesting a link between placental ischemia and PDE-5 expression. To determine whether PDE-5 antagonism could correct the defects in angiogenic balance seen in response to the RUPP procedure, the placental levels of both sFlt-1 and VEGF were determined by ELISA. As seen in Fig. 2C, the RUPP procedure significantly increased the production of placental sFlt-1 (643 ± 44 vs. 809 ± 81 pg/mg, P < 0.05). A similar significant increase was seen between normal pregnant and RUPP rats receiving sildenafil (651 ± 71 vs. 849 ± 75 pg/mg; P < 0.05), suggesting that sildenafil does not affect sFlt-1 production in RUPP rats. Likewise, as shown in Fig. 2B, the RUPP procedure produced a significant increase in placental VEGF compared with controls (30 ± 2 pg/mg vs. 43 ± 5 pg/mg; P < 0.05), which was closely mimicked by comparative groups receiving sildenafil (29 ± 1 vs. 44 ± 4 pg/mg; P < 0.05), suggesting no effect on VEGF production. When levels of bioavailable VEGF in the maternal circulation were examined, as previously shown, there was a significant reduction in RUPP rats (1,017 ± 95 vs. 670 ± 68 pg/ml; P < 0.05). Sildenafil had no statistical effect in either normal pregnant (773 ± 28 pg/ml) or RUPP rats (704 ± 52 pg/ml), although there was a marked trend for decreased free VEGF in normal pregnant rats.
Fig. 2.
A: placental phosphodiesterase-5 (PDE-5) expression was observed in placentas from normal pregnant (NP) and RUPP animals. RUPP animals exhibited ∼50 increase in PDE-5 expression (1 ± 0.05 vs. 1.55 ± 0.15). n = 4 per group. B and C: the RUPP procedure increased placental vascular endothelial growth factor (VEGF) (30 ± 2 vs. 43 ± 5 pg/mg) and sFlt-1 (643 ± 44 vs. 809 ± 81 pg/mg). Sildenafil had no significant effect on placental VEGF or sFlt-1 in either NP or RUPP rats. D: free, bioavailable VEGF in the maternal circulation was measured by ELISA. In response to the RUPP procedure, there was a significant decrease in free VEGF (1,017 ± 95 vs. 670 ± 68 pg/ml). Sildenafil treatment caused a trend for decreased free VEGF (773 ± 28 pg/ml), although this failed to reach significance. Sildenafil had no effect on free VEGF in RUPP animals. n = 6 per group. Statistically significant (P < 0.05) comparisons are indicated by brackets.
RUPP and sildenafil administration increase medullary but not cortical PDE-5 expression.
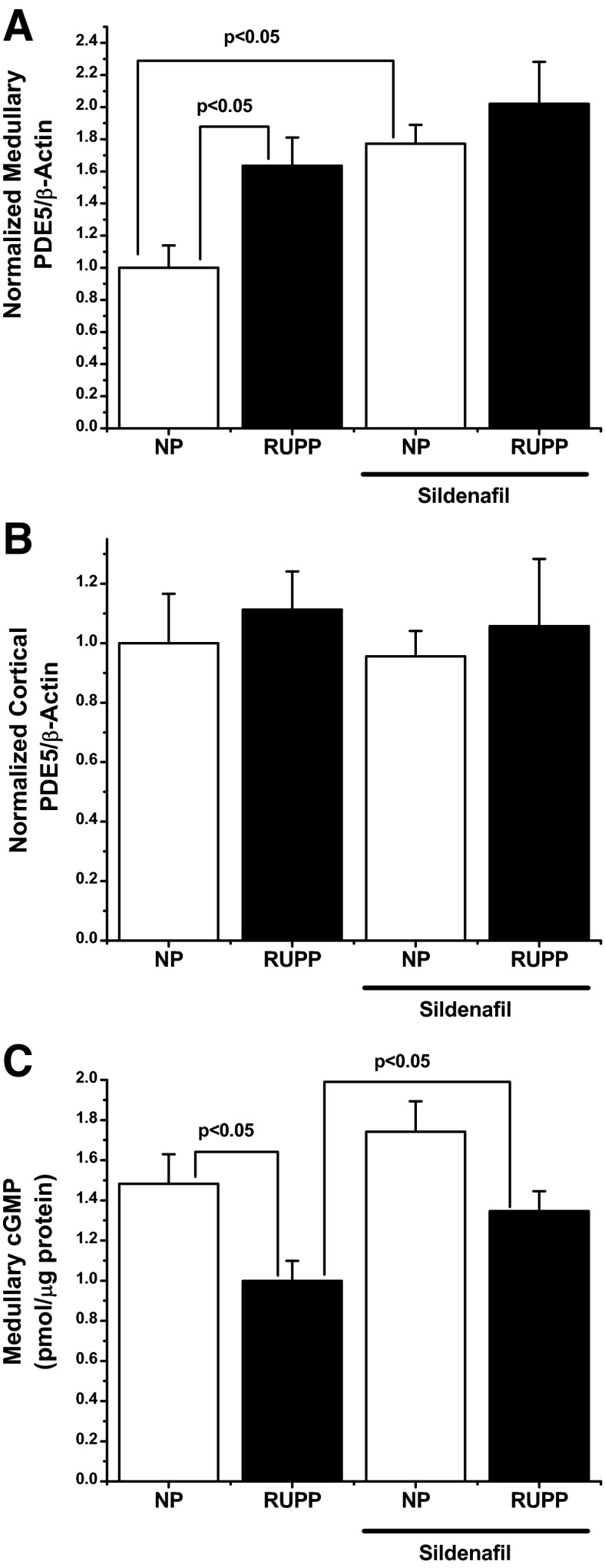
To determine possible effects of RUPP and sildenafil administration on renal PDE-5 levels, both cortical and medullary levels of the enzyme were determined by Western blot analysis. As seen in Fig. 2B, cortical levels of PDE-5 were unaffected by either the RUPP procedure or sildenafil administration. In contrast, however, the RUPP procedure induced a significant increase in the PDE-5/β-actin ratio when normalized to control levels (1 ± 0.14 