Abstract
Acupuncture or electroacupuncture (EA) potentially offers a nonpharmacological approach to reduce high blood pressure (BP). However, ∼70% of the patients and animal subjects respond to EA, while 30% do not. EA acts, in part, through an opioid mechanism in the rostral ventrolateral medulla (rVLM) to inhibit sympathoexcitatory reflexes induced by gastric distention. CCK-8 opposes the action of opioids during analgesia. Therefore, we hypothesized that CCK-8 in the rVLM antagonizes EA modulation of sympathoexcitatory cardiovascular reflex responses. Male rats anesthetized with ketamine and α-chloralose subjected to repeated gastric distension every 10 min were examined for their responsiveness to EA (2 Hz, 0.5 ms, 1–4 mA) at P5-P6 acupoints overlying median nerve. Repeated gastric distension every 10 min evoked consistent sympathoexcitatory responses. EA at P5-P6 modulated gastric distension-induced responses. Microinjection of CCK-8 in the rVLM reversed the EA effect in seven responders. The CCK1 receptor antagonist devazepide microinjected into the rVLM converted six nonresponders to responders by lowering the reflex response from 21 ± 2.2 to 10 ± 2.9 mmHg (first vs. second application of EA). The EA modulatory action in rats converted to responders with devazepide was reversed with rVLM microinjection of naloxone (n = 6). Microinjection of devazepide in the absence of a second application of EA did not influence the primary pressor reflexes of nonresponders. These data suggest that CCK-8 antagonizes EA modulation of sympathoexcitatory cardiovascular responses through an opioid mechanism and that inhibition of CCK-8 can convert animals that initially are unresponsive to EA to become responsive.
Keywords: sympathoexcitation, acupuncture, nonresponders, rostral ventrolateral medulla
the lifetime risk of developing hypertension, an important underlying cause of stroke and heart attacks, increases with age for middle-aged adults (2013 AHA Statistical Fact Sheet). The 7th Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure report recommends lifestyle modification (i.e., nontraditional approaches) with or without pharmacological intervention (10). Acupuncture or electroacupuncture (EA) potentially offers a nonpharmacological approach to reduce high blood pressure (BP). Recently, we and others have shown that in mildly hypertensive patients, EA applied once weekly over an 8-wk course of therapy lowers BP by 10–12 mmHg, a response that persists for an additional 4 wk after termination of EA stimulation (18, 41). Notably, the hypotensive action of acupuncture, including manual and EA, is absent in a subgroup (nonresponders) comprising ∼30% of clinical and experimental subjects undergoing treatment (40, 41, 72). The mechanisms of the cardiovascular nonresponsiveness to EA are unknown.
The rostral ventrolateral medulla (rVLM) serves as an important site of regulation for respiration, circulation, and pain, and it is an essential region controlling sympathetic outflow (24, 48). Our previous study showed that μ- and δ-opioid receptors in the rVLM play an important role in EA's cardiovascular action (45). In addition, enkephalinergic neurons (22) in the rVLM are activated by EA, suggesting that this opioid neuromodulator participates in EA suppression of sympathoexcitatory activity in this brain stem region. Moreover, the opioid-mediated inhibitory actions of EA occur in the hypothalamus, midbrain, and the brain stem, including the rVLM (44, 45). Endorphins in the arcuate nucleus of the ventral hypothalamus are transported to the rVLM through long projections (23, 44). Recently, we have shown that the expression of preproenkephalin, the precursor of met-enkephalin, is increased late (90 min) in the rVLM after a single, as well as 24 h, after repetitive EA stimulation in anesthetized (38) and conscious rats (37), suggesting that molecular changes of enkephalin gene expression contribute to EA's prolonged cardiovascular actions. Hence, enkephalins, endorphins, as well as their related receptors in the rVLM, importantly participate in cardiovascular modulation by EA.
Cholecystokinin (CCK) originally found to be located in the gastrointestinal tract more recently has been identified in the central nervous system (CNS) as a neurotransmitter that is involved in many important functions, including satiety, pain, cognition, and emotion (13, 55). The sulfated C-terminal octapeptide of cholecystokinin (CCK-8) is the predominant form of CCK in the CNS. Neurons containing CCK and its receptors are localized in several regions of the brain, including the brain stem (3, 4, 51–53). CCK-8 in the rVLM modulates sympathetic outflow (61, 62). This peptide antagonizes the opioid regulation of pain (26), mood disorders (27), as well as acupuncture's action through stimulation of CCK1 and CCK2 receptors (25, 31, 32, 34, 35, 63, 76). In the past two decades, the CCK system has been shown to reduce responsiveness to the analgesic effect of EA (25, 31, 32, 34, 35, 63, 76). Exogeneous CCK injected intracerebroventricularly was used in one study (25). Other studies have relied on molecular rather than physiological evidence (31, 32, 34, 35, 76). A single study evaluating low responders has shown that reducing tissue levels of CCK in the cortex, hippocampus, and midbrain is associated with an increase in the responsiveness to EA analgesia (63). The specific nuclear location of this action and the mechanism by which lower levels of CCK improved EA analgesia have not been assessed. Furthermore, CCK's role in EA modulation of cardiovascular function has not been evaluated nor has the mechanism underlying the complete absence of responsiveness to EA. Thus, in the present study, we hypothesized that CCK-8 in the rVLM antagonizes EA modulation of sympathoexcitatory reflex pressor responses. A preliminary report of this work has been published (39).
MATERIALS AND METHODS
All procedures were carried out in accordance with the Society for Neuroscience and guidelines developed by the National Institutes of Health. The minimum possible number of rats was used to obtain reproducible results in this study. In addition, every effort was made to minimize discomfort and suffering. Surgical and experimental protocols were approved by the Animal Use and Care Committee at the University of California, Irvine. This study focused on nonresponders to EA, although a few responders were used to evaluate the action of CCK-8 during EA inhibition of the excitatory reflex. Since we found more responders than nonresponders, the responsive animals not used in protocols of the present study were utilized in other ongoing studies in our laboratory.
Anatomical Study
Immunohistochemical staining for enkephalin and CCK1 receptors: surgical preparation.
Adult male Sprague-Dawley rats (400–500 g) were used to microinject colchicine into the subarachnoid space. Briefly, a mixture of ketamine/xylazine (80/12 mg/ml; Sigma) was used to induce (0.3–0.4 ml im) and maintain (0.1–0.2 ml im) anesthesia. Body temperature was monitored with a rectal probe and maintained at 37°C. Heart rate and oxygen saturation were monitored using a pulse oximeter (Nonin Medical, Plymouth, MN). Following induction, the head of the rat was placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) and flexed ∼30° forward in the frame. A one-inch midline incision was made from the external occipital protuberance located at the base of the skull. After exposing the foramen magnum near the brain stem, a 27-gauge, 1.25-inch-long hypodermic needle attached to a 1.0-ml syringe was inserted into the subarachnoid space through the atlanto-occipital membrane overlying the fourth ventricle. We injected colchicine (80 μg/kg; Sigma, St. Louis, MO) in 0.08–0.13 ml of solution (3,000 μg/ml) dissolved in 0.9% normal saline. The dose of colchicine used in the present study was determined on the basis of previous studies (6, 11, 17, 22). Following administration of colchicine, the incision was closed, and the rats were allowed to recover.
Tissue preparation.
