Abstract
Cleft palate is one of the most common birth defects in humans. Whereas gene knockout studies in mice have shown that both the Osr2 and Pax9 transcription factors are essential regulators of palatogenesis, little is known about the molecular mechanisms involving these transcription factors in palate development. We report here that Pax9 plays a crucial role in patterning the anterior-posterior axis and outgrowth of the developing palatal shelves. We found that tissue-specific deletion of Pax9 in the palatal mesenchyme affected Shh expression in palatal epithelial cells, indicating that Pax9 plays a crucial role in the mesenchyme-epithelium interactions during palate development. We found that expression of the Bmp4, Fgf10, Msx1 and Osr2 genes is significantly downregulated in the developing palatal mesenchyme in Pax9 mutant embryos. Remarkably, restoration of Osr2 expression in the early palatal mesenchyme through a Pax9Osr2KI allele rescued posterior palate morphogenesis in the absence of Pax9 protein function. Our data indicate that Pax9 regulates a molecular network involving the Bmp4, Fgf10, Shh and Osr2 pathways to control palatal shelf patterning and morphogenesis.
Keywords: Cleft palate, Bmp4, Osr2, Pax9, Shh, Mouse
INTRODUCTION
The mammalian secondary palate initiates from the oral side of the maxillary processes as a pair of outgrowths that grow vertically down the sides of the developing tongue. At a precise developmental stage, the palatal shelves re-orient to the horizontal position above the dorsum of the tongue, and grow towards and subsequently fuse with each other at the midline. In addition, the palatal shelves fuse anteriorly with the primary palate, which is derived from the embryonic medial nasal processes, to form the intact roof of the oral cavity. Disturbances of the growth, elevation or fusion of the palatal shelves could result in cleft palate, one of the most common birth defects in humans (Bush and Jiang, 2012; Ferguson, 1988; Gritli-Linde, 2007).
The developing palatal shelves are composed of neural crest-derived mesenchyme (Ito et al., 2003) covered by a thin layer of oral epithelium, and exhibit distinct stereotyped shapes along the anterior-posterior (AP) axis (Bush and Jiang, 2012). Recent studies have demonstrated that many genes are differentially expressed along the AP axis in the developing palate (Baek et al., 2011; Hilliard et al., 2005; Li and Ding, 2007; Liu et al., 2008; Welsh and O’Brien, 2009). For example, the short stature homeobox 2 (Shox2), Msh homeobox 1 (Msx1) and fibroblast growth factor 10 (Fgf10) genes are preferentially expressed in the anterior palatal mesenchyme (Alappat et al., 2005; Rice et al., 2004; Yu et al., 2005; Zhang et al., 2002), whereas the BarH-like homeobox 1 (Barx1), meningioma 1 (Mn1), mesenchyme homeobox 2 (Meox2) and T-box transcription factor 22 (Tbx22) genes are preferentially expressed in the posterior palatal mesenchyme (Barlow et al., 1999; Li and Ding, 2007; Liu et al., 2008), with the AP gene expression boundary coinciding with the first formed palatal ruga (Welsh and O’Brien, 2009). Moreover, anterior and posterior palatal mesenchyme cells exhibit distinct responses to epithelial signals. Exogenous Bmp4 induced Msx1 mRNA expression in the anterior, but not posterior, palatal mesenchyme, whereas Fgf8 induced Pax9 mRNA expression in the posterior, but not anterior, palatal mesenchyme (Hilliard et al., 2005). In addition, the anterior palatal epithelium has the unique ability to induce Shox2 mRNA expression in the palatal mesenchyme (Yu et al., 2005). These data indicate that the developing palate is patterned along the AP axis by restricted gene expression in both the epithelium and mesenchyme.
Recent studies have shown that the anteriorward outgrowth of palatal shelves is associated with periodic formation of palatal rugae and is controlled by reciprocal epithelial-mesenchymal crosstalk along the AP axis (Lan and Jiang, 2009; Pantalacci et al., 2008; Welsh and O’Brien, 2009). The palatal rugae are the thickened epithelial ridges on the oral side of the secondary palate, and are formed in a defined sequence during palate outgrowth (Welsh and O’Brien, 2009). Palatal rugae express high levels of Shh and act as signaling centers involved in epithelial-mesenchymal interactions required to coordinate palate outgrowth and patterning (Lan and Jiang, 2009; Rice et al., 2006; Welsh and O’Brien, 2009). Shh from palatal epithelial cells stimulates cell proliferation and the expression of Fgf10 in the developing palatal mesenchyme (Lan and Jiang, 2009), and Shh and Fgf10 function in a positive-feedback loop that drives the outgrowth of the palatal shelves (Bush and Jiang, 2012; Lan and Jiang, 2009; Rice et al., 2004). In addition, cross-regulation of the Shh and bone morphogenetic protein (Bmp) signaling pathways has also been detected in the developing palate (Baek et al., 2011; Lan and Jiang, 2009; Zhang et al., 2002).
We have reported previously that mice homozygous for a targeted null mutation in the Osr2 gene had complete cleft palate (Lan et al., 2004). Osr2 encodes a zinc-finger protein with extensive sequence similarity to the Drosophila Odd-skipped family transcription factors that regulate multiple developmental processes during embryogenesis and tissue morphogenesis (Coulter and Wieschaus, 1988; Green et al., 2002; Hao et al., 2003; Hart et al., 1996; Lan et al., 2001; Saulier-Le Dréan et al., 1998; Wang and Coulter, 1996). Osr2-/- mutant mice exhibited domain-specific defect in palatal mesenchyme cell proliferation and delay in palatal shelf elevation (Lan et al., 2004). In addition to cleft palate, Osr2-/- mutant mice developed supernumerary teeth lingual to their molar teeth (Zhang et al., 2009). Osr2 mRNA expression exhibited a lingual-to-buccal gradient, complementary to that of Bmp4, in the mesenchyme surrounding the early developing tooth buds and patterns the tooth developmental field by suppressing the propagation of mesenchymal odontogenic activity driven by the Msx1-Bmp4 positive-feedback loop (Jia et al., 2013; Zhang et al., 2009).
Paired-box gene 9, also known as Pax9, is a member of the transcription factor family characterized by the Paired-class DNA-binding domain (Stapleton et al., 1993). Pax9-deficient mice die shortly after birth, exhibiting complete cleft palate (Peters et al., 1998). Little is known about the molecular and cellular mechanisms involving Pax9 in palate development, however. We recently generated a mouse strain (Pax9fneo/+) that allows the Cre/loxP-mediated inactivation of the Pax9 gene and subsequent activation of transgenic Osr2 expression from the endogenous Pax9 locus (Zhou et al., 2011). We report here that Pax9 is required for maintenance of Osr2 expression in the developing palatal mesenchyme and that expression of Osr2 from the Pax9 locus partially rescued palate morphogenesis in Pax9-null mutant mice.
RESULTS
Pax9 exhibits differential expression patterns along the AP axis of the developing palate
To study the mechanism by which Pax9 regulates palate development, we first examined the expression patterns of Pax9 during palate development by whole-mount and section in situ hybridization. From E12.5 to E13.5, when the palate shelves grew vertically, Pax9 exhibited a posterior-to-anterior gradient expression pattern in the palatal mesenchyme (Fig. 1A,B). Moreover, whereas Pax9 mRNAs were expressed throughout the oral-nasal axis of the palatal mesenchyme in the posterior region (Fig. 1E), Pax9 mRNA expression exhibited apparently lower levels in the oral half than in the nasal half of the palatal mesenchyme in the anterior region (Fig. 1C,E).
Fig. 1.
