Abstract
The proper formation and morphogenesis of dendrites is fundamental to the establishment of neural circuits in the brain. Following cell cycle exit and migration, neurons undergo organized stages of dendrite morphogenesis, which include dendritic arbor growth and elaboration followed by retraction and pruning. Although these developmental stages were characterized over a century ago, molecular regulators of dendrite morphogenesis have only recently been defined. In particular, studies in Drosophila and mammalian neurons have identified numerous cell-intrinsic drivers of dendrite morphogenesis that include transcriptional regulators, cytoskeletal and motor proteins, secretory and endocytic pathways, cell cycle-regulated ubiquitin ligases, and components of other signaling cascades. Here, we review cell-intrinsic drivers of dendrite patterning and discuss how the characterization of such crucial regulators advances our understanding of normal brain development and pathogenesis of diverse cognitive disorders.
Keywords: Cell-intrinsic driver, Dendrite development, Dendrite morphogenesis, Dendrite patterning, Transcription factor, Ubiquitin ligases
Introduction
With their tremendous complexity and diversity, dendrites are one of nature’s architectural masterpieces. More than a century ago, Ramón y Cajal proposed an important role for dendrites (referred to at that time as protoplasmic processes) as specialized morphological structures that receive neuronal input (Ramón y Cajal, 1995). Further studies using a variety of neuronal cell types (see Glossary, Box 1) have vastly improved our understanding of dendrite development (Scott and Luo, 2001; Grueber and Jan, 2004). The development of new approaches for studying dendrite morphogenesis (see Box 2) has led to the view that axons and dendrites work in concert to define neuronal connectivity. A key concept that has emerged from such functional studies is that the particular shapes of dendrites are intimately tied to the proper wiring of neuronal circuits and their function (Häusser et al., 2000; Parrish et al., 2007b; Branco et al., 2010; Branco and Häusser, 2011; Gidon and Segev, 2012; Lavzin et al., 2012).
Box 1. Glossary
Anterodorsal and lateral projection neurons (aPNs and lPNs). These neurons of the Drosophila antennal lobe are crucial for olfactory processing. They receive excitatory input from olfactory receptor neurons in glomeruli and transmit signals to the mushroom body and lateral horn.
Cerebellar granule neurons. The most numerous neurons of the brain, these offer an ideal system for biochemical, morphological and physiological studies. Granule neurons undergo typified stages of development, which can be studied in dissociated culture, slices and in vivo. These neurons form specialized structures for synaptic input known as dendritic claws, which receive inputs from mossy fiber terminals and Golgi neuron axons.
Cortical pyramidal neurons. These vary in morphology depending on the layer they occupy, but typically have a multipolar morphology with a single apical dendrite, multiple basal dendrites and a single axon. Like granule neurons, cortical neurons can be studied in dissociated culture, slices and in vivo.
Dendritic arborization (da) neurons. These are lateral periphery sensory neurons that cover the Drosophila body wall. They have a typified branching pattern depending on their subtype. Class I da neurons have the simplest arbors, whereas Class IV have the most complex dendritic arbors covering larger dendritic fields. Class-specific variation allows analysis of the factors driving simple and complex dendritic arbors.
External sensory (ES) neurons. These neurons originate from a single precursor cell after a series of asymmetrical divisions, ultimately forming the Drosophila external sensory organ. ES neurons have been used to analyze the Drosophila peripheral nervous system, and deficits in ES neurons can be studied in behavioral assays.
γ neurons. These neurons are found in Drosophila mushroom bodies (structures involved in olfactory memory). During the first day of pupal life, γ neuron dendrites undergo extensive degeneration with loss of dendrites branching into the larval vertical and medial lobes. Dendrites then regrow as adult projection patterns are established.
Hippocampal pyramidal neurons. These neurons have numerous synaptic inputs and specialized protrusions known as dendritic spines along their dendrite shafts. They have been used for electrophysiology and morphology studies, although analyses typically require methods such as Scholl analysis because of the density of dendrites.
Multidendritic (md) sensory neurons. Also known as type II neurons of the peripheral nervous system, these are divided into three subtypes: tracheal dendrite (md-td), bipolar dendrite (md-bd) and dendritic arborization (da). They are located along the body wall, where they serve as touch receptors and proprioceptors.
Optic tectal neurons. Xenopus optic tectal neurons receive and integrate visual as well as auditory, somatosensory and vestibular inputs. In addition to electrophysiological analyses, these neurons can easily be labeled and visualized in vivo allowing time-lapse studies of dendrite morphogenesis.
Retinal ganglion cells (RGCs). These neurons are located in the ganglion cell layer of the retina, which receives inputs from bipolar and amacrine cells. The primary output of these cells is to higher order centers in the brain, such as the thalamus and hypothalamus, as well as midbrain structures.
Vertical system neurons. These are present in the lobula plate of the Drosophila optic lobe, where they are responsible for motion detection and stabilization reflexes during flight. They have a complex and highly elaborate set of dendrites and an axon that travels medially towards the esophagus.
Box 2. Techniques and culture systems for studying dendrite morphogenesis
Single-cell labeling in combination with genetic manipulation in several culture systems and organisms has been used to assess gene function in neurons. In particular, methods including biolistic transfection (Karlsgodt et al., 2008), DiI labeling (Arnold et al., 1994; Lo et al., 1994) and viral transfection (Gan et al., 2000) have facilitated studies of dendrite development. In addition, expression of genes/markers from specific promoters has been used to visualize subpopulations of neurons (Nedivi et al., 1998). Genetic mosaic methods have been used to label and genetically manipulate individual neurons (Gao et al., 1999; Holtmaat et al., 2009).
Many studies have been carried out in Drosophila due to the well-characterized nature of specific neuron populations, the ability to carry out forward genetic screens, and the ease of studying unique aspects of dendrite morphogenesis. C. elegans also offers an elegant system for genetic studies of proteins involved in dendrite morphogenesis, and several major findings in the field have originated in nematodes. However, the characterization of dendrite morphogenesis in specific neuronal populations in nematodes lags behind that of flies and mammals. The vast majority of studies in mammalian systems have been carried out in rodents, including mice and rats, mostly using cortical, hippocampal or cerebellar granule neurons. All three populations can be studied in dissociated cultures or using an ex vivo approach with slice cultures, as well as in vivo using electroporation, viral transduction or genetic knockouts. Furthermore, behavioral assays in mammalian systems offer the advantage of studying the complex behaviors and pathologies seen in humans. However, compared with Drosophila and C. elegans, characterizing the regulators of dendrite morphogenesis in mammals and their effects on behavior and neuronal connectivity are technically more arduous and time consuming. Thus, the combination of Drosophila, C. elegans and mammalian systems offers a complementary approach to the study of dendrite morphogenesis.
Prior to the elaboration of dendrites, neurons undergo axo-dendritic polarization, whereby the morphologically and functionally distinct axonal and dendritic compartments (see Box 3) are specified. In most neurons, including retinal ganglion neurons, forebrain pyramidal neurons and cerebellar granule neurons, the generation of an axon precedes the development and elaboration of dendrites (Ramón y Cajal, 1995). Although individual neuronal cell types have specific programs of dendrite development, the critical steps in dendrite morphogenesis can be broadly defined (Fig. 1).
Box 3. Structurally and functionally defining axons and dendrites
Neuronal polarization follows a strictly orchestrated and tightly controlled sequence of events (Lee and Luo, 1999; Barnes and Polleux, 2009), with distinct axonal and dendritic compartments defined by much more than their functional differences as the output and input processes, respectively, of the neuron. Structurally, dendrites are supported by an intricate scaffold of microtubules and filamentous actin (F-actin). Microtubules fill the interior of dendrites providing structural integrity, while F-actin is distributed along the cortex (Tahirovic and Bradke, 2009; Cáceres et al., 2012). In mammalian neurons, the structural protein Tau-1 (also known as Mapt) is typically localized in axons, whereas dendrites can be identified based on enrichment of microtubule-associated protein 2 (Map2) (Peters et al., 1991). Furthermore, in contrast to axons, which have unidirectional plus-end-distal microtubules, dendrites have both plus- and minus-end-distal populations (Baas et al., 1991; Baas and Lin, 2011). Thus, structural as well as functional differences define the distinct axonal and dendritic compartments in neurons.
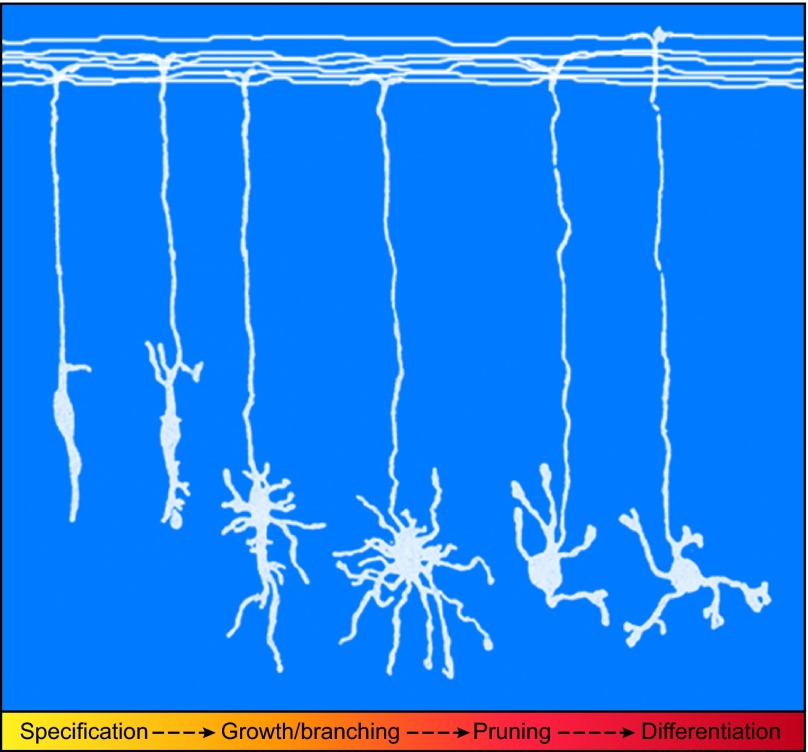
Fig. 1.
Critical stages of dendrite morphogenesis in mammalian neurons. The morphogenesis of granule neuron dendrites in the cerebellar cortex, like that of dendrites in other areas of the brain and in other organisms, occurs via distinct stages mediated by a variety of molecular regulators. After exiting the cell cycle, neural progenitors undergo polarization, whereby an axon is specified and subsequently extends, and this is followed by the specification of additional processes as dendrites. Dendrite morphogenesis then begins with dendrite growth and branching. Exuberant dendrite arbors are then pruned with the elimination of some processes but not others, yielding the dendrites that will persist after development. These remaining dendrites undergo a process of differentiation and maturation, whereby they develop specialized structures suited to the formation of synapses and contact with axons. Although the exact order of these steps and their timing varies between individual neuronal types and organisms, these fundamental steps are generally conserved. The image shown depicts granule neurons of the rat cerebellar cortex at distinct stages of dendrite development as initially drawn and characterized by Ramón y Cajal (Ramón y Cajal, 1995). In the last stage, specialized structures for synaptic input known as dendritic claws are pictured as cup-like extensions at the ends of the dendrites.
First, dendrites extend away from the soma into their target field using guidance cues to steer towards or away from their targets. During this time, dendrites grow and attain length, diameter, growth rate and molecular characteristics that are distinct from those of axons (Craig and Banker, 1994). Second, as dendrites grow farther away from the soma, branching is necessary to cover the target field. Dendrites can branch numerous times, with extensive secondary and tertiary branching. Dendrite branching occurs primarily via interstitial branching, whereby branches emerge from the side of existing dendrite shafts; branches initially appear as filopodia then morph into growth cone-like structures and extend to become stable branches (Dailey and Smith, 1996). Third, dendrite growth is restrained as the dendrite arbor reaches defined borders, giving rise to the mature shape of the dendritic tree (Wässle et al., 1981; Gao et al., 1999). For example, retinal ganglion cells (RGCs, see Glossary, Box 1) stop growing upon contact with neighboring RGCs of the same type (Wässle et al., 1981), allowing each functional group of RGCs to non-redundantly cover the entire retina. Such dendritic tiling occurs in diverse populations of neurons (Perry and Linden, 1982; Kramer and Kuwada, 1983; Amthor and Oyster, 1995; Grueber et al., 2002; Sagasti et al., 2005; Millard and Zipursky, 2008; Huckfeldt et al., 2009) and is induced by repulsive interactions between dendrites of the same neuron type. In some systems, dendritic fields may be spatially restricted to a two-dimensional plane, thereby facilitating these repulsive contacts (Han et al., 2012). Self-avoidance ensures that dendritic branches of the same neuron spread out evenly within a territory (Corty et al., 2009). In other neurons, such as Drosophila motoneurons, dendrites have a domain organization that relies on molecular boundaries defined during segmentation of the embryo (Landgraf et al., 2003). Together, these mechanisms of dendritic tiling and self-avoidance are essential for limiting dendrite growth and establishing non-redundant coverage of distinct territories. Fourth, dendrites differentiate and develop specialized structures that house synapses. In hippocampal pyramidal neurons (see Glossary, Box 1), dendrites generate small specialized protrusions termed dendritic spines (Peters et al., 1991), whereas in cerebellar granule neurons (see Glossary, Box 1) dendrites form cup-like structures termed dendritic claws at their ends (Palay and Chan-Palay, 1974). These steps in dendrite patterning are necessary for the accurate formation of neuronal circuitry. Finally, dendrite pruning is an important step in establishing the mature dendritic arbor. In Drosophila, dendrites can undergo substantial remodeling during metamorphosis from larva to adults. These changes occur through programmed degeneration of the dendrite arbor, with molecular pathways governing the fragmentation of dendrites and clearance by phagocytosis (Williams and Truman, 2005). The soma then undergoes a process whereby a new dendritic arbor is regrown. By contrast, pruning in mammalian neurons refers to the modification of arbors via retraction and elimination of dendrite branches. For example, in the rodent cerebellum, following the stage of exuberant arbors, dendrites are pruned to establish their mature shape and subsequently undergo postsynaptic differentiation (Ramón y Cajal, 1995; Okazawa et al., 2009). The process of pruning may therefore ensure that only dendrites that are properly innervated undergo maturation (Wingate and Thompson, 1994; Wong et al., 2000; Ramos et al., 2007).