Rats were reanesthetized with a large dose of ketamine/xylazine (0.6–1.0 ml im) 22–24 h following administration of colchicine. The animals then were perfused transcardially with 0.9% saline and cold 4% paraformaldehyde in phosphate buffer (PB; pH 7.2). The medulla oblongata was removed and stored in 4% paraformaldehyde for 2 h and subsequently in 30% sucrose for 48 h to prevent ice crystallization. Coronal sections of the brain (30 μm) were cut with a cryostat microtome (Leica CM1850; Leica, Nussloch, Germany) and serially placed in cold cryoprotectant solution (7).
Double-fluorescent labeling.
Free-floating sections were used for immunohistochemical staining, as described previously (21). Briefly, after rinsing three times (10 min each) with PBS containing 0.3% Triton X-100 (PBST; pH = 7.4), brain sections were placed in 1% normal donkey serum (Jackson Immunoresearch Laboratories, West Grove, PA) for 1 h and incubated with primary antibodies at 4°C for 48 h. PBST solution containing two primary antibodies, including a goat anti-CCK1 receptor (1:250; Santa Cruz Biotechnology, lot no. E1067, Santa Cruz, CA) and a mouse anti-met- and anti-leu-enkephalin antibody (1:400, no. MAB350; Chemicon International, Atlanta, GA). Sections then were incubated with rhodamine-conjugated donkey anti-goat and fluorescein-conjugated donkey anti-mouse antibodies (all 1:100; Jackson Immunoresearch Laboratories) in PBST at 4°C for 24 h. These secondary antibodies raised in the donkey are made for multiple labels. They have minimal cross-reactivity to other nonspecific species (2010 catalog specializing in secondary antibodies; Jackson Immunoresearch Laboratories). Sections were mounted on slides and air dried after washing with PBS (pH = 7.4) for 30 min (10 min × 3 times). Slides were covered with glass slips using mounting medium (Vector Laboratories, Burlingame, CA). In addition, immunohistochemical control studies were performed by omission of the primary or secondary antibodies and by preabsorption with excess met- and leu-enkephalin peptide (both 10 μg/ml, nos. 0537500 and ZN233; Bachem Peninsula Laboratories, San Carlos, CA). No labeling was detected under these conditions.
Imaging analysis.
Brain sections were scanned and examined with a laser-scanning confocal microscope (Zeiss LSM 710, Meta system; Zeiss, Thornwood, NY). This apparatus was equipped with HeNe and Argon lasers and allowed operation of multiple channels. Lasers of 488- and 543-nm wavelengths were used to excite fluorescein (green) and rhodamine (red). Each confocal section analyzed was limited to 0.5-μm thickness in the Z-plane. Digital images of the labels were captured and analyzed with software (Zeiss LSM) provided with this microscope. Images in two colors in the same plane were merged to reveal the relationship between two labels. Single- and double-labeled neurons were evaluated.
Physiological Studies
Drugs.
The opioid receptor antagonist, naloxone (100 nM) (69) and the CCK1 receptor antagonist devazepide (alternative names: MK-329 or l-364718, 0.5 mM) (12) were purchased from Sigma Aldrich (St. Louis, MO). The sulfated CCK-8 agonist (0.2 mM) (26) was bought from American Peptide (Sunnyvale, CA). Devazepide powder (25 mg) was rinsed in 500 μl ethanol, dissolved in the same amount of polyethylene glycol 400 (Sigma Aldrich) to achieve a concentration of 25 mg/ml, and stored before use at 4°C. On the day of the experiment, we mixed 1.6 μl of the devazepide solution with 1.6 μl Tocrisolve 100 (Tocris, Ellisville, MS) to achieve a concentration of 12.5 mg/ml that was diluted further with saline to yield a final concentration of 0.2 mg/ml (0.5 mM). The vehicle for both CCK-8 and naloxone was normal saline, while 0.4% ethanol, 0.4% polyethylene glycol 400, and 0.8% Tocrisolve 100 in saline were used to dissolve devazepide. Cited references describe affinities, specificities, and dosages of drugs (12, 26, 69).
Anesthesia and surgical preparation.
Studies were performed on adult Sprague-Dawley male rats (retired breeders, 450–700 g) following an overnight fast. Anesthesia was induced with ketamine (100 mg/kg im). Additional doses of α-chloralose (25–30 mg/kg iv) were administered to maintain an adequate level of anesthesia. A femoral vein was cannulated for the administration of fluids, and a femoral artery was cannulated and connected to a pressure transducer (P23XL, IBP) to monitor blood pressure. Heart rate was derived from the pulsatile blood pressure signal. The trachea was isolated and intubated to provide artificial ventilation using a respirator (model 661; Harvard Apparatus). Arterial blood gases and pH were measured periodically with a blood gas analyzer (ABL5; Radiometer America, Westlake, OH) to assess each animal's physiological condition. Blood gases were maintained within the normal physiological range: Po2 >100 mmHg, Pco2 (30–40 mmHg), and arterial pH (7.35–7.4). Body temperature was monitored with a rectal thermometer (model 44TD), and maintained between 36 and 38°C with a heating pad.
Gastric distention.
An unstressed 2-cm diameter latex balloon (catalog no.: 391766, www.Amscan.com) was attached to a polyurethane tube (3-mm diameter) and inserted into the stomach through the mouth and esophagus. Transmural pressure was determined by measuring the pressure required to inflate the balloon with various volumes of air before it was inserted into the stomach (43). The balloon was palpated manually from the surface of the body during insertion, as it was passed through the esophagus into the stomach to confirm positioning of the balloon inside the stomach. A syringe was attached to the cannula to inflate and deflate the balloon with air, while a manometer through a T-connection was used to monitor balloon pressure. Distention pressures were selected to fall within the range that a rat normally experiences during ingestion of food and fluids in a single meal (2, 15). To induce increases in blood pressure, the balloon was inflated inside the stomach. Increases in blood pressure were observed within 30 s of inflation. The balloon was deflated within 30 s after reaching the maximal increase in blood pressure. We did not include animals in the study when the balloon was verified post mortem to be in the esophagus.
Microinjections.
Animals were placed in a stereotaxic head frame to position their heads with the floor of the fourth ventricle in a horizontal position. A partial craniotomy was performed to expose the medulla to allow access to the rVLM. A modified CMA microdialysis probe that was 14 mm long (tip diameter 0.24 mm; CMA Microdialysis, Stockholm, Sweden) and lacked the microdialysis membrane (65, 66) was inserted unilaterally (side chosen randomly) into the medulla with visual approximation at a 90° angle relative to the dorsal surface of the medulla, 1.8–2.3 mm lateral from the midline, 1–1.5 mm rostral to the obex, and advanced 3.0–3.3 mm from dorsal toward the ventral surface (56). These coordinates provide access to a region in the rVLM that has been found to contain premotor sympathoexcitatory cells (24). Proper positioning of probes in the rVLM was confirmed by noting a 5–10-mmHg elevation in arterial pressure following probe insertion. These changes are similar to those observed during investigation of neurotransmitter actions in the rVLM (29, 50, 64). The probe was connected to a UMP3 microsyringe injector (World Precision Instruments, Sarasota, FL) and a 25-μl Hamilton syringe to deliver 50 nl at a rate of 25 nl/s. Of note, several of our previous studies (14, 43, 64, 66) have demonstrated significant blockade of EA's actions following unilateral administration of drugs that is similar in magnitude to that observed with bilateral microinjection (64). Microinjection of vehicle into the rVLM and drug into surrounding regions provided chemical and anatomical controls.
Electroacupuncture.
