Abstract
Developmental processes such as morphogenesis, patterning and differentiation are continuously active in the adult Hydra polyp. We carried out a small molecule screen to identify compounds that affect patterning in Hydra. We identified a novel molecule, DAC-2-25, that causes a homeotic transformation of body column into tentacle zone. This transformation occurs in a progressive and polar fashion, beginning at the oral end of the animal. We have identified several strains that respond to DAC-2-25 and one that does not, and we used chimeras from these strains to identify the ectoderm as the target tissue for DAC-2-25. Using transgenic Hydra that express green fluorescent protein under the control of relevant promoters, we examined how DAC-2-25 affects tentacle patterning. Genes whose expression is associated with the tentacle zone are ectopically expressed upon exposure to DAC-2-25, whereas those associated with body column tissue are turned off as the tentacle zone expands. The expression patterns of the organizer-associated gene HyWnt3 and the hypostome-specific gene HyBra2 are unchanged. Structure-activity relationship studies have identified features of DAC-2-25 that are required for activity and potency. This study shows that small molecule screens in Hydra can be used to dissect patterning processes.
Keywords: Cnidaria, Hydra, Pattern formation, Pyridone, Regeneration, Small molecule
INTRODUCTION
The adult Hydra polyp has a simple body plan - it is organized along a single axis with two concentrically arranged epithelial cell layers (the endoderm and ectoderm) that form a hollow tube (Campbell and Bode, 1983). At the oral end of the axis is a head, consisting of a structure called the hypostome that contains the mouth opening, and a ring of tentacles. The aboral end of the tube consists of the basal disk (foot) that allows the animal to stick to the substratum. The two epithelial layers are separated by a basal lamina and interspersed between the epithelial cells are cells of a multipotent lineage called interstitial cells (i-cells), which give rise to four differentiated cell types: gametes, nerves, secretory cells and nematocytes (David, 2012). All of the epithelial cells in the Hydra body column are mitotically active, whereas those in the tentacles and the foot are arrested in G2 of the cell cycle (Dübel et al., 1987). In order to maintain its size and axial pattern in the face of constant cell production, Hydra must get rid of excess cells. This is accomplished mainly by shunting of cells into buds, which is the asexual form of reproduction in Hydra, and by sloughing of cells at the tips of the tentacles and at the foot. The effect of these tissue dynamics is that cells are constantly changing position (Campbell, 1967a; Campbell, 1967b). This requires cells to monitor their positions and respond appropriately, for example by changing their morphology (Philipp et al., 2009) and gene expression pattern (Smith et al., 2000) as they are displaced from the body column into the tentacles.
In addition to its dynamic patterning processes, Hydra has a remarkable ability to regenerate (Bode and Bode, 1980). Studies of the molecular control of patterning and regeneration have focused on expression of candidate genes in experimentally manipulated Hydra (e.g. Philipp et al., 2009) and on responses to molecules that perturb known pathways (Broun et al., 2005; Münder et al., 2010; Philipp et al., 2009). These approaches have led to important discoveries, e.g. that the canonical Wnt signaling pathway is a key component of the Hydra axial organizer (Broun and Bode, 2002; Broun et al., 2005; Gee et al., 2010). Genetic screens for identifying genes involved in patterning are not feasible with Hydra because sexual reproduction is not efficient in the laboratory and embryos go through a period of developmental arrest that can be long (Sugiyama, 1983). Nonetheless, some interesting Hydra mutants have been identified through sexual crosses (Sugiyama, 1983; Sugiyama and Fujisawa, 1977; Sugiyama and Fujisawa, 1978a). Despite our inability to carry out genetic screens in Hydra, several tools and aspects of its biology make it a compelling model organism. As the sister group to Bilateria, Cnidaria can offer important insights into the evolution of metazoan development. The three cell lineages of Hydra are well characterized (Bode, 1996; Sugiyama and Fujisawa, 1978b), and are maintained independently of each other with no transdifferentiation (Bode, 1996), making experiments of lineage tracing straightforward. In addition, the production of transgenic Hydra is relatively easy (Wittlieb et al., 2006).
Several features of Hydra suggested that it would be amenable to screens for small molecules that affect patterning. Hydra is kept in a simple, non-sterile culture medium, and can go without feeding for a week or more. Hydra propagates clonally by asexual budding; thus, it is easy to obtain large numbers of genetically identical animals. Due to its simple body composition, all of the cell types are easily exposed to small molecules in the culture medium. The sequenced Hydra genome and the set of gene models generated from it (Chapman et al., 2010) can be used in identifying the protein targets of small molecules. Finally, studies using small molecule inhibitors of known targets have provided important insights into Hydra patterning (Broun et al., 2005). Thus, small molecule screening appeared to be a promising approach for identifying effectors of developmental processes in Hydra. We initiated a screen on Hydra undergoing head regeneration and identified a novel molecule, 6-(4-dimethylaminophenyl)-4-methylpyridin-2(1H)-one (DAC-2-25), that induces extra tentacles during regeneration. In chronically treated polyps, DAC-2-25 causes a homeotic transformation of body column into tentacle zone. This is, as far as we are aware, the first report of an unbiased small molecule screen for modulators of patterning in a whole-animal system.
RESULTS
Screening a small molecule library for modulators of Hydra head regeneration
Most studies of Hydra regeneration have focused on the head, the site of the axial organizer (Broun and Bode, 2002). Thus, we screened for small molecules that perturbed head regeneration, using a small molecule library generated in the UC Irvine Department of Chemistry. We used transversely bisected H. vulgaris polyps (strain AEP) exposed to the small molecules at a concentration of about 100 μg/ml in Hydra medium (HM) for 1 week at 18°C in 24-well plates. Each well contained oral and aboral halves from three polyps. The animals were not fed and the medium was not changed during the course of exposure. By using cut animals, we hoped to identify molecules affecting regeneration and/or developmental patterning. Among the first 60 molecules screened, we identified one (DAC-2-25, Fig. 1A) that induced the formation of extra tentacles on the regenerating oral end (Fig. 1B). There was no effect on foot regeneration. DAC-2-25 was retested on a larger number of animals and found to reproducibly cause animals to regenerate twice the normal number of tentacles (Fig. 1C).
Fig. 1.