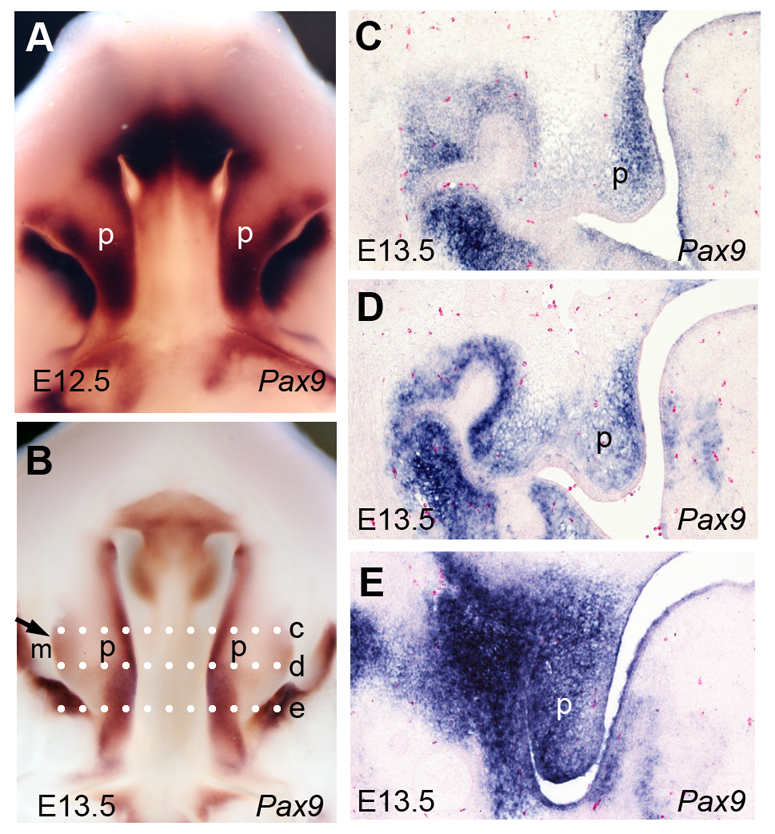
Pax9 mRNA expression during palate development. (A,B) Whole-mount in situ hybridization detection of Pax9 mRNA in the developing palatal shelves in E12.5 (A) and E13.5 (B) mouse embryos. (C-E) Frontal sections through distinct planes along the anterior-posterior axis of the E13.5 palatal shelves showing Pax9 mRNA expression pattern in the anterior (C), middle (D) and posterior (E) regions. Dotted white lines marked c-e in B indicate the section planes for C-E, respectively. m, molar tooth germ; p, palatal shelf.
Defects in palatal shape and AP patterning in Pax9 mutant mice
We recently generated mice carrying a deletion of the first two exons of the Pax9 gene (Pax9del) and showed that Pax9del/del mutant mice displayed similar developmental defects, as reported previously in another Pax9-deficient mouse strain, including cleft palate and tooth developmental arrest (Peters et al., 1998; Zhou et al., 2011). Detailed histological analysis of Pax9del/del mutant embryos showed that the mutant palatal shelves had an aberrant shape and lacked the lateral indentation between the maxillary molar tooth germ and the palatal shelf at E13.5, in comparison with the wild-type littermates (Fig. 2A-F). At E14.5, the palatal shelves had elevated to the horizontal position above the developing tongue and initiated fusion at the midline in the control embryos (Fig. 2G-I). By contrast, the mutant embryos exhibited delay in palatal shelf elevation (Fig. 2J-L). By E15.5, the midline epithelial seam between the palatal shelves had mostly disappeared in the control embryos (Fig. 2M-O). By contrast, the mutant palatal shelves remained separate from each other (Fig. 2P-R). In addition to the lack of palatal fusion, scanning electron microscopy studies showed that the mutant palatal shelves had defects in formation of the palatal rugae, which are the thickened epithelial ridges on the oral side of the palatal shelves, and deficiency in primary palate outgrowth (Fig. 3A-D).
Fig. 2.
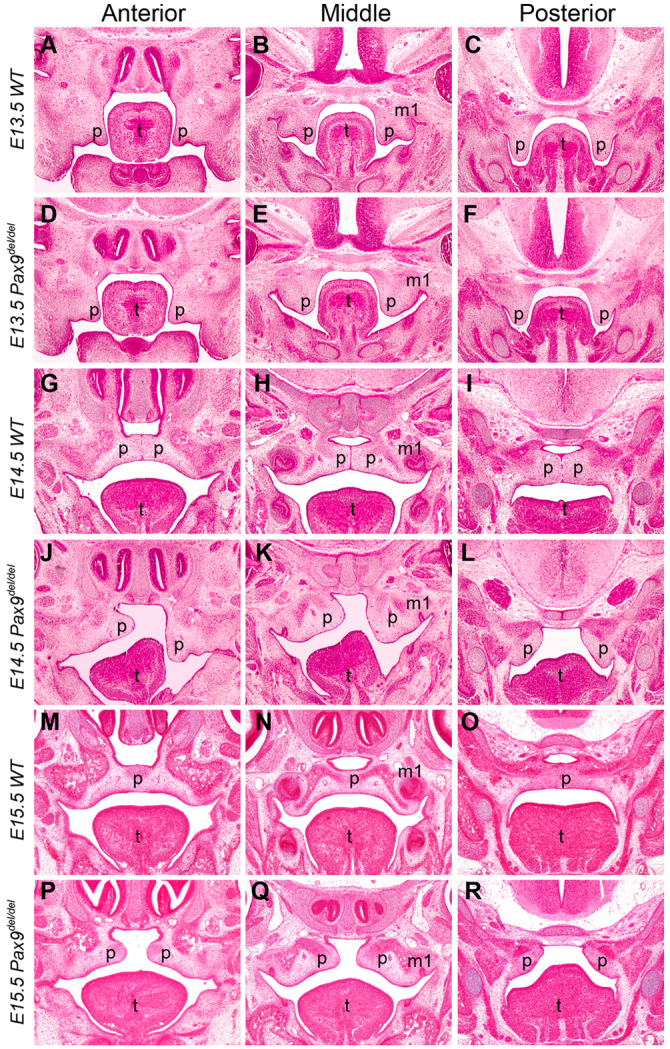
Histological analysis of palate developmental defects in Pax9del/del mutant mouse embryos. Representative frontal sections from the anterior, middle and posterior regions of the developing palatal shelves are shown. (A-F) E13.5 control (A-C) and mutant (D-F) littermates. (G-L) E14.5 control (G-I) and mutant (J-L) littermates. (M-R) E15.5 control (M-O) and mutant (P-R) littermates. m1, first molar tooth germ; p, palatal shelf; t, tongue.
Fig. 3.
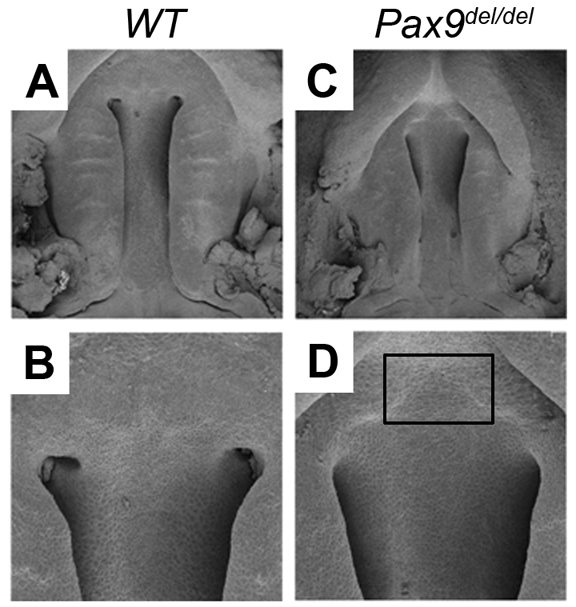
Scanning electron microscopy analysis of palate developmental defects in Pax9del/del mutant mice. (A-D) Oral view of the developing palate in wild-type (A,B) and Pax9del/del mutant (C,D) embryos at E13.5. Rectangle in D marks the region of deficiency in primary palate.
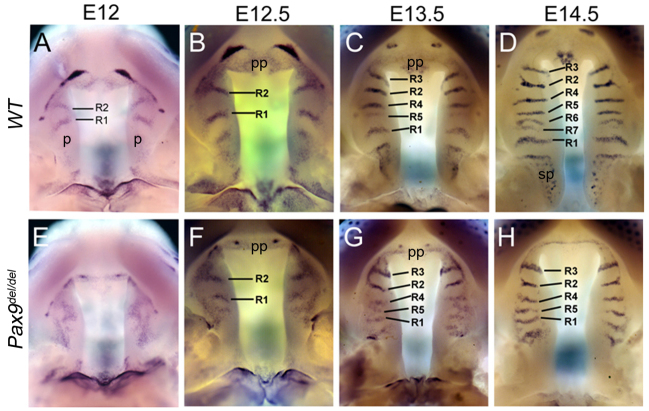
Recent studies showed that the palatal rugae express high levels of Shh mRNAs and are formed in a defined temporal sequence during palate outgrowth (Baek et al., 2011; Pantalacci et al., 2008; Welsh and O’Brien, 2009). Our whole-mount in situ hybridization analyses confirmed that the Pax9del/del mutant embryos had defects in palatal rugae formation. At E12.0, when two pairs of clearly defined rugae had formed in wild-type embryos (Fig. 4A), the Pax9del/del mutant embryos showed diffuse Shh mRNA expression in the palatal epithelium and lacked clearly demarcated rugae structures (Fig. 4E). From E12.5 to E14.5, the palatal rugae formed sequentially in a defined sequence as the palatal shelves grew rostrally (Fig. 4B-D). In the mutant palate, although two pairs of strong Shh-expressing rugae were detected in the anterior most region, corresponding to R2 and R3 in the control palate, Shh expression was much weaker and not distinctly localized in discrete rugae structures in the middle region of the palatal shelves at E13.5 in the mutant embryos (Fig. 4G). By E14.5, the Shh-expressing rugae structures were better demarcated but there were fewer rugae and the distance between R1 and R5 was significantly shorter in the mutant embryos than in the control littermates (compare Fig. 4D with 4H), indicating impairment in anteriorward outgrowth of the palatal shelves in the mutant embryos. In addition, the punctate Shh expression pattern observed in the developing sensory papilla overlying the posterior soft palate was absent in the Pax9del/del mutant embryos (Fig. 4D,H). The mutant embryos also exhibited reduced Shh expression in the primary palate, in comparison with the control littermates (Fig. 4D,H).