vs. 1.63 ± 0.18; P < 0.05), as can be seen in Fig. 2A. Sildenafil administration to control animals also leads to an increase in normalized PDE-5/β-actin compared with control rats (1.77 ± 0.12; P < 0.05) but did not significantly augment medullary PDE-5 when compared either to RUPP or sildenafil administration alone (2.02 ± 0.26), although there was a trend to increased expression over both RUPP and sildenafil administration.
Renal medullary cGMP is reduced in RUPP rats, but it is significantly increased by sildenafil administration.
To determine the effect of sildenafil on renal medullary cGMP, total cGMP was determined by competitive EIA. As seen in Fig. 3C, in response to the RUPP procedure, medullary cGMP was significantly decreased compared with normal pregnant animals (1.5 ± 0.15 vs. 0.99 ± 0.1 pmol/μg of protein; P < 0.05). Sildenafil had no significant effect on normal pregnant controls compared with untreated normal pregnant animals (1.7 ± 0.15; P = 0.25), although a slight trend for increased levels was noted. However, RUPP rats receiving sildenafil exhibited a significant increase in medullary cGMP compared with RUPP controls (1.3 ± 0.1; P < 0.05), although this failed to fully normalize to normal pregnant levels.
Fig. 3.
A: renal medullary expression of PDE-5 was monitored by Western blot analysis. In response to the RUPP procedure, the normalized PDE-5/β-actin level was increased ∼60% (1 ± 0.14 vs. 1.63 ± 0.18) over NP controls. Sildenafil also increased PDE-5 expression in the medulla (1.77 ± 0.12). Sildenafil had no significant effect in either NP or RUPP rats. B: in contrast, neither the RUPP procedure nor sildenafil administration had any effect on cortical PDE-5 in any experimental group. C: medullary cGMP was significantly decreased (1.5 ± 0.15 vs. 0.99 ± 0.1 pmol/μg of protein) compared with NP rats. Sildenafil had no effect in NP rats, but significantly increased medullary cGMP when administered to RUPP animals (1.3 ± 0.1 pmol/μg protein). n = 5 for each group. Statistically significant (P < 0.05) comparisons are indicated by brackets.
DISCUSSION
One of the most commonly recognized mechanisms of vascular dysfunction in preeclampsia is an overall decrease in the bioavailablity of NO. Normal pregnancy is associated with an increase in endothelium-derived NO, which is concomitant with overall increases in vasodilation and vascular compliance, which seems to be regulated by increased nitric oxide synthase activity (1–3, 16). Indeed, pregnancy induces a significant increase in circulating levels of cGMP in the mother, which as the second messenger of NO, suggests increased nitric oxide synthase (NOS) activity. It was logical then, that a decrease in NO could be a major contributor to the endothelial dysfunction, which is a classical hallmark of preeclampsia.