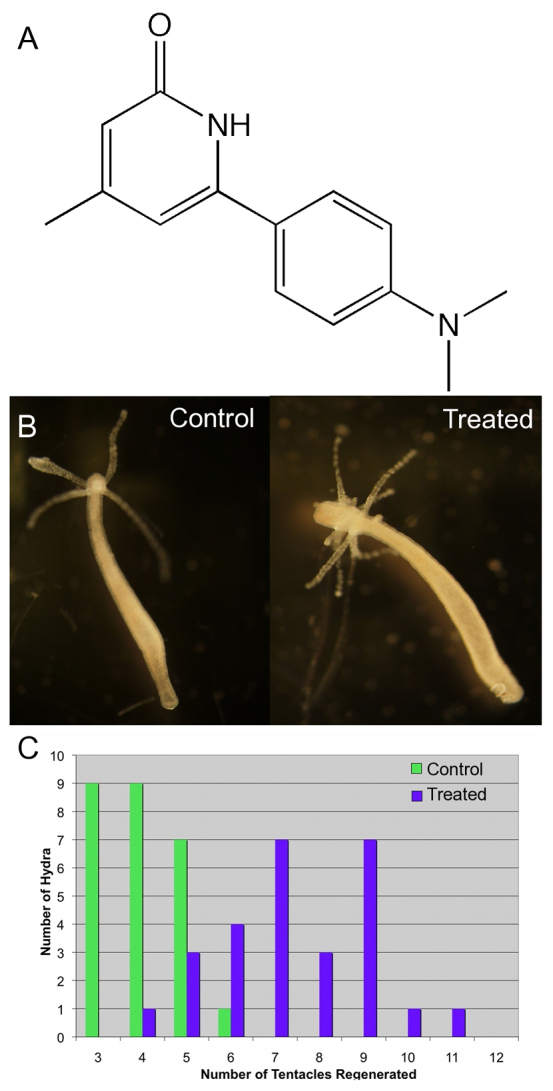
Regeneration in the presence of DAC-2-25 induces extra tentacles. (A) The structure of DAC-2-25. (B) Animals of the AEP strain of H. vulgaris were allowed to regenerate in HM only or in the presence of HM containing 5 μM DAC-2-25. (C) The mean number of tentacles regenerated by control animals (blue) and animals treated with 5 μM DAC-2-25 (green). These data are from three independent experiments in which a total of 60 animals underwent head regeneration in the presence (30 animals) or absence (30 animals) of DAC-2-25. Error bars indicate s.d.
Chronic treatment with DAC-2-25 induces ectopic tentacles on the body column
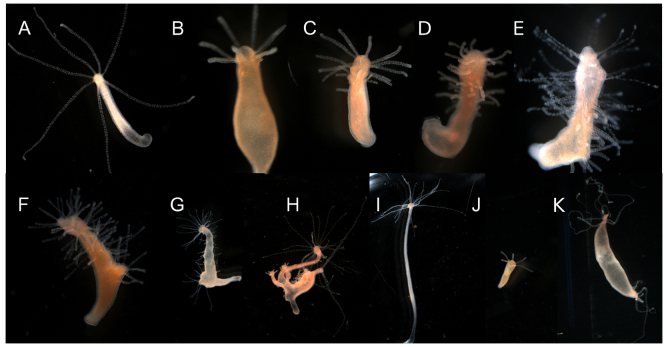
We next examined the effect of chronic exposure of intact Hydra polyps to DAC-2-25. Over the course of 3 weeks of DAC-2-25 treatment, ectopic tentacles first appeared just below the existing tentacle ring after 7-10 days of treatment. With continued exposure, ectopic tentacles emerged progressively farther down the body column, eventually covering the entire length of the animal (Fig. 2A-E), with the exception of the foot. Even after 8 weeks of treatment the foot remained free of tentacles. Buds that formed during treatment also had extra tentacles. DAC-2-25 has no visible side effects at concentrations that cause formation of extra tentacles. Animals that have been chronically exposed and are covered with ectopic tentacles gradually lose the extra tentacles following removal of DAC-2-25 (Fig. 2F-I). Buds that formed after cessation of treatment had no ectopic tentacles (Fig. 2J). Exposures to higher concentrations of DAC-2-25 were tried, including 10 and 20 μM, and no variation was observed in either the pace at which the ectopic tentacles spread, or the polarity with which they arose.
Fig. 2.
Chronic exposure to DAC-2-25 leads to ectopic tentacle formation that spreads towards the aboral end of the polyp. Hydra morphology before (A) and after (E) 3 weeks of exposure to 5 μM DAC-2-25; all pictures are of the same animal. Seven tentacles can be seen on the untreated animal (A). After 9 days of exposure the animal has produced two ectopic tentacles (B). After 13 days of exposure (C) and 16 days of exposure (D), the animal has an increased number of tentacles. After 21 days of exposure (E) the animal is covered in tentacles. This animal was then removed from DAC-2-25 and placed in HM. Tentacles were gradually lost, as seen after 8 days (F) and 12 days (G). Buds formed normally in the recovering animal (H) and had normal morphology upon detachment from the parent (J). After 54 days (I), the animal had re-established its original axial pattern. (K) Hydra morphology after 3 weeks of exposure to 50 nM ALP.
Phylochemogenetic profiling reveals DAC-2-25 responsive and non-responsive strains of Hydra
Our small-molecule screen was carried out using the AEP strain of H. vulgaris, the strain that is used to produce transgenic Hydra (Wittlieb et al., 2006). We identified additional strains of H. vulgaris that responded as robustly as the AEP strain (Fig. 3), and one strain of H. vulgaris (collected in Zürich, Switzerland by Pierre Tardent in the 1960s), that did not respond. We tested the ability of the Zürich strain to respond by exposing it to a variety of concentrations and for various treatment durations. No treatment condition was capable of inducing the formation of ectopic tentacles in this strain. We also tested H. viridissima and found that it responded. The last common ancestor of H. vulgaris and H. viridissima was the ancestor of all extant members of the genus Hydra (Martínez et al., 2010). Thus, the lack of response in the Zürich strain is most parsimoniously explained by loss of the ability to respond. To determine whether the failure of the Zürich strain to respond to DAC-2-25 could be due to a reduced inability to take up compounds from the medium, we treated it with alsterpaullone (ALP), a potent GSK3-β inhibitor that causes ectopic activation of the canonical Wnt pathway and ectopic tentacle formation (Broun et al., 2005). The Zürich strain was responsive to ALP treatment.
Fig. 3.
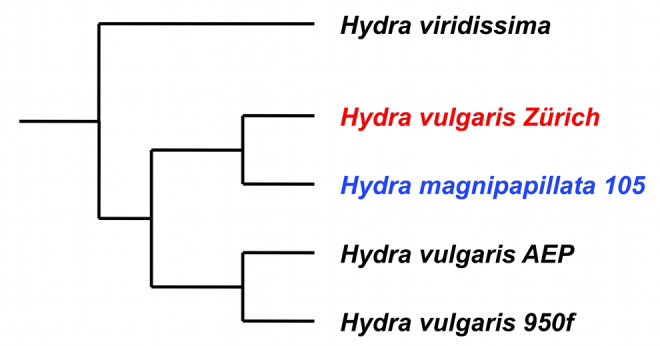
Phylochemogenetic profiling of Hydra with DAC-2-25. Various species and strains of Hydra were tested for their ability to respond to DAC-2-25 and alsterpaullone (ALP). All strains responded to ALP. All strains of H. vulgaris responded to DAC-2-25, except for H. vulgaris Zürich. H. magnipapillata strain 105 was categorized as a ‘partial responder’ (blue): buds that formed during treatment of this strain had many ectopic tentacles, whereas the parent occasionally produced one ectopic tentacle. The outgroup species, Hydra viridissima, is robustly responsive to both compounds.
The formation of ectopic tentacles in polyps chronically treated with DAC-2-25 is reminiscent of the phenotype seen when Hydra is treated transiently with 5 μM ALP (Broun et al., 2005). There are, however, clear differences between the two phenotypes. First, treatment with ALP leads to the appearance of ectopic tentacles simultaneously all over the body column, whereas treatment with DAC-2-25 leads to progressive ectopic tentacle formation, beginning at the oral end. Treatment with 5 μM ALP yields a rapid response, with ectopic tentacles appearing 4 days after exposure. Chronic treatment with 5 μM ALP is not possible due to the toxicity of the molecule. However, animals can be treated for at least 3 weeks at 50 nM with no toxicity. These animals did not produce ectopic tentacles, but the aboral end was repatterned into a second head (Fig. 2K). Despite these differences, we further explored the possibility that DAC-2-25 targets GSK3-β by sequencing cDNAs for GSK3-β from the AEP and Zürich strains. The coding sequences of the two cDNAs (GenBank Accession Numbers JN083831 and JN083832) have 18 nucleotide differences, all of them silent. Thus, the GSK3-β proteins of the two strains are identical. This result is inconsistent with the two strains being differentially responsive if the DAC-2-25 target is GSK3-β.