Fig. 4.
Analysis of palatal ruga formation and anteriorward palatal outgrowth in Pax9del/del mutant embryos. (A-H) Whole-mount in situ hybridization detection of patterns of Shh mRNA expression in the developing palate in wild-type control (A-D) and Pax9del/del mutant (E-H) embryos from E12.0 to E14.5. Strong Shh mRNA expression marks the palatal rugae, which increase in number from two at E12.0 to seven pairs by E14.5 in the wild-type embryos (A-D), whereas the mutant had formed only five pairs by E14.5 (E-H). p, palatal shelf; pp, primary palate; sp, sensory papilla.
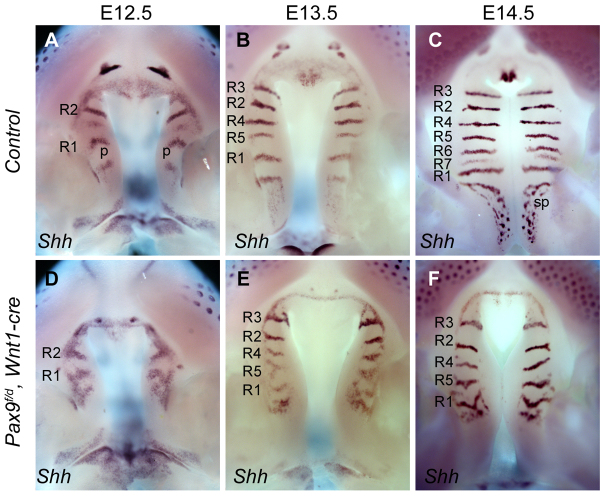
Pax9 was expressed in the palatal mesenchyme, as well as in the posterior palatal epithelium (Fig. 1). To investigate whether defects in Shh expression were due to loss of Pax9 function in the epithelium, we inactivated Pax9 specifically in neural crest-derived mesenchymal cells by crossing the Pax9fneo mice with Wnt1-Cre transgenic mice (Danielian et al., 1998). We found that the Pax9fneo/delWnt1-Cre mutant embryos showed similar defects in Shh mRNA expression to the Pax9del/del mutant embryos in both the primary and secondary palate (compare Fig. 5 with Fig. 4). These results suggest that Pax9 plays an important role in regulating the mesenchymal-epithelial interactions during palate development.
Fig. 5.
Palatal Shh mRNA expression pattern in Pax9fneo/del;Wnt1Cre mutant embryos. (A-F) Patterns of Shh mRNA expression in the palate in wild-type (A-C) and Pax9fneo/del;Wnt1-Cre mutant (D-F) embryos from E12.5 to E14.5. p, palatal shelf; sp, sensory papilla.
In addition to defects in the anteriorward outgrowth of the palate shelves, we found that the anterior-posterior boundary of the palatal mesenchyme was disturbed in the Pax9del/del mutant embryos. As shown in Fig. 6, expression of Shox2 was restricted anterior to R1, whereas Barx1 expression was restricted posterior to R1 in the E13.5 wild-type palate (Fig. 6A,C). By contrast, both the posterior boundary of Shox2 expression and the anterior boundary of Barx1 expression appeared diffuse and anteriorly shifted in the Pax9del/del mutant palate (Fig. 6B,D). Taken together, these results indicate that deletion of Pax9 resulted in disruption of the AP boundary of the palatal mesenchyme, which correlated with the misshapen palatal shelves and defects in expansion of the palatal shelves along the AP axis in the Pax9del/del mutant embryos.
Fig. 6.
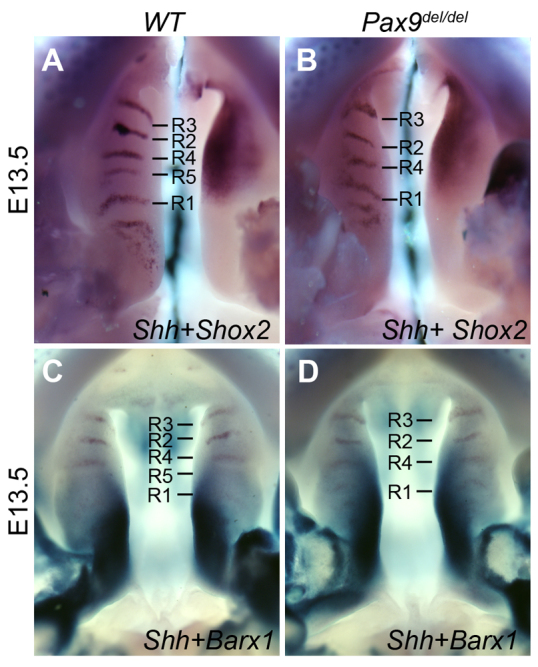
Disruption of the anterior-posterior boundary in the secondary palate in Pax9del/del mutant embryos. (A,B) Direct comparison of Shh (left half) and Shox2 (right half) mRNA expression patterns in the palatal shelf pairs in wild-type (A) and Pax9del/del mutant (B) embryos at E13.5. (C,D) Dual color in situ hybridization detection of Shh (brown) and Barx1 (turquoise) mRNAs in the palatal shelves of E13.5 wild-type (C) and Pax9del/del mutant (D) embryos.
Pax9 acts upstream of Bmp4, Fgf10, Msx1 and Osr2 in the palatal mesenchyme
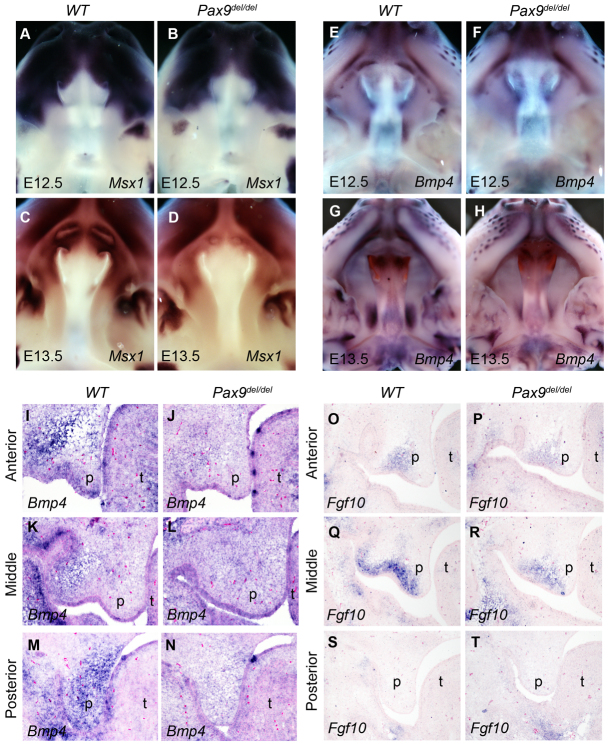
The defects in Shh expression in the palatal epithelium in both the Pax9del/del and Pax9fneo/del;Wnt1-Cre mouse embryos indicate that Pax9 regulates mesenchymal-epithelial crosstalk during palate development. To investigate the molecular mechanism that mediates Pax9 function in palate development, we carried out extensive molecular marker analyses by using both quantitative real-time RT-PCR and in situ hybridization analyses of E12.5 and E13.5 embryos. Expression of Bmp4 and Msx1 mRNAs were consistently significantly reduced in the Pax9del/del mutant palate (Fig. 7A,B). Whereas previous studies using section in situ hybridization analysis suggested that expression of both Msx1 and Bmp4 was restricted in the anterior palate (Zhang et al., 2002), our whole-mount in situ hybridization assays demonstrated that, although Msx1 mRNA expression was restricted in the anterior palate (Fig. 8A-D), the Bmp4 mRNA expression pattern in the developing palatal shelves consisted of an anterior and a posterior domain separated by a Bmp4-negative middle palatal region (Fig. 8E,G). We further confirmed this unique pattern of Bmp4 mRNA expression by section in situ hybridization of the whole series of frontal sections through the developing palatal shelves of E13.5 mouse embryos and found that expression of Bmp4 mRNAs was significantly reduced in both the anterior and posterior regions of the developing palate of Pax9del/del mutant embryos in comparison with control littermates (Fig. 8E-N).