Acupuncture needles (32 gauge stainless steel) were placed bilaterally at P5-P6 acupoints at a depth of ∼3 mm (14, 43). This region of the pericardial meridian located just above the flexor crease in the paw overlies the median nerve. Stimulation of these acupoints has been shown to evoke strong input into the rVLM (68). The needles were connected to a constant current stimulator with stimulus isolation unit and stimulator (model no. S88, Grass, West Warwick, RI). Each set of electrodes was stimulated separately, so that current did not flow from one location to the contralateral forelimb. Correct placement of the needles at the P5-P6 acupoints was confirmed by observing slight repetitive paw twitches at or near motor threshold during EA. The twitches were important observations to confirm stimulation of motor fibers in the median nerves (8, 42, 43). Gallamine triethiodide (4 mg/kg) was administered intravenously before application of 30 min EA (2 Hz, 0.5 ms, 1–4 mA) to avoid muscle movement during stimulation of the median nerves. Of note, motor nerve stimulation does not participate in the EA-cardiovascular response since we have shown that EA inhibition of reflex cardiovascular responses does not change following muscle paralysis (46). Application of EA lasted 30 min, while gastric distention during MN afferent stimulation was repeated every 10 min.
Histological confirmation of microinjection sites.
The injection sites were marked with 50 nl of Chicago Sky Blue dye (5% in 0.5 M sodium acetate) at the end of each experiment. Thereafter, rats were euthanized under deep anesthesia with additional α-chloralose, followed by saturated KCl. The stomach was exposed to confirm placement of the balloon. The medulla was removed and submerged in 4% paraformaldehyde for at least 72 h. Frozen 40-μm coronal sections were cut with a CM 1850 cryostat microtome (Leica) to confirm histologically the microinjection sites. Dye spots were identified with a binocular microscope. Using the atlas of Paxinos and Watson as a guide, sites of microinjections in the medulla were plotted with Corel Presentation software on reconstructed coronal sections (56).
Experimental Protocols
EA actions on gastric reflex responses.
Arterial blood gases, pH, and body temperature were kept within normal limits throughout the study. Gastric distention was induced by slowly inflating the balloon over a 10-s period by injecting 8–10 ml of air. Once the maximal increase of blood pressure was attained (generally within 30 s), the injected air was withdrawn slowly from the balloon. Peak excitatory blood pressure responses were noted typically within 20 to 30 s of inflation. Ten-minute recovery intervals were necessary to generate repeatable cardiovascular reflex responses (14, 43, 79). In seven other rats, the response to 30 min of EA at P5-P6 acupoints was evaluated during repeated gastric distention.
Role of CCK-8 in pressor reflex and EA response.
The action of CCK on the pressor reflex induced by GD was examined by microinjecting CCK into rVLM in the absence of EA in four rats. In 13 rats initially responsive to EA, 50 nl CCK-8 (n = 7) or saline (n = 6) was microinjected into the rVLM immediately after 30 min of EA. Thus, after recording two repeatable responses to gastric distention, EA at P5-P6 was applied bilaterally for 30 min during three additional gastric distensions. The effect of CCK-8 or saline microinjected into the rVLM at the end of EA was evaluated during eight additional gastric distensions. Thus, a total of 13 gastric distensions were evaluated to examine influence of exogenous CCK-8 on the EA response.
Role of CCK1 receptor antagonism in rVLM of nonresponders.
Nonresponders were identified by the inability of EA to inhibit sympathoexcitatory reflex responses. The response to rVLM microinjection of the CCK1 receptor antagonist devazepide immediately after termination of the initial 30-min period of EA was evaluated by applying EA a second time. The action of the vehicle control also was evaluated in five additional nonresponsive rats during repeated EA.
Role of opioids in nonresponders converted to responders.
The action of naloxone, an opioid receptor antagonist, in the rVLM was evaluated during EA inhibition of excitatory cardiovascular responses in nonresponders that had been converted to responders with devazepide. Blockade of opioid receptors in the rVLM, thus, was performed 2 min prior to the tenth gastric distention. Four additional reflex responses were measured after delivery of naloxone. Responses to vehicles (devazepide and naloxone solvents) microinjected into the rVLM were examined in five other nonresponders.
Statistical Analysis
Reflex responses are expressed as the difference in mean arterial blood pressure comparing steady-state baseline BP and pressure at peak response. Changes in mean arterial pressure are presented as bar histograms. Data are presented as means ± SE. The increases in blood pressures before and after delivery of experimental drug, vehicle, or saline were compared by a one-way repeated-measures ANOVA followed post hoc by the Student-Newman-Keuls test. Additionally, a two-way repeated-measures ANOVA followed post hoc by the Student-Newman-Keuls test was used to compare the inhibitory responses between control and treatment groups. Data are plotted and analyzed with the Kolmogorov-Smirnov test for normal data distribution and normalized when necessary with SigmaPlot (Jandel Scientific). All statistical analyses were performed with SigmaPlot/Stat (Jandel Scientific). The 0.05 probability level was used to detect significant differences.
RESULTS
Anatomical Study
Double labeling of enkephalin and CCK1 receptors in rostral ventrolateral medulla.
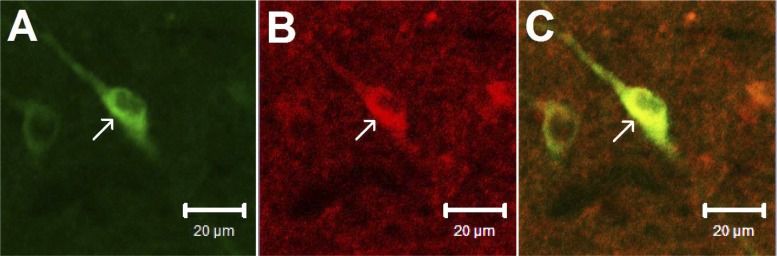
Consistent with our previous findings (22), perikarya containing enkephalin were found in the rVLM of two rats. In addition, labeling of CCK1 receptors was identified in the rVLM, suggesting the presence of CCK1 receptors in this area. More important, we noted colocalization of CCK1 receptors with neurons containing enkephalin in both rats. Approximately 28 enkephalin-containing neurons were noted in each section of the rat's rVLM. Seventeen of these neurons colabeled with CCK1 receptors. Thus, ∼60% of the enkephalinergic neurons contained CCK1 receptors. Fig. 1 demonstrates confocal images of a neuron double-labeled with enkephalin and CCK1 receptors in the rVLM of a rat.
Fig. 1.
Confocal microscopic images show fluorescent labeling of neurons containing enkephalin (green) and CCK1 receptors (red) in the rVLM of a rat. Merged image from A and B is shown in C. Arrows in A, B, and C indicate a neuron stained with enkephalin, CCK1 receptors, and the two labels, respectively. Scale bars: 20 μm.
Physiological Studies
EA actions at P5-P6 acupoints on gastric distention reflex responses.
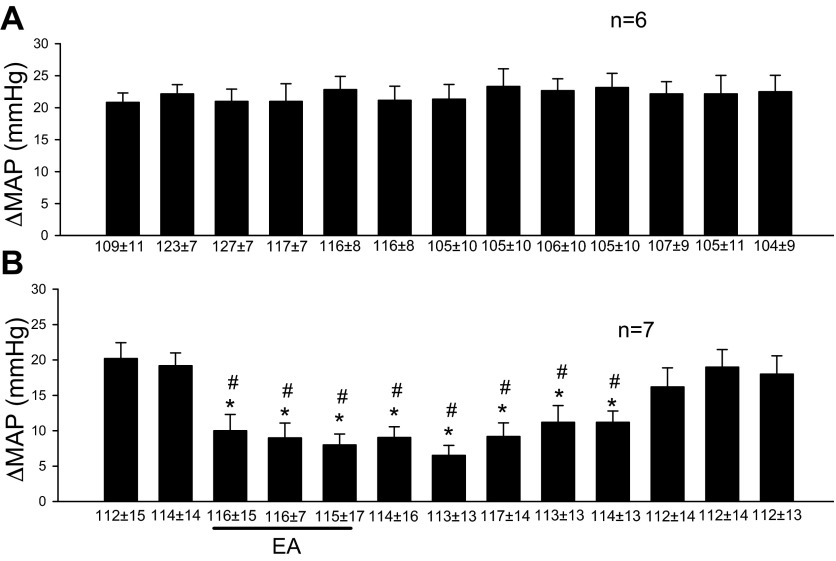
Repeated gastric distension evoked consistent sympathoexcitatory responses every 10 min (Fig. 2A). In the responders, a single 30-min application of EA modulated the excitatory cardiovascular responses by about 50% (Fig. 2B). This observation is consistent with our previous studies (14, 43). In contrast, rats that did not respond to 30 min of EA were classified as nonresponders (n = 26).