The molecular mechanisms regulating dendrite morphogenesis can be broadly divided into cell-extrinsic and cell-intrinsic mechanisms. Chemoattractive and chemorepulsive cues, such as ephrins, semaphorins and neurotrophins, are important examples of cell-extrinsic regulators of dendrite morphogenesis (Whitford et al., 2002; Jan and Jan, 2003; Miller and Kaplan, 2003; Kim and Chiba, 2004). Cell-intrinsic control refers to mechanisms that do not strictly depend on external cues, although external cues may influence their activity (Goldberg, 2004; Kim and Bonni, 2007; Stegmüller and Bonni, 2010; Yang et al., 2010; de la Torre-Ubieta and Bonni, 2011; Puram and Bonni, 2011; Yamada et al., 2013). The cell-extrinsic regulators of dendrite morphogenesis, including cell surface receptors and other membrane-bound proteins, have been reviewed (Scott and Luo, 2001; Whitford et al., 2002; Grueber and Jan, 2004; Konur and Ghosh, 2005; Corty et al., 2009) and will not be discussed here. Rather, we focus our attention on the major cell-intrinsic mechanisms, namely the regulators operating within the cell downstream or independently of cell surface receptors and other neurons, and their role in dendrite morphogenesis in both invertebrates and mammals. In doing so, we provide a comprehensive review of dendrite biology, offering insights into potential areas of future investigation and conveying broader themes within the field.
The importance of cell-intrinsic control of dendrite patterning
During development, different neuronal cell types encounter similar environmental factors. However, intrinsic pathways within each neuron control the cellular interpretation of these extrinsic cues, thus allowing neurons to generate distinct patterns of dendrite development. For example, distinct cortical pyramidal neurons (see Glossary, Box 1) respond differently to the same neurotrophin: exposure of cortical slices to neurotrophin 4 (NT-4, or Ntf4) induces dendrite arborization and complexity in layer V pyramidal neurons, but has little or no effect on layer IV neurons (McAllister et al., 1995), whereas brain-derived neurotrophic factor (BDNF) strongly stimulates dendrite growth in layer IV neurons with moderate effects on layer V neurons. Distinct neurotrophin receptor expression patterns may thus explain the unique response of each cortical layer to different neurotrophins, highlighting that cell-intrinsic mechanisms determine the neuronal response to particular extrinsic cues in the environment.
In addition to controlling the specificity of dendrite patterning, cell-intrinsic mechanisms also coordinate the timing of dendrite morphogenesis. Neonatal RGCs rapidly lose the ability to extend axons upon the onset of dendrite development (Goldberg et al., 2002; Goldberg, 2004). Furthermore, RGCs from postnatal day (P) 8 animals extend significantly more dendrites than RGCs cultured from embryonic day (E) 20 animals under identical culture conditions, suggesting that a precisely timed cell-intrinsic program enables neurons to rapidly develop and elaborate dendrites. Together, these studies demonstrate the important role of cell-intrinsic regulators in defining neuronal responses to extrinsic cues and driving dendrite patterning in the nervous system.
Transcriptional control of dendritic patterning
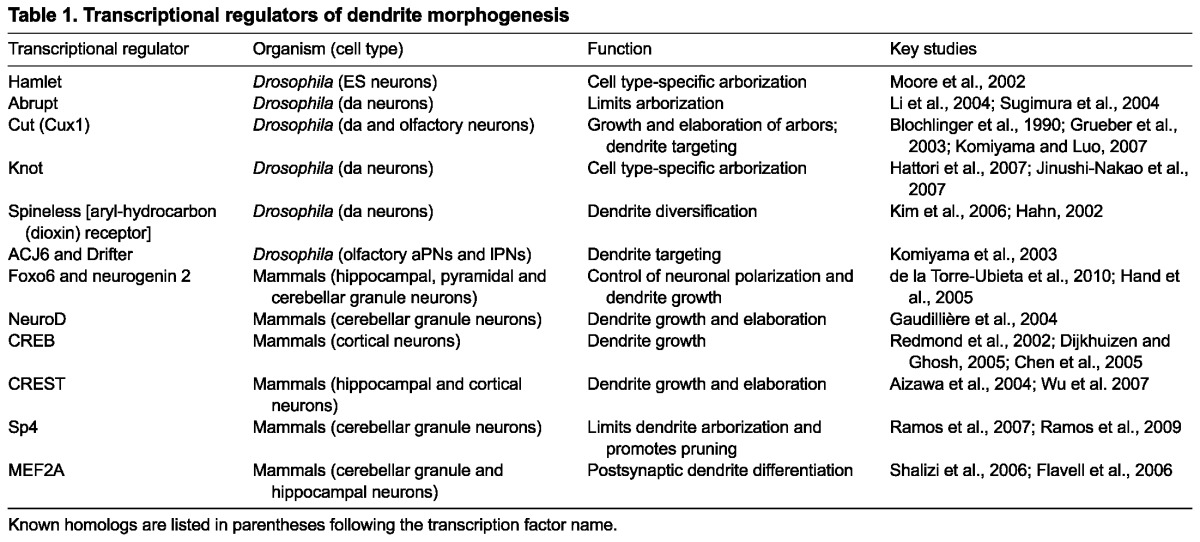
Transcriptional regulators contribute to the specification of neuronal dendrite arbors. In some cases, distinct dendrite morphology can be attained by varying the levels of a single transcriptional regulator, whereas in other cases multiple transcriptional regulators operate synergistically to define dendrite arbors. As we discuss below, studies in both flies and mammals have identified a number of transcription factors that can positively and negatively regulate distinct aspects of dendrite morphogenesis (summarized in Table 1).
Table 1.
Transcriptional regulators of dendrite morphogenesis
Insights from flies: individual and combinatorial actions of transcription factors regulate dendrite patterning
Studies of the Drosophila peripheral nervous system have revealed that transcription factors can induce dramatic changes in dendrite patterning. For example, Jan and colleagues identified the zinc-finger (ZnF)-containing transcription factor Hamlet as a key regulator of dendrite branching (Moore et al., 2002). In loss-of-function hamlet mutants, the single, unbranched dendritic arbor of external sensory (ES) neurons (see Glossary, Box 1) mimics the highly branched arbor that is characteristic of multidendritic (md) sensory neurons (see Glossary, Box 1). Expression of Hamlet in md neuron precursors has the opposite effect, yielding dendrite morphologies similar to those of ES neurons. Together, these findings emphasize that a single transcription factor can drive cell type-specific differentiation and dendrite arborization.
Forward genetic screens in Drosophila class I dendritic arborization (da) neurons (see Glossary, Box 1) have identified more than 70 transcription factors that may control dendrite growth (Parrish et al., 2006). Among these, a gene encoding the BTB-ZnF protein Abrupt is uniquely expressed in class I da neurons. Ectopic expression of Abrupt in other classes of da neurons reduces their complexity and size, suggesting that Abrupt limits dendrite growth and elaboration in class I da neurons (Li et al., 2004; Sugimura et al., 2004). By contrast, the homeodomain-containing transcription factor Cut is expressed at different levels in class I, II, III and IV da neurons; the four classes exhibit undetectable, low, medium and high levels of Cut expression, respectively (Blochlinger et al., 1990; Grueber et al., 2003). Strikingly, loss-of-function cut mutations in neurons that normally express cut causes simplification of dendrites, whereas overexpression of cut in class I neurons switches arbors toward the dendrite pattern of class III neurons (Grueber et al., 2003). Cut appears to induce actin-rich filopodia-like protrusions that may influence branch dynamics and allow neurons to elaborate more complex dendritic trees. Like Cut, the bHLH-PAS transcription factor Spineless is expressed in all four classes of da neurons. Spineless appears to enable dendrite diversification, perhaps by endowing da neurons with the ability to respond to other transcription factors and signaling molecules. spineless mutant flies have more complex class I and II da neurons, whereas class III and IV da neurons develop simpler dendritic arbors (Kim et al., 2006).
Transcription factors have also been implicated in dendritic patterning in the Drosophila olfactory system, in which distinct cell lineages non-redundantly target their dendrites to specific glomeruli. For example, in addition to specifying da neuron arborization, Cut has also been implicated in dendrite targeting in the Drosophila olfactory system (Komiyama and Luo, 2007). The POU homeodomain transcription factors ACJ6 and Drifter (also known as Ventral veins lacking) also appear to define the specificity of dendrite targeting in anterodorsal and lateral projection neurons (aPNs and lPNs, respectively, see Glossary, Box 1). Misexpression of Drifter in aPNs, which normally express ACJ6, or misexpression of ACJ6 in lPNs, which normally express Drifter, causes dendrites to target the incorrect glomeruli, suggesting that these transcription factors specify dendrite targeting in these neurons (Komiyama et al., 2003). More recent studies have identified a role for the BTB-ZnF transcription factor Lola, the chromatin remodeling factor Bap55 operating through the TIP60 complex, and the histone deacetylase Rpd3 acting via the transcription factor Prospero in the wiring and targeting of Drosophila olfactory projection neurons (Komiyama and Luo, 2007; Spletter et al., 2007; Tea et al., 2010; Tea and Luo, 2011).
Transcription factors may also function in a combinatorial fashion to specify dendrite patterning (Corty et al., 2009). The transcription factor Knot (also known as Collier) is expressed specifically in class IV da neurons of Drosophila, where it suppresses Cut-induced filopodia-like protrusions (Hattori et al., 2007; Jinushi-Nakao et al., 2007; Crozatier and Vincent, 2008). The combined expression of Cut and Knot is essential for the correct patterning of class IV da neurons. By contrast, Cut but not Knot is expressed in class III neurons, which uniquely harbor actin-rich terminal branchlets known as spiky protrusions. These terminal branchlets can be specifically marked by Fascin (also known as Singed), which is also required downstream of Cut for these spiky protrusions to form correctly (Nagel et al., 2012). Thus, the pattern of expression of Cut and Knot dictates neuronal type-specific dendrite morphology. Together, these studies in Drosophila establish the theme that individual transcription factors, acting either alone or in combination with other transcription factors, may drive specific aspects of dendrite patterning and arborization in a cell type-specific manner.
Transcriptional control of dendrite morphogenesis in the mammalian brain
A few of the transcriptional regulators of dendrite morphogenesis in Drosophila appear to have conserved functions in mammalian neurons. Mammalian orthologs of Cut, termed Cut-like 1 and 2 (Cux1 and Cux2), have been characterized and may have conserved functions in dendrite morphogenesis. In mammalian cortical pyramidal neurons, Cux1 but not Cux2 appears to reduce dendrite complexity by suppressing the expression of p27Kip1 (also known as Cdkn1b) and regulating RhoA (Li et al., 2010b). Other studies suggest that Cux1 and Cux2 operate in a cell-intrinsic manner to stimulate the growth and branching of dendrites in upper layer cortical neurons (Cubelos et al., 2010). In addition to Cux1 and Cux2, other homologs of Drosophila have been identified in mammals. However, many of these, such as the Spineless homolog aryl-hydrocarbon (dioxin) receptor (AHR), have undefined roles in dendrite morphogenesis in mammals (Hahn, 2002).
Several transcription factors have been implicated in dendrite morphogenesis in the mammalian brain (Gaudillière et al., 2004; Hand et al., 2005; Shalizi et al., 2006; Ramos et al., 2007; Shalizi et al., 2007; de la Torre-Ubieta et al., 2010). An overarching principle of these studies is that distinct transcription factors may be dedicated to different phases of dendrite development. For example, de la Torre-Ubieta et al. identified a crucial role for the brain-enriched FOXO transcription factor Foxo6 in the establishment of neuronal polarity (de la Torre-Ubieta et al., 2010; Christensen et al., 2011). In both primary cerebellar granule and hippocampal neurons, as well as in the cerebellar cortex in vivo, knockdown of FOXO proteins leads to an unpolarized neuronal morphology. Foxo6 directly stimulates the expression of Pak1, which then acts locally to promote neuronal polarization (Bokoch, 2003; Jacobs et al., 2007; Causeret et al., 2009). At later stages of neuronal morphogenesis, Foxo6 inhibits the growth of differentiated dendrites, thereby contributing to the characteristic morphology of neurons with long axons and shorter dendrites. In other studies, the bHLH transcription factor neurogenin 2 (Ngn2) has been implicated in the specification of unipolar apical dendrite morphology in cortical pyramidal neurons (Hand et al., 2005). Expression of wild-type Ngn2, but not the Y241F phosphorylation mutant, induces multipolar dendrite morphology with no apical dendrite, suggesting that Tyr241 phosphorylation is required for Ngn2 to regulate dendrites. Together, these findings establish transcriptional mechanisms that both control the specification of dendrites and license further steps in dendrite development.