Fig. 7.
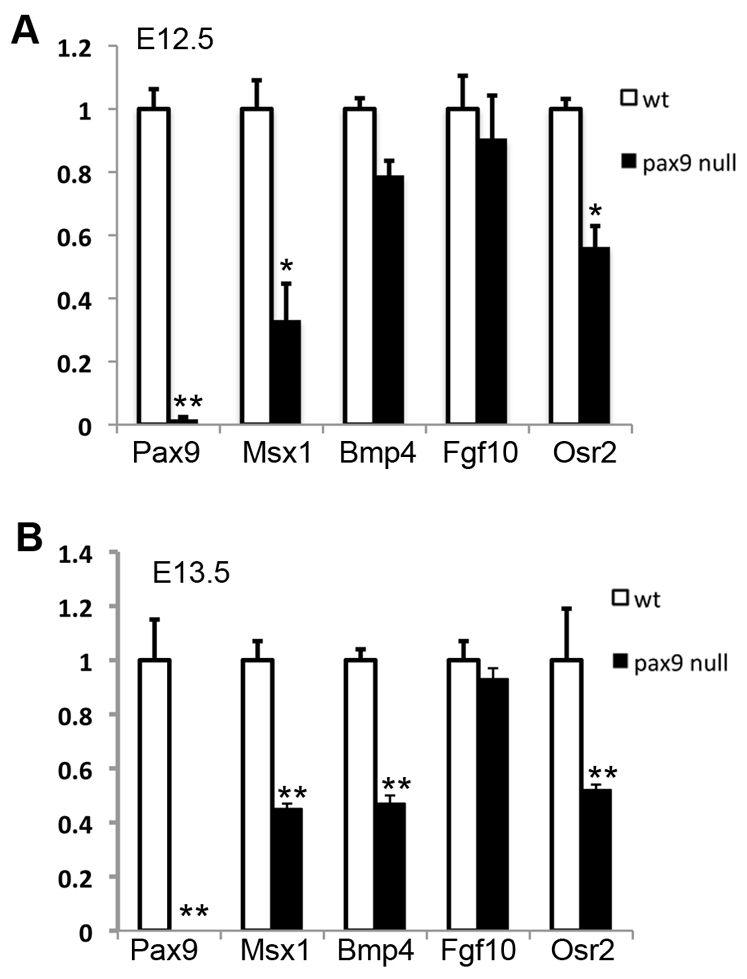
Quantitative RT-PCR analysis of the levels of expression of Pax9, Msx1, Bmp4, Fgf10 and Osr2 mRNAs in E12.5 and E13.5 palatal shelves in wild-type and Pax9del/del mutant embryos. (A) E12.5, (B) E13.5. Error bars represent s.e.m. *P<0.05; **P<0.01.
Fig. 8.
Comparison of expression of Bmp4, Fgf10 and Msx1 mRNAs in the palatal shelves in Pax9del/del mutant and control embryos. (A-D) Whole-mount in situ hybridization results of Msx1 mRNA expression in wild-type (A,C) and Pax9del/del mutant (B,D) embryos at E12.5 (A,B) and E13.5 (C,D). (E-H) Whole-mount in situ hybridization results of Bmp4 mRNA expression in wild-type (E,G) and Pax9del/del mutant (F,H) embryos at E12.5 (E,F) and E13.5 (G,H). (I-N) Frontal sections showing expression of Bmp4 mRNAs in the anterior, middle and posterior regions of the developing palate in E13.5 wild-type (I,K,M) and Pax9del/del mutant (J,L,N) embryos. (O-T) Frontal sections showing expression of Fgf10 mRNA in the anterior, middle and posterior regions of the developing palate in E13.5 wild-type (O,Q,S) and Pax9del/del mutant (P,R,T) embryos. p, palatal shelf; t, tongue.
Our real-time RT-PCR analyses also consistently detected a reduction in the amount of Fgf10 mRNAs in the microdissected E12.5 and E13.5 mutant palatal tissues in comparison with the control samples, although the difference was not statistically significant (Fig. 7A,B). This was probably due to the overall low abundance and highly restricted domain of Fgf10 mRNA expression in the developing palatal shelves, as Fgf10 mRNA expression was restricted to only the anterior and middle oral quarters of the developing palatal mesenchyme (Fig. 8O,Q,S). Indeed, section in situ hybridization assays consistently showed reduced Fgf10 mRNA expression in the Pax9del/del mutant palate in comparison with control littermates (Fig. 8O-T).
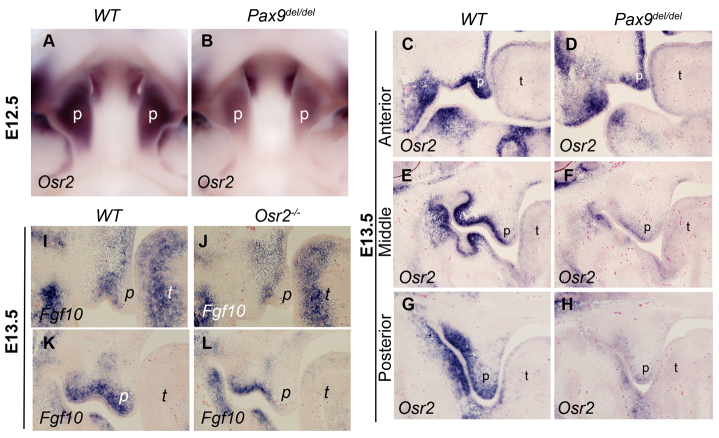
We have previously shown that Pax9 is required for maintenance of Osr2 mRNA expression in the developing tooth mesenchyme (Zhou et al., 2011). In this study, we found that Osr2 mRNA expression was significantly reduced in the developing palate in the Pax9del/del mutant embryos, as detected by both real-time RT-PCR and whole-mount in situ hybridization assays (Fig. 7A,B; Fig. 9A,B). We further verified the changes in Osr2 mRNA expression by using section in situ hybridization assay and found that Osr2 mRNA expression was dramatically reduced in the middle and posterior regions of the palatal shelves, whereas a higher level of Osr2 mRNA expression was present in the very anterior region of the mutant palatal mesenchyme at E13.5 (Fig. 9C-H). Moreover, we found that expression of Fgf10 mRNAs was also downregulated in the developing palatal shelves in Osr2-/- mutant embryos, in comparison with wild-type littermates (Fig. 9I-L). Together, these results suggest that Osr2 might act downstream of Pax9 to maintain Fgf10 expression in the palatal mesenchyme during palatal outgrowth.
Fig. 9.
Pax9 is required to maintain Osr2 mRNA expression in the developing palatal shelves. (A,B) Whole-mount in situ hybridization results of Osr2 mRNA expression in the palatal shelves in E12.5 wild-type control (A) and Pax9 mutant (B) littermates. (C-H) Frontal sections showing Osr2 mRNA expression in the anterior (C,D), middle (E,F) and posterior (G,H) regions of the developing palatal shelves in E13.5 wild-type (C,E,G) and Pax9del/del mutant (D,F,H) embryos. (I-L) Frontal sections showing Fgf10 mRNA expression in the anterior (I,J) and middle (K,L) regions of the developing palatal shelves in E13.5 wild-type (I,K) and Osr2-/- mutant (J,L) embryos. p, palatal shelf; t, tongue.