Sensitivity to DAC-2-25 is not broadly conserved. DAC-2-25 has no effect on regeneration in the anthozoan Nematostella vectensis (Dr Aissam Ikmi, Stowers Institute for Medical Research, personal communication) or the planarians Dugesia tigrina and Schmidtea mediterranea (Dr Eva-Maria S. Collins, UC San Diego, personal communication).
DAC-2-25 targets ectodermal epithelial cells
To determine what cells are targeted by DAC-2-25, we took advantage of the ability to create chimeric and mosaic Hydra. Chimeras are animals where the ectoderm, endoderm or i-cell lineage of one strain is completely replaced with the corresponding lineage from another strain (Marcum and Campbell, 1978). A mosaic animal is one in which populations of cells from the two contributing strains exist within the same lineage. We produced three types of AEP/Zürich chimeras and various mosaic animals. The three chimeric lines were: (1) a line with AEP ectoderm and Zürich endoderm (ecto:AEP/endo:Zürich); (2) a line with AEP endoderm and Zürich ectoderm (ecto:Zürich/endo:AEP); (3) a line in which AEP i-cells were introduced into a Zürich epithelium (i-cell chimera). The mosaics contained various proportions and arrangements of AEP and Zürich tissue. To track the source of the cells in these animals, we used a transgenic Hydra line expressing green fluorescent protein (GFP) in the endoderm and red fluorescent protein (DsRed2) in the ectoderm, and a line in which all three lineages express DsRed2 (see Materials and methods).
To investigate the role of ectodermal epithelial cells in producing the ectopic tentacle phenotype, we treated the ecto:AEP/endo:Zürich chimera with DAC-2-25 (Fig. 4A). Ectopic tentacles arose in this animal in a manner similar to that seen when the AEP strain is exposed (Fig. 4B). The ecto:Zürich/endo:AEP chimera did not respond to DAC-2-25 (Fig. 4C). From these results, we concluded that the endoderm is not the target of DAC-2-25.
Fig. 4.
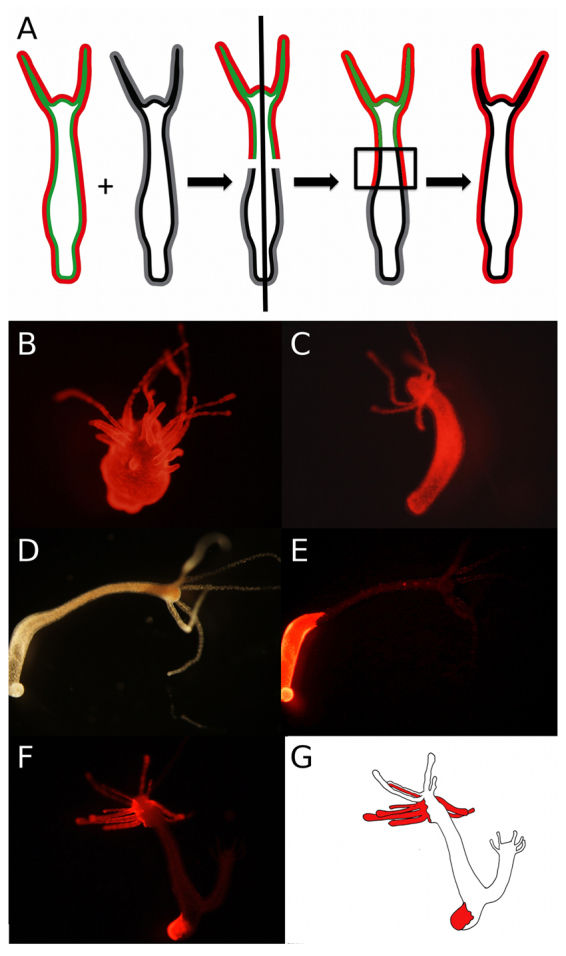
DAC-2-25 targets ectodermal epithelial cells in a cell-autonomous manner. (A) A schematic representation of the method used to produce chimeric Hydra (see Materials and methods for more details). (B) The chimera described in A was exposed to DAC-2-25 and ectopic tentacles arose in a manner similar to that seen in AEP animals. (C) A chimera with Zürich ectoderm and AEP endoderm did not respond to DAC-2-25. (D,E) Ectopic tentacles did not form in an animal with AEP i-cells and Zürich epithelial layers. (F,G) Mosaic Hydra were produced when chimeras (shown in D,E) budded. These mosaics were exposed to DAC-2-25 and ectopic tentacles were seen only in epithelial tissue derived from the AEP donor.
To determine the role of the i-cell lineage, we tested the ability of the i-cell chimera to respond to DAC-2-25. These chimeras were treated with DAC-2-25 for 3 weeks. Ectopic tentacles were not seen in any cases (Fig. 4D,E), indicating that AEP i-cells are not sufficient, in the numbers seen in our experiments, to induce the ectopic tentacle phenotype in Zürich epithelial tissue.
In some cases, buds formed during the course of DAC-2-25 treatment on animals in which both AEP and Zürich epithelium occupied the budding zone, yielding buds composed, to varying degrees, of both AEP and Zürich epithelium. These buds gave us an opportunity to examine the response of mosaic animals with varying compositions of source tissues. We obtained longitudinally mosaic animals, animals where a ‘finger’ of AEP tissue extended into Zürich epithelium, and animals in which the hypostome was Zürich and the rest of the animal AEP (Fig. 4F,G). We found that ectopic tentacles formed only in regions containing AEP ectoderm. These results indicate that the phenotype produced by DAC-2-25 is cell-autonomous.
To further examine the cell autonomy of the response to DAC-2-25, we grafted the upper half of a Zürich animal to the lower half of a transgenic AEP animal. When exposed to DAC-2-25, ectopic tentacles were not formed in the Zürich region of the animal and did not appear in the AEP region of the body column of the mosaics until ectopic tentacles began to appear in the corresponding region in the control animal. These results indicate that the temporal component of the DAC response is an autonomous feature of the AEP tissue.