The bHLH transcription factor NeuroD also stimulates dendrite growth and arborization (Gaudillière et al., 2004). Knockdown of NeuroD in primary cerebellar granule neurons and organotypic cerebellar slices dramatically reduces dendrite growth and arborization, but does not inhibit the growth of axons. The function of NeuroD in dendrite growth persists into adulthood, as shown for adult-born granule neurons of the hippocampus in NeuroD (Neurod1) knockout mice (Gao et al., 2009). Granule neurons from NeuroD null mice have significantly shorter dendrites than neurons from wild-type animals. Together, these findings establish an essential function for NeuroD in dendrite morphogenesis.
Transcriptional mediators of activity-dependent dendrite morphogenesis
Interestingly, NeuroD activity appears to be regulated by calcium signaling and neuronal activity (Fig. 2). The activity-regulated protein kinase CaMKIIα stimulates NeuroD phosphorylation at Ser336, thereby triggering NeuroD-dependent transcription and dendrite growth (Gaudillière et al., 2004). Accordingly, disruption of NeuroD largely abrogates the effects of CaMKIIα on dendrite development. Thus, NeuroD acts downstream of neuronal activity to regulate dendrite elaboration. However, the transcriptional targets of NeuroD that mediate these effects on dendrite morphogenesis remain to be elucidated.
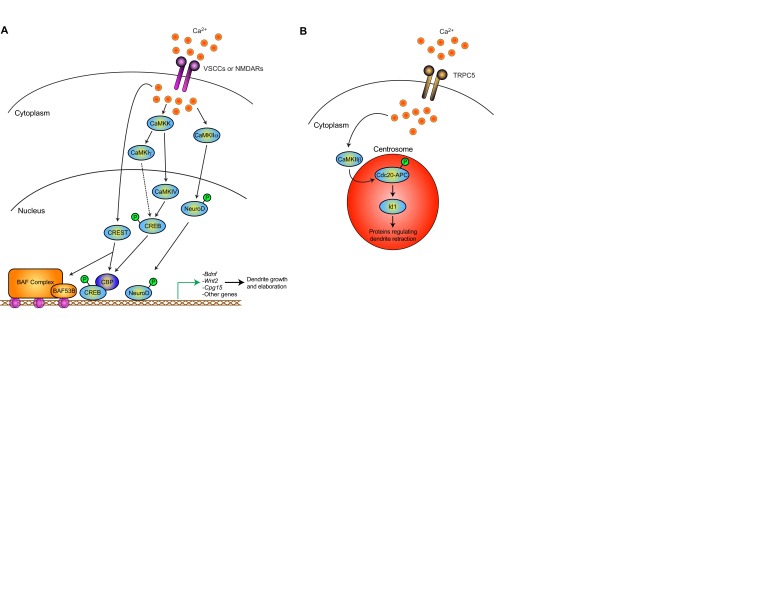
Fig. 2.
Calcium-mediated regulation of dendrite morphogenesis. Calcium-regulated control occurs throughout dendrite morphogenesis - during growth and elaboration as well as during dendrite pruning and retraction. (A) Neuronal depolarization with subsequent calcium entry via voltage-sensitive calcium channels (VSCCs) or NMDA receptors (NMDARs) triggers the activation of calcium/calmodulin-dependent kinase (CaMK) family members, thereby directing transcription factor activity in the nucleus. CREB functions downstream of CaMKIγ or CaMKIV, while the transcription factor NeuroD is phosphorylated and activated by CaMKIIα. Other transcriptional regulators such as CBP bind to CREB and influence transcription. At the level of chromatin, the chromatin remodeling complex nBAF plays a role in regulating activity-dependent dendrite growth. CREST binds to the nBAF complex and, in turn, controls gene expression. Together, these diverse mechanisms provide complex, yet tightly regulated, control of gene expression relevant for dendrite growth, including Bdnf, Wnt2 and Cpg15 (Nrn1). (B) During later stages of dendrite development, calcium regulates ubiquitin signaling at the centrosome to drive dendrite retraction and pruning. Calcium influx via the membrane channel TRPC5 activates CaMKIIβ, which phosphorylates and inhibits the major ubiquitin ligase Cdc20-APC at the centrosome. As a result, the Cdc20-APC substrate Id1 accumulates at the centrosome leading to dendrite retraction and pruning.
Like NeuroD, the transcription factor cAMP responsive element binding protein (CREB) mediates activity-dependent dendrite morphogenesis in mammalian brain neurons (Fig. 2) (Dijkhuizen and Ghosh, 2005). In addition to the protein kinase CaMKIV, the small GTP-binding protein Rap1 appears to contribute to calcium-dependent activation of CREB signaling, suggesting that multiple pathways might link calcium influx with CREB-dependent transcription and dendrite growth (Chen et al., 2005). Expression of dominant-negative CREB suppresses voltage-gated calcium channel- and CaMKIV-induced dendrite growth (Redmond et al., 2002), suggesting that CREB-dependent transcription is required for activity-dependent dendrite growth and that CREB acts as a node in activity-dependent signaling. Although the specific targets of CREB that control dendrite growth remain to be fully characterized, BDNF has been identified as one target that promotes dendrite growth in both cortical and cerebellar neurons (McAllister et al., 1995; McAllister et al., 1996; Schwartz et al., 1997; Horch et al., 1999; Mertz et al., 2000).
The SYT-related nuclear protein calcium-responsive transactivator (CREST, also known as Ss18l1) also mediates calcium-induced dendrite growth (Fig. 2). Using a transactivator trap approach, Ghosh and colleagues identified CREST as a key downstream effector of calcium influx (Aizawa et al., 2004). Crest knockout mice have impaired dendrite growth in the cortex and hippocampus, and cortical neurons cultured from these mice fail to elaborate dendrites in response to neuronal activity. CREST operates as a transcriptional regulator, and studies from Crabtree and colleagues revealed that CREST binds to subunits of the neuron-specific chromatin remodeling Brg/Brm-associated factor complex (nBAF) to drive activity-dependent dendrite growth (Wu et al., 2007). CREST and nBAF interact in neurons, where, in combination with the key subunit nBAF53b (also known as Actl6b), they control the expression of genes involved in neuronal morphogenesis. Using Baf53b knockout mice, Gap43, Stmn2, Rap1a, Gprin1 and Ephexin1 (also known as Ngef) were identified as targets of this complex. Expression of Ephexin1 corrected dendrite defects in Baf53b knockout neurons and reversed their impairment in activity-dependent dendrite growth (Wu et al., 2007), consistent with a role of Ephexin1 in balancing Rho and Rac/Cdc42 signaling (Shamah et al., 2001; Sahin et al., 2005). Several homologs of nBAF have been identified in Drosophila as important regulators of dendrite development (Parrish et al., 2006), suggesting that this signaling pathway might be conserved.
As dendrites are pruned, they begin to form postsynaptic structures specialized for contact with axons. In the cerebellum, for example, granule neurons form cup-like structures called dendritic claws (Hámori and Somogyi, 1983). One transcription factor implicated in dendrite differentiation is myocyte enhancer factor 2A (MEF2A). Knockdown analyses reveal that MEF2A is required for the morphogenesis of dendritic claws in the cerebellar cortex in vivo (Shalizi et al., 2006). Neuronal activity stimulates calcineurin, which induces the dephosphorylation of MEF2A at Ser408 and promotes a sumoylation-to-acetylation switch at Lys403, thereby activating MEF2A and inhibiting dendritic claw differentiation. The SUMO E3 ligase PIASx (also known as Pias2) induces MEF2 sumoylation and consequently stimulates dendritic claw differentiation in the cerebellar cortex in vivo (Shalizi et al., 2007). Biochemical and nuclear magnetic resonance (NMR) structural studies suggest that Ser408 phosphorylation stimulates the ability of the SUMO E2 enzyme Ubc9 (also known as Ube2i) to trigger SUMO conjugation at Lys403 (Mohideen et al., 2009). These findings establish a calcium-regulated MEF2 sumoylation switch that transcriptionally controls dendrite differentiation. Although several transcription factors have emerged as key regulators of dendrite patterning in invertebrates and mammals, it will be essential to understand how these distinct pathways are ultimately integrated to sculpt the mature dendrite arbor.
Role of other transcriptional regulators
In granule neurons of the cerebellar cortex, the transcriptional regulator SnoN (also known as Skil) controls neuronal branching, including dendrite branching, in an isoform-specific manner (Huynh et al., 2011). In Drosophila, polycomb proteins, which are broadly involved in transcriptional silencing, have been implicated in the maintenance of Drosophila sensory neuron dendrites (Parrish et al., 2007a).
Transcription factors may also repress dendrite branching. The ZnF transcription factor Sp4, for example, has been implicated in the patterning of granule neuron dendrites (Ramos et al., 2007). Knockdown of Sp4 in primary granule neurons and in organotypic cerebellar slices leads to the exuberant branching of dendrites (Ramos et al., 2007). In addition, activity-induced dendritic remodeling is blocked by Sp4 knockdown, suggesting that Sp4 might restrict branch formation and promote activity-dependent pruning. Follow-up studies revealed that Sp4 binds to the promoter of neurotrophin 3 (NT-3, or Ntf3) and represses its activity, thereby reducing NT-3 expression and limiting dendrite branching in neurons (Ramos et al., 2009). Thus, distinct transcription factors may positively or negatively regulate dendrite arborization in the mammalian brain, offering a highly specific but complex layer of control over dendrite morphogenesis.
The role of steroid hormones
Like transcription factors, steroid hormones operate in the nucleus to regulate dendrite development. The γ neurons of the Drosophila mushroom body (see Glossary, Box 1), for example, appear to be differentially regulated by the nuclear receptor Ftz-f1 and its homolog Hr39 (Boulanger et al., 2011). Although these analyses primarily focused on axonal pruning and remodeling, the authors suggest a role for Ftz-f1 in triggering expression of the steroid hormone receptor Ecr-B1 and downregulating expression of Hr39, thereby inducing pruning of γ neuron dendrites. By contrast, Hr39 competes with endogenous Ftz-f1 and thereby decreases Ecr-B1 levels to disrupt pruning. Thus, Ftz-f1 and Hr39 exert opposing effects on dendrite arbors by acting as a rheostat for Ecr-B1 expression. Furthermore, recent studies of Drosophila da neurons suggest that, in the presence of the steroid hormone ecdysone, Ecr-B1 binds to CREB-binding protein (CBP) and, in collusion with the epigenetic factor Brm, induces the acetylation of H3K27 at the Sox14 gene locus (Kirilly et al., 2011). Thus, steroid hormones may operate through epigenetic mechanisms to regulate dendrite morphogenesis, although the biochemical links remain unknown.
Cytoskeleton-mediated control of dendritic morphogenesis
Cytoskeletal regulators act on structural proteins within the soma and dendrites to control dendrite morphogenesis throughout development. Early in development, these regulators drive fundamental changes in dendrite growth and arborization, whereas at later stages they provide mechanisms for the finely tuned control of the dendrite arbor. By restructuring the actin and microtubule skeleton, these regulators can mediate direct changes in dendrite arborization and length.
Rho family GTPases and actin cytoskeletal regulators
The Rho family of GTPases modulates the cytoskeleton to regulate dendrite growth and branching in invertebrate and mammalian neurons (Leemhuis et al., 2004; Newey et al., 2005; Chen and Firestein, 2007). These proteins cycle between an activated GTP-bound state and an inactive GDP-bound state. The small GTPase RhoA limits dendrite growth, whereas Ras-related C4 botulinum toxin substrate 1 (Rac1) and Cdc42 appear to drive dendrite elaboration (Scott and Luo, 2001). Constitutively active RhoA expression in Drosophila central nervous system neurons, Xenopus RGC and central neurons, chick RGC explants, and rat hippocampal slices reduces dendrite length and the volume of the dendritic field (Ruchhoeft et al., 1999; Lee et al., 2000; Li et al., 2000; Nakayama et al., 2000; Wong et al., 2000). RhoA loss-of-function mutations in flies cause mushroom body neurons to overshoot their boundaries, leading to abnormal dendritic fields (Lee et al., 2000). In contrast to RhoA mutants, loss of Rac1 in flies reduces dendrite complexity and size in mushroom body neurons (Ng et al., 2002). Similarly, in larval class IV da neurons, Rac1 and the actin-stabilizing protein tropomyosin regulate dendrite growth and branching (Lee et al., 2003; Li and Gao, 2003). Loss of Cdc42 in vertical system neurons (see Glossary, Box 1) of the Drosophila visual system interferes with typical branching patterns and tapering of dendrites (Scott et al., 2003), whereas hyperactivation of Cdc42 in mice bearing mutations in the Cdc42 GAP NOMA-GAP (also known as Arhgap33) leads to simplified cortical dendrites in vivo by regulating the actin regulator cofilin (Rosário et al., 2012). In other systems, including Xenopus optic tectal neurons (see Glossary, Box 1) and mammalian RGCs, Rac1 and to a lesser extent Cdc42 selectively increase dendrite branch extension and retraction (Li et al., 2000; Wong et al., 2000).