Expression of Osr2 from the Pax9 locus rescued posterior palate morphogenesis in Pax9-deficient mice
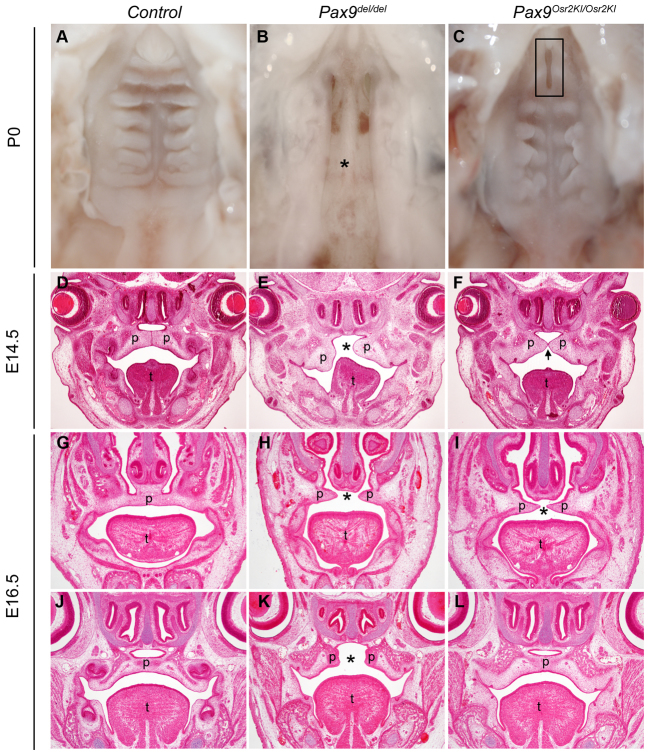
To further investigate the role of Osr2 in Pax9-mediated regulation of palate development, we examined palatogenesis in the Pax9Osr2KI/Osr2KI mice, in which an Osr2 cDNA replaced the first two exons of the endogenous Pax9 gene (Zhou et al., 2011). Whereas Pax9del/del mutant newborn pups had complete cleft secondary palate (Fig. 10B), 50% of the Pax9Osr2KI/Osr2KI newborn pups exhibited fused posterior secondary palate, with a partial cleft in the anterior palate region only (n=24) (Fig. 10C). Histological analyses of E14.5 embryos showed that the Pax9Osr2KI/Osr2KI mutant embryos exhibited rescue of the defects in palatal shelf elevation (n=4), in comparison with the Pax9del/del mutant embryos that always showed defects in palatal shelf elevation (Fig. 10D-F). By E16.5, 50% of the Pax9Osr2KI/Osr2KI mutant embryos showed a fused posterior palate (n=6) (Fig. 10L), but the anterior region of the palate shelves failed to make contact at the midline (Fig. 10I).
Fig. 10.
Partial rescue of palate morphogenesis in the Pax9Osr2KI/Osr2KI mutant mice. (A-C) Oral view of the palate in control (A), Pax9del/del mutant (B) and Pax9Osr2KI/Osr2KI mutant (C) newborn pups. In contrast to complete cleft secondary palate in the Pax9del/del mutant (asterisk in B), Pax9Osr2KI/Osr2KI mutant pups had a partial cleft of the anterior palate (rectangle in C). (D-F) Histology of frontal sections through the middle regions of the developing palate in E14.5 control (D), Pax9del/del mutant (E) and Pax9Osr2KI/Osr2KI mutant (F) embryos. Arrow in F indicates the midline epithelial seam of the palatal shelves. (G-L) Frontal sections through the anterior (G-I) and posterior (J-L) regions of the developing palate in E16.5 control (G,J), Pax9del/del mutant (H,K) and Pax9Osr2KI/Osr2KI mutant (I,L) embryos. Asterisk indicates the gap between the palatal shelves. p, palatal shelf; t, tongue.
Osr2 plays an important role in regulating palatal mesenchyme cell proliferation (Lan et al., 2004). We analyzed palatal cell proliferation in Pax9del/del embryos and control littermates by using BrdU labeling and found that the posterior palate region, but not the anterior palate region, had significant reduction in cell proliferation in E13.5 Pax9del/del embryos in comparison with their control littermates (Fig. 11A-D; Fig. 11I). This posterior palatal growth defect was rescued in the Pax9Osr2KI/Osr2KI embryos (Fig. 11E-I), confirming a crucial role for Osr2 in palatal shelf growth and indicating that the reduction in palatal growth contributed to cleft palate pathogenesis in the Pax9del/del mice.
Fig. 11.
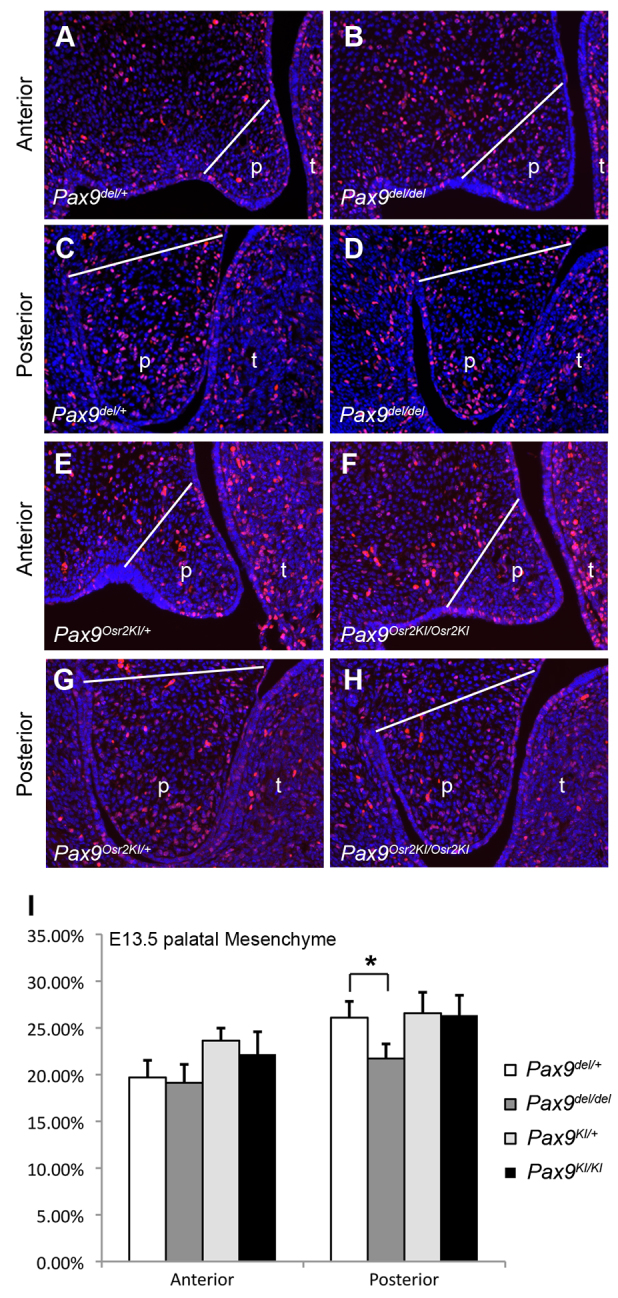
Analysis of cell proliferation in the developing palate in E13.5 Pax9del/del and Pax9Osr2KI/Osr2KI embryos. (A-D) Representative images of sections through the anterior (A,B) and posterior (C,D) regions of the palate in Pax9del/+ control (A,C) and Pax9del/del mutant (B,D) embryos showing distribution of immunostained BrdU-labeled nuclei (red). Sections were counterstained with Hoechst (blue). (E-H) Representative images of sections through the anterior (E,F) and posterior (G,H) regions of the palate in Pax9Osr2KI/+ (E,G) and Pax9Osr2KI/Osr2KI mutant (F,H) embryos showing distribution of immunostained BrdU-labeled nuclei (red). White line indicates approximate position below which the palatal mesenchyme cells were counted. (I) The percentage of BrdU-labeled cells in the E13.5 palatal mesenchyme. Error bar represents s.d. *P<0.01.
We investigated further whether the rescued morphogenesis of the posterior palate in the Pax9Osr2KI/Osr2KI mutant embryos correlated with restoration of Bmp4, Msx1, Shh and Fgf10 mRNA expression during palate development. We examined more than four pairs of E13.5 embryos for each probe and all Pax9Osr2KI/Osr2KI mutant embryos exhibited reduced expression of Msx1 and Bmp4 mRNAs in the developing palatal shelves, in comparison with the wild-type littermates (Fig. 12A-D). Moreover, the Pax9Osr2KI/Osr2KI mutant embryos showed loss of Shh mRNA expression in the posterior palate and decreased Shh mRNA expression in the rugae in the mid-palate region, similar to the Pax9del/del mutant embryos (compare Fig. 12E,F and Fig. 4C,G). The domain of Shox2 mRNA expression in the palatal shelves was also similarly shifted anteriorly in the Pax9Osr2KI/Osr2KI embryos as in the Pax9del/del mutant embryos (compare Fig. 12E,F and Fig. 6A,B). However, we found that two out of five Pax9Osr2KI/Osr2KI mutant embryos had restored Fgf10 mRNA expression in the anterior and middle palate regions at E13.5 (Fig. 13A-D). Together, these results suggest that Osr2 acts downstream of or in parallel with the Bmp4 and Shh pathways and cooperates with Pax9 to maintain Fgf10 expression during palate development.
Fig. 12.