Fig. 2.
Electroacupuncture at P5-P6 modulates gastric distension-induced sympathoexcitatory responses. A: consistent evoked responses with repeated gastric distension every 10 min. B: displays reduced reflex during and after stimulation of P5-P6 acupoints overlying the median nerve. Line underneath bar histogram represents 30 min of EA. ΔMAP, change of mean arterial blood pressure. *Significant difference, P < 0.05, compared with control before EA. #Significant difference, P < 0.05, compared with control group. In this and other figures, mean ± SE below each bar represents baseline MAP before gastric distension.
CCK-8 in EA modulation of cardiovascular excitatory reflex.
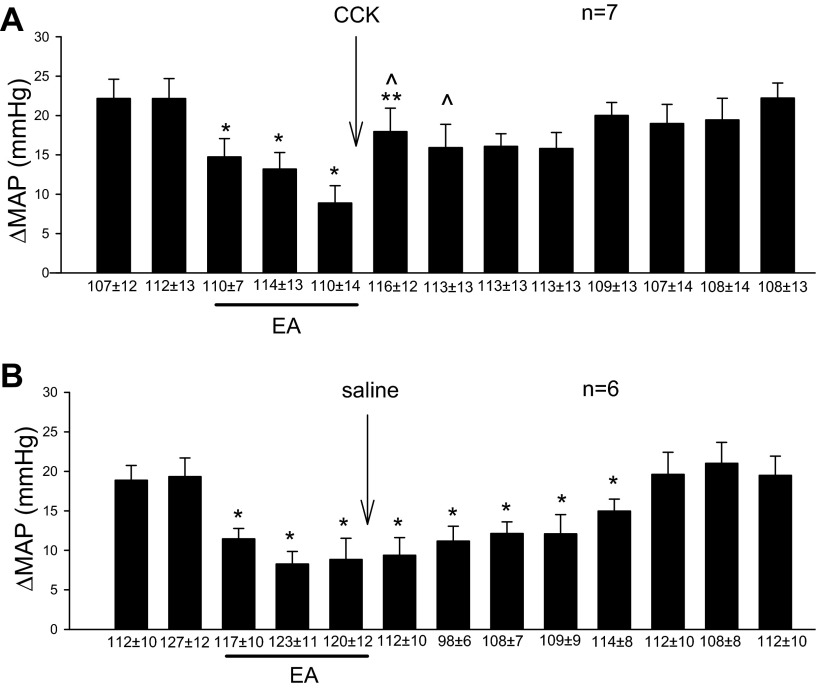
The role of CCK-8 in EA's inhibitory action on pressor reflex was examined in the rVLM of a subgroup of animals that were initially responsive to a single 30-min period of EA. Microinjection of CCK-8 immediately after acupuncture reversed EA inhibition of reflex elevations of mean arterial pressure (Fig. 3A). CCK in the absence of EA (n = 4) did not influence the pressor responses (22 ± 1 mmHg) induced by gastric distension or basal BP. Thus, the reflex-induced change in blood pressure was unaltered by CCK when EA was not applied. Furthermore, saline did not alter the EA response (Fig. 3B).
Fig. 3.
CCK-8 in the rVLM plays an important role in EA inhibition of sympathoexcitatory cardiovascular reflex responses. CCK-8 microinjected into the rVLM reversed the EA-related reduction of the cardiovascular reflex responses (A) in contrast to microinjection of saline, which did not alter the response (B). *Significant differences compared with control, P < 0.05. **Significant difference compared with the preceding response, P < 0.05. ^Significant difference compared with saline group.
CCK1 receptor antagonist in nonresponders.
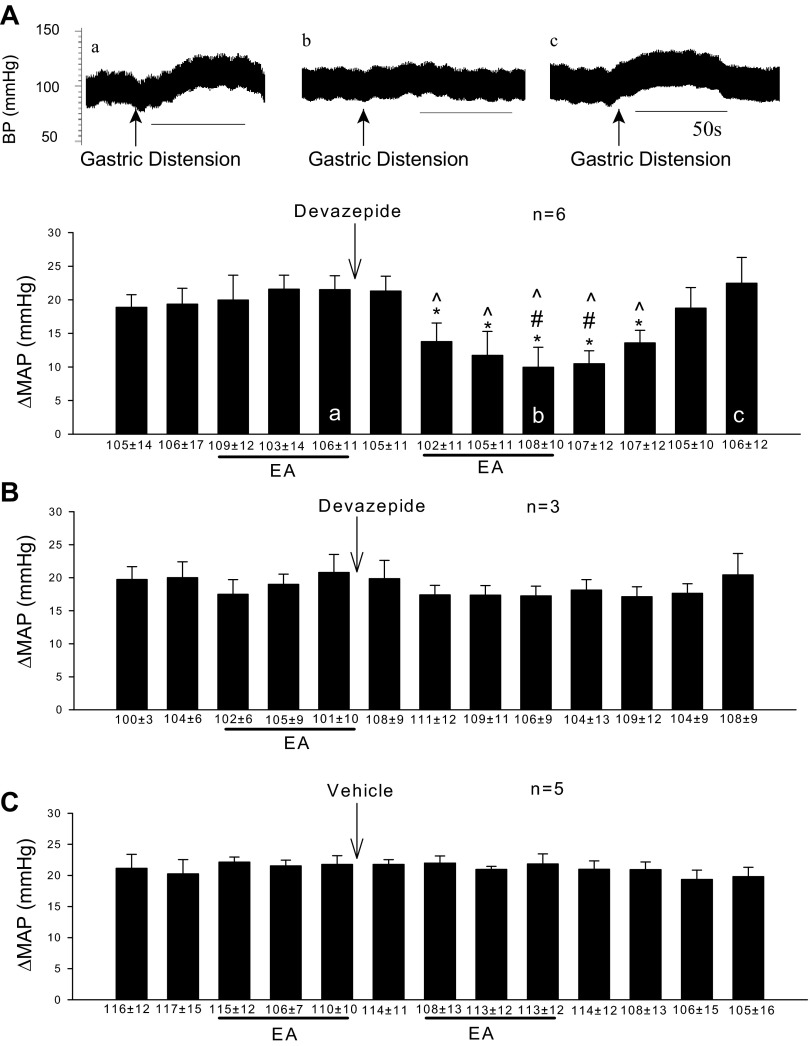
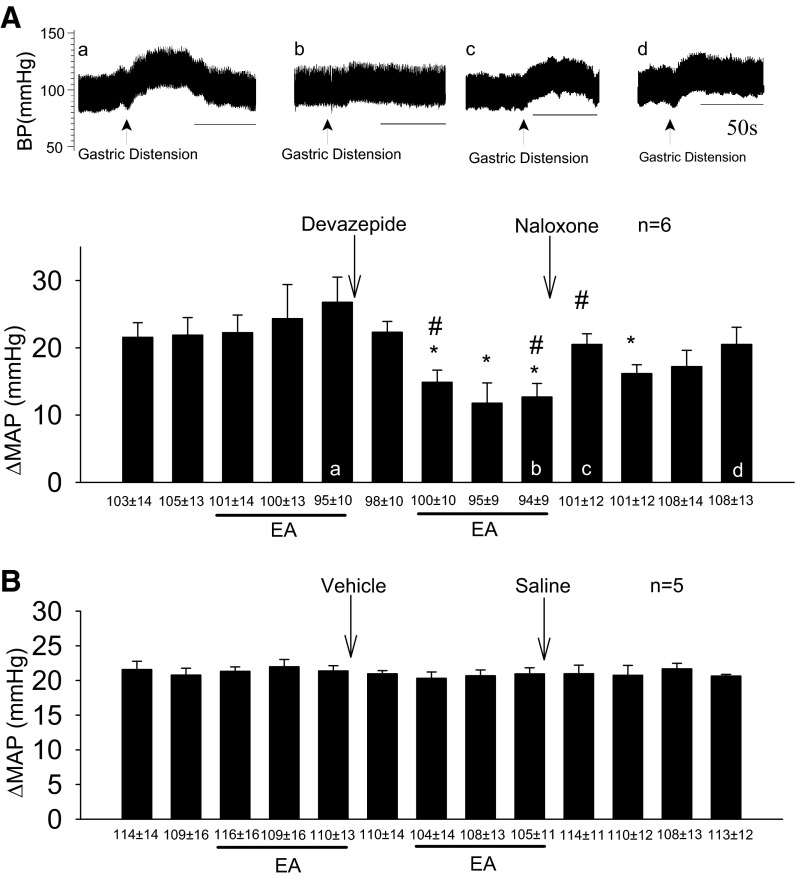
Rats not responsive to a single application of EA received microinjection of devazepide into the rVLM. The CCK antagonist converted all nonresponders into responders during a second application of 30 min EA (Fig. 4A). The converted group exhibited a reduced reflex pressor response of about 50%, similar to the extent of EA inhibition in animals that were initially responsive to acupuncture. On the other hand, in the absence of a second application of EA, devazepide did not change the primary gastric distension reflex (Fig. 4B). Microinjection of the vehicle control for devazepide into the rVLM also did not alter nonresponsiveness to EA (Fig. 4C).
Fig. 4.
Blockade of CCK1 receptors with devazepide converts animals unresponsive to EA into responders. A: after CCK blockade in the rVLM, nonresponders were converted to responders during a second 30-min EA treatment. Letters a–c displayed in the bars of the histogram correspond with blood pressures a to c shown above. Arrows indicate time of gastric distension application. B: in the absence of second EA, devazepide did not affect the primary gastric distension reflex. C: vehicle into the rVLM did not modify the nonresponsiveness to EA. *Significant difference, P < 0.05 compared with control before EA. #Significant difference, P < 0.05 compared with CCK blockade without the second EA. ^Significant difference, P < 0.05 compared with vehicle group.
Role of opioids in nonresponders converted to responders.
The action of opioids in nonresponders that were converted to responders with devazepide was examined at the end of a second 30-min period of EA. Similar to our previous observations in animals initially responsive to EA (43, 45), opioid receptor blockade with naloxone transiently reversed EA modulation of the excitatory hemodynamic responses in the converted group (Fig. 5A). The significantly reduced blood pressure response represented by 3rd bar from right in Fig. 5A demonstrates that the effect of naloxone subsided within 20 min and that the acupuncture effect outlasted the action of naloxone, consistent with our previous study (69). On the other hand, sequential microinjections of vehicle and saline in the nonresponders did not alter either nonresponsiveness to EA or the primary gastric distension reflex response (Fig. 5B).