How might Rho and Rac activity be regulated? Interestingly, neuronal depolarization induced by NMDA and glutamate receptors in the retina appears to regulate dendrite dynamics through Rho and Rac (Wong et al., 2000). Studies in hippocampal neurons have identified the GEF Tiam1 as a crucial molecular link between NMDA signaling and Rac1 (Tolias et al., 2005). Similarly, the GEF family of Ephexins regulates the activity of RhoA, Rac and Cdc42 (Shamah et al., 2001; Sahin et al., 2005; Margolis et al., 2010). Interestingly, different Ephexin proteins operate downstream of distinct Eph receptors and have divergent effects on Rho and Rac activation (Shamah et al., 2001; Margolis et al., 2010). For example, Ephexin5 (also known as Arhgef15) specifically stimulates RhoA activity with little or no effect on Rac and Cdc42 (Margolis et al., 2010). Although Ephexins regulate axon growth cone dynamics and synaptic development (Shamah et al., 2001; Sahin et al., 2005; Margolis et al., 2010), a function for Ephexins, specifically Ephexin1, in dendrite morphogenesis has been described (Wu et al., 2007). Beyond GEF-dependent activation, Rac activity has also been linked to non-canonical Wnt signaling via catenins in hippocampal neurons (Yu and Malenka, 2003; Rosso et al., 2005; Peng et al., 2009). This pathway is regulated by the postsynaptic protein Shank and the origin recognition core complex, both of which have functions in dendrite morphogenesis (Huang et al., 2005; Quitsch et al., 2005). Together, these findings suggest that Rho family GTPases might be essential in transducing extracellular cues and other signals to direct structural changes within neurons.
Several studies have aimed to identify the key downstream effectors of Rho family GTPases. RhoA activates the Rho-associated kinase (ROK), and in hippocampal neurons ROK inhibition suppresses the reduction in dendrite length induced by constitutively active RhoA expression (Nakayama et al., 2000), suggesting that ROK is required for RhoA function in dendrite morphogenesis. ROK, in turn, controls the phosphorylation of myosin light chains and actomyosin contractility (Kimura et al., 1996; Hirose et al., 1998; Winter et al., 2001). Compared with RhoA, less is known about the downstream effectors of Rac1 and Cdc42 in the control of dendrite morphogenesis. Rac1 and Cdc42 might converge on a common signaling pathway. Consistent with this model, the serine/threonine kinase Pak1 appears to be activated by Rac1 and Cdc42 and induces dendrite elaboration in immature cortical neurons (Hayashi et al., 2007). Rac1 and Cdc42 may also activate the Arp2/3 complex (Chhabra and Higgs, 2007). However, further research is required to elucidate additional upstream regulators and downstream effectors of Rho family GTPases.
Like Rho GTPases, the actin polymerization factor Enabled (Ena) appears to regulate the actin cytoskeleton to drive dendrite patterning. In Drosophila md sensory neurons, loss-of-function mutations in ena cause dendrites to turn dorsally, and these dendrites fail to reach segment boundaries (Gao et al., 1999). Interestingly, Ena and the tyrosine kinase Ableson (Abl) appear to act downstream of the guidance receptor Roundabout (Robo) and the receptor tyrosine phosphatase Dlar (also known as Lar) (Wills et al., 1999; Bashaw et al., 2000). Although Ena and its homologs form a complex with actin cytoskeletal proteins and regulate actin dynamics (Lanier and Gertler, 2000), the specific molecular effectors of Ena in dendrite morphogenesis remain to be elucidated.
Motor proteins and microtubule regulators
Following extension of the actin cytoskeleton, dendrites must be stabilized by microtubules to maintain adequate structural integrity, a process that involves a number of microtubule motor and transport proteins. In contrast to those in axons, microtubules within dendrites support transport in both directions (Baas et al., 1988; Baas et al., 1989). As demonstrated by depletion of the motor protein CHO1 (also known as MKLP1 or KIF23) in sympathetic neurons, these bidirectional microtubules are essential for the formation and maintenance of dendrites (Sharp et al., 1997; Yu et al., 2000). In addition to CHO1, many other microtubule transport proteins contribute to dendrite development. For example, in Drosophila, loss-of-function mutations in the genes encoding the minus-end-directed dynein motor protein Dhc64 and its associated protein Lissencephaly1 (Lis1) inhibit dendrite growth, branching, and maturation in mushroom body neurons (Liu et al., 2000; Smith et al., 2000). Interestingly, LIS1 (also known as PAFAH1B1) mutations in humans result in the loss of gyri and sulci, which leads to a smooth appearance of the cortex known as lissencephaly. Although this pathology is primarily due to defects in the migration of cortical neurons, heterotropic pyramidal neurons of the hippocampus and early cortical neurons in heterozygous Lis1 (Pafah1b1) mutant mice exhibit reduced dendrite length and branching (Fleck et al., 2000; Cahana et al., 2001). Additional motor proteins, including the kinesin family member Kif5, have been implicated in the trafficking of proteins required for dendrite growth (Hoogenraad et al., 2005). Other regulators appear to control the activity of motor proteins: Nna1 (also known as Agtpbp1), for example, regulates microtubule stability through intranuclear lysyl oxidase propeptide and NF-κB RelA signaling to direct Purkinje cell dendrite development (Li et al., 2010a). Collectively, these studies establish the important role of microtubule motor proteins in the formation and maintenance of dendrites.
Several molecules that link microtubule dynamics to the actin cytoskeleton are also emerging as important regulators of dendrite growth, supporting the idea that changes in the actin cytoskeleton of dendrites must subsequently be stabilized by microtubules. For instance, in the case of Lis1 and dynein, these proteins form a complex with Nudel, which is a p35/Cdk5 substrate (p35 is a neuronal-specific activator of Cdk5) (Niethammer et al., 2000; Sasaki et al., 2000). Interestingly, p35/Cdk5 activity can be regulated by Rac (Nikolic et al., 1998), suggesting that p35/Cdk5 might act as signaling link between the actin cytoskeleton and microtubules in neurons. In Drosophila, the large cytoskeletal linker protein Kakapo (Kak, also known as Short stop), which has the vertebrate homolog MACF, contains domains that bind to actin and microtubules (Gregory and Brown, 1998; Strumpf and Volk, 1998). In kak mutant flies, microtubule structure is disrupted in numerous cell types, and, accordingly, dendrites in md neurons and motoneurons exhibit defective branching (Prokop et al., 1998; Gao et al., 1999). Together, these studies suggest that linking actin and microtubule dynamics is crucial for the growth and branching of dendrites.
Trafficking and membrane remodeling during dendrite morphogenesis: a role for Golgi and endoplasmic reticulum proteins
The growth and elaboration of dendrites requires large amounts of plasma membrane and protein, demanding dedicated mechanisms for polarized trafficking of cargo into new branches (Corty et al., 2009). Compartmentalized Golgi, known as Golgi outposts, are important components of the secretory pathway that are found in invertebrate and mammalian dendrites (Horton and Ehlers, 2003; Horton et al., 2005; Ye et al., 2007). In rat hippocampal neurons, Golgi outposts tend to be found in longer, highly branched dendrites, and perturbations of Golgi trafficking in these neurons disrupts dendrite growth and maintenance (Horton et al., 2005; Pfenninger, 2009). Moreover, local ablation of Golgi outposts reduces the branch dynamics of Drosophila da neurons (Ye et al., 2007). How might Golgi outposts control dendrite morphogenesis? A recent study suggests that Golgi outposts directly nucleate microtubules via the proteins γ-tubulin and CP309, a Drosophila homolog of the mammalian centrosomal matrix protein AKAP450 (also known as Akap9) (Ori-McKenney et al., 2012). There are likely to be additional mechanisms and functions downstream of Golgi outposts that remain to be defined.
Recent studies suggest that the dendritic endoplasmic reticulum (ER) might also play a role in the localization of essential proteins at branch points, highlighting an important role for protein kinase C (PKC) and the ER protein CLIMP-63 (also known as Ckap4) in spatially limiting AMPA receptors in response to type I metabotropic glutamate receptor (mGluR) signaling (Cui-Wang et al., 2012). Remarkably, local zones of ER complexity reside at branch points that work with these proteins to concentrate AMPA receptors. Thus, active mechanisms for localizing the secretory machinery, including the Golgi and ER, at sites of dendrite growth and remodeling may regulate dendrite development.
In forward genetic screens in flies, several proteins that mediate ER to Golgi transport, such as Dar2, Dar3 and Dar6, which are orthologs of the yeast proteins Sec23, Sar1 and Rab1, respectively, are required for dendrite elaboration in class IV da neurons (Ye et al., 2007). For example, Dar3 is necessary for vesicle formation as proteins traffic from the ER to the Golgi. Correspondingly, Dar3 mutants have diffuse Golgi outposts and disrupted dendrite growth. Knockdown of the Dar3 homolog Sar1 in rat hippocampal neurons has similar effects and specifically disrupts dendrite but not axon development (Ye et al., 2007), suggesting that flies and mammals use evolutionarily conserved mechanisms to control dendritic secretory trafficking.
How might Golgi proteins localize at sites of active dendrite remodeling? In Drosophila, the golgin coiled-coil adaptor protein Lava lamp (Lva) controls Golgi outpost distribution by associating with the microtubule-based motor complex dynein-dynactin. Consistent with this function, dominant-negative Lva causes Golgi outposts to shift to proximal dendrites, leading to a distal to proximal shift of dendrite branching in da neurons (Ye et al., 2007). Follow-up studies have found that additional mutations in dynein light intermediate chain 2 and dynein intermediate chain phenocopy the effect of lva mutations on Golgi outposts and dendrite morphogenesis (Satoh et al., 2008; Zheng et al., 2008). Because the dynein complex is a minus-end-directed motor, these findings raise the intriguing possibility that dynein functions to traffic key components of the branching machinery to expanding dendrite arbors.
Key regulators of dendrite morphogenesis may act locally at the Golgi apparatus to control dendrite morphogenesis. Litterman et al. have uncovered the E3 ubiquitin ligase Cul7-Fbxw8 as a crucial regulator of both Golgi morphogenesis and dendrite development (Litterman et al., 2011). Cul7-Fbxw8 localizes at the Golgi apparatus in neurons, and inhibition of Cul7-Fbxw8 impairs Golgi morphogenesis and function in granule neurons and consequent dendrite arbor elaboration in the rodent cerebellar cortex in vivo (Litterman et al., 2011). The cytoskeletal regulator Obsl1 interacts with Cul7-Fbxw8 and localizes Cul7 to the Golgi apparatus in neurons, and thus promotes dendrite growth (Litterman et al., 2011). Remarkably, mutations of CUL7 and OBSL1 cause the human developmental disorder 3M syndrome (Huber et al., 2005; Maksimova et al., 2007; Hanson et al., 2009), raising the question of whether dendrite abnormalities might occur in this disorder. Taken together, these findings suggest that protein complexes might act locally at the Golgi apparatus to direct dendrite morphogenesis.
Along with the secretory pathway, the endocytic pathway may regulate dendrite growth and branching by influencing the density of cell surface receptors involved in dendrite morphogenesis (Jan and Jan, 2010). For example, components of the early endocytic pathway, such as the small GTPase Rab5, facilitate arborization of Drosophila da neurons in a dynein-dependent manner (Satoh et al., 2008). By contrast, mutations in the coiled-coil protein Shrub, the Drosophila homolog of yeast Snf7, which mediates endosome to lysosome trafficking via the ESCRT-III complex, cause exuberant branching of dendrites in da neurons (Sweeney et al., 2006). Interestingly, in yeast, Snf7 is essential for the formation of multivesicular bodies (Teis et al., 2008; Saksena et al., 2009), raising the question of whether these endosomal compartments have a role in dendrite patterning. What are the specific cell surface molecules that regulate dendrite morphogenesis? Recent data implicate the clathrin adaptor-associated kinase Nak in higher order dendrite growth (Yang et al., 2011). Nak appears to interact with aspects of the endocytic pathway to direct the dendritic localization of clathrin puncta at branch points and dendritic tips, where it facilitates the internalization of the Drosophila L1CAM homolog Neuroglian. In the future, in addition to clarifying the endocytic machinery relevant to dendrite morphogenesis, it will be essential to identify additional cargo of this pathway that directs dendrite development.
Factors that regulate dendritic field size and scaling
In order for information to be transmitted with high fidelity, dendrite arbors must appropriately cover their target receptive fields of innervation. Dendrites must also grow to an appropriate size to avoid the overlap of processes from similar neuron types. As we discuss below, Hippo family members and PI3K-mTor signaling proteins have emerged as important drivers of dendritic field size and the scaling of dendritic arbors.
Hippo family members
The Ste20 family kinase Hippo regulates organ size in mammals and flies (Pan, 2007). In Drosophila, Hippo is positively regulated by Salvador (Sav) and phosphorylates and activates Warts (Wts), a nuclear DBF2-related (NDR) kinase. In Drosophila da neurons, this complex inhibits components of the polycomb repressor complex (PRC) to block transcriptional silencing (Parrish et al., 2007a). Consistent with this model, mutations in Hippo pathway members or Polycomb group (PcG) genes impair dendrite maintenance in class IV da neurons, leading to gaps in dendritic fields (Emoto et al., 2006; Parrish et al., 2007a), establishing a crucial role for Hippo-regulated Sav-Wts signaling in dendrite maintenance.