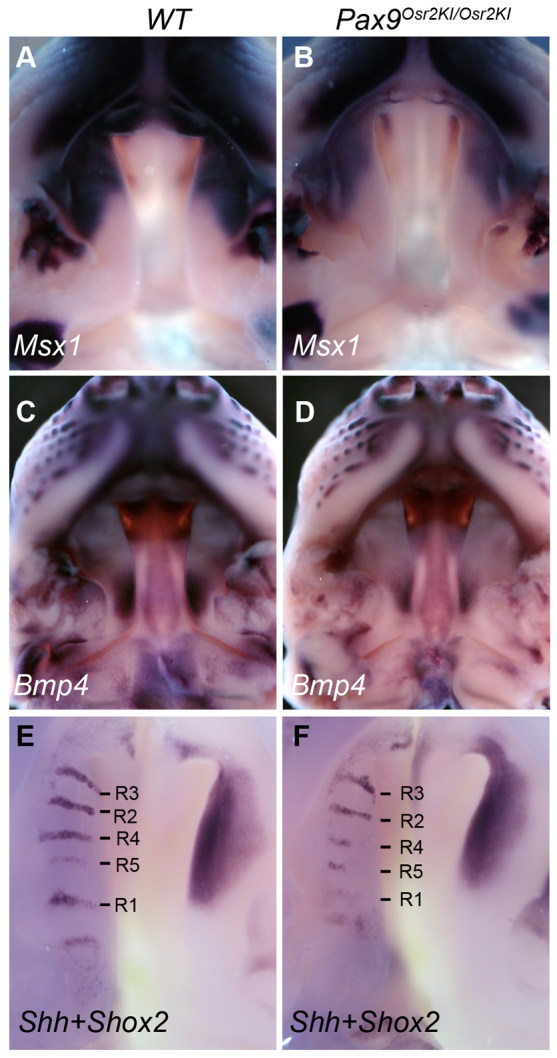
Comparison of patterns of expression of Msx1, Bmp4, Shh and Shox2 mRNAs in E13.5 wild-type and Pax9Osr2KI/Osr2KI mutant palate. (A,B) Whole-mount view of Msx1 mRNA expression in the palate in control (A) and Pax9Osr2KI/Osr2KI mutant (B) embryos. (C,D) Whole-mount view of Bmp4 mRNA expression in the palate in control (C) and Pax9Osr2KI/Osr2KI mutant (D) embryos. (E,F) Whole-mount in situ hybridization results for Shh (left half) and Shox2 (right half) mRNA expression in bisected upper jaw samples of control (E) and Pax9Osr2KI/Osr2KI mutant (F) embryos. R1 to R5 mark the rugae according to the temporal sequence of formation.
Fig. 13.
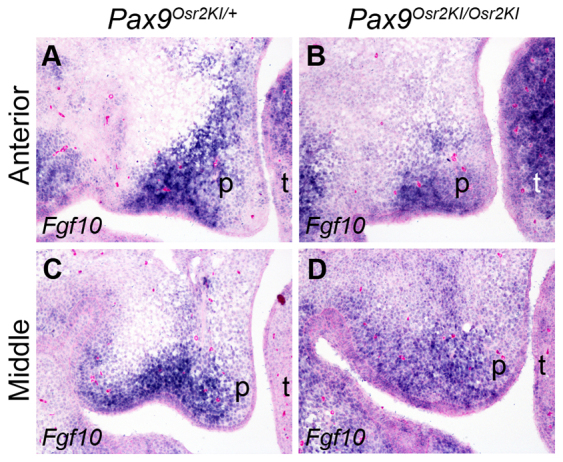
Comparison of Fgf10 mRNA expression in the developing palate in E13.5 control and Pax9Osr2KI/Osr2KI mutant embryos. (A-D) Representative frontal sections through the anterior (A,B) and middle (C,D) regions of the developing palatal shelves were used for detection of Fgf10 mRNA expression by section in situ hybridization. p, palatal shelf; t, tongue.
DISCUSSION
Although cleft palate in Pax9-deficient mice was first reported more than 15 years ago (Peters et al., 1998), the molecular mechanisms involving Pax9 in palate development have not been documented. In this study, we found that Pax9 regulates mesenchymal-epithelial signaling and AP patterning during palate outgrowth and that Pax9 acts upstream of Osr2 to regulate palatal shelf growth and elevation.
Reciprocal signaling between the epithelium and neural crest derived mesenchyme plays a crucial role in the growth of the secondary palate (Bush and Jiang, 2012; Lan and Jiang, 2009; Rice et al., 2004; Welsh and O’Brien, 2009). We found that disruption of patterns of Shh expression in the palatal epithelium was among the earliest detectable defects during palate development in the Pax9del/del mutant mice. Remarkably, Shh mRNA expression in the palatal epithelium was also disrupted in the Pax9fNeo/delWnt1-Cre mutant embryos (Fig. 5), indicating that Pax9-mediated transcriptional regulation in the neural crest-derived palatal mesenchyme indirectly regulates Shh expression in the palatal epithelium. Previous studies of cleft palate pathogenesis in the Msx1-/- mutant mice implicated a role for Bmp4 acting downstream of Msx1 in the palatal mesenchyme but upstream of Shh expression in the palatal epithelium in the anterior palate (Zhang et al., 2002). Whereas Zhang et al. (Zhang et al., 2002), using only the section in situ hybridization assay, suggested that expression of both Msx1 and Bmp4 was restricted to the anterior palate region, our whole-mount in situ hybridization assays clearly detected strong Bmp4 mRNA expression in a discrete domain in the posterior palate at both E12.5 and E13.5 (Fig. 8E,G). Levi et al. (Levi et al., 2006) reported whole-mount in situ hybridization results for Bmp4 mRNA expression in the E13.5 palate and also clearly showed a strong Bmp4 expression domain in the posterior palate (Levi et al., 2006). We carried out section in situ hybridization of the whole series of frontal sections of E13.5 mouse palate and confirmed the separate Bmp4 mRNA expression domains in the anterior and posterior regions of the palatal shelves (Fig. 8I,K,M). Moreover, Levi et al. (Levi et al., 2006) showed that Bmp4 mRNA expression in the anterior palate, but not in the posterior palate, was reduced in the Msx1-/- embryos in comparison with wild-type control embryos (Levi et al., 2006). We found that the posterior domain of Bmp4 mRNA expression was significantly downregulated in the Pax9del/del mutant embryos, indicating that Pax9 regulates Bmp4 expression in the posterior palate independently of Msx1. Previous biochemical studies have shown that Pax9 could activate the luciferase reporter expression driven by either the Msx1 or Bmp4 gene promoter in co-transfected COS7 cells (Ogawa et al., 2006). Together, these data suggest that Pax9 directly regulates both Msx1 and Bmp4 expression in the palatal mesenchyme and that Bmp4 expression in the posterior palate may also be involved in maintenance of Shh expression in the palatal epithelium.
In addition to Bmp4, mesenchymally expressed Fgf10 is involved in maintenance of Shh expression in the palatal epithelium, with Fgf10 and Shh acting in a positive-feedback loop to regulate palatal outgrowth (Lan and Jiang, 2009; Rice et al., 2004). Fgf10 mRNA expression was downregulated in the Pax9del/del mutant palatal shelves. Interestingly, we found that Fgf10 mRNA expression was also downregulated in the Osr2-/- mutant palatal shelves and that Osr2 mRNA expression was significantly downregulated in Pax9del/del mutant palatal shelves. Moreover, we found that Fgf10 mRNA expression was restored in a subset of Pax9Osr2KI/Osr2KI mutant palate, which correlated with partial rescue of palate morphogenesis in the Pax9Osr2KI/Osr2KI mutant embryos. Together, these data suggest that Pax9 may regulate Fgf10 expression in the developing palatal mesenchyme through Osr2.
Whereas Osr2-/- and Pax9del/del mutant mice each had cleft palate, the palatal phenotypes were not the same. Osr2-/- mutant embryos had reduced mesenchyme cell proliferation throughout the AP axis of the palatal shelves at E13.5 (Lan et al., 2004), whereas Pax9del/del mutant embryos showed reduced mesenchymal cell proliferation only in the posterior region and exhibited malformed palatal shelves lacking the lateral indentation. However, both Osr2-/- and Pax9del/del mutant embryos had defects in palatal shelf elevation (Lan et al., 2004; and this study). We found that Osr2 mRNA expression was significantly downregulated in Pax9del/del mutant palatal shelves as early as E12.5 and that restoration of Osr2 expression in the palatal mesenchyme through the Pax9Osr2KI allele rescued palatal elevation and posterior palate morphogenesis in ∼50% of the Pax9Osr2KI/Osr2KI mutant mice (Fig. 10). Whether Osr2 is a direct target of Pax9 transcriptional regulation requires further investigation, however, because maintenance of Osr2 mRNA expression in the developing palatal shelves requires active Shh signaling (Lan and Jiang, 2009) but Shh expression was significantly perturbed in the Pax9del/del mutant palate. Moreover, although posterior palate morphogenesis was rescued in the Pax9Osr2KI/Osr2KI embryos in comparison with Pax9del/del mutants, the restoration of Osr2 to the palatal mesenchyme through the Pax9Osr2KI allele was insufficient to rescue the effects of loss of Pax9 on expression of Bmp4, Msx1 and Shh in the developing palate. Furthermore, we found that Osr2 mRNA expression was more dramatically reduced in the middle and posterior regions of the Pax9del/del mutant palate, which correlated with the reduced expression of Shh mRNA expression in the middle and posterior regions of the palatal epithelium in these mutant embryos. Together, these results suggest that Pax9 plays a primary role in regulating the mesenchymal-epithelial interactions involving the Bmp4 and Shh signaling, and that Osr2 acts downstream of these pathways to regulate palatal cell proliferation and palatal shelf elevation.