Fig. 5.
Opioid blockade reverses EA inhibition of visceral induced elevations in BP following conversion to responders by devazepide. A: opioid blockade eliminated EA inhibition of viscerally induced elevations in BP following conversion to responders by devazepide. Letters a–d displayed in the bars of the histogram correspond with blood pressure tracings a to d. Arrows indicate time of gastric distension application. B: vehicle and saline microinjected into the rVLM do not modify the nonresponsiveness to EA. *Significant difference, P < 0.05, compared with controls before EA. #Significant difference, P < 0.05, compared with vehicle group.
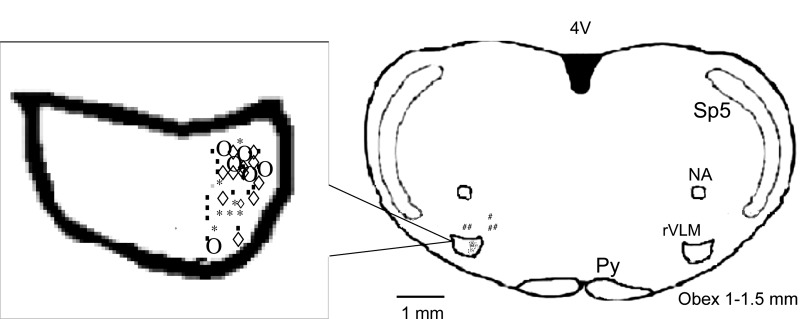
Confirmation of microinjection sites.
Examination of the rat brain slices verified that all injections located within the rVLM significantly influenced the responses to gastric distension, while the five injections outside the rVLM did not alter the reflex cardiovascular responses (Fig. 6). Microinjection sites were observed 1.0 to 1.5 mm rostral to the obex, 0.5 to 1.0 mm from the ventral surface, and 1.8 to 2.3 mm lateral to the midline, a region consistent with the location of the rVLM, according to the atlas of Paxinos and Watson (56).
Fig. 6.
Composite map shows microinjection sites relative to the obex in rostral ventrolateral medulla (rVLM). Symbols represent microinjection of saline (○), vehicle (◇), devazepide (■), CCK-8 (*), and injections outside the rVLM (#). All the injections were unilateral (side chosen randomly), although for ease of display, all injection sites are superimposed on the left. Py, pyramidal tract; Sp5, spinal trigeminal nucleus; NA, nucleus ambiguus; 4V, 4th ventricle.
DISCUSSION
We have shown in a number of experimental studies that EA inhibits sympathoexcitatory cardiovascular reflex responses (14, 43, 44, 78, 79). However, approximately one-third of the animals do not respond to 30 min EA at P5-P6. The present study is the first to demonstrate that CCK-8 in the rVLM antagonizes EA modulation of sympathoexcitatory hemodynamic responses. Perhaps more importantly, the current study demonstrates that nonresponders can be converted into an EA-responsive group, following blockade of CCK1 receptors. We also examined the mechanism associated with the inhibitory action of EA in the converted group and showed that similar to animals that are initially responsive to EA (14, 43, 44, 78, 79), the opioid system contributes to EA modulation of cardiovascular excitatory responses in nonresponders that have been converted into responders by CCK1 receptor blockade.
Of note, in a number of studies, we have found that unilateral blockade significantly diminishes the EA response, which quite likely involves bilateral activation of brain stem nuclei (67, 69). In the present study, we observed less of a response to EA inhibition comparing devazepide converted-responders to rats that responded initially to EA without devazepide. In this regard, we observed that the EA-associated reduction in the pressor reflex in the converted-responder group was less than that observed in the group that responded initially (35 vs. 48%). Furthermore, we found that the duration of EA's inhibitory action in the converted responders was shorter than in rats that responded initially (50 vs. 80 min). These results suggest that unilateral blockade of CCK's action partially (but significantly) blocks CCK function. The important point, however, is that unilateral blockade with devazepide in the rVLM was sufficient to observe a substantial action of EA on the excitatory reflex response. These results support our working hypothesis.