Hippo is also a key regulator of the NDR kinase Tricornered (Trc), which is activated by Furry (Fry). In Drosophila class IV da neurons, mutations in trc or fry were initially believed to render dendrites insensitive to contact-mediated suppression of outgrowth, leading to overlapping dendritic fields and loss of dendritic tiling (Emoto et al., 2006). However, new analyses from Han et al. demonstrate that trc and fry mutants fail to confine dendrites in a two-dimensional plane, allowing expansion of dendrites in three dimensions (Han et al., 2012). Consequently, dendrites in these mutants are able to avoid contact-mediated repulsion, resulting in overlapping receptive fields and the loss of tiling. Interestingly, components of TOR complex 2 (TORC2), including Sin1, Rapamycin-insensitive companion of Tor (Rictor) and Tor, form a complex with Trc and trigger its activation and phosphorylation. Accordingly, mutations in these genes disrupt the tiling of class IV da neurons by failing to restrict dendrites to a two-dimensional plane (Koike-Kumagai et al., 2009; Han et al., 2012). Importantly, these mechanisms appear to be evolutionary conserved: SAX-1 and SAX-2, the Caenorhabditis elegans homologs of Trc and Fry, respectively, also drive dendritic tiling. Together, these studies suggest that Hippo signaling regulates Trc-Fry and Wts-Sav signaling to coordinate dendrite tiling and maintenance (Jan and Jan, 2010).
In the mammalian brain, NDR kinases 1 and 2 (also known as Stk38 and Stk38l) regulate dendrite arborization and dendritic spine development (Ultanir et al., 2012). Knockdown of NDR1/2 or expression of dominant-negative NDR mutants increases dendrite arborization and proximal branching in pyramidal neurons. AP2 associated kinase (AAK1) and the GEF Rabin8 have been identified as substrates of NDR1/2 (Ultanir et al., 2012). Accordingly, these intracellular vesicular trafficking proteins drive dendrite growth and spine development, respectively.
PI3K-mTOR signaling proteins
Several studies in both flies and mammals have suggested a function for phosphoinositide 3-kinase (PI3K) signaling in dendrite scaling and development. In flies, the PI3K-mammalian target of rapamycin (mTOR) pathway restricts dendrite development (Parrish et al., 2009); however, this pathway is regulated extrinsically by expression of the microRNA bantam by neighboring epithelial cells, which in turn dampens Akt activity in the da neurons. In mammalian neurons, the PI3K-Akt-mTOR pathway promotes dendrite growth (Jaworski et al., 2005; Kumar et al., 2005). Additional studies using neurons cultured from Reeler mice and wild-type littermates reveal that reelin stimulates mTOR-S6 kinase 1 signaling in a Dab1-dependent manner (Jossin and Goffinet, 2007). Strikingly, pharmacologic inhibition of PI3K, Akt or mTOR in hippocampal neurons blocks the stimulatory effects of reelin on dendrite growth (Jossin and Goffinet, 2007). Together, these findings suggest that reelin operates upstream of PI3K and target of rapamycin complex 1 and 2 (TORC1/2) signaling to regulate dendrite morphogenesis. Interestingly, mutations in components of target of TORC2, including Sin1, Rictor and Tor, have also been implicated in dendrite patterning in Drosophila. These TORC2 components form a physical complex with Trc to drive class IV da neuron dendritic tiling (Koike-Kumagai et al., 2009). Collectively, these studies suggest that Ras-PI3K-mTOR as well as TORC2 signaling regulate dendrite development, with potential roles in dendrite scaling and tiling.
Cell cycle-regulated ubiquitin ligases and dendrite development
A growing body of literature has identified novel functions for cell cycle proteins in postmitotic neurons (Kim and Bonni, 2007; Yang et al., 2010; Puram and Bonni, 2011). Nearly a decade ago, the major ubiquitin ligase Cdh1-APC was shown to restrict axon growth in postmitotic neurons (Konishi et al., 2004), a finding that triggered numerous analyses of the regulation and substrates of this protein complex (Huynh et al., 2009; Lasorella et al., 2006; Stegmuller et al., 2006). In light of Cdh1-APC function in axons, the role of the related ubiquitin ligase Cdc20-APC has been also characterized in neurons (Kim et al., 2009; Puram et al., 2010). These studies revealed that components of the Cdc20-APC complex are expressed in the developing brain, where it promotes dendrite growth and elaboration (Kim et al., 2009). The centrosome-associated histone deacetylase 6 (Hdac6) promotes the polyubiquitylation of Cdc20, thereby stimulating the ubiquitin ligase activity of Cdc20-APC. In turn, centrosomal Cdc20-APC triggers the polyubiquitylation and degradation of the HLH protein inhibitor of DNA binding 1 (Id1) to stimulate dendrite development. Centrosomal Id1 controls dendrite development by interacting with the ubiquitin receptor Rpn10 (also known as S5a or Psmd4) and thereby inhibiting proteasome activity at the centrosome in neurons (Puram et al., 2013). The Cdc20-APC cell-intrinsic pathway of dendrite morphogenesis appears to be regulated upstream through cell-extrinsic cues such as calcium signaling via the canonical calcium channel TRPC5 and the major protein kinase CaMKIIβ (Fig. 2) (Puram et al., 2011a; Puram et al., 2011b). In the future, it will be essential to determine whether additional regulators of centrosomal signaling can mediate the integration of cell-extrinsic cues and cell-intrinsic signaling driven by cell cycle proteins.
RNA targeting and local protein translation in dendrites
In order to rapidly extend and maintain dendrite branches, neurons must rapidly synthesize proteins locally within dendrites. Local translation appears to have a central role in dendrite morphogenesis (Chihara et al., 2007). The RNA-binding proteins Pumilio and Nanos, which were originally identified as mRNA targeting proteins in Drosophila embryos, are required for dendrite patterning in class III and IV da neurons but not in class I and II neurons (Ye et al., 2004). In addition, following the period of initial dendrite growth, the maintenance and further branching of class IV da neurons in larva depends on dendritic targeting of nanos mRNA along with Glorund and Smaug, which regulate nanos translation by recognizing stem loops in its 3′ untranslated region (UTR) (Brechbiel and Gavis, 2008). Similarly, staufen 1 (Stau1), a double-stranded RNA-binding protein, has been linked to dendritic RNA localization in neurons, translational control and mRNA decay. Cultured hippocampal neurons from mutant mice with truncated Stau1 show defective dendritic targeting of Stau1 and β-actin (Actb) mRNA-containing ribonucleoproteins, and simplified dendritic arbors (Vessey et al., 2008). However, these animals have no obvious behavioral deficits, suggesting that Stau1 is likely to act redundantly with other local translation mechanisms. What might label specific mRNAs for dendritic targeting? Buckley et al. have identified ID element retrotransposons, a retained class of short interspersed repetitive elements (SINEs), within the introns of several dendritically targeted mRNAs (Buckley et al., 2011). These sequences are sufficient for targeting both endogenous and exogenous transcripts to dendrites, and, accordingly, appear to influence protein distribution within the cell. Thus, sequence-specific elements as well as RNA-protein interactions may direct local dendritic translation.
In Xenopus, the mRNA-binding protein cytoplasmic polyadenylation element binding protein 1 (CPEB1) regulates activity-dependent dendrite morphogenesis in the visual system. Using morpholino-mediated knockdown and mutant expression studies, Bestman and Cline proposed a role for CPEB1 in the local translation of mRNAs (Bestman and Cline, 2008). Consistent with these findings, CaMKII phosphorylates CPEB in hippocampal neurons, which induces the interaction of CPEB with cytoplasmic polyadenylation element (CPE)-like sequences in mRNA, thus stimulating translation (Atkins et al., 2004). A targeting function for CPEB has been proposed. Upon depolarization, CPEB is recruited to CPE-like sequences in the 3′ UTR of BDNF mRNA, targeting the mRNA to hippocampal neuron dendrites (Oe and Yoneda, 2010). Like CPEB1, Fragile X mental retardation protein (FMRP) regulates the trafficking and translation of mRNAs to the neuronal periphery, and thereby influences dendrite morphogenesis (Bagni and Greenough, 2005). However, the precise mechanism by which FMRP controls dendrite patterning remains unclear. These studies suggest that mRNA-binding proteins such as CPEB1 and FMRP are crucial regulators of mRNA targeting and translation in dendrites. Accordingly, defects in these cellular mechanisms may have dramatic consequences for the proper generation of neuronal circuitry and brain function.
Conclusions and perspectives
Recent studies reveal an enormous degree of complexity in the signaling mechanisms that control dendrite growth, patterning and maintenance. Although numerous cell-intrinsic regulators, ranging from transcription factors to cytoskeletal proteins, orchestrate dendrite morphogenesis (summarized in Fig. 3), there are several salient themes. It is clear that proteins involved in dendrite morphogenesis have functions that may be completely divergent or unrelated in non-neuronal cell types. The ubiquitin ligase Cdc20-APC is perhaps the quintessential example of this principle; Cdc20-APC drives dendrite growth and elaboration in neurons, but in mitotic cells is responsible for the transition from metaphase to anaphase (Kim et al., 2009; Puram et al., 2010). Thus, simply limiting our analyses of dendrite morphogenesis to proteins known to regulate morphology more generally is not sufficient. Forward genetic screens in Drosophila over the past 15 years have demonstrated the utility of unbiased approaches in identifying novel drivers of dendrite patterning (Scott and Luo, 2001; Jan and Jan, 2003; Grueber and Jan, 2004; Parrish et al., 2006). In the future, it will be useful to extend this approach to mammalian systems, where RNAi libraries and similar approaches can be utilized to comprehensively identify regulators of dendrite morphogenesis. Although implementation of such an approach will be challenging given the arduous methods for quantifying dendrite arbors, optimizing this hypothesis-generating approach will open up the possibility of uncovering additional pathways that regulate dendrite morphogenesis in higher order vertebrates.
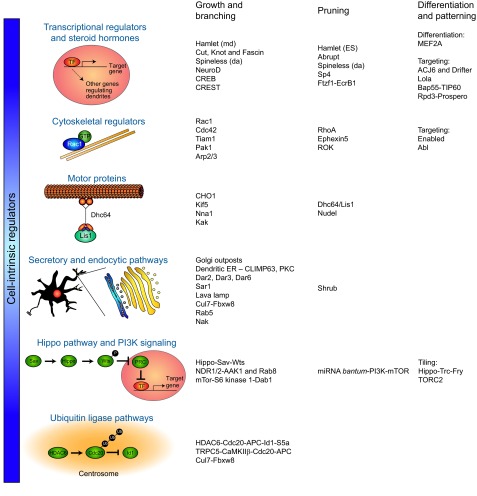
Fig. 3.
A summary of the key cell-intrinsic regulators of distinct stages of dendrite morphogenesis. Individual proteins or their signaling cassettes involved at each stage of dendrite morphogenesis are indicated, as described in the main text. In cases in which a given factor has opposing effects on two different populations of neurons, the neuron type is listed in parentheses.
Regulators of dendrite patterning also appear to have dedicated roles in driving specific phases of dendrite morphogenesis. For example, AMP-activated protein kinase (AMPK) phosphorylates the motor protein Kif5a to specify dendrites and establish their identity early in morphogenesis (Parrish et al., 2006). Later in development, NeuroD stimulates early stages of dendrite growth and elaboration, while the transcription factors Sp4 and MEF2 trigger the pruning and maturation of dendrites, respectively (Gaudillière et al., 2004; Shalizi et al., 2006; Ramos et al., 2007). Together, our survey of the literature reveals the emerging concept of specific cell-intrinsic regulators mapping onto distinct temporal phases of dendrite development. Interestingly, several molecules, such as the NDR kinases, have roles in both dendrite morphogenesis and spine formation (Ultanir et al., 2012), raising the intriguing possibility that the same regulators of dendrite patterning have additional functions in other phases of neuronal development. In the future, exploring cross-talk between signaling cascades that are active during distinct phases of development will be important in understanding the transitions from one phase to another.
Although the diverse regulators of dendrite morphogenesis must ultimately converge on the dendrite itself, leading to changes such as extension or retraction and branching or pruning, molecular integration is likely to occur at earlier steps in signaling. For example, the cell-extrinsic regulators Wnt and Dishevelled (Dvl) modulate the activity of the Rho GTPase Rac and JNK, whereas the secreted cue Sema3A triggers protein kinase A (PKA) activation, together providing a glimpse of how cell-extrinsic and cell-intrinsic regulators may ultimately be integrated. An important aspect of the fine-tuned control of dendrite morphogenesis appears to arise from regulators working in synergy to offer homeostatic regulation. The Drosophila transcription factors Cut, Knot and Spineless provide an elegant example of this combinatorial approach to signaling and its effects on dendrite patterning. However, the opposing effects of Ftz-f1 and Hr39 on steroid hormone pathways or Rac1 and Cdc42 on cytoskeletal proteins demonstrate that this integrative approach is not restricted to transcription factors. Rigorous investigation of the downstream convergence of signaling pathways on individual dendrites offers a fruitful avenue for understanding the complex dynamics that mediate formation of the mature dendritic arbor.
Despite the extensive research into dendrite patterning during the past two decades, new and exciting areas of general biology will have a major impact on our understanding of dendrite morphogenesis in the years to come. For example, a recent study has identified a role for the microRNA bantam in repressing Akt activity and blocking the regeneration of dendrites in Drosophila da neurons (Ultanir et al., 2012). Similarly, the role of organelles such as the primary cilium in dendrite morphogenesis remains to be elucidated. Defects in dendrite morphogenesis have been observed in neurons with conditional deletion of cilia (Song et al., 2012), but the underlying signaling mechanisms remain poorly characterized. The authors suggest that Wnt signaling might be dysregulated, thereby mediating aberrations in dendrite patterning. There has also been an explosion of interest in the secretory pathway and its functions in diverse aspects of biology, and the role of the secretory pathway machinery in dendrite morphogenesis is no exception (Kumamoto et al., 2012).