Defects in palatal shelf elevation have been attributed to malformation of or physical obstruction by the tongue in several mutant mouse strains (Barrow et al., 2000; Dudas et al., 2004; Gendron-Maguire et al., 1993; Murray et al., 2007; Wang et al., 2003). In the Pax9del/del mutant embryos, failure of palatal shelf elevation was also accompanied by abnormal morphology of the tongue (Fig. 2K,L,R). However, we found that Pax9del/del mutant embryos had defects in expression of Shh, Msx1 and Bmp4 by E12.5, and exhibited abnormally broadened mid-palate region by E13.5, indicating that the mutant palatal shelves were already malformed 1 day before the time of normal palatal shelf elevation. The abnormal broadening of the palatal shelves in the mid-palate region correlated with the disruption of the anterior-posterior boundary of the palatal shelves, whereas the lack of indentation lateral to the palatal shelves correlated with the early developmental arrest of the molar tooth germs in the mutant embryos. As all Pax9Osr2KI/Osr2KI embryos also had early developmental arrest of the molar tooth germs (Zhou et al., 2011), but about half of those mutants developed fused posterior secondary palate (Fig. 10L), the tooth developmental defect was unlikely to be a determining factor in palatal shelf elevation. However, we found that some, but not all, of the Pax9Osr2KI/Osr2KI embryos had restored Fgf10 mRNA expression in the palatal mesenchyme, which correlated with partial rescue of palate morphogenesis in those mutants. Thus, although we have not ruled out possible contribution by abnormalities of surrounding structures, including the tooth germs and tongue, to the impairment of palatal shelf elevation in the Pax9del/del mutant embryos, our data support a crucial role for Pax9-dependent gene expression within the developing palatal shelves in palatal shelf elevation.
Whereas 50% of late-term Pax9Osr2KI/Osr2KI embryos showed fusion of the posterior palate, all of the mutant embryos had cleft of the anterior palate. The anterior cleft palate defect correlated with the reduction in Msx1 expression in the anterior palate in both Pax9del/del and Pax9Osr2KI/Osr2KI embryos (Fig. 12C,D). As Msx1 is not expressed in the posterior palate, these results indicate that Pax9 regulates palate development through distinct molecular pathways in the anterior and posterior regions. Similar to the anterior palatal mesenchyme, the developing tooth bud mesenchyme exhibits partially overlapping expression of Msx1, Pax9 and Osr2 (Zhang et al., 2009; Zhou et al., 2011). We recently discovered that Msx1 and Osr2 interact physically but act antagonistically in the regulation of mesenchymal odontogenic activity (Jia et al., 2013; Zhang et al., 2009; Zhou et al., 2011). It is possible that the Msx1-Osr2 interaction may play an important role in the development of the anterior palate. In the Pax9Osr2KI/Osr2KI embryos, the reduction of Msx1 expression affects the pathways downstream of Msx1-Osr2 interactions, which would account for the anterior cleft palate defect. Together, these results indicate that both Osr2 and Pax9 interact with the distinct molecular pathways in the anterior and posterior palate. The Pax9Osr2KI/Osr2KI mutant mice provide a valuable tool for comprehensive analysis of the gene regulatory network controlling palate development and patterning.
MATERIALS AND METHODS
Mouse strains
Pax9fneo/+, Pax9del/+ and Pax9Osr2KI/+ mice have been described previously (Zhou et al., 2011). Pax9del/+ and Pax9Osr2KI/+ mice were maintained in the C57BL/6J strain background and were intercrossed to generate homozygous mutants for analyses. Pax9fneo/+ mice were crossed with Wnt1-Cre transgenic mice (Danielian et al., 1998) to generate Pax9fneo/+Wnt1-Cre mice, which were then crossed with Pax9del/+ mice to generate Pax9fneo/delWnt1-Cre mutant embryos for analyses.
Scanning electron microscopy
Embryos were fixed with 2% glutaraldehyde/2% paraformaldehyde in PBS followed by OsO4 treatment and dehydration through an ethanol series. The embryos were critical point dried under CO2 and sputter coated with 30 nm gold particles. Images were obtained using Zeiss Supra 40VP Field Emission at the Electron Microscope Research Core of the University of Rochester Medical Center (NY, USA).
Histology and in situ hybridization
Embryos were collected at desired developmental stages and fixed in 4% paraformaldehyde overnight at 4°C. For whole-mount in situ hybridization, embryos were dehydrated through graded methanol. For section in situ hybridization, embryos were dehydrated through graded alcohols, embedded in paraffin and sectioned at 7 μm. Whole-mount (Baek et al., 2011; Lan et al., 2001) and section in situ hybridization (Zhang et al., 1999) were carried out as described previously with digoxygenin or fluorescein-labeled antisense RNA probes. For section in situ hybridization, frontal sections through palatal shelves were selected from the anterior, middle and posterior regions, with the middle region consisting of sections through the maxillary first molar tooth buds.
For histology, embryos were fixed in either Bouin’s fixative or 4% paraformaldehyde. Fixed embryos were dehydrated through a graded series of ethanol and embedded in paraffin wax. Serial sections of 7 μm were stained with Hematoxylin and Eosin.
BrdU labeling and cell proliferation assay
For detection of cell proliferation in the palatal shelves, timed-mated pregnant female mice were injected once intraperitoneally at gestational day 13.5 with the BrdU Labeling Reagent (Roche, 15 μl/g body weight). One hour after injection, embryos were dissected, fixed in 4% paraformaldehyde at 4°C overnight, embedded in paraffin wax and sectioned at 5 μm in the coronal plane. The sections were rehydrated and submerged in pre-cooled acetone at -20°C for 30 minutes. After washing with PBS, the specimens were treated with 20 μg/ml proteinase K at room temperature for 10 minutes. After washing with PBS, sections were treated with 2 M HCl for 1 hour at room temperature to denature the genomic DNA. Samples were rinsed in PBS for three times, then incubated in blocking solution (2% BSA, 10% goat serum, 0.1% Tween in PBS) at room temperature for 30 minutes, followed by incubation with Alexa Fluor 594-conjugated anti-BrdU antibody (Invitrogen, #B35132) solution (1:50 in blocking solution) at 4°C overnight in a humid atmosphere. The sections were rinsed in PBS, counterstained with Hoechst and mounted with anti-fade mounting gel. Images were obtained by using a Zeiss fluorescence microscope and analyzed with Imaris software. The numbers of BrdU-labeled nuclei and total nuclei were recorded for two independent control and mutant littermate pairs. The cell proliferation data were recorded and analyzed separately for the anterior and posterior regions of the palatal shelves, with data from 15-20 sections from each region of each palatal shelf. Student’s t-test was used to analyze the differences in the datasets and P<0.01 was considered statistically significant.
Real-time RT-PCR
Palatal shelves were micro-dissected from E12.5 and E13.5 embryos, quickly frozen in liquid nitrogen and stored individually at -80°C. Following identification of the genotypes by allele-specific PCR, two pairs of palatal shelf samples were pooled by genotype. Total RNA was extracted using the RNeasy Micro Kit (Qiagen). First-strand cDNA synthesis was achieved using the SuperScript First-Strand Synthesis System (Invitrogen). Quantitative PCR amplifications were performed in a C1000 Touch Thermal Cycler (Bio-Rad) using the SYBR GreenER qPCR Supermix (Invitrogen). Gene-specific primers were: Hprt-F (5′-TGCTGGTGAAAAGGACCTCTCG-3′) and Hprt-R (5′-CTGGCAACATCAACAGGACTCC-3′); Pax9-F (5′-TATTCTGCGCAACAAGATCG-3′) and Pax9-R (5′-GGTGGTGTAGGCACCTTAGC-3′); Bmp4-F (5′-GAGGGATCTTTACCGGCTCC-3′) and Bmp4-R (5′-GTTGAAGAGGAAACGAAAAGCAG-3′); Msx1-F (5′-AAGATGCTCTGGTGAAGGC-3′) and Msx1-R (5′-TGGTCTTGTGCTTGCGTA-3′); Fgf10-F (5′-TTTGAGCCATAGAGTTTCCCC-3′) and Fgf10-R (5′-CGGGACCAAGAATGAAGACTG-3′); Osr2-F (5′-TCTTTACACATCCCGCTTCC-3′) and Osr2-R (5′-GGAAAGGTCATGAGGTCCAA-3′). For each sample, the relative levels of target mRNAs were normalized to that of HPRT using the standard curve method. Four sets of samples were analyzed for each gene. Student’s t-test was used to analyze the difference and P<0.05 was considered statistically significant.