DAC-2-25 transforms the body column into tentacle zone
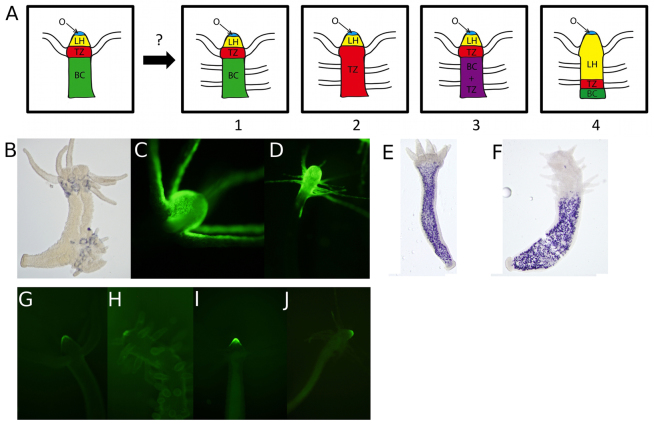
We considered four explanations for the origin of ectopic tentacles in DAC-2-25-treated animals: (1) formation of tentacles on tissue that retains its body column identity; (2) conversion of body column tissue into tentacle zone tissue (i.e. a homeotic transformation); (3) conversion of body column into a combination of body column and tentacle zone; or (4) expansion of the hypostome with concomitant accumulation of displaced tentacles (Fig. 5A). To distinguish among these possibilities, we used genes whose expression patterns define tissue identity along the oral/aboral axis. These include: (1) a marker of the organizer, the Hydra Wnt3 ortholog (HyWnt3), which is expressed at the tip of the hypostome (Hobmayer et al., 2000); (2) a marker of hypostome, the Hydra T-box gene HyBra2, which is expressed throughout the hypostome, but not in the tentacle zone (Bielen et al., 2007); (3) a marker of tentacles, HyAlx, an aristaless-related homeobox gene that is expressed in rings at the bases of the tentacles (Smith et al., 2000); (4) a marker for tentacle zone, Hym-301 (Hobmayer et al., 2000; Smith et al., 2000; Takahashi et al., 2005); and (5) a marker for body column tissue, a gene for a novel secreted protein (GenBank Accession Number XP_002165635) (Hwang et al., 2007). This gene was described as being expressed in body column endodermal epithelial cells by Hwang et al. (Hwang et al., 2007), but we have found that it is expressed in gland cells in the body column (Fig. 5E).
Fig. 5.
Markers of positional identity show conversion of body column to tentacle zone upon chronic treatment with DAC-2-25. (A) Four models to explain the identity of the tissue containing ectopic tentacles produced in response to DAC-2-25. (Far left) An untreated Hydra has four tissue identities associated with the head and upper body column: O, organizer; LH, lower hypostome; TZ, tentacle zone; BC, body column. We hypothesized that: (1) ectopic tentacles could form within tissue that has retained body column identity; (2) ectopic tentacles could arise within tissue that has been transformed into tentacle zone; (3) ectopic tentacles could arise in tissue that has both body column and tentacle zone identities; or (4) that the lower hypostome expands, pushing the tentacle zone down the body column, while previously formed tentacles are maintained. (B) In situ hybridization for HyAlx in a polyp treated for 11 days; ectopic tentacles have rings of HyAlx expression in their ectoderm, as seen in untreated controls [not shown, described elsewhere (Smith et al., 2000)]. H301p::GFP::H301t, a transgenic line in which the Hym-301 promoter drives GFP expression in the ectoderm (C), after 27 days of exposure to DAC-2-25 (D), the zone of GFP expression has expanded. (E) An in situ hybridization for a body column-specific gene in an untreated animal and after 23 days of exposure to DAC-2-25 (F). The transgenic line Ec(HyWnt3FL::GFP) created by Nakamura et al. (Nakamura et al., 2011), in which the Wnt3 promoter drives expression of GFP in the ectoderm (G). (H) The region of GFP expression in ec(HyWnt3FL::GFP) did not expand upon exposure to DAC-2-25. The expression pattern of GFP in a transgenic reporter for HyBra2 in the ectoderm (I) does not change in response to DAC-2-25 (J).
The ectopic tentacles in DAC-2-25-treated animals show rings of HyAlx expression at their bases, as is seen with normal tentacles (Fig. 6B), indicating that HyAlx functions in formation of ectopic tentacles as it does in normal tentacles (Smith et al., 2000). We produced a transgenic line in which the Hym-301 promoter drives GFP expression (H301p::GFP::H301t), such that GFP expression is activated after cells are displaced from the body column into the head (Fig. 6C), similar to endogenous Hym-301 expression (Takahashi et al., 2005). The presence of GFP in the tentacles and the upper region of the hypostome of the transgenic animals, where Hym-301 gene expression is not detected by in situ hybridization, is probably due to the long half-life of GFP, allowing it to persist in cells that have been displaced into the tentacles and upper hypostome. In H301p::GFP::H301t animals chronically exposed to DAC-2-25, the aboral boundary of GFP expression moved down the animal in synchrony with the formation of ectopic tentacles (Fig. 6D). This result is consistent with at least a partial transformation of body column tissue into tentacle zone and rules out ectopic tentacle formation from what is otherwise body column tissue.
Fig. 6.
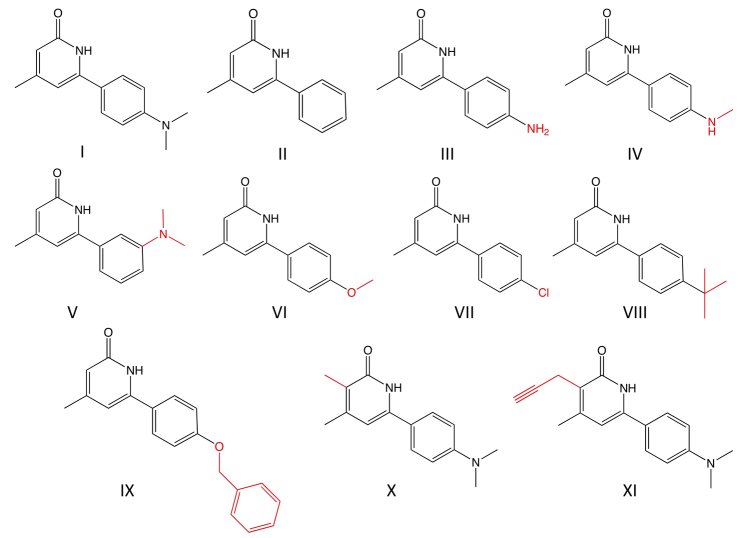
DAC-2-25 and derivatives for SAR studies. A subset of the 44 derivatives of DAC-2-25 (I) that were used for SAR studies is shown. Those that are not shown in the figure are listed in supplementary material Appendix S1. Compounds II, III, V, VII and IX did not induce ectopic tentacles. Compounds IV, VI, VIII, X and XI induced ectopic tentacles.
When expression of the body column marker was examined using animals that had been chronically treated with DAC-2-25, we found that the oral expression boundary of the marker moved down the animal in synchrony with ectopic tentacle formation (Fig. 6F). This result indicates that the tissue containing ectopic tentacles has lost features of body column tissue and, together with the Hym-301 result, supports the hypothesis that the body column is transformed into tentacle zone.
We investigated the expression pattern of HyWnt3 in DAC-2-25-treated animals using two different transgenic HyWnt3 promoter::GFP transgenic lines obtained from the Holstein lab: in one, expression of the transgene is restricted to the ectoderm [ec(HyWnt3FL::GFP)]; and in the other, expression is restricted to the endoderm [en(HyWnt3FL::GFP)] (Nakamura et al., 2011). Chronic exposure to DAC-2-25 did not alter the GFP expression pattern in either line (Fig. 6G,H and data not shown). If DAC-2-25 causes expansion of the hypostome at the expense of body column, such elongation would be accompanied by continuous displacement of the tentacle zone downwards. Existing tentacles would then be displaced upwards into the elongating hypostome and new tentacles would emerge at the front of the downward moving tentacle zone. To test this possibility, we produced a transgenic line in which the HyBra2 promoter drives GFP expression, [ec(HyBra2::GFP]. This line faithfully recapitulates HyBra2 expression as described by Bielen et al. (Bielen et al., 2007) (Fig. 6I). When this line was exposed to DAC-2-25, the transgene expression domain was not altered (Fig. 6J). Taken together, the data from the various transgenic lines lead to the conclusion that DAC-2-25 treatment causes the body column to be transformed into tentacle zone.