CCK-8 antagonizes the actions of opioids (26), GABA (49), dopamine (28), endocannabinoids (9), vasoactive-intestinal-polypeptide (33), and 5-hydroxytryptamine (71). Through these actions, this octapeptide may participate in regulation of satiety, feeding, learning and memory, nociception, and mood disorders, by stimulation of CCK1 and CCK2 receptors. To this list, we now can add a role for CCK in EA modulation of sympathoexcitatory events.
The sulfated C-terminal CCK-8, the predominant form of CCK in the CNS, is observed in high levels in the hippocampus, amygdala, septum, olfactory tubercles, caudate nucleus, paraventricular nucleus and dorsal medial region of the hypothalamus, and the nucleus of the solitary tract in the brain stem (3, 13). CCK containing neurons and receptors are localized in the brain stem, although the rVLM has not previously been examined specifically (4, 51–53). Several studies have suggested that the CCK system may contribute to the mechanism underlying decreased responsiveness to EA-induced analgesia (25, 31, 32, 34, 35, 63, 76). For example, CCK1 and CCK2 receptor gene expression in the hypothalamus has been demonstrated to have an inverse relationship with the individual variations to analgesia during high-frequency (100 Hz) EA in rats (32). Furthermore, CCK1 receptor expression is higher in low responders than high responders to 2-Hz EA treatment (34). Similarly, the analgesic effect induced by 2-Hz EA is enhanced in CCK1 receptor-deficient rats compared with the wild-type rats (31, 35). None of these experiments actually prove that CCK is the cause of decreased responsiveness to acupuncture, since they rely strictly on associations. However, Han's group has shown that intracerebroventricular or intrathecal CCK-8 reverses EA analgesia (25). They also have shown that the endogenous level of CCK-8 in the periaqueductal gray is higher in the brain of low responders compared with high responders (63). Additionally, intracerebroventricular injection of a CCK-8 antisense vector increases analgesia during EA in low responders (63). However, there are limitations to only limiting CCK production, which may limit its interaction with a number of downstream mediators and neurotransmitters. These early approaches did not identify the receptor mechanism through which CCK might act. Furthermore, while these studies suggest that CCK may be negatively involved in EA-related analgesia, no studies have evaluated the role of CCK in EA modulation of cardiovascular function or specific regions in the brain where CCK acts in any circumstance, and none have evaluated animals that are completely unresponsive to acupuncture.
CCK's antagonistic influence on the action of opioids in the rVLM infers that CCK receptors are associated with enkephalinergic neurons. Although it has been established that the distribution of CCK (3) and CCK receptors (60) is similar to that of opioid peptides and related receptors (1) in several brain regions (19, 20, 77), the present study is the first to show colocalization of enkephalin with CCK1 receptors in the rVLM. This anatomical evidence suggests that CCK1 receptors have the potential to regulate blood pressure through an interaction with enkephalins produced in the rVLM (22).
CCK receptor blockade facilitates EA inhibition of sympathoexcitatory responses. Exogenous CCK likewise diminishes the cardiovascular action of acupuncture in animals that are responsive to EA. CCK receptor stimulation influences the release of neurochemicals, including opioids (16, 30, 36, 54, 70). Taken together, our anatomical and physiological data suggest that endogenous CCK through CCK1 receptor stimulation interferes either with the release or the production of opioids in the rVLM during EA to, in turn, limit EA's cardiovascular action. Our previous data show that a single 30-min period of EA stimulation does not increase preproenkephalin during and for at least 20 min after acupuncture stimulation (38), indicating that the cardiovascular response to EA applied once depends on constitutive (preformed) enkephalin. Thus, it is likely that CCK's action during a brief single application of EA is through its action on the release of enkephalin rather than its production.
Three cellular mechanisms could be involved in the CCK-8 antagonism to opioid function. First, deletion of CCK2 receptors in mice upregulates the endogenous opioid system (57), suggesting that CCK receptor activation may inhibit opioid availability, i.e., a presynaptic process. Second, CCK-8 binding to CCK receptors reduces opioid receptor binding (58, 73, 75). This interaction between CCK receptors and opioid receptors likely occurs postsynaptically. Third, CCK receptor activation counteracts opioid inhibition of Ca2+ channel currents (5, 59). In this latter regard, CCK-8 reverses opioid receptor-mediated depression of calcium currents in rat dorsal root ganglia (47, 74). Although any of these three mechanisms could be playing a role in the current observations, our physiological and anatomical data suggest that CCK receptor stimulation likely influences the availability of enkephalin.
Perspectives and Significance
Approximately 77.9 million (1 out of every 3) adults have high blood pressure in the United States. Approximately 69% of people with their first heart attack, 77% who experience their first stroke, and 74% with congestive heart failure have blood pressures above 140/90 mmHg. High blood pressure was either the primary or a contributing cause of death in about 348,102 of the 2.4 million U.S. deaths in 2009 (2013 AHA Statistical Fact Sheet). It is clear that hypertension and its consequences represent an enormous public health problem. Acupuncture represents a viable nonpharmacological treatment option. However, its hypotensive action is absent in ∼30% of clinical and experimental subjects undergoing treatment. The current study demonstrates that nonresponders can be converted to responders following blockade CCK1 receptor in rVLM. This study thus provides not only a better understanding of the complex mechanisms underlying EA's brain stem modulatory action on reflex elevations in blood pressure but also suggests a potential method for converting EA nonresponders into responders to enhance EA's efficacy in treating hypertension.
CCK-8 in the rVLM antagonizes EA modulation of reflex sympathoexcitatory cardiovascular responses. Blockade of CCK1 receptors in the rVLM of nonresponders increases the animals' responsiveness to EA. Following conversion, EA modulates blood pressure through an opioid mechanism in the rVLM.
GRANTS
This study was supported by National Institutes of Health Grants HL-63313 and HL-072125 and American Heart Association Grant 10POST4190125.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.L., S.C.T.-AL., and J.C.L. conception and design of research; M.L. and Z.-L.G. performed experiments; M.L. analyzed data; M.L. interpreted results of experiments; M.L. and Z.-L.G. prepared figures; M.L. and S.C.T.-AL. drafted manuscript; M.L., S.C.T.-AL., Z.-L.G., and J.C.L. edited and revised manuscript; M.L., S.C.T.-AL., Z.-L.G., and J.C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We are sincerely thankful to Emma Choi, Zheyan Xu, Jonathan Chou, and Keun Kim for their technical assistance.