Characterizing the pathways regulating dendrite morphogenesis is likely to have profound consequences for our understanding of developmental disorders of cognition. Abnormalities in dendrite morphogenesis have been described in diverse neurological disorders including autism spectrum disorders (ASD), Down syndrome and Fragile X (Al-Bassam et al., 2012), as well as neurodegenerative disease (Takashima et al., 1981; Becker et al., 1986; Armstrong, 1995; Irwin et al., 2000; Kaufmann and Moser, 2000; Dierssen and Ramakers, 2006; Pardo and Eberhart, 2007). Psychiatric disorders such as schizophrenia may also be characterized by dendritic abnormalities (Graveland et al., 1985; Selkoe et al., 1987). In all these disorders, it remains unclear whether dendrite defects represent the cause or effect of the disease; however, it is tempting to speculate that reversing dendrite abnormalities in these disorders might prove at least partially clinically beneficial. Although dendrite development is likely to require a delicate balance between numerous molecular pathways, improving our understanding of these diverse regulators might render the manipulation of dendrite patterning a real possibility in the future.
Acknowledgments
We thank Luis Mejia, Luis de la Torre and other members of the A.B. laboratory for helpful discussions and critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing financial interests.
Funding
Work in the authors’ laboratories is supported by a grant from the National Institutes of Health (NIH) to A.B. and from the NIH Medical Scientist Training Program to S.V.P. Deposited in PMC for release after 12 months.
References
- Aizawa H., Hu S. C., Bobb K., Balakrishnan K., Ince G., Gurevich I., Cowan M., Ghosh A. (2004). Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science 303, 197–202 [DOI] [PubMed] [Google Scholar]
- Al-Bassam S., Xu M., Wandless T. J., Arnold D. B. (2012). Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Rep. 2, 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor F. R., Oyster C. W. (1995). Spatial organization of retinal information about the direction of image motion. Proc. Natl. Acad. Sci. USA 92, 4002–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. D. (1995). The neuropathology of Rett syndrome—overview 1994. Neuropediatrics 26, 100–104 [DOI] [PubMed] [Google Scholar]
- Arnold D., Feng L., Kim J., Heintz N. (1994). A strategy for the analysis of gene expression during neural development. Proc. Natl. Acad. Sci. USA 91, 9970–9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C. M., Nozaki N., Shigeri Y., Soderling T. R. (2004). Cytoplasmic polyadenylation element binding protein-dependent protein synthesis is regulated by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 24, 5193–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Lin S. (2011). Hooks and comets: The story of microtubule polarity orientation in the neuron. Dev. Neurobiol. 71, 403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Deitch J. S., Black M. M., Banker G. A. (1988). Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. USA 85, 8335–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Black M. M., Banker G. A. (1989). Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J. Cell Biol. 109, 3085–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Slaughter T., Brown A., Black M. M. (1991). Microtubule dynamics in axons and dendrites. J. Neurosci. Res. 30, 134–153 [DOI] [PubMed] [Google Scholar]
- Bagni C., Greenough W. T. (2005). From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat. Rev. Neurosci. 6, 376–387 [DOI] [PubMed] [Google Scholar]
- Barnes A. P., Polleux F. (2009). Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32, 347–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashaw G. J., Kidd T., Murray D., Pawson T., Goodman C. S. (2000). Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101, 703–715 [DOI] [PubMed] [Google Scholar]
- Becker L. E., Armstrong D. L., Chan F. (1986). Dendritic atrophy in children with Down’s syndrome. Ann. Neurol. 20, 520–526 [DOI] [PubMed] [Google Scholar]
- Bestman J. E., Cline H. T. (2008). The RNA binding protein CPEB regulates dendrite morphogenesis and neuronal circuit assembly in vivo. Proc. Natl. Acad. Sci. USA 105, 20494–20499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K., Bodmer R., Jan L. Y., Jan Y. N. (1990). Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 4, 1322–1331 [DOI] [PubMed] [Google Scholar]
- Bokoch G. M. (2003). Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781 [DOI] [PubMed] [Google Scholar]
- Boulanger A., Clouet-Redt C., Farge M., Flandre A., Guignard T., Fernando C., Juge F., Dura J. M. (2011). ftz-f1 and Hr39 opposing roles on EcR expression during Drosophila mushroom body neuron remodeling. Nat. Neurosci. 14, 37–44 [DOI] [PubMed] [Google Scholar]
- Branco T., Häusser M. (2011). Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron 69, 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T., Clark B. A., Häusser M. (2010). Dendritic discrimination of temporal input sequences in cortical neurons. Science 329, 1671–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechbiel J. L., Gavis E. R. (2008). Spatial regulation of nanos is required for its function in dendrite morphogenesis. Curr. Biol. 18, 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley P. T., Lee M. T., Sul J. Y., Miyashiro K. Y., Bell T. J., Fisher S. A., Kim J., Eberwine J. (2011). Cytoplasmic intron sequence-retaining transcripts can be dendritically targeted via ID element retrotransposons. Neuron 69, 877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres A., Ye B., Dotti C. G. (2012). Neuronal polarity: demarcation, growth and commitment. Curr. Opin. Cell Biol. 24, 547–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahana A., Escamez T., Nowakowski R. S., Hayes N. L., Giacobini M., von Holst A., Shmueli O., Sapir T., McConnell S. K., Wurst W., et al. (2001). Targeted mutagenesis of Lis1 disrupts cortical development and LIS1 homodimerization. Proc. Natl. Acad. Sci. USA 98, 6429–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causeret F., Terao M., Jacobs T., Nishimura Y. V., Yanagawa Y., Obata K., Hoshino M., Nikolic M. (2009). The p21-activated kinase is required for neuronal migration in the cerebral cortex. Cereb. Cortex 19, 861–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Firestein B. L. (2007). RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J. Neurosci. 27, 8378–8386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang P. Y., Ghosh A. (2005). Regulation of cortical dendrite development by Rap1 signaling. Mol. Cell. Neurosci. 28, 215–228 [DOI] [PubMed] [Google Scholar]
- Chhabra E. S., Higgs H. N. (2007). The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 9, 1110–1121 [DOI] [PubMed] [Google Scholar]
- Chihara T., Luginbuhl D., Luo L. (2007). Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat. Neurosci. 10, 828–837 [DOI] [PubMed] [Google Scholar]
- Christensen R., de la Torre-Ubieta L., Bonni A., Colón-Ramos D. A. (2011). A conserved PTEN/FOXO pathway regulates neuronal morphology during C. elegans development. Development 138, 5257–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corty M. M., Matthews B. J., Grueber W. B. (2009). Molecules and mechanisms of dendrite development in Drosophila. Development 136, 1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. M., Banker G. (1994). Neuronal polarity. Annu. Rev. Neurosci. 17, 267–310 [DOI] [PubMed] [Google Scholar]
- Crozatier M., Vincent A. (2008). Control of multidendritic neuron differentiation in Drosophila: the role of Collier. Dev. Biol. 315, 232–242 [DOI] [PubMed] [Google Scholar]
- Cubelos B., Sebastián-Serrano A., Beccari L., Calcagnotto M. E., Cisneros E., Kim S., Dopazo A., Alvarez-Dolado M., Redondo J. M., Bovolenta P., et al. (2010). Cux1 and Cux2 regulate dendritic branching, spine morphology, and synapses of the upper layer neurons of the cortex. Neuron 66, 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui-Wang T., Hanus C., Cui T., Helton T., Bourne J., Watson D., Harris K. M., Ehlers M. D. (2012). Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell 148, 309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey M. E., Smith S. J. (1996). The dynamics of dendritic structure in developing hippocampal slices. J. Neurosci. 16, 2983–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre-Ubieta L., Bonni A. (2011). Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron 72, 22–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre-Ubieta L., Gaudillière B., Yang Y., Ikeuchi Y., Yamada T., DiBacco S., Stegmüller J., Schüller U., Salih D. A., Rowitch D., et al. (2010). A FOXO-Pak1 transcriptional pathway controls neuronal polarity. Genes Dev. 24, 799–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierssen M., Ramakers G. J. (2006). Dendritic pathology in mental retardation: from molecular genetics to neurobiology. Genes Brain Behav. 5 Suppl., 48–60 [DOI] [PubMed] [Google Scholar]
- Dijkhuizen P. A., Ghosh A. (2005). Regulation of dendritic growth by calcium and neurotrophin signaling. Prog. Brain Res. 147, 15–27 [DOI] [PubMed] [Google Scholar]
- Emoto K., Parrish J. Z., Jan L. Y., Jan Y. N. (2006). The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature 443, 210–213 [DOI] [PubMed] [Google Scholar]
- Flavell S. W., Cowan C. W., Kim T. K., Greer P. L., Lin Y., Paradis S., Griffith E. C., Hu L. S., Chen C., Greenberg M. E. (2006). Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311, 1008–1012 [DOI] [PubMed] [Google Scholar]
- Fleck M. W., Hirotsune S., Gambello M. J., Phillips-Tansey E., Suares G., Mervis R. F., Wynshaw-Boris A., McBain C. J. (2000). Hippocampal abnormalities and enhanced excitability in a murine model of human lissencephaly. J. Neurosci. 20, 2439–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W. B., Grutzendler J., Wong W. T., Wong R. O., Lichtman J. W. (2000). Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron 27, 219–225 [DOI] [PubMed] [Google Scholar]
- Gao F. B., Brenman J. E., Jan L. Y., Jan Y. N. (1999). Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 13, 2549–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Ure K., Ables J. L., Lagace D. C., Nave K. A., Goebbels S., Eisch A. J., Hsieh J. (2009). Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 12, 1090–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudillière B., Konishi Y., de la Iglesia N., Yao G., Bonni A. (2004). A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron 41, 229–241 [DOI] [PubMed] [Google Scholar]
- Gidon A., Segev I. (2012). Principles governing the operation of synaptic inhibition in dendrites. Neuron 75, 330–341 [DOI] [PubMed] [Google Scholar]
- Goldberg J. L. (2004). Intrinsic neuronal regulation of axon and dendrite growth. Curr. Opin. Neurobiol. 14, 551–557 [DOI] [PubMed] [Google Scholar]
- Goldberg J. L., Klassen M. P., Hua Y., Barres B. A. (2002). Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296, 1860–1864 [DOI] [PubMed] [Google Scholar]
- Graveland G. A., Williams R. S., DiFiglia M. (1985). Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science 227, 770–773 [DOI] [PubMed] [Google Scholar]
- Gregory S. L., Brown N. H. (1998). kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 143, 1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber W. B., Jan Y. N. (2004). Dendritic development: lessons from Drosophila and related branches. Curr. Opin. Neurobiol. 14, 74–82 [DOI] [PubMed] [Google Scholar]
- Grueber W. B., Jan L. Y., Jan Y. N. (2002). Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 129, 2867–2878 [DOI] [PubMed] [Google Scholar]
- Grueber W. B., Jan L. Y., Jan Y. N. (2003). Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell 112, 805–818 [DOI] [PubMed] [Google Scholar]
- Hahn M. E. (2002). Aryl hydrocarbon receptors: diversity and evolution. Chem. Biol. Interact. 141, 131–160 [DOI] [PubMed] [Google Scholar]
- Hámori J., Somogyi J. (1983). Differentiation of cerebellar mossy fiber synapses in the rat: a quantitative electron microscope study. J. Comp. Neurol. 220, 365–377 [DOI] [PubMed] [Google Scholar]
- Han C., Wang D., Soba P., Zhu S., Lin X., Jan L. Y., Jan Y. N. (2012). Integrins regulate repulsion-mediated dendritic patterning of drosophila sensory neurons by restricting dendrites in a 2D space. Neuron 73, 64–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R., Bortone D., Mattar P., Nguyen L., Heng J. I., Guerrier S., Boutt E., Peters E., Barnes A. P., Parras C., et al. (2005). Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron 48, 45–62 [DOI] [PubMed] [Google Scholar]
- Hanson D., Murray P. G., Sud A., Temtamy S. A., Aglan M., Superti-Furga A., Holder S. E., Urquhart J., Hilton E., Manson F. D., et al. (2009). The primordial growth disorder 3-M syndrome connects ubiquitination to the cytoskeletal adaptor OBSL1. Am. J. Hum. Genet. 84, 801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., Sugimura K., Uemura T. (2007). Selective expression of Knot/Collier, a transcriptional regulator of the EBF/Olf-1 family, endows the Drosophila sensory system with neuronal class-specific elaborated dendritic patterns. Genes Cells 12, 1011–1022 [DOI] [PubMed] [Google Scholar]
- Häusser M., Spruston N., Stuart G. J. (2000). Diversity and dynamics of dendritic signaling. Science 290, 739–744 [DOI] [PubMed] [Google Scholar]
- Hayashi K., Ohshima T., Hashimoto M., Mikoshiba K. (2007). Pak1 regulates dendritic branching and spine formation. Dev. Neurobiol. 67, 655–669 [DOI] [PubMed] [Google Scholar]