Acknowledgments
We thank Samantha Brugmann, Yang Chai and Steven Potter for comments and suggestions.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
Y.L. and R.J. conceived and designed this research project. J.Z., Y.G., Y.L. and S.J. carried out the experiments. All authors participated in analysis and discussion of the experimental data. J.Z. and Y.G. wrote the initial draft of the manuscript. All authors participated in editing and finalizing the manuscript.
Funding
This work was supported by National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) [DE013681 and DE015207 to R.J.]. Deposited in PMC for release after 12 months.
References
- Alappat S. R., Zhang Z., Suzuki K., Zhang X., Liu H., Jiang R., Yamada G., Chen Y. (2005). The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev. Biol. 277, 102–113 [DOI] [PubMed] [Google Scholar]
- Baek J. A., Lan Y., Liu H., Maltby K. M., Mishina Y., Jiang R. (2011). Bmpr1a signaling plays critical roles in palatal shelf growth and palatal bone formation. Dev. Biol. 350, 520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A. J., Bogardi J. P., Ladher R., Francis-West P. H. (1999). Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Dev. Dyn. 214, 291–302 [DOI] [PubMed] [Google Scholar]
- Barrow J. R., Stadler H. S., Capecchi M. R. (2000). Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse. Development 127, 933–944 [DOI] [PubMed] [Google Scholar]
- Bush J. O., Jiang R. (2012). Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development 139, 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter D. E., Wieschaus E. (1988). Gene activities and segmental patterning in Drosophila: analysis of odd-skipped and pair-rule double mutants. Genes Dev. 2, 1812–1823 [DOI] [PubMed] [Google Scholar]
- Danielian P. S., Muccino D., Rowitch D. H., Michael S. K., McMahon A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323–1326 [DOI] [PubMed] [Google Scholar]
- Dudas M., Sridurongrit S., Nagy A., Okazaki K., Kaartinen V. (2004). Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech. Dev. 121, 173–182 [DOI] [PubMed] [Google Scholar]
- Ferguson M. W. (1988). Palate development. Development 103 Suppl., 41–60 [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M., Mallo M., Zhang M., Gridley T. (1993). Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 75, 1317–1331 [DOI] [PubMed] [Google Scholar]
- Green R. B., Hatini V., Johansen K. A., Liu X. J., Lengyel J. A. (2002). Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development 129, 3645–3656 [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A. (2007). Molecular control of secondary palate development. Dev. Biol. 301, 309–326 [DOI] [PubMed] [Google Scholar]
- Hao I., Green R. B., Dunaevsky O., Lengyel J. A., Rauskolb C. (2003). The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev. Biol. 263, 282–295 [DOI] [PubMed] [Google Scholar]
- Hart M. C., Wang L., Coulter D. E. (1996). Comparison of the structure and expression of odd-skipped and two related genes that encode a new family of zinc finger proteins in Drosophila. Genetics 144, 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard S. A., Yu L., Gu S., Zhang Z., Chen Y. P. (2005). Regional regulation of palatal growth and patterning along the anterior-posterior axis in mice. J. Anat. 207, 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Yeo J. Y., Chytil A., Han J., Bringas P., Jr, Nakajima A., Shuler C. F., Moses H. L., Chai Y. (2003). Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 130, 5269–5280 [DOI] [PubMed] [Google Scholar]
- Jia S., Zhou J., Gao Y., Baek J. A., Martin J. F., Lan Y., Jiang R. (2013). Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development 140, 423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Jiang R. (2009). Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development 136, 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Kingsley P. D., Cho E. S., Jiang R. (2001). Osr2, a new mouse gene related to Drosophila odd-skipped, exhibits dynamic expression patterns during craniofacial, limb, and kidney development. Mech. Dev. 107, 175–179 [DOI] [PubMed] [Google Scholar]
- Lan Y., Ovitt C. E., Cho E. S., Maltby K. M., Wang Q., Jiang R. (2004). Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development 131, 3207–3216 [DOI] [PubMed] [Google Scholar]
- Levi G., Mantero S., Barbieri O., Cantatore D., Paleari L., Beverdam A., Genova F., Robert B., Merlo G. R. (2006). Msx1 and Dlx5 act independently in development of craniofacial skeleton, but converge on the regulation of Bmp signaling in palate formation. Mech. Dev. 123, 3–16 [DOI] [PubMed] [Google Scholar]
- Li Q., Ding J. (2007). Gene expression analysis reveals that formation of the mouse anterior secondary palate involves recruitment of cells from the posterior side. Int. J. Dev. Biol. 51, 167–172 [DOI] [PubMed] [Google Scholar]
- Liu W., Lan Y., Pauws E., Meester-Smoor M. A., Stanier P., Zwarthoff E. C., Jiang R. (2008). The Mn1 transcription factor acts upstream of Tbx22 and preferentially regulates posterior palate growth in mice. Development 135, 3959–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S. A., Oram K. F., Gridley T. (2007). Multiple functions of Snail family genes during palate development in mice. Development 134, 1789–1797 [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kapadia H., Feng J. Q., Raghow R., Peters H., D’Souza R. N. (2006). Functional consequences of interactions between Pax9 and Msx1 genes in normal and abnormal tooth development. J. Biol. Chem. 281, 18363–18369 [DOI] [PubMed] [Google Scholar]
- Pantalacci S., Prochazka J., Martin A., Rothova M., Lambert A., Bernard L., Charles C., Viriot L., Peterkova R., Laudet V. (2008). Patterning of palatal rugae through sequential addition reveals an anterior/posterior boundary in palatal development. BMC Dev. Biol. 8, 116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H., Neubüser A., Kratochwil K., Balling R. (1998). Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12, 2735–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R., Spencer-Dene B., Connor E. C., Gritli-Linde A., McMahon A. P., Dickson C., Thesleff I., Rice D. P. (2004). Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Invest. 113, 1692–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R., Connor E., Rice D. P. (2006). Expression patterns of Hedgehog signalling pathway members during mouse palate development. Gene Expr. Patterns 6, 206–212 [DOI] [PubMed] [Google Scholar]
- Saulier-Le Dréan B., Nasiadka A., Dong J., Krause H. M. (1998). Dynamic changes in the functions of Odd-skipped during early Drosophila embryogenesis. Development 125, 4851–4861 [DOI] [PubMed] [Google Scholar]
- Stapleton P., Weith A., Urbánek P., Kozmik Z., Busslinger M. (1993). Chromosomal localization of seven PAX genes and cloning of a novel family member, PAX-9. Nat. Genet. 3, 292–298 [DOI] [PubMed] [Google Scholar]
- Wang L., Coulter D. E. (1996). bowel, an odd-skipped homolog, functions in the terminal pathway during Drosophila embryogenesis. EMBO J. 15, 3182–3196 [PMC free article] [PubMed] [Google Scholar]
- Wang T., Tamakoshi T., Uezato T., Shu F., Kanzaki-Kato N., Fu Y., Koseki H., Yoshida N., Sugiyama T., Miura N. (2003). Forkhead transcription factor Foxf2 (LUN)-deficient mice exhibit abnormal development of secondary palate. Dev. Biol. 259, 83–94 [DOI] [PubMed] [Google Scholar]
- Welsh I. C., O’Brien T. P. (2009). Signaling integration in the rugae growth zone directs sequential SHH signaling center formation during the rostral outgrowth of the palate. Dev. Biol. 336, 53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Gu S., Alappat S., Song Y., Yan M., Zhang X., Zhang G., Jiang Y., Zhang Z., Zhang Y., et al. (2005). Shox2-deficient mice exhibit a rare type of incomplete clefting of the secondary palate. Development 132, 4397–4406 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhao X., Hu Y., St Amand T., Zhang M., Ramamurthy R., Qiu M., Chen Y. (1999). Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev. Dyn. 215, 45–53 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Song Y., Zhao X., Zhang X., Fermin C., Chen Y. (2002). Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 129, 4135–4146 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lan Y., Chai Y., Jiang R. (2009). Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science 323, 1232–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Gao Y., Zhang Z., Zhang Y., Maltby K. M., Liu Z., Lan Y., Jiang R. (2011). Osr2 acts downstream of Pax9 and interacts with both Msx1 and Pax9 to pattern the tooth developmental field. Dev. Biol. 353, 344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]