Structure-activity relationship (SAR) studies identify features of DAC-2-25 required for biological activity
The ultimate goal of these studies is to identify the protein target of DAC-2-25, which could potentially be achieved by affinity chromatography coupled with mass spectrometry. This approach requires structure-activity relationship (SAR) studies to identify appropriate sites for immobilization of the molecule on a solid support and to identify the most active derivative of the molecule. Forty-three derivatives of DAC-2-25 were synthesized (supplementary material Appendix S1) and one was purchased (Sigma-Aldrich), and subsequently tested on the AEP strain (the strain used in the original screen) using the chronic exposure assay. The studies described above were carried out at a DAC-2-25 concentration of 5 μM. All derivatives were first tested at 5 μM, and if no phenotype was produced within 3 weeks of exposure, the derivative was tested at 10 and 20 μM. If ectopic tentacles were not seen at these higher concentrations, the compound was considered inactive. If a derivative induced ectopic tentacles at 5 μM, it was retested at 1 μM, 500 nM, 100 nM and 50 nM.
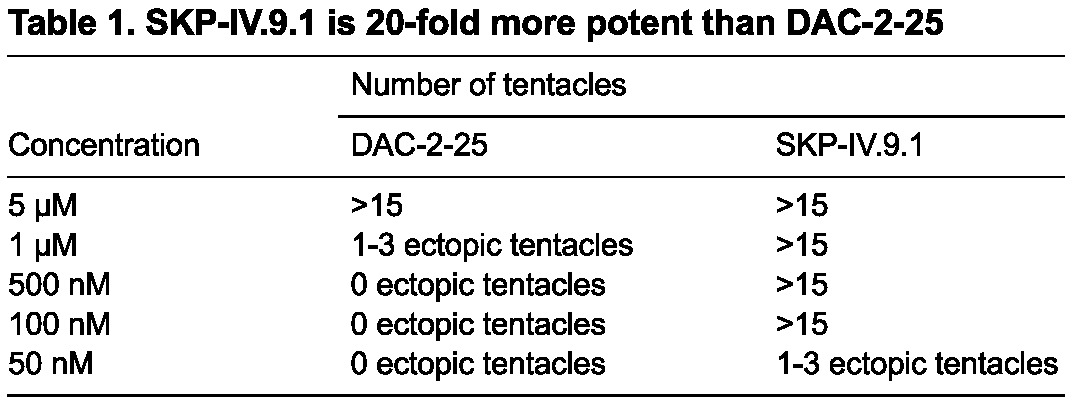
Our SAR study identified several features of DAC-2-25 that are essential for activity and lack of toxicity. We have found that the aryl ring at the C6 position of the 2-pyridone is required to produce the phenotype and that an alkyl substituent is required in the para position of the aryl ring. We have also found that the addition of a methyl group on the pyridone ring at the C3 position increases the activity of the compound 20-fold (Table 1).
Table 1.
SKP-IV.9.1 is 20-fold more potent than DAC-2-25
To investigate the possibility that inactive derivatives bind to the target molecule and abrogate activity of DAC-2-25 by competition for binding, animals were treated with equimolar concentrations of DAC-2-25 and the inactive derivative DAC-1-217 (Fig. 6, compound II), and twofold excess DAC-1-217. Ectopic tentacles were formed in a time frame similar to that for DAC-2-25 treatment alone in both experiments, indicating that the inactive compound does not compete for binding with DAC-2-25.
One of the derivatives, SKP-IV.9.1 (Fig. 6, compound X), initiated the formation of ectopic tentacles 1 day faster than DAC-2-25. An end-point dilution experiment was performed to measure the potency of SKP-IV.9.1 (Table 1). Animals were exposed to either DAC-2-25 or SKP-IV.9.1 at concentrations of 5 μM, 1 μM, 500 nM, 100 nM and 50 nM. Ten animals were exposed for 3 weeks and the number of ectopic tentacles was then counted. DAC-2-25 induces the formation of ectopic tentacles robustly at 5 μM but only one or two ectopic tentacles are produced at 1 μM, and none at lower concentrations. Ectopic tentacles were robustly produced when the animals were treated with SKP-IV.9.1 at all concentrations except 50 nM, where one or two ectopic tentacles were produced. The threshold between inactive and minimal activity (characterized as one or two ectopic tentacles) is between 1 μM and 500 nM for DAC-2-25, and less than 50 nM for SKP-IV.9.1; thus, SKP-IV.9.1 is at least 20-fold more potent than DAC-2-25. Ectopic tentacle formation at the oral end of buds is more sensitive to the compounds than ectopic tentacle formation on the body column. Ectopic tentacles formed at the oral ends of buds at all concentrations of SKP-IV.9.1 tested, but only at 5 μM, 1 μM and 500 nM for DAC-2-25.
DISCUSSION
The mechanisms that underlie pattern formation in metazoans are still far from being fully understood. Because of its single axis, its ability to regenerate from pieces of the adult polyp or from aggregates of cells, and its ability to be mathematically modeled (Gierer and Meinhardt, 1972; Meinhardt, 2012), Hydra is an important organism for studies of pattern formation. However, the inability to carry out genetic screens has prevented unbiased searches for genes that affect patterning in Hydra. Forward genetic screens in Hydra are precluded due to the difficulty of obtaining large numbers of embryos and the long and variable period between fertilization and hatching seen in most strains. Although the availability of the Hydra genome sequence potentially allows large-scale reverse genetic screens using RNAi, such screens are not feasible using the currently published methods for carrying out RNAi in Hydra (Chera et al., 2006; Lohmann et al., 1999; Smith et al., 2000). An unbiased screen for genes involved in Hydra patterning using differential display RT-PCR on RNA samples from regenerating aggregates of Hydra cells led to identification of a gene encoding a novel peptide expressed during head regeneration (Takahashi et al., 1997). However, there have been no additional reports of genes identified using this approach. We were intrigued by the possibility of applying small molecule screens to Hydra owing to the ease with which whole animals can be exposed to chemicals in the culture medium. The sequencing of the Hydra genome would facilitate the identification of the targets of small molecules, and the ability to make transgenic animals allows for the production of reporter lines for characterization of the changes in gene expression that result from small molecule exposure.
Our screen for small molecules that affect patterning in Hydra was simple - three bisected adult polyps were placed in each well of a 24-well plate together with 1 ml of medium containing a small molecule. After 7 days of exposure, examination under a dissecting microscope was carried out. Among the first 60 molecules screened, we identified DAC-2-25. The robustness and specificity of the response to DAC-2-25 shows that it is possible to identify small molecules that yield informative patterning phenotypes in Hydra.