REFERENCES
- 1. Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW. delta-Opioid receptor immunoreactivity: distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci 15: 1215– 1235, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baird J, Travers J, Travers S. Parametric analysis of gastric distention responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1568– R1580, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res 212: 51– 57, 1981 [DOI] [PubMed] [Google Scholar]
- 4. Beitz AJ, Clements JR, Ecklund LJ, Mullett MM. The nuclei of origin of brainstem enkephalin and cholecystokinin projections to the spinal trigeminal nucleus of the rat. Neuroscience 20: 409– 425, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem 278: 18776– 18784, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Ceccatelli S, Millhorn DE, Hokfelt T, Goldstein M. Evidence for the occcurrence of an enkephalin-like peptide in adrenaline and noradrenaline neurons of the rat medulla oblongata. Exp Brain Res 74: 631– 640, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Chan RK, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol 348: 433– 460, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Chao DM, Shen LL, Tjen-A-Looi SC, Pitsillides KF, Li P, Longhurst JC. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am J Physiol Heart Circ Physiol 276: H2127– H2134, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology 34: 509– 521, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206– 1252, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Ciriello J, Caverson MM. Relation of enkephalin-like immunoreactive neurons to other neuropeptide and monoamine-containing neurons in the ventrolateral medulla. Brain Res 81: 3– 15, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Crawley JN. Subtype-selective cholecystokinin receptor antagonists block cholecystokinin modulation of dopamine-mediated behaviors in the rat mesolimbic pathway. J Neurosci 12: 3380– 3391, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides 15: 731– 755, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Crisostomo M, Li P, Tjen-A-Looi SC, Longhurst JC. Nociceptin in rVLM mediates electroacupuncture inhibition of cardiovascular reflex excitatory response in rats. J Appl Physiol 98: 2056– 2063, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Davison JS, Grundy D. Modulation of single vagal efferent fibre discharge by gastrointestinal afferents in the rat. J Physiol 284: 82, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deng PY, Xiao Z, Jha A, Ramonet D, Matsui T, Leitges M, Shin HS, Porter JE, Geiger JD, Lei S. Cholecystokinin facilitates glutamate release by increasing the number of readily releasable vesicles and releasing probability. J Neurosci 30: 5136– 5148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finley JC, Lindstreom P, Petrusz P. Immunocytochemical localization of beta-endorphin-containing neurons in the rat brain. Neuroendocrinology 33: 28– 42, 1981 [DOI] [PubMed] [Google Scholar]
- 18. Flachskampf FA, Gallasch J, Gefeller O, Gan J, Mao J, Pfahlberg AB, Wortmann A, Klinghammer L, Pflederer W, Daniel WG. Randomized trial of acupuncture to lower blood pressure. Circulation 115: 3121– 3129, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Gall C, Lauterborn J, Burks D, Seroogy K. Co-localization of enkephalin and cholecystokinin in discrete areas of rat brain. Brain Res 403: 403– 408, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Gibbins IL, Furness JB, Costa M. Pathway-specific patterns of the co-existence of substance P, calcitonin gene-related peptide, cholecystokinin and dynorphin in neurons of the dorsal root ganglia of the guinea-pig. Cell Tissue Res 248: 417– 437, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Guo ZL, Li P, Longhurst J. Central pathways in the pons and midbrain involved in cardiac sympathoexcitatory reflexes in cats. Neuroscience 113: 433– 444, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Guo ZL, Moazzami AR, Longhurst JC. Electroacupuncture induces c-Fos expression in the rostral ventrolateral medulla and periaqueductal gray in cats: relation to opioid-containing neurons. Brain Res 1030: 103– 115, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Guo Z, Longhurst J. Expression of c-Fos in arcuate nucleus induced by electroacupuncture: Relations to neurons containing opioids and glutamate. Brain Res 1166: 65– 76, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyenet PG. Role of ventral medulla oblongata in blood pressure regulation. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. New York: Oxford University Press, 1990, p. 145–167 [Google Scholar]
- 25. Han JS, Ding XZ, Fan SG. Cholecystokinin octapeptide (CCK-8): antagonism to electroacupuncture analgesia and a possible role in electroacupuncture tolerance. Pain 27: 101– 115, 1986 [DOI] [PubMed] [Google Scholar]
- 26. Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol 85: 280– 286, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Hernando F, Fuentes JA, Fournie-Zaluski MC, Roques BP, Ruiz-Gayo M. Antidepressant-like effects of CCK(B) receptor antagonists: involvement of the opioid system. Eur J Pharmacol 318: 221– 229, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature 285: 476– 478, 1980 [DOI] [PubMed] [Google Scholar]
- 29. Huber DA, Schreihofer AM. Altered regulation of the rostral ventrolateral medulla in hypertensive obese Zucker rats. Am J Physiol Heart Circ Physiol 301: H230– H240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kendrick K, Leng G, Higuchi T. Noradrenaline, dopamine and serotonin release in the paraventricular and supraoptic nuclei of the rat in response to intravenous cholecystokinin injections. J Neuroendocrinol 3: 139– 144, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Kim S, Moon H, Park J, Lee G, Shin M, Hong M, Bae H, Jin Y, Min B. The maintenance of individual differences in the sensitivity of acute and neuropathic pain behaviors to electroacupuncture in rats. Brain Res Bull 74: 357– 360, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Ko ES, Kim SK, Kim JT, Lee G, Han JB, Rho SW, Hong MC, Bae H, Min BI. The difference in mRNA expressions of hypothalamic CCK and CCK-A and -B receptors between responder and non-responder rats to high frequency electroacupuncture analgesia. Peptides 27: 1841– 1845, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Kosaka T, Kosaka K, Tateishi K, Hamaoka Y, Yanaihara N, Wu JY, Hama K. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J Comp Neurol 239: 420– 430, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Lee G, Rho S, Shin M, Hong M, Min B, Bae H. The association of cholecystokinin-A receptor expression with the responsiveness of electroacupuncture analgesic effects in rat. Neurosci Lett 325: 17– 20, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Lee GS, Han JB, Shin MK, Hong MC, Kim SW, Min BI, Bae H. Enhancement of electroacupuncture-induced analgesic effect in cholecystokinin-A receptor-deficient rats. Brain Res Bull 62: 161– 164, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Lee SH, Soltesz I. Requirement for CB1 but not GABAB receptors in the cholecystokinin-mediated inhibition of GABA release from cholecystokinin-expressing basket cells. J Physiol 589: 891– 902, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li M, Tjen A-Looi S, Guo ZL, Longhurst JC. Repetitive electroacupuncture causes prolonged increased met-enkephalin expression in the rVLM of conscious rats. Auton Neurosci 170: 30– 35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li M, Tjen A-Looi S, Longhurst JC. Electroacupuncture enhances preproenkephalin mRNA expression in rostral ventrolateral medulla of rats. Neurosci Lett 477: 61– 65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li M, Tjen-A-Looi SC, Choi ES, Xu Z, Ho J, Longhurst JC. Cholecystokinin antagonizes opioid function during electroacupuncture modulation of reflex hypertension in rats (Abstract). FASEB 26: 1091.74, 2012 [Google Scholar]
- 40. Li P, Ayannusi O, Reed C, Longhurst JC. Inhibitory effect of electroacupuncture (EA) on the pressor response induced by exercise stress. Clin Auton Res 14: 182– 188, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Li P, Longhurst JC. Long-lasting inhibitory effect of EA on blood pressure in patients with mild to moderate hypertension (Abstract). Soc Neurosci 37: 417.15/CCC26, 2007 [Google Scholar]