Indeed, when the levels of NO metabolites in the amniotic fluid and umbilical vein of preeclamptic pregnancies are compared with healthy pregnancies, there is a significant decrease observed (22). The role of NO and NOS in placental ischemia has been extensively studied in experimental placental-ischemia-induced hypertension. Ex vivo studies on the peripheral vasculature of RUPP rats demonstrated impaired relaxation in response to ACh and a decrease in observed NO metabolites. There is also a differential response to NOS and cGMP inhibition, suggesting a correlation between NO/cGMP and placental ischemia-induced vascular dysfunction (5). Perhaps more convincing, inhibition of NOS in rodents causes a pathological state, which mimics preeclampsia in several key ways and which is more exaggerated in pregnant animals than in nonpregnant controls (16). These animals have been shown to exhibit not only hypertension, but fetal growth restriction, proteinuria, and renal vasoconstriction (7, 26, 41). It is logical then that a restoration of NO bioavailability or cGMP could have a palliative effect in the preeclampsia patient.
With the important role of PDE-5 in the regulation of cGMP and the preliminary data showing maternal uterine specific expression of PDE-5, we hypothesized that PDE-5 inhibition would enhance placental function and attenuate the symptoms of placental ischemia. Promisingly, when we examined PDE-5 in the uterine tissue of RUPP animals, we found ∼50% increase in the protein expression (Fig. 2A), strengthening the rationale for the hypothesis. Indeed, when RUPP rats were given sildenafil citrate for 5 days (GD14–19), their MAP was significantly decreased, while healthy control pregnancies were unaffected (Fig. 1A). Unfortunately, this was unaccompanied by attenuation of fetal growth restriction, but importantly sildenafil administration seemed to have no detrimental effects on either placental or fetal growth. In fact, it appeared to enhance placental mass in control animals (Fig. 1, B and C).
One of the best-established pathogenic factors induced by placental ischemia is the soluble vascular endothelial growth factor (VEGF) receptor, soluble fms-like tyrosine kinase-1 (sFlt-1) (15), the infusion or expression of which causes symptoms strongly reminiscent of a preeclamptic state (24, 27). Given the attenuation in symptoms, if the hypothesis were correct and placental function was being enhanced, the antiangiogenic imbalance induced by placental ischemia would likely be reversed. Surprisingly, when the placental levels of both VEGF and sFlt-1 were examined by ELISA, the ischemia-induced increases seen in RUPP animals were unaffected by sildenafil administration (Fig. 2, B and C). Likewise, the decrease in bioavailable VEGF induced by the RUPP procedure was not reversed by sildenafil, and sildenafil itself decreased free VEGF in control animals (Fig. 2D). Together, these data suggest that placental hypoxia is not being attenuated by sildenafil in the RUPP animals. This is perhaps not surprising, as the RUPP model relies on mechanical restriction of the uterine blood supply, and it is possible that the treatment may not be able to increase placental perfusion due to this mechanical constriction. What then is the mechanism by which blood pressure is being decreased?
Interestingly, there is strong evidence that PDE-5 has an important role in the regulation of fluid homeostasis and sodium excretion during pregnancy. During pregnancy, PDE-5 in the renal medulla increases significantly (28). Extensive work from the Baylis laboratory had demonstrated that medullary PDE-5 blunts the natriuretic response to both NO and atrial natriuretic peptide, apparently through direct tubular actions, which is hypothesized to be an important regulator of the volume expansion, which is a normal part of a healthy pregnancy (18, 34). Indeed, marked PDE-5 inhibition results in decreased maternal plasma volume and sodium retention (33). In order to determine whether this medullary PDE-5 was being affected by sildenafil administration in the RUPP animals, we examined PDE-5 expression in both the cortex and medulla. While the cortical levels of PDE-5 were unaffected by the RUPP procedure or sildenafil administration (Fig. 2B), the medulla had a very different response. The RUPP procedure alone caused ∼60% increase in medullary PDE-5 (Fig. 2A). Sildenafil administration caused a similar increase in PDE-5, although the two treatments together did not augment the protein expression further. To see what effect sildenafil would have on downstream medullary cGMP concentration, we measured the cGMP levels by competitive ELISA. In response to the RUPP procedure, medullary cGMP concentration was significantly reduced (Fig. 3C). In agreement with previous studies (33), the dose of sildenafil used in this study had no effect on medullary cGMP in control animals. However, in the RUPP animals, medullary cGMP was significantly increased by sildenafil. One potential interpretation is that tubular natriuresis is impaired in response to the RUPP procedure and partially recovered by PDE-5 inhibition. This, in turn, could be the changes in MAP seen in these animals. This is the first reported link between placental ischemia and changes in renal PDE-5 expression. Although the mechanistic link between the two is not clear, it suggests a new and novel mechanism linking placental ischemia to changes in renal NO. Future studies examining the effects of both RUPP and sildenafil administration on sodium regulation in the tubules should prove enlightening.