The initial phenotype we obtained with DAC-2-25, regeneration of extra tentacles, suggested that this molecule was affecting some aspect of the patterning process during head regeneration. Animals chronically exposed to DAC-2-25 were particularly informative in this regard. Chronic treatment gave rise to no visible side effects, indicating that DAC-2-25 has high specificity for its target. The most intriguing aspects of the phenotype seen in chronically treated animals are the pace at which extra tentacles were formed and the polarity of their appearance. These features of the phenotype suggest that elaboration of the phenotype might be connected to the graded competence component of Hydra patterning described by Meinhardt (Meinhardt, 2012). Tissue competence leads to the polarity of regeneration and is relatively stable, as demonstrated by transplantation experiments in which the probability of polarity reversal of regenerating body columns increases when grafted hypostome and tentacle zone were in contact with the former aboral end of a host body column for more than 96 hours (Wilby and Webster, 1970a; Wilby and Webster, 1970b). And more recently, transplantation experiments have shown that explants from ALP-treated animals retained high competence for head formation for 8 days after removal from ALP, measured by the ability to induce the formation of a secondary axis (Gee et al., 2010). Although phenotypic determinants of organizer tissue remained, the expression levels of HyWnt3 and of other organizer-associated genes returned to normal within 48 hours of the end of ALP treatment (Gee et al., 2010). The polarity and temporal aspects of the response to DAC-2-25, and its cell autonomy, suggest that DAC-2-25 acts on a pathway within the cell whose ability to respond to the molecule is established by the competence gradient.
By examining expression of several marker genes in animals chronically exposed to DAC-2-25, we found that the body column was converted into tentacle zone. The phenotype is not the result of expansion of the tentacle zone while maintaining the existing body column, i.e. the animal is not longer. Nor is the hypostome noticeably larger in the treated animals. Rather, the enlarged tentacle zone is produced at the expense of body column tissue. Thus, we describe this phenotype as a homeotic transformation because it meets Bateson’s definition (Bateson, 1894), i.e. that ‘something has been changed into the likeness of something else’. Because of its dynamic patterning, Hydra is capable of reversing homeotic transformations, as is seen when animals are removed from DAC-2-25.
Animals treated chronically with DAC-2-25 and animals treated with a pulse of ALP share the feature of forming ectopic tentacles. This similarity suggested that the two molecules might target the same pathway, the canonical Wnt signaling pathway, and perhaps the same molecule, GSK3-β. Sequences of GSK3-β cDNAs from the AEP and Zürich strains rule out the possibility that GSK3-β is the direct target of DAC-2-25 as they encode proteins with identical amino acid sequences. We cannot, however, rule out the possibility that DAC-2-25 targets some other component of the canonical Wnt signaling pathway or a target of this pathway.
To understand the mechanism by which DAC-2-25 affects patterning, we obviously need to know the identity of its target, which we assume to be a protein. A standard approach for identifying the protein target of a small molecule is to immobilize the small molecule and use it to affinity purify the target, which is then identified by mass spectrometry. We have carried out SAR studies to identify sites on DAC-2-25 that could be used for immobilization. As part of the SAR study, we explored the possibility of using click chemistry (Best, 2009) to facilitate the identification of the target of DAC-2-25. In particular, we wanted to determine whether we could produce a bioactive alkyene-containing derivative of DAC-2-25 onto which we could click a biotin-tagged azide molecule to allow affinity purification of the target using streptavidin beads. To this end, a derivative was produced in which a propargyl group was added at the C3 position (supplementary material Appendix S1; Fig. 6, compound XI). This compound is active at 5 μM in the chronic exposure assay. Thus, using this derivative and click chemistry in an attempt to purify the DAC-2-25 target seems reasonable.
We are intrigued by the possibility of using genomic approaches to aid in the identification of the protein targeted by DAC-2-25. By looking for non-synonymous changes in the exons of the genomes of the AEP and Zürich strains, we can potentially identify candidate target proteins for DAC-2-25.
MATERIALS AND METHODS
Hydra strains and culture
Experiments were carried out using H. vulgaris AEP (Martin et al., 1997; Technau et al., 2003), H. vulgaris Zürich (provided by Dr Monika Hassel, Philipps-Universität Marburg), H. vulgaris 950f (Dr Richard Campbell, University of California, Irvine), H. viridissima 1695c (Dr Daniel Martínez, Pomona College) and H. magnipapillata strain 105 (Chapman et al., 2010). Animals were cultured as previously described (Lenhoff, 1983).
Small molecule screening
Small molecules from the UC Irvine Department of Chemistry library were supplied frozen in 10 μl aliquots in 96-well deep-well plates at a concentration of 10 mg/ml in DMSO. Stock solutions were dissolved in 1 ml of Hydra medium (HM) and the resulting solutions were then transferred to a 24-well plate. Each well contained the pieces from three bisected adult AEP polyps. The plate was incubated for 1 week at 18°C in the dark. The animals were not fed nor was the medium changed during the incubation period. The plates were then examined under a dissecting microscope.
Treatment of Hydra with DAC-2-25 and its derivatives
Free base forms of DAC-2-25 and its derivatives were dissolved in DMSO at a concentration of 5 mM and diluted in HM to the desired concentration immediately before use. Hydrochloride salts of DAC-2-25 and its derivatives were dissolved in Nanopure water at a concentration of 5 mM and diluted in HM immediately before use. Chronically treated animals were fed three times/week and transferred to medium containing freshly diluted compound following feeding.
Construction of a transgenic Hydra line expressing green fluorescent protein in the endoderm and red fluorescent protein in the ectoderm
This line was made by grafting two lines that we had previously produced, in which GFP was expressed in the endoderm of one line and DsRed2 was expressed in the ectoderm of the other line. In both lines, expression of the reporter gene was under the control of an actin gene promoter (Böttger et al., 2002; Wittlieb et al., 2006). Animals from these two lines were bisected and grafted according to standard methods. During the tissue displacement that occurs in Hydra, the two epithelial layers can move at slightly different rates, leading to the two layers getting out of register at the graft boundary. When this occurred, we excised the region of the grafted animal in which the DsRed2-expressing tissue had come to overlie GFP-expressing tissue. Regeneration from the excised piece of tissue led to an animal in which the endoderm was green and the ectoderm was red. This line was named (ecto[β-act::RFP]/endo[β-act::GFP]).
Construction of a transgenic Hydra line expressing red fluorescent protein in all tissue lineages
We constructed a transgenic line in which DsRed2 is expressed in all three lineages. This was carried out by injecting embryos of the AEP strain with the pHyVec5 plasmid, which contains the gene for DsRed2 driven by an actin gene promoter (Dana et al., 2012). A transgenic line was obtained in which only the i-cells expressed DsRed2. This line was clonally propagated and then self-crossed to produce lines that are homozygous diploid for the transgene and express DsRed2 in all three lineages. Genome sequencing has confirmed that these lines contain a single integrated copy of the transgene (R.E.S., unpublished).
Construction and treatment of chimeras and mosaics
For each transgenic chimera, a single founder animal was produced and propagated clonally and representative animals were treated and observed. A chimera with AEP ectoderm and Zürich endoderm was constructed by grafting of halves of ecto[β-act::RFP]/endo[β-act::GFP] polyps with halves of Zürich strain polyps. As described in the above section, when the two epithelial layers got out of register, part of the body column was cut out and allowed to regenerate. The presence of a completely red ectoderm and the absence of GFP expression in the endoderm indicated a fully chimeric animal.
A chimera with AEP i-cells within Zürich epithelium (AEP i-cell chimera) was constructed by grafting the oral half from a Zürich polyp and the aboral half from a DsRed2 (all-red) polyp. DsRed2-expressing nematocytes and nerve cells could be seen in the upper (Zürich) region of the chimera within 48 hours.