- Hirose M., Ishizaki T., Watanabe N., Uehata M., Kranenburg O., Moolenaar W. H., Matsumura F., Maekawa M., Bito H., Narumiya S. (1998). Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J. Cell Biol. 141, 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A., Bonhoeffer T., Chow D. K., Chuckowree J., De Paola V., Hofer S. B., Hübener M., Keck T., Knott G., Lee W. C., et al. (2009). Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad C. C., Milstein A. D., Ethell I. M., Henkemeyer M., Sheng M. (2005). GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat. Neurosci. 8, 906–915 [DOI] [PubMed] [Google Scholar]
- Horch H. W., Krüttgen A., Portbury S. D., Katz L. C. (1999). Destabilization of cortical dendrites and spines by BDNF. Neuron 23, 353–364 [DOI] [PubMed] [Google Scholar]
- Horton A. C., Ehlers M. D. (2003). Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J. Neurosci. 23, 6188–6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. C., Rácz B., Monson E. E., Lin A. L., Weinberg R. J., Ehlers M. D. (2005). Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48, 757–771 [DOI] [PubMed] [Google Scholar]
- Huang Z., Zang K., Reichardt L. F. (2005). The origin recognition core complex regulates dendrite and spine development in postmitotic neurons. J. Cell Biol. 170, 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C., Dias-Santagata D., Glaser A., O’Sullivan J., Brauner R., Wu K., Xu X., Pearce K., Wang R., Uzielli M. L., et al. (2005). Identification of mutations in CUL7 in 3-M syndrome. Nat. Genet. 37, 1119–1124 [DOI] [PubMed] [Google Scholar]
- Huckfeldt R. M., Schubert T., Morgan J. L., Godinho L., Di Cristo G., Huang Z. J., Wong R. O. (2009). Transient neurites of retinal horizontal cells exhibit columnar tiling via homotypic interactions. Nat. Neurosci. 12, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M. A., Stegmüller J., Litterman N., Bonni A. (2009). Regulation of Cdh1-APC function in axon growth by Cdh1 phosphorylation. J. Neurosci. 29, 4322–4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M. A., Ikeuchi Y., Netherton S., de la Torre-Ubieta L., Kanadia R., Stegmüller J., Cepko C., Bonni S., Bonni A. (2011). An isoform-specific SnoN1-FOXO1 repressor complex controls neuronal morphogenesis and positioning in the mammalian brain. Neuron 69, 930–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin S. A., Galvez R., Greenough W. T. (2000). Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb. Cortex 10, 1038–1044 [DOI] [PubMed] [Google Scholar]
- Jacobs T., Causeret F., Nishimura Y. V., Terao M., Norman A., Hoshino M., Nikolić M. (2007). Localized activation of p21-activated kinase controls neuronal polarity and morphology. J. Neurosci. 27, 8604–8615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. (2003). The control of dendrite development. Neuron 40, 229–242 [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. (2010). Branching out: mechanisms of dendritic arborization. Nat. Rev. Neurosci. 11, 316–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J., Spangler S., Seeburg D. P., Hoogenraad C. C., Sheng M. (2005). Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J. Neurosci. 25, 11300–11312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi-Nakao S., Arvind R., Amikura R., Kinameri E., Liu A. W., Moore A. W. (2007). Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron 56, 963–978 [DOI] [PubMed] [Google Scholar]
- Jossin Y., Goffinet A. M. (2007). Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol. Cell. Biol. 27, 7113–7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt K. H., Sun D., Jimenez A. M., Lutkenhoff E. S., Willhite R., van Erp T. G., Cannon T. D. (2008). Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev. Psychopathol. 20, 1297–1327 [DOI] [PubMed] [Google Scholar]
- Kaufmann W. E., Moser H. W. (2000). Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 10, 981–991 [DOI] [PubMed] [Google Scholar]
- Kim A. H., Bonni A. (2007). Thinking within the D box: initial identification of Cdh1-APC substrates in the nervous system. Mol. Cell. Neurosci. 34, 281–287 [DOI] [PubMed] [Google Scholar]
- Kim S., Chiba A. (2004). Dendritic guidance. Trends Neurosci. 27, 194–202 [DOI] [PubMed] [Google Scholar]
- Kim M. D., Jan L. Y., Jan Y. N. (2006). The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev. 20, 2806–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. H., Puram S. V., Bilimoria P. M., Ikeuchi Y., Keough S., Wong M., Rowitch D., Bonni A. (2009). A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell 136, 322–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., et al. (1996). Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245–248 [DOI] [PubMed] [Google Scholar]
- Kirilly D., Wong J. J., Lim E. K., Wang Y., Zhang H., Wang C., Liao Q., Wang H., Liou Y. C., Wang H., et al. (2011). Intrinsic epigenetic factors cooperate with the steroid hormone ecdysone to govern dendrite pruning in Drosophila. Neuron 72, 86–100 [DOI] [PubMed] [Google Scholar]
- Koike-Kumagai M., Yasunaga K., Morikawa R., Kanamori T., Emoto K. (2009). The target of rapamycin complex 2 controls dendritic tiling of Drosophila sensory neurons through the Tricornered kinase signalling pathway. EMBO J. 28, 3879–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T., Luo L. (2007). Intrinsic control of precise dendritic targeting by an ensemble of transcription factors. Curr. Biol. 17, 278–285 [DOI] [PubMed] [Google Scholar]
- Komiyama T., Johnson W. A., Luo L., Jefferis G. S. (2003). From lineage to wiring specificity. POU domain transcription factors control precise connections of Drosophila olfactory projection neurons. Cell 112, 157–167 [DOI] [PubMed] [Google Scholar]
- Konishi Y., Stegmüller J., Matsuda T., Bonni S., Bonni A. (2004). Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 303, 1026–1030 [DOI] [PubMed] [Google Scholar]
- Konur S., Ghosh A. (2005). Calcium signaling and the control of dendritic development. Neuron 46, 401–405 [DOI] [PubMed] [Google Scholar]
- Kramer A. P., Kuwada J. Y. (1983). Formation of the receptive fields of leech mechanosensory neurons during embryonic development. J. Neurosci. 3, 2474–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto N., Gu Y., Wang J., Janoschka S., Takemaru K., Levine J., Ge S. (2012). A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat. Neurosci. 15, 399–405, S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Zhang M. X., Swank M. W., Kunz J., Wu G. Y. (2005). Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 25, 11288–11299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M., Jeffrey V., Fujioka M., Jaynes J. B., Bate M. (2003). Embryonic origins of a motor system: motor dendrites form a myotopic map in Drosophila. PLoS Biol. 1, E41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. M., Gertler F. B. (2000). From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility. Curr. Opin. Neurobiol. 10, 80–87 [DOI] [PubMed] [Google Scholar]
- Lasorella A., Stegmüller J., Guardavaccaro D., Liu G., Carro M. S., Rothschild G., de la Torre-Ubieta L., Pagano M., Bonni A., Iavarone A. (2006). Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 442, 471–474 [DOI] [PubMed] [Google Scholar]
- Lavzin M., Rapoport S., Polsky A., Garion L., Schiller J. (2012). Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature 490, 397–401 [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 [DOI] [PubMed] [Google Scholar]
- Lee T., Winter C., Marticke S. S., Lee A., Luo L. (2000). Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron 25, 307–316 [DOI] [PubMed] [Google Scholar]
- Lee A., Li W., Xu K., Bogert B. A., Su K., Gao F. B. (2003). Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development 130, 5543–5552 [DOI] [PubMed] [Google Scholar]
- Leemhuis J., Boutillier S., Barth H., Feuerstein T. J., Brock C., Nürnberg B., Aktories K., Meyer D. K. (2004). Rho GTPases and phosphoinositide 3-kinase organize formation of branched dendrites. J. Biol. Chem. 279, 585–596 [DOI] [PubMed] [Google Scholar]
- Li W., Gao F. B. (2003). Actin filament-stabilizing protein tropomyosin regulates the size of dendritic fields. J. Neurosci. 23, 6171–6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Van Aelst L., Cline H. T. (2000). Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat. Neurosci. 3, 217–225 [DOI] [PubMed] [Google Scholar]
- Li W., Wang F., Menut L., Gao F. B. (2004). BTB/POZ-zinc finger protein abrupt suppresses dendritic branching in a neuronal subtype-specific and dosage-dependent manner. Neuron 43, 823–834 [DOI] [PubMed] [Google Scholar]
- Li J., Gu X., Ma Y., Calicchio M. L., Kong D., Teng Y. D., Yu L., Crain A. M., Vartanian T. K., Pasqualini R., et al. (2010a). Nna1 mediates Purkinje cell dendritic development via lysyl oxidase propeptide and NF-κB signaling. Neuron 68, 45–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Zhao C. T., Wang Y., Yuan X. B. (2010b). The transcription factor Cux1 regulates dendritic morphology of cortical pyramidal neurons. PLoS ONE 5, e10596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litterman N., Ikeuchi Y., Gallardo G., O’Connell B. C., Sowa M. E., Gygi S. P., Harper J. W., Bonni A. (2011). An OBSL1-Cul7Fbxw8 ubiquitin ligase signaling mechanism regulates Golgi morphology and dendrite patterning. PLoS Biol. 9, e1001060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Steward R., Luo L. (2000). Drosophila Lis1 is required for neuroblast proliferation, dendritic elaboration and axonal transport. Nat. Cell Biol. 2, 776–783 [DOI] [PubMed] [Google Scholar]
- Lo D. C., McAllister A. K., Katz L. C. (1994). Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron 13, 1263–1268 [DOI] [PubMed] [Google Scholar]
- Maksimova N., Hara K., Miyashia A., Nikolaeva I., Shiga A., Nogovicina A., Sukhomyasova A., Argunov V., Shvedova A., Ikeuchi T., et al. (2007). Clinical, molecular and histopathological features of short stature syndrome with novel CUL7 mutation in Yakuts: new population isolate in Asia. J. Med. Genet. 44, 772–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis S. S., Salogiannis J., Lipton D. M., Mandel-Brehm C., Wills Z. P., Mardinly A. R., Hu L., Greer P. L., Bikoff J. B., Ho H. Y., et al. (2010). EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell 143, 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister A. K., Lo D. C., Katz L. C. (1995). Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15, 791–803 [DOI] [PubMed] [Google Scholar]
- McAllister A. K., Katz L. C., Lo D. C. (1996). Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17, 1057–1064 [DOI] [PubMed] [Google Scholar]
- Mertz K., Koscheck T., Schilling K. (2000). Brain-derived neurotrophic factor modulates dendritic morphology of cerebellar basket and stellate cells: an in vitro study. Neuroscience 97, 303–310 [DOI] [PubMed] [Google Scholar]
- Millard S. S., Zipursky S. L. (2008). Dscam-mediated repulsion controls tiling and self-avoidance. Curr. Opin. Neurobiol. 18, 84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. D., Kaplan D. R. (2003). Signaling mechanisms underlying dendrite formation. Curr. Opin. Neurobiol. 13, 391–398 [DOI] [PubMed] [Google Scholar]
- Mohideen F., Capili A. D., Bilimoria P. M., Yamada T., Bonni A., Lima C. D. (2009). A molecular basis for phosphorylation-dependent SUMO conjugation by the E2 UBC9. Nat. Struct. Mol. Biol. 16, 945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. W., Jan L. Y., Jan Y. N. (2002). hamlet, a binary genetic switch between single- and multiple-dendrite neuron morphology. Science 297, 1355–1358 [DOI] [PubMed] [Google Scholar]
- Nagel J., Delandre C., Zhang Y., Förstner F., Moore A. W., Tavosanis G. (2012). Fascin controls neuronal class-specific dendrite arbor morphology. Development 139, 2999–3009 [DOI] [PubMed] [Google Scholar]
- Nakayama A. Y., Harms M. B., Luo L. (2000). Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 20, 5329–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedivi E., Wu G. Y., Cline H. T. (1998). Promotion of dendritic growth by CPG15, an activity-induced signaling molecule. Science 281, 1863–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey S. E., Velamoor V., Govek E. E., Van Aelst L. (2005). Rho GTPases, dendritic structure, and mental retardation. J. Neurobiol. 64, 58–74 [DOI] [PubMed] [Google Scholar]
- Ng J., Nardine T., Harms M., Tzu J., Goldstein A., Sun Y., Dietzl G., Dickson B. J., Luo L. (2002). Rac GTPases control axon growth, guidance and branching. Nature 416, 442–447 [DOI] [PubMed] [Google Scholar]
- Niethammer M., Smith D. S., Ayala R., Peng J., Ko J., Lee M. S., Morabito M., Tsai L. H. (2000). NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron 28, 697–711 [DOI] [PubMed] [Google Scholar]
- Nikolic M., Chou M. M., Lu W., Mayer B. J., Tsai L. H. (1998). The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature 395, 194–198 [DOI] [PubMed] [Google Scholar]
- Oe S., Yoneda Y. (2010). Cytoplasmic polyadenylation element-like sequences are involved in dendritic targeting of BDNF mRNA in hippocampal neurons. FEBS Lett. 584, 3424–3430 [DOI] [PubMed] [Google Scholar]
- Okazawa M., Abe H., Katsukawa M., Iijima K., Kiwada T., Nakanishi S. (2009). Role of calcineurin signaling in membrane potential-regulated maturation of cerebellar granule cells. J. Neurosci. 29, 2938–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney K. M., Jan L. Y., Jan Y. N. (2012). Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay S., Chan-Palay V. (1974). Cerebellar Cortex: Cytology and Organization. New York, NY: Springer-Verlag; [Google Scholar]
- Pan D. (2007). Hippo signaling in organ size control. Genes Dev. 21, 886–897 [DOI] [PubMed] [Google Scholar]