- 42. Li P, Pitsillides KF, Rendig SV, Pan HL, Longhurst JC. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation 97: 1186– 1194, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Li P, Rowshan K, Crisostomo M, Tjen-A-Looi SC, Longhurst JC. Effect of electroacupuncture on pressor reflex during gastric distention. Am J Physiol Regul Integr Comp Physiol 283: R1335– R1345, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Li P, Tjen-A-Looi SC, Guo ZL, Fu LW, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J Appl Physiol 106: 620– 630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li P, Tjen-A-Looi SC, Longhurst JC. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Auton Neurosci 89: 38– 47, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Li P, Tjen-A-Looi SC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol 290: H2535– H2542, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Liu NJ, Xu T, Xu C, Li CQ, Yu YX, Kang HG, Han JS. Cholecystokinin octapeptide reverses mu-opioid-receptor-mediated inhibition of calcium current in rat dorsal root ganglion neurons. J Pharmacol Exp Ther 275: 1293– 1299, 1995 [PubMed] [Google Scholar]
- 48. Lovick T, Li P. Integrated function of neurones in the rostral ventrolateral medulla. Prog Brain Res 81: 223– 231, 1989 [PubMed] [Google Scholar]
- 49. Ma KT, Si JQ, Zhang ZQ, Zhao L, Fan P, Jin JL, Li XZ, Zhu L. Modulatory effect of CCK-8S on GABA-induced depolarization from rat dorsal root ganglion. Brain Res 1121: 66– 75, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Mandel DA, Schreihofer AM. Modulation of the sympathetic response to acute hypoxia by the caudal ventrolateral medulla in rats. J Physiol 587: 461– 475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mantyh PW, Hunt SP. Evidence for cholecystokinin-like immunoreactive neurons in the rat medulla oblongata which project to the spinal cord. Brain Res 291: 49– 54, 1983 [DOI] [PubMed] [Google Scholar]
- 52. Mercer LD, Beart PM. Immunolocalization of CCK1R in rat brain using a new anti-peptide antibody. Neurosci Lett 359: 109– 113, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Mercer LD, Le VQ, Nunan J, Jones NM, Beart PM. Direct visualization of cholecystokinin subtype2 receptors in rat central nervous system using anti-peptide antibodies. Neurosci Lett 293: 167– 170, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Millington WR, Mueller GP, Lavigne GJ. Cholecystokinin type A and type B receptor antagonists produce opposing effects on cholecystokinin-stimulated beta-endorphin secretion from the rat pituitary. J Pharmacol Exp Ther 261: 454– 461, 1992 [PubMed] [Google Scholar]
- 55. Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP. International Union of Pharmacology. XXI Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev 51: 745– 781, 1999 [PubMed] [Google Scholar]
- 56. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2009 [Google Scholar]
- 57. Pommier B, Beslot F, Simon A, Pophillat M, Matsui T, Dauge V, Roques BP, Noble F. Deletion of CCK2 receptor in mice results in an upregulation of the endogenous opioid system. J Neurosci 22: 2005– 2011, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Quesada A, Micevych P. Estrogen and CCK1 receptor modification of mu-opioid receptor binding in the cortex of female rats. Brain Res 1073–1074: 316– 320, 2006. [DOI] [PubMed] [Google Scholar]
- 59. Rhim H, Miller RJ. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J Neurosci 14: 7608– 7615, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saito A, Sankaran H, Goldfine ID, Williams JA. Cholecystokinin receptors in the brain: characterization and distribution. Science 208: 1155– 1156, 1980 [DOI] [PubMed] [Google Scholar]
- 61. Sartor DM, Verberne AJ. Cholecystokinin selectively affects presympathetic vasomotor neurons and sympathetic vasomotor outflow. Am J Physiol Regul Integr Comp Physiol 282: R1174– R1184, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Sartor DM, Verberne AJ. The sympathoinhibitory effects of systemic cholecystokinin are dependent on neurons in the caudal ventrolateral medulla in the rat. Am J Physiol Regul Integr Comp Physiol 291: R1390– R1398, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Tang NM, Dong HW, Wang XM, Tsui ZC, Han JS. Cholecystokinin antisense RNA increases the analgesic effect induced by electroacupuncture or low-dose morphine: conversion of low responder rats into high responders. Pain 71: 71– 80, 1997 [DOI] [PubMed] [Google Scholar]
- 64. Tjen-A-Looi SC, Guo ZL, Li M, Longhurst JC. Medullary GABAergic mechanisms contribute to electroacupuncture modulation of cardiovascular depressor responses during gastric distension in rats. Am J Physiol Regul Integr Comp Physiol 304: R321– R332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tjen-A-Looi SC, Hsiao AF, Longhurst JC. Central and peripheral mechanisms underlying gastric distention inhibitory reflex responses in hypercapnic-acidotic rats. Am J Physiol Heart Circ Physiol 300: H1003– H1012, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tjen-A-Looi SC, Li P, Longhurst JC. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: Roles of endocannabinoids and GABA. J Appl Physiol 106: 1793– 1799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tjen-A-Looi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neuron by electroacupuncture in cats. Auton Neurosci 106: 119– 131, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Tjen-A-Looi SC, Li P, Longhurst JC. Medullary substrate and differential cardiovascular response during stimulation of specific acupoints. Am J Physiol Regul Integr Comp Physiol 287: R852– R862, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Tjen-A-Looi SC, Li P, Longhurst JC. Role of medullary GABA, opioids, and nociceptin in prolonged inhibition of cardiovascular sympathoexcitatory reflexes during electroacupuncture in cats. Am J Physiol Heart Circ Physiol 293: H3627– H3635, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Tobin VA, Leng G, Ludwig M, Douglas AJ. Increased sensitivity of monoamine release in the supraoptic nucleus in late pregnancy: region- and stimulus-dependent responses. J Neuroendocrinol 22: 430– 437, 2010 [DOI] [PubMed] [Google Scholar]
- 71. van der KD, Hunt SP, Steinbusch HW, Verhofstad AA. Separate populations of cholecystokinin and 5-hydroxytryptamine-containing neuronal cells in the rat dorsal raphe, and their contribution to the ascending raphe projections. Neurosci Lett 26: 25– 30, 1981 [DOI] [PubMed] [Google Scholar]
- 72. Vogel J, Bolling SF, Costello R, Guarneri E, Krucoff M, Longhurst JC. Integrating complementary medicine into cardiovascular medicine. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (Writing Committee to Develop an Expert Consensus Document on Complementary and Integrative Medicine). J Am Coll Cardiol 46: 184– 221, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Wang XJ, Han JS. Modification by cholecystokinin octapeptide of the binding of mu-, delta-, and kappa-opioid receptors. J Neurochem 55: 1379– 1382, 1990 [DOI] [PubMed] [Google Scholar]
- 74. Xu T, Liu NJ, Li CQ, Shangguan Y, Yu YX, Kang HG, Han JS. Cholecystokinin octapeptide reverses the kappa-opioid-receptor-mediated depression of calcium current in rat dorsal root ganglion neurons. Brain Res 730: 207– 211, 1996 [DOI] [PubMed] [Google Scholar]
- 75. Zhang L, Wang X, Han J. Modification of opioid receptors and uncoupling of receptors from G proteins as possible mechanisms underlying suppression of opioid binding by cholecystokinin octapeptide. Chin Med Sci J 8: 1– 4, 1993 [PubMed] [Google Scholar]
- 76. Zhang LX, Li XL, Wang L, Han JS. Rats with decreased brain cholecystokinin levels show increased responsiveness to peripheral electrical stimulation-induced analgesia. Brain Res 745: 158– 164, 1997 [DOI] [PubMed] [Google Scholar]
- 77. Zhang X, de Araujo LG, Elde R, Wiesenfeld-Hallin Z, Hokfelt T. Effect of morphine on cholecystokinin and mu-opioid receptor-like immunoreactivities in rat spinal dorsal horn neurons after peripheral axotomy and inflammation. Neuroscience 95: 197– 207, 2000 [DOI] [PubMed] [Google Scholar]
- 78. Zhou W, Tjen-A-Looi S, Longhurst JC. Brain stem mechanisms underlying acupuncture modality-related modulation of cardiovascular responses in rats. J Appl Physiol 99: 851– 860, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Zhou W, Fu LW, Guo ZL, Longhurst JC. Role of glutamate in rostral ventrolateral medulla in acupuncture-related modulation of visceral reflex sympathoexcitation. Am J Physiol Heart Circ Physiol 292: H1868– H1875, 2007 [DOI] [PubMed] [Google Scholar]