The chimera with AEP endoderm and Zürich ectoderm was obtained from a bud generated by the AEP i-cell/Zürich ectoderm chimera described in the previous paragraph. This animal was propagated clonally. Mosaic animals were obtained in the same manner.
Production of transgenic reporter Hydra lines
Transgenic Hydra lines were generated as previously described (Wittlieb et al., 2006). Transgenic Hydra are initially mosaic, with only some of the cells containing the transgene. To produce reporter Hydra that were fully transgenic, hatchlings were monitored for GFP expression, and clonally propagated until all buds that were produced showed GFP expression in the expected locations for Hym-301 and HyBra2.
Construction of H301p::GFP::H301t was carried out using standard recombinant DNA methods, the actin gene promoter and 3’ UTR in the plasmid HyEGFP described by Böttger et al. (Böttger et al., 2002) were replaced respectively with a 1212 bp fragment containing the Hym-301 promoter (ending at -9 relative to the Hym-301 translation start codon) and an 840 bp fragment containing the Hym-301 3’ UTR and polyadenylation site (both amplified from Hydra magnipapillata strain 105 genomic DNA).
To construct a plasmid in which the HyBra2 promoter drives expression of GFP (HyBra2::GFP) a DNA segment consisting of 1456 bp upstream of the HyBra2 start codon (Chapman et al., 2010) fused to the sequence encoding the first nine amino acids of a Hydra actin gene (Fisher and Bode, 1989) was commercially synthesized (Genewiz). This DNA segment was cloned upstream of GFP in pHyVec13 (GenBank Accession Number JN982438) to yield ec(HyBra2::GFP).
In situ hybridization
In situ hybridization was carried out as previously described (Bode et al., 2008). Some steps were automated using an InsituPro VS in situ hybridization instrument (Intavis Bioanalytical Instruments).
Treatment of Hydra with alsterpaullone
ALP treatment was performed as previously described (Broun et al., 2005). ALP (Sigma-Aldrich) was dissolved in DMSO at a stock concentration of 20 mM and aliquots were stored at -80°C. An aliquot of the stock solution was diluted to the desired concentration in HM immediately prior to use.
Sources of DAC-2-25 derivatives
The initial hit, DAC-2-25, and several related pyridones in the initial screen were provided as part of the UCI Bioactive Fragment Library, a collection of total synthesis intermediates contributed by the UC Irvine Department of Chemistry research groups and compiled by the synthesis facility in the UC Irvine Department of Pharmaceutical Sciences. Additional analogues were synthesized by employing Parra’s simple process in which the dienediolate of an α,β-unsaturated carboxylic acid, formed by deprotonation of the corresponding enoic acid, undergoes addition to an aryl- or alkyl-nitrile followed by spontaneous cyclization to form the 2-pyridone ring system in one operation (Brun et al., 1999; Brun et al., 2000). This methodology is well suited to for the preparation of a wide variety of C3-, C4- and C6-substituted pyridones in which the substitution pattern is reliably determined by the respective enoic acid and nitrile starting materials. The sheer simplicity of this one-step procedure is counterbalanced by low to moderate yields (20-80%) in which the rest of the mass balance is unreacted starting materials, but it nonetheless is the method of choice for preparing such pyridone analogues. The experimental details and analytical data for the synthetic derivatives are described in detail in supplementary material Appendix S1. One of the derivatives, 4-(dimethylamino)benzoic acid, was purchased from Sigma-Aldrich (catalog number 275654-25G).
RT-PCR
RT-PCR of GSK3-β transcripts from the H. vulgaris AEP and Zürich strains was performed as previously described (Dana et al., 2012) using the primers GSK3_L (ATGGTTATTTTACGAAACTATATCC) and GSK3_R (TTAAGAAGCTTCTGTTGAAATATTG).
Supplementary Material
Acknowledgments
We are very grateful to Dr Felix Grün for providing information on the small molecule library and advice on its use. We thank Dr Thomas Holstein for providing the Ec(HyWnt3FL::GFP) and En(HyWnt3FL::GFP) transgenic lines. We thank Dr Peter Fuhrer for his expert technical assistance in the preparation of this manuscript. We greatly appreciate the thoughtful critiques of the manuscript provided by Drs Annalise Nawrocki, Eva-Maria Schoetz Collins and Celina Juliano. We thank Dr Ira Blitz for insightful discussions regarding possible mechanisms of DAC-2-25 action.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
K.M.G., C.E.D., S.S.P., D.A.C., Y.N., T.F., A.R.C. and R.E.S. conceived and designed the experiments; K.M.G., C.E.D., S.S.P., D.A.C. and Y.N. performed the experiments; K.M.G., C.E.D., S.S.P., Y.N., T.F., A.R.C. and R.E.S. analyzed the data; K.M.G., A.R.C. and R.E.S. wrote the paper.
Funding
This work was supported by grants from the National Institute of General Medical Sciences [R24-GM080537 to R.E.S. and R01-GM057550 to A.R.C.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.094490/-/DC1
References
- Bateson W. (1894). Materials for the Study of Variation. New York, NY: Macmillan; [Google Scholar]
- Best M. D. (2009). Click chemistry and bioorthogonal reactions: unprecedented selectivity in the labeling of biological molecules. Biochemistry 48, 6571–6584 [DOI] [PubMed] [Google Scholar]
- Bielen H., Oberleitner S., Marcellini S., Gee L., Lemaire P., Bode H. R., Rupp R., Technau U. (2007). Divergent functions of two ancient Hydra Brachyury paralogues suggest specific roles for their C-terminal domains in tissue fate induction. Development 134, 4187–4197 [DOI] [PubMed] [Google Scholar]
- Bode H. R. (1996). The interstitial cell lineage of hydra: a stem cell system that arose early in evolution. J. Cell Sci. 109, 1155–1164 [DOI] [PubMed] [Google Scholar]
- Bode P. M., Bode H. R. (1980). Formation of pattern in regenerating tissue pieces of hydra attenuata. I. Head-body proportion regulation. Dev. Biol. 78, 484–496 [DOI] [PubMed] [Google Scholar]
- Bode H., Lengfeld T., Hobmayer B., Holstein T. W. (2008). Detection of expression patterns in Hydra pattern formation. Methods Mol. Biol. 469, 69–84 [DOI] [PubMed] [Google Scholar]
- Böttger A., Alexandrova O., Cikala M., Schade M., Herold M., David C. N. (2002). GFP expression in Hydra: lessons from the particle gun. Dev. Genes Evol. 212, 302–305 [DOI] [PubMed] [Google Scholar]
- Broun M., Bode H. R. (2002). Characterization of the head organizer in hydra. Development 129, 875–884 [DOI] [PubMed] [Google Scholar]
- Broun M., Gee L., Reinhardt B., Bode H. R. (2005). Formation of the head organizer in hydra involves the canonical Wnt pathway. Development 132, 2907–2916 [DOI] [PubMed] [Google Scholar]
- Brun E. M., Gil S., Mestres R., Parra M. (1999). Dienediolates of α, β-unsaturated carboxylic acids in synthesis: a new synthetic method for 2-pyridones. Synlett 1999, 1088–1090 [Google Scholar]