- Pardo C. A., Eberhart C. G. (2007). The neurobiology of autism. Brain Pathol. 17, 434–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish J. Z., Kim M. D., Jan L. Y., Jan Y. N. (2006). Genome-wide analyses identify transcription factors required for proper morphogenesis of Drosophila sensory neuron dendrites. Genes Dev. 20, 820–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish J. Z., Emoto K., Jan L. Y., Jan Y. N. (2007a). Polycomb genes interact with the tumor suppressor genes hippo and warts in the maintenance of Drosophila sensory neuron dendrites. Genes Dev. 21, 956–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish J. Z., Emoto K., Kim M. D., Jan Y. N. (2007b). Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu. Rev. Neurosci. 30, 399–423 [DOI] [PubMed] [Google Scholar]
- Parrish J. Z., Xu P., Kim C. C., Jan L. Y., Jan Y. N. (2009). The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in drosophila sensory neurons. Neuron 63, 788–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y. R., He S., Marie H., Zeng S. Y., Ma J., Tan Z. J., Lee S. Y., Malenka R. C., Yu X. (2009). Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron 61, 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Linden R. (1982). Evidence for dendritic competition in the developing retina. Nature 297, 683–685 [DOI] [PubMed] [Google Scholar]
- Peters A., Palay S. L., Webster H. D. (1991). The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. New York, NY: Oxford University Press; [Google Scholar]
- Pfenninger K. H. (2009). Plasma membrane expansion: a neuron’s Herculean task. Nat. Rev. Neurosci. 10, 251–261 [DOI] [PubMed] [Google Scholar]
- Prokop A., Uhler J., Roote J., Bate M. (1998). The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. J. Cell Biol. 143, 1283–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram S. V., Bonni A. (2011). Novel functions for the anaphase-promoting complex in neurobiology. Semin. Cell Dev. Biol. 22, 586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram S. V., Kim A. H., Bonni A. (2010). An old dog learns new tricks: a novel function for Cdc20-APC in dendrite morphogenesis in neurons. Cell Cycle 9, 482–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram S. V., Kim A. H., Ikeuchi Y., Wilson-Grady J. T., Merdes A., Gygi S. P., Bonni A. (2011a). A CaMKIIβ signaling pathway at the centrosome regulates dendrite patterning in the brain. Nat. Neurosci. 14, 973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram S. V., Riccio A., Koirala S., Ikeuchi Y., Kim A. H., Corfas G., Bonni A. (2011b). A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes Dev. 25, 2659–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram S. V., Kim A. H., Park H. Y., Anckar J., Bonni A. (2013). The ubiquitin receptor S5a/Rpn10 links centrosomal proteasomes with dendrite development in the mammalian brain. Cell Rep. 4, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitsch A., Berhörster K., Liew C. W., Richter D., Kreienkamp H. J. (2005). Postsynaptic shank antagonizes dendrite branching induced by the leucine-rich repeat protein Densin-180. J. Neurosci. 25, 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. (1995). Histology of the Nervous System of Man and Vertebrates. New York, NY: Oxford University Press; [Google Scholar]
- Ramos B., Gaudillière B., Bonni A., Gill G. (2007). Transcription factor Sp4 regulates dendritic patterning during cerebellar maturation. Proc. Natl. Acad. Sci. USA 104, 9882–9887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos B., Valín A., Sun X., Gill G. (2009). Sp4-dependent repression of neurotrophin-3 limits dendritic branching. Mol. Cell. Neurosci. 42, 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond L., Kashani A. H., Ghosh A. (2002). Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron 34, 999–1010 [DOI] [PubMed] [Google Scholar]
- Rosário M., Schuster S., Jüttner R., Parthasarathy S., Tarabykin V., Birchmeier W. (2012). Neocortical dendritic complexity is controlled during development by NOMA-GAP-dependent inhibition of Cdc42 and activation of cofilin. Genes Dev. 26, 1743–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S. B., Sussman D., Wynshaw-Boris A., Salinas P. C. (2005). Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 8, 34–42 [DOI] [PubMed] [Google Scholar]
- Ruchhoeft M. L., Ohnuma S., McNeill L., Holt C. E., Harris W. A. (1999). The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J. Neurosci. 19, 8454–8463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagasti A., Guido M. R., Raible D. W., Schier A. F. (2005). Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr. Biol. 15, 804–814 [DOI] [PubMed] [Google Scholar]
- Sahin M., Greer P. L., Lin M. Z., Poucher H., Eberhart J., Schmidt S., Wright T. M., Shamah S. M., O’connell S., Cowan C. W., et al. (2005). Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46, 191–204 [DOI] [PubMed] [Google Scholar]
- Saksena S., Wahlman J., Teis D., Johnson A. E., Emr S. D. (2009). Functional reconstitution of ESCRT-III assembly and disassembly. Cell 136, 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Shionoya A., Ishida M., Gambello M. J., Yingling J., Wynshaw-Boris A., Hirotsune S. (2000). A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron 28, 681–696 [DOI] [PubMed] [Google Scholar]
- Satoh D., Sato D., Tsuyama T., Saito M., Ohkura H., Rolls M. M., Ishikawa F., Uemura T. (2008). Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat. Cell Biol. 10, 1164–1171 [DOI] [PubMed] [Google Scholar]
- Schwartz P. M., Borghesani P. R., Levy R. L., Pomeroy S. L., Segal R. A. (1997). Abnormal cerebellar development and foliation in BDNF-/- mice reveals a role for neurotrophins in CNS patterning. Neuron 19, 269–281 [DOI] [PubMed] [Google Scholar]
- Scott E. K., Luo L. (2001). How do dendrites take their shape? Nat. Neurosci. 4, 359–365 [DOI] [PubMed] [Google Scholar]
- Scott E. K., Reuter J. E., Luo L. (2003). Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J. Neurosci. 23, 3118–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Bell D. S., Podlisny M. B., Price D. L., Cork L. C. (1987). Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science 235, 873–877 [DOI] [PubMed] [Google Scholar]
- Shalizi A., Gaudillière B., Yuan Z., Stegmüller J., Shirogane T., Ge Q., Tan Y., Schulman B., Harper J. W., Bonni A. (2006). A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 311, 1012–1017 [DOI] [PubMed] [Google Scholar]
- Shalizi A., Bilimoria P. M., Stegmüller J., Gaudillière B., Yang Y., Shuai K., Bonni A. (2007). PIASx is a MEF2 SUMO E3 ligase that promotes postsynaptic dendritic morphogenesis. J. Neurosci. 27, 10037–10046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamah S. M., Lin M. Z., Goldberg J. L., Estrach S., Sahin M., Hu L., Bazalakova M., Neve R. L., Corfas G., Debant A., et al. (2001). EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105, 233–244 [DOI] [PubMed] [Google Scholar]
- Sharp D. J., Yu W., Ferhat L., Kuriyama R., Rueger D. C., Baas P. W. (1997). Identification of a microtubule-associated motor protein essential for dendritic differentiation. J. Cell Biol. 138, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Niethammer M., Ayala R., Zhou Y., Gambello M. J., Wynshaw-Boris A., Tsai L. H. (2000). Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2, 767–775 [DOI] [PubMed] [Google Scholar]
- Song Y., Ori-McKenney K. M., Zheng Y., Han C., Jan L. Y., Jan Y. N. (2012). Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 26, 1612–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spletter M. L., Liu J., Liu J., Su H., Giniger E., Komiyama T., Quake S., Luo L. (2007). Lola regulates Drosophila olfactory projection neuron identity and targeting specificity. Neural Dev. 2, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmüller J., Bonni A. (2010). Destroy to create: E3 ubiquitin ligases in neurogenesis. F1000 Biol. Rep. 2, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmüller J., Konishi Y., Huynh M. A., Yuan Z., Dibacco S., Bonni A. (2006). Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron 50, 389–400 [DOI] [PubMed] [Google Scholar]
- Strumpf D., Volk T. (1998). Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, vein, at the muscle-tendon junction site. J. Cell Biol. 143, 1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K., Satoh D., Estes P., Crews S., Uemura T. (2004). Development of morphological diversity of dendrites in Drosophila by the BTB-zinc finger protein abrupt. Neuron 43, 809–822 [DOI] [PubMed] [Google Scholar]
- Sweeney N. T., Brenman J. E., Jan Y. N., Gao F. B. (2006). The coiled-coil protein shrub controls neuronal morphogenesis in Drosophila. Curr. Biol. 16, 1006–1011 [DOI] [PubMed] [Google Scholar]
- Tahirovic S., Bradke F. (2009). Neuronal polarity. Cold Spring Harb. Perspect. Biol. 1, a001644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S., Becker L. E., Armstrong D. L., Chan F. (1981). Abnormal neuronal development in the visual cortex of the human fetus and infant with down’s syndrome. A quantitative and qualitative Golgi study. Brain Res. 225, 1–21 [DOI] [PubMed] [Google Scholar]
- Tea J. S., Luo L. (2011). The chromatin remodeling factor Bap55 functions through the TIP60 complex to regulate olfactory projection neuron dendrite targeting. Neural Dev. 6, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tea J. S., Chihara T., Luo L. (2010). Histone deacetylase Rpd3 regulates olfactory projection neuron dendrite targeting via the transcription factor Prospero. J. Neurosci. 30, 9939–9946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teis D., Saksena S., Emr S. D. (2008). Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev. Cell 15, 578–589 [DOI] [PubMed] [Google Scholar]
- Tolias K. F., Bikoff J. B., Burette A., Paradis S., Harrar D., Tavazoie S., Weinberg R. J., Greenberg M. E. (2005). The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45, 525–538 [DOI] [PubMed] [Google Scholar]
- Ultanir S. K., Hertz N. T., Li G., Ge W. P., Burlingame A. L., Pleasure S. J., Shokat K. M., Jan L. Y., Jan Y. N. (2012). Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 uncovers their roles in dendrite arborization and spine development. Neuron 73, 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey J. P., Macchi P., Stein J. M., Mikl M., Hawker K. N., Vogelsang P., Wieczorek K., Vendra G., Riefler J., Tübing F., et al. (2008). A loss of function allele for murine Staufen1 leads to impairment of dendritic Staufen1-RNP delivery and dendritic spine morphogenesis. Proc. Natl. Acad. Sci. USA 105, 16374–16379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H., Peichl L., Boycott B. B. (1981). Dendritic territories of cat retinal ganglion cells. Nature 292, 344–345 [DOI] [PubMed] [Google Scholar]
- Whitford K. L., Dijkhuizen P., Polleux F., Ghosh A. (2002). Molecular control of cortical dendrite development. Annu. Rev. Neurosci. 25, 127–149 [DOI] [PubMed] [Google Scholar]
- Williams D. W., Truman J. W. (2005). Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development 132, 3631–3642 [DOI] [PubMed] [Google Scholar]
- Wills Z., Bateman J., Korey C. A., Comer A., Van Vactor D. (1999). The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron 22, 301–312 [DOI] [PubMed] [Google Scholar]
- Wingate R. J., Thompson I. D. (1994). Targeting and activity-related dendritic modification in mammalian retinal ganglion cells. J. Neurosci. 14, 6621–6637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C. G., Wang B., Ballew A., Royou A., Karess R., Axelrod J. D., Luo L. (2001). Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81–91 [DOI] [PubMed] [Google Scholar]
- Wong W. T., Faulkner-Jones B. E., Sanes J. R., Wong R. O. (2000). Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J. Neurosci. 20, 5024–5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. I., Lessard J., Olave I. A., Qiu Z., Ghosh A., Graef I. A., Crabtree G. R. (2007). Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron 56, 94–108 [DOI] [PubMed] [Google Scholar]
- Yamada T., Yang Y., Bonni A. (2013). Spatial organization of ubiquitin ligase pathways orchestrates neuronal connectivity. Trends Neurosci. 36, 218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Kim A. H., Bonni A. (2010). The dynamic ubiquitin ligase duo: Cdh1-APC and Cdc20-APC regulate neuronal morphogenesis and connectivity. Curr. Opin. Neurobiol. 20, 92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. K., Peng Y. H., Li H., Lin H. C., Lin Y. C., Lai T. T., Suo H., Wang C. H., Lin W. H., Ou C. Y., et al. (2011). Nak regulates localization of clathrin sites in higher-order dendrites to promote local dendrite growth. Neuron 72, 285–299 [DOI] [PubMed] [Google Scholar]
- Ye B., Petritsch C., Clark I. E., Gavis E. R., Jan L. Y., Jan Y. N. (2004). Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr. Biol. 14, 314–321 [DOI] [PubMed] [Google Scholar]
- Ye B., Zhang Y., Song W., Younger S. H., Jan L. Y., Jan Y. N. (2007). Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130, 717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Malenka R. C. (2003). Beta-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 6, 1169–1177 [DOI] [PubMed] [Google Scholar]
- Yu W., Cook C., Sauter C., Kuriyama R., Kaplan P. L., Baas P. W. (2000). Depletion of a microtubule-associated motor protein induces the loss of dendritic identity. J. Neurosci. 20, 5782–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Wildonger J., Ye B., Zhang Y., Kita A., Younger S. H., Zimmerman S., Jan L. Y., Jan Y. N. (2008). Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 10, 1172–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]