Sildenafil has been suggested as a possible therapeutic for preeclampsia for some time. Promisingly, several animal models of preeclampsia, which each partially mimics the human syndrome, have demonstrated beneficial improvements in fetal outcome when treated with sildenafil (14, 29, 30, 37). Herraiz et al. (14) and Ramesar et al. (29, 30) both demonstrated that the detrimental effect of NOS inhibition during pregnancy could be significantly attenuated by sildenafil administration. These studies, however, relied on whole body NOS inhibition, which may not necessarily mimic the pathophysiological manifestation of human preeclampsia. In particular, the RUPP model shares a number of physiological and molecular similarities with human preeclampsia, which are not recapitulated by NOS inhibition. Specifically, at the physiological level, RUPP rats demonstrate hypertension, proteinuria, maternal endothelial dysfunction, and reduced glomerular filtration rate (13, 36). Furthermore, RUPP rats demonstrate maternal elevated sFlt-1, decreased free VEGF, increased soluble endoglin, increased oxidative stress, production of agonistic AT-1 receptor autoantibodies, production of inflammatory cytokines, and increased vascular and renal endothelin (9–11, 19–21, 35).
Stanley et al. (37) using the catechol-O-methyltransferase knockout mouse, demonstrated that fetal growth restriction in this model could be significantly attenuated by sildenafil, likely through decreased resistance of the fetoplacental circulation. It should be noted that this model is a better model of fetal growth restriction rather than preeclampsia, as in this study, there was no effect of the knockout on blood pressure in late gestation. Although these preliminary studies provided support for sildenafil, the present study suggests that sildenafil could have a palliative effect in placental ischemia-induced hypertension, a central underlying cause of severe preeclampsia.
Enthusiasm for sildenafil has been tempered by results of a small-scale, placebo-controlled human study, which found no significant benefits in pregnancy duration or fetal development. However, for safety reasons, the study used a graduated dosing schedule, with relatively low doses of sildenafil in the earliest time points, which were at the low end of the human therapeutic range. Additionally, the administration of sildenafil was begun relatively late in the gestation program. These and other objections (8) have left open the possibility of using PDE-5 inhibitors in the preeclamptic patient. The present study, demonstrating a beneficial effect of sildenafil administration in a clinically relevant model of placental ischemia-induced hypertension supports continued research into sildenafil as a potential therapy for preeclampsia patients. Although the results presented here are promising, some caution is necessary, as decreasing blood pressure alone without altering the maternal vasculature could, in fact, cause further underperfusion of the placental and exacerbate the ischemia, which underlies the disorder. However, here, we have shown that the decrease in maternal blood pressure had no detrimental effect on fetal growth restriction, placental mass, fetal demise, or further shifts in angiogenic balance, possible because of PDE-5 inhibitor-induced changes in vascular dilation. Future work will focus on the vascular and renal effects of PDE-5 inhibitors under conditions of placental ischemia. The reduction in blood pressure without detrimental fetal effects is promising, as increased maternal blood pressure is usually the deciding factor in early induction of labor. An intervention that could bring down maternal blood pressure without harm to the fetus would allow for longer gestation times and increased fetal development—an effect that cannot be determined in the defined time points of the RUPP model. Further studies in both preclinical models and human populations are warranted to settle whether this could truly be a useful therapeutic for the preeclampsia patient.
Perspectives and Significance
Current management of preeclampsia is severely hampered by a lack of effective therapeutic agents, and there has been long-term interest in using PDE-5 inhibitors in this patient population. Here, we have demonstrated a palliative effect of sildenafil citrate in a clinically relevant rodent model of preeclampsia. Furthermore, we demonstrate for the first time a link between placental ischemia and increased renal medullary PDE-5 expression. Further preclinical research to determine the molecular agents responsible for this effect are certainly warranted and may provide a new therapeutic mechanism for PDE-5 inhibitors in preeclampsia patients. These results also suggest a potential for clinical studies using PDE-5 inhibitors in managing hypertension in the preeclampsia patient.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.M.G. and J.P.G. conception and design of research; E.M.G., A.C.P., and E.A.D. performed experiments; E.M.G. and A.C.P. analyzed data; E.M.G. interpreted results of experiments; E.M.G. prepared figures; E.M.G. drafted manuscript; E.M.G. and J.P.G. edited and revised manuscript; E.M.G., A.C.P., E.A.D., and J.P.G. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by National Heart, Lung, and Blood Institute Grants R21-HL109763, P01-HL51971, K99-HL116774, and a postdoctoral fellowship from the American Heart Association.
REFERENCES
- 1.Abram SR, Alexander BT, Bennett WA, Granger JP. Role of neuronal nitric oxide synthase in mediating renal hemodynamic changes during pregnancy. Am J Physiol Regul Integr Comp Physiol 281: R1390–R1393, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Anumba DO, Robson SC, Boys RJ, Ford GA. Nitric oxide activity in the peripheral vasculature during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol 277: H848–H854, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Chavez JE, Mosher MD. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J 7: 566–571, 1993 [PubMed] [Google Scholar]
- 4.Coppage KH, Sun X, Baker RS, Clark KE. Expression of phosphodiesterase 5 in maternal and fetal sheep. Am J Obstet Gynecol 193: 1005–1010, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35: 367–372, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Dai ZK, Tan MS, Chai CY, Chou SH, Lin PC, Yeh JL, Jeng AY, Chang CI, Chen IJ, Wu JR. Effects of sildenafil on pulmonary hypertension and levels of ET-1, eNOS, and cGMP in aorta-banded rats. Exp Biol Med (Maywood) 231: 942–947, 2006 [PubMed] [Google Scholar]
- 7.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest 96: 482–490, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downing J. Sildenafil for the treatment of preeclampsia. Hypertens Pregnancy 29: 248–250; author reply 251–242, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48: 711–716, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 122: 383–392, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Herraiz S, Pellicer B, Serra V, Cauli O, Cortijo J, Felipo V, Pellicer A. Sildenafil citrate improves perinatal outcome in fetuses from pre-eclamptic rats. BJOG 119: 1394–1402, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Karumanchi SA, Epstein FH. Placental ischemia and soluble fms-like tyrosine kinase 1: cause or consequence of preeclampsia? Kidney Int 71: 959–961, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 31: 1065–1069, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Khong Y, Brosens I. Defective deep placentation. Best Pract Res Clin Obstet Gynaecol 25: 301–311, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Knight S, Snellen H, Humphreys M, Baylis C. Increased renal phosphodiesterase-5 activity mediates the blunted natriuretic response to ANP in the pregnant rat. Am J Physiol Renal Physiol 292: F655–F659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension 52: 1161–1167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52: 1168–1172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lyall F, Young A, Greer IA. Nitric oxide concentrations are increased in the fetoplacental circulation in preeclampsia. Am J Obstet Gynecol 173: 714–718, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 71: 977–984, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meis PJ, Goldenberg RL, Mercer BM, Iams JD, Moawad AH, Miodovnik M, Menard MK, Caritis SN, Thurnau GR, Bottoms SF, Das A, Roberts JM, McNellis D. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol 178: 562–567, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Molnar M, Suto T, Toth T, Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol 170: 1458–1466, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55: 394–398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni XP, Safai M, Rishi R, Baylis C, Humphreys MH. Increased activity of cGMP-specific phosphodiesterase (PDE5) contributes to resistance to atrial natriuretic peptide natriuresis in the pregnant rat. J Am Soc Nephrol 15: 1254–1260, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol 157: 136–140, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate improves fetal outcomes in pregnant, l-NAME treated, Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol 149: 22–26, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 41: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, Baker PN. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy 28: 369–382, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Sasser JM, Baylis C. Effects of sildenafil on maternal hemodynamics and fetal growth in normal rat pregnancy. Am J Physiol Regul Integr Comp Physiol 298: R433–R438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasser JM, Ni XP, Humphreys MH, Baylis C. Increased renal phosphodiesterase-5 activity mediates the blunted natriuretic response to a nitric oxide donor in the pregnant rat. Am J Physiol Renal Physiol 299: F810–F814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Stanley JL, Andersson IJ, Poudel R, Rueda-Clausen CF, Sibley CP, Davidge ST, Baker PN. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension 59: 1021–1028, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Turner JA. Diagnosis and management of pre-eclampsia: an update. Int J Womens Health 2: 327–337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wareing M, Myers JE, O'Hara M, Kenny LC, Warren AY, Taggart MJ, Skillern L, Machin I, Baker PN. Effects of a phosphodiesterase-5 (PDE5) inhibitor on endothelium-dependent relaxation of myometrial small arteries. Am J Obstet Gynecol 190: 1283–1290, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Xie YP, Chen B, Sanders P, Guo A, Li Y, Zimmerman K, Wang LC, Weiss RM, Grumbach IM, Anderson ME, Song LS. Sildenafil prevents and reverses transverse-tubule remodeling and Ca2+ handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension 59: 355–362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol 169: 1316–1320, 1993 [DOI] [PubMed] [Google Scholar]