- Brun E. M., Gil S., Mestres R., Parra M. (2000). A new synthetic method to 2-pyridones. Synthesis 2000, 273–280 [Google Scholar]
- Campbell R. D. (1967a). Tissue dynamics of steady state growth in Hydra littoralis. I. Patterns of cell division. Dev. Biol. 15, 487–502 [DOI] [PubMed] [Google Scholar]
- Campbell R. D. (1967b). Tissue dynamics of steady state growth in Hydra littoralis. II. Patterns of tissue movement. J. Morphol. 121, 19–28 [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Bode H. R. (1983). Terminology for morphology and cell types. In Hydra: Research Methods, (ed. Lenhoff H. M.), pp. 5–14 New York, NY: Plenum Press; [Google Scholar]
- Chapman J. A., Kirkness E. F., Simakov O., Hampson S. E., Mitros T., Weinmaier T., Rattei T., Balasubramanian P. G., Borman J., Busam D., et al. (2010). The dynamic genome of Hydra. Nature 464, 592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chera S., de Rosa R., Miljkovic-Licina M., Dobretz K., Ghila L., Kaloulis K., Galliot B. (2006). Silencing of the hydra serine protease inhibitor Kazal1 gene mimics the human SPINK1 pancreatic phenotype. J. Cell Sci. 119, 846–857 [DOI] [PubMed] [Google Scholar]
- Dana C. E., Glauber K. M., Chan T. A., Bridge D. M., Steele R. E. (2012). Incorporation of a horizontally transferred gene into an operon during cnidarian evolution. PLoS ONE 7, e31643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C. N. (2012). Interstitial stem cells in Hydra: multipotency and decision-making. Int. J. Dev. Biol. 56, 489–497 [DOI] [PubMed] [Google Scholar]
- Dübel S., Hoffmeister S. A. H., Schaller H. C. (1987). Differentiation pathways of ectodermal epithelial cells in hydra. Differentiation 35, 181–189 [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Bode H. R. (1989). Nucleotide sequence of an actin-encoding gene from Hydra attenuata: structural characteristics and evolutionary implications. Gene 84, 55–64 [DOI] [PubMed] [Google Scholar]
- Gee L., Hartig J., Law L., Wittlieb J., Khalturin K., Bosch T. C., Bode H. R. (2010). beta-catenin plays a central role in setting up the head organizer in hydra. Dev. Biol. 340, 116–124 [DOI] [PubMed] [Google Scholar]
- Gierer A., Meinhardt H. (1972). A theory of biological pattern formation. Kybernetik 12, 30–39 [DOI] [PubMed] [Google Scholar]
- Hobmayer B., Rentzsch F., Kuhn K., Happel C. M., von Laue C. C., Snyder P., Rothbächer U., Holstein T. W. (2000). WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature 407, 186–189 [DOI] [PubMed] [Google Scholar]
- Hwang J. S., Ohyanagi H., Hayakawa S., Osato N., Nishimiya-Fujisawa C., Ikeo K., David C. N., Fujisawa T., Gojobori T. (2007). The evolutionary emergence of cell type-specific genes inferred from the gene expression analysis of Hydra. Proc. Natl. Acad. Sci. USA 104, 14735–14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhoff H. M. (1983). Hydra: Research Methods. New York, NY: Plenum Press; [Google Scholar]
- Lohmann J. U., Endl I., Bosch T. C. (1999). Silencing of developmental genes in Hydra. Dev. Biol. 214, 211–214 [DOI] [PubMed] [Google Scholar]
- Marcum B. A., Campbell R. D. (1978). Developmental roles of epithelial and interstitial cell lineages in hydra: analysis of chimeras. J. Cell Sci. 32, 233–247 [DOI] [PubMed] [Google Scholar]
- Martin V. J., Littlefield C. L., Archer W. E., Bode H. R. (1997). Embryogenesis in hydra. Biol. Bull. 192, 345–363 [DOI] [PubMed] [Google Scholar]
- Martínez D. E., Iñiguez A. R., Percell K. M., Willner J. B., Signorovitch J., Campbell R. D. (2010). Phylogeny and biogeography of Hydra (Cnidaria: Hydridae) using mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 57, 403–410 [DOI] [PubMed] [Google Scholar]
- Meinhardt H. (2012). Modeling pattern formation in hydra: a route to understanding essential steps in development. Int. J. Dev. Biol. 56, 447–462 [DOI] [PubMed] [Google Scholar]
- Münder S., Käsbauer T., Prexl A., Aufschnaiter R., Zhang X., Towb P., Böttger A. (2010). Notch signalling defines critical boundary during budding in Hydra. Dev. Biol. 344, 331–345 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Tsiairis C. D., Özbek S., Holstein T. W. (2011). Autoregulatory and repressive inputs localize Hydra Wnt3 to the head organizer. Proc. Natl. Acad. Sci. USA 108, 9137–9142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp I., Aufschnaiter R., Ozbek S., Pontasch S., Jenewein M., Watanabe H., Rentzsch F., Holstein T. W., Hobmayer B. (2009). Wnt/beta-catenin and noncanonical Wnt signaling interact in tissue evagination in the simple eumetazoan Hydra. Proc. Natl. Acad. Sci. USA 106, 4290–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. M., Gee L., Bode H. R. (2000). HyAlx, an aristaless-related gene, is involved in tentacle formation in hydra. Development 127, 4743–4752 [DOI] [PubMed] [Google Scholar]
- Sugiyama T. (1983). Isolating Hydra mutants by sexual inbreeding. In Hydra: Research Methods (ed. Lenhoff H. M.), pp. 211–221 New York, NY: Plenum Press; [Google Scholar]
- Sugiyama T., Fujisawa T. (1977). Genetic analysis of developmental mechanisms in hydra. III. Characterization of a regeneration-deficient strain. J. Embryol. Exp. Morphol. 42, 65–77 [Google Scholar]
- Sugiyama T., Fujisawa T. (1978a). Genetic analysis of developmental mechanisms in Hydra. II. Isolation and characterization of an interstitial cell-deficient strain. J. Cell Sci. 29, 35–52 [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Fujisawa T. (1978b). Genetic analysis of developmental mechanisms in hydra. V. Cell lineage and development of chimera hydra. J. Cell Sci. 32, 215–232 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Muneoka Y., Lohmann J., Lopez de Haro M. S., Solleder G., Bosch T. C., David C. N., Bode H. R., Koizumi O., Shimizu H., et al. (1997). Systematic isolation of peptide signal molecules regulating development in hydra: LWamide and PW families. Proc. Natl. Acad. Sci. USA 94, 1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Hatta M., Yum S., Gee L., Ohtani M., Fujisawa T., Bode H. R. (2005). Hym-301, a novel peptide, regulates the number of tentacles formed in hydra. Development 132, 2225–2234 [DOI] [PubMed] [Google Scholar]
- Technau U., Miller M. A., Bridge D., Steele R. E. (2003). Arrested apoptosis of nurse cells during Hydra oogenesis and embryogenesis. Dev. Biol. 260, 191–206 [DOI] [PubMed] [Google Scholar]
- Wilby O. K., Webster G. (1970a). Experimental studies on axial polarity in hydra. J. Embryol. Exp. Morphol. 24, 595–613 [PubMed] [Google Scholar]
- Wilby O. K., Webster G. (1970b). Studies on the transmission of hypostome inhibition in hydra. J. Embryol. Exp. Morphol. 24, 583–593 [PubMed] [Google Scholar]
- Wittlieb J., Khalturin K., Lohmann J. U., Anton-Erxleben F., Bosch T. C. G. (2006). Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc. Natl. Acad. Sci. USA 103, 6